Abstract
Orbitides are plant-derived small cyclic peptides with a wide range of biological activities. Phytochemical investigation of the whole plants of Dianthus chinensis was performed with the aim to discover new bioactive orbitides. Five undescribed proline-containing orbitides, dianthiamides A–E (1–5), were isolated from a methanolic extract of Dianthus chinensis. Their structures were elucidated by extensive analysis of 1D and 2D NMR and HRESI–TOF–MS as well as ESI–MS/MS fragmentation data. The absolute configuration of the amino acid residues of compounds 1–5 was determined by Marfey’s method. All compounds were tested for their cytotoxic activity, and dianthiamide A (1) exhibited weak activity against A549 cell line with IC50 value of 47.9 μM.
1. Introduction
Dianthus chinensis L. is a perennial herbaceous plant belonging to the Caryophyllaceae family, and is distributed widely in Europe and Eastern Asia. The whole plant of D. chinensis is commonly used as a traditional medicine in Korea for treating diuretic, carcinoma, urethritis, and carbuncles [1,2,3,4,5,6]. Previous investigation on the phytochemical constituents of the genus Dianthus led to the isolation of cyclopeptides [4,5,6,7,8,9], dianthramides [5,10,11], triterpenoidal saponins [1,2,3,12,13], anthocyanins [14], and pyran-type glycosides [15]. Orbitides, formerly known as Caryophyllaceae-type cyclic peptides, are N-to-C cyclized plant peptides lacking disulfide bonds, which possess 5 to 12 amino acid residues. Orbitides are ribosomally synthesized and post-translationally modified cyclic peptides, which have been discovered in many plants of the families such as Annonaceae, Asteraceae, Caryophyllaceae, Euphorbiaceae, Lamiaceae, Linaceae, Phytolaccaceae, Rutaceae, Schizandraceae, and Verbenaceae. Recently, orbitides have gained increasing attention owed to a wide range of biological activities including cytotoxic, antimalarial, immunomodulatory, and antiproliferative activities [16,17,18,19]. The Dianthus genus is a rich source of proline-containing orbitides, some of which showed cytotoxic activity against several cancer cell lines [5,6,18,20]. Therefore, we have embarked on a research program for the isolation of new bioactive orbitides from medicinal plant, and five undescribed orbitides, dianthiamides A–E (1–5) (Figure 1), were isolated from a MeOH extract of the whole plants of the D. chinensis. Herein, the isolation and structure determination as well as their cytotoxic activity against A549 cell line are described.
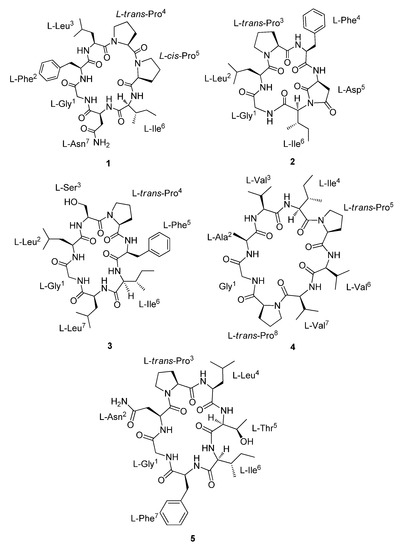
Figure 1.
Structures of compounds 1–5.
2. Results and Discussion
Dianthiamide A (1) was obtained as a yellow amorphous powder. Its molecular formula of C37H54N8O8 was determined from the HRESI–TOF–MS data (m/z 739.4144 [M + H]+; calcd for 739.4137). The 1H and 13C NMR in conjunction with HSQC data of 1 displayed the presence of 37 carbon signals assigned to eight amide carbonyl carbons (δC 168.8, 169.8, 170.8, 170.9, 171.1, 171.4, 172.3, and 173.4), seven α-amino acid carbons [δC 44.4 (CH2), 48.6 (CH), 49.6 (CH), 56.0 (CH), 59.6 (CH), 60.8 (CH), and 61.6 (CH)], six aromatic carbons [δC 126.8, 128.8, (2C), 129.1 (2C), and 138.4], two methines, ten methylenes, and four methyls (Table 1), suggesting that 1 is a heptapeptide. Furthermore, HSQC, HMBC, and COSY spectra showed the identification of seven amino acid residues including phenylalanine (F), glycine (G), isoleucine (I), asparagine (N), leucine (L), and two prolines (Pa and Pb).

Table 1.
1H and 13C NMR data for dianthiamide A (1) (DMSO-d6, 700 MHz, d in ppm, J in Hz).
In the HMBC and ROESY experiments, the cyclic feature and amino acid sequence of 1 were elucidated by the correlations observed between the amino acid Hα and continuous amide group (CONH). Therefore, the linear sequence of 1 was identified as G-F-L-Pa-Pb-I-N. Also, the HMBC correlation from Gly-Hα (δH 3.40 and 3.55) to Asn-C=O (δC 172.3) as well as the ROESY correlation between Gly-NH (δH 8.70) and Asn-Hα (δH 4.18) established the cyclic heptapeptide as cyclo-G-F-L-Pa-Pb-I-N (Figure 2). The amino acid sequence of 1 was further confirmed by analysis of the ESI–MS/MS fragment ions. Presumably, though there were several ring-opening sites, it occurred at two preferred positions at Proa4-Prob5 and Ile6-Asn7, respectively. Each the linear sequences, Prob5-Ile6-Asn7-Gly1-Phe2-Leu3-Proa4 (b7PbPa) and Asn7-Gly1-Phe2-Leu3-Proa4-Prob5-Ile6 (b7NI) was certified by acylium ions (bnPbPa and bnNI) and after loss of CO (anNI) at m/z 642 (b6PbPa), 626 (b6NI), 529 (b5PbPa and b5NI), 404 (a4NI), 364 (b4PbPa-H2O), 319 (b3NI), and 211 (b2PbPa), corresponding to the successive loss of amino acid residues (Figure S9).
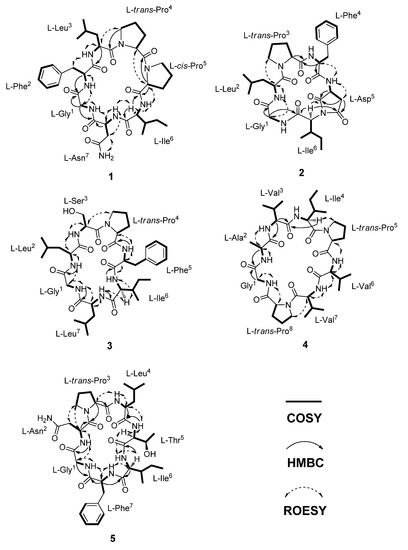
Figure 2.
Key COSY, HMBC, and ROESY correlations of 1–5.
The geometry of proline residues was assigned on the basis of the ΔδCβ-Cγ values and the presence of a ROE correlations between the proline Hα or Hδ and the Hα of previous amino acid. The ΔδCβ-Cγ value (3.2 ppm) of the Proa4 and the ROE correlation between the Hα (δH 4.48) of Leu3 and the Hδ (δH 3.44) of Proa4 indicated that the amide bond in the Proa4 was a trans. However, the ΔδCβ-Cγ value (9.0 ppm) of the Prob5 and the ROE correlation between the Hα (δH 4.45) of Proa4 and the Hα (δH 4.55) of Prob5 indicated that the geometry of Prob5 was a cis (Figure 3) [21,22,23]. The absolute configuration of amino acid residues in 1 were identified as L configuration, which was deduced by acid hydrolysis and Marfey’s derivatization, followed by HPLC analysis [24,25,26,27]. The N-α-(2,4-dinitro-5-fluorophenyl)-l-alaninamide (l-FDAA)-derivatives of 1 gave peaks at tR (min) 15.0 (l-Asp, m/z 386), 20.8 (l-Pro, m/z 368), 29.5 (l-Leu, m/z 384), 29.7 (l-Phe, m/z 418), and 30.2 (l-Ile, m/z 384) (Figure S10). Therefore, dianthiamide A (1) was established as cyclo-(Gly1-l-Phe2-l-Leu3-l-trans-Proa4-l-cis-Prob5-l-Ile6-l-Asn7).
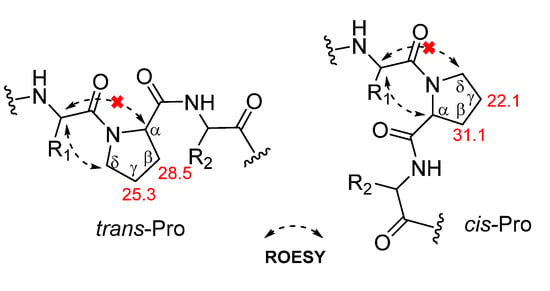
Figure 3.
Proline isomerization of 1.
Dianthiamide B (2) was isolated as a yellow amorphous powder, the HRESI–TOF–MS data were consistent with the molecular formula C32H44N6O7 (m/z 647.3161 [M + Na]+; calcd for 647.3163). The 1H, 13C and HSQC NMR spectra of 2 showed 32 carbons, consisting of seven amide carbonyl carbons, six α-amino acid carbons, six aromatic carbons, two methines, seven methylenes, and four methyls (Table 2). HSQC, HMBC, and COSY spectra demonstrated the presence of six amino acid residues including aspartic acid (D), phenylalanine (F), glycine (G), isoleucine (I), leucine (L), and proline (P). Moreover, HMBC, COSY, and ROESY spectra indicated that the sequence and connectivity of the hexapeptide was cyclo-G-L-P-F-D-I (Figure 2). The HMBC correlations between Hα (δH 4.19) of Ile6 and two carbonyls (δC 176.2 and 177.2) of Asp5 showed that dehydration of NH-Ile6 and COOH-Asp5 formed an additional five membered ring system (pyrrolidine-2,5-dione). The amino acid sequence in 2 was further supported by the fragmentation pattern of ESI–MS/MS data, in which the preferred ring-opening of 2 occurred at the amide bond between leucine and proline (Figure S19). The geometry of amide bond of Pro3 residue in 2 was assigned the trans configuration, on the basis of the difference of the 13C NMR chemical shift (ΔδCβ-Cγ = 3.9 ppm) [21,22,23] as well as the ROE correlation between the Hα (δH 4.51) of Leu2 and the Hδ (δH 3.68 and 3.42) of Pro3 residue. In addition, the absolute configuration of six amino acid residues in 2 were all assigned as L, which was determined by HPLC analysis of the acid hydrolysate after Marfey’s derivatization (Figure S20). Therefore, dianthiamide B (2) was determined as cyclo-(Gly1-l-Leu2-l-trans-Pro3-l-Phe4-l-Asp5-l-Ile6).

Table 2.
1H and 13C NMR data for dianthiamide B (2) (DMSO-d6, 700 MHz, d in ppm, J in Hz).
Dianthiamide C (3) was obtained as a yellow amorphous powder, showed a molecular formula of C37H57N7O8 as determined by its HRESI–TOF–MS data (m/z 750.4165 [M + Na]+; calcd 750.4160). The 13C and HSQC NMR data of 3 displayed the presence of 37 carbon signals including seven amide carbonyls, as well as seven α-amino acid carbons, suggesting a heptapeptide (Table 3). Full assignments of 1H and 13C NMR data for each amino acid residue were accomplished by combined analysis of COSY, HSQC, and HMBC spectra and suggested that 3 was composed of seven amino acid such as phenylalanine (F), glycine (G), isoleucine (I), two leucines (La and Lb), proline (P), and serine (S) residue (Table 3). The HMBC and ROESY spectra indicated that the amino acid sequence was cyclo-G-La-S-P-F-I-Lb (Figure 2), which was further confirmed by ESI–MS/MS fragmentation analysis (Figure S29). The observed ΔδCβ-Cγ value (3.2 ppm) of the Pro4 and the ROE correlation from the Hα (δH 4.89) of Ser3 to the Hδ (δH 3.91 and 3.42) of Pro4 revealed that the geometry of Pro4 of 3 was a trans configuration [21,22,23]. The absolute configuration of 3 was determined by Marfey’s method [24,25,26,27], which indicated that all the amino acids were L configuration (Figure S30). Therefore, dianthiamide C (3) was confirmed as cyclo-(Gly1-l-Leu2-l-Ser3-l-trans-Pro4-l-Phe5-l-Ile6-l-Leu7).

Table 3.
1H and 13C NMR data for dianthiamide C (3) (DMSO-d6, 700 MHz, d in ppm, J in Hz).
Dianthiamide D (4), a yellow amorphous powder, gave the molecular formula C36H60N8O8, based on the HRESI–TOF–MS data (m/z 755.4448 [M + Na]+; calcd 755.4426). Detailed analyses of the 1D and 2D (COSY, HSQC, and HMBC) NMR data revealed that 4 was a octapeptide containing alanine (A), glycine (G), isoleucine (I), two prolines (Pa and Pb) and three valines (Va, Vb, and Vc) residues (Table 4). The amino acid sequence of 4 was established as cyclo-G-A-Va-I-Pa-Vb-Vc-Pb by analysis of HMBC and ROESY data (Figure 2). This conclusion was also supported by the ESI–MS/MS sequence analysis (Figure S39). The observed ΔδCβ-Cγ values of the Proa5 (2.6 ppm) and Prob8 (4.0 ppm) and the ROE correlations from the Hα of Ile4 (δH 4.48) to the Hδ of Proa5 (δH 3.79 and 3.62), and from the Hα of Valc7 (δH 4.43) to the Hδ of Prob8 (δH 3.76 and 3.53) indicated that the geometry of both Proa5 and Prob8 of 4 were trans configuration [21,22,23]. Moreover, the absolute configuration of 4 was assigned by Marfey’s method [24,25,26,27], which indicated that all the amino acids had L configuration (Figure S40). Therefore, dianthiamide D (4) was established as cyclo-(Gly1-l-Ala2-l-Vala3-l-Ile4-l-trans-Proa5-l-Valb6-l-Valc7-l-trans-Prob8).

Table 4.
1H and 13C NMR data for dianthiamide D (4) (DMSO-d6, 700 MHz, d in ppm, J in Hz).
Dianthiamide E (5), a yellow amorphous powder. Its molecular formula of C36H54N8O9 was determined from the HRESI–TOF–MS data (m/z 743.4097 [M + H]+; calcd for 743.4086). Analysis of 1D and 2D (COSY, HSQC, and HMBC) NMR data (Table 5 and Figure 2) as well as ESI–MS/MS sequence data (Figure S49) demonstrated that the seven amino acid residues were phenylalanine (F), glycine (G), isoleucine (I), asparagine (N), leucine (L), proline (P), and threonine (T). The sequence of these amino acid residues was assigned as cyclo-G-N-P-L-T-I-F by the observed HMBC and ROESY data (Figure 2).

Table 5.
1H and 13C NMR data for dianthiamide E (5) (DMSO-d6, 700 MHz, d in ppm, J in Hz).
The small difference of the ΔδCβ-Cγ values of the Pro3 (4.4 ppm) and the ROE correlations between the Hα of Asn2 (δH 4.79) and the Hδ of Pro3 (δH 3.73 and 3.56) revealed that the geometry of Pro3 of 5 was a trans [21,22,23]. Furthermore, Marfey’s analysis assigned L configurations to all the amino acid residues in 5 (Figure S50) [24,25,26,27]. Therefore, dianthiamide E (5) was established as cyclo-(Gly1-l-Asn2-l-trans-Pro3-l-Leu4-l-Thr5-l-Ile6-l-Phe7).
Recently, it has been reported that cyclic peptides isolated from the genus Dianthus exhibited cytotoxic activity against several cancer cell lines [5,6,18]. Therefore, all isolates were tested for their cytotoxic activity against human non-small cell lung cancer A549 and human stomach adenocarcinoma MKN-28 cells, with docetaxel as a positive control. However, dianthiamide A (1) only showed weak activity against A549 cell line with IC50 value of 47.9 μM, and docetaxel was used as a positive control (IC50: 0.08 μM). The other compounds 2–5 were inactive against A549 and MKN-28 cells (IC50: >200 μM).
3. Materials and Methods
3.1. Chemicals
HPLC grade acetonitrile was purchased from m Fisher Chemical (Loughborough, UK) and all other chromatographic solvents were purchased from Duksan Pure Chemicals Co., Cheongju, Korea). Paclitaxel as a positive control was obtained from LC Laboratories (Woburn, MA, USA). MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) was purchased from Sigma-Aldrich Corp. (St. Louis, MO, USA).
3.2. General Experimental Procedures
Optical rotations were measured on a JASCO DIP-1000 polarimeter. UV spectra were recorded on a JASCO UV-550 spectrophotometer. ECD spectra were obtained on a JASCO J-715 spectrometer, and IR spectra were measured on a JASCO FT-IR 4100 spectrometer (JASCO, Tokyo, Japan). NMR spectra were recorded on a Bruker AVANCE 700 MHz spectrometer (Bruker, MA, USA) using DMSO-d6 as solvent. ESI–MS and HRESI–TOF–MS were obtained with LCQ Fleet (Thermo Fisher Scientific, San Jose, CA, USA) and maXis 4G mass spectrometers (Bruker, Bremen, Germany), respectively. Column chromatography was performed on silica gel (70–230 mesh, Merck, Darmstadt, Germany) and Lichroprep RP-18 (40–63 μm, Merck, Darmstadt, Germany). MPLC was performed on a Biotage Isolera Prime chromatography system (Biotage, Uppsala, Sweden). Preparative HPLC was performed using Waters HPLC system equipped with two Waters 515 pumps with a 2996 photodiode-array detector (Waters Corporation, Milford, MA, USA) using an YMC J’sphere ODS-H80 column (4 μm, 150 × 20 mm, i.d., Kyoto, Japan, flow rate 6 mL/min). TLC was performed using precoated silica gel 60 F254 (0.25 mm, Merck, Darmstadt, Germany) plates, and spots were detected by a 10% vanillin-H2SO4 in water spray reagent.
3.3. Plant Material
The dried whole plants of Dianthus chinensis L. (Caryophyllaceae) were purchased from Kyungdong herbal market in Seoul, Korea, in June 2014. A voucher specimen (CBNU-2014-06-DC) was authenticated by B.Y.H. and deposited at the Herbarium of the College of Pharmacy, Chungbuk National University, Korea.
3.4. Isolation and Purification of Compounds 1–5
The dried and powdered whole plants of D. chinensis (3.0 kg) were extracted with MeOH (3 × 16 L) at room temperature. The extract was evaporated under reduced pressure, and the residue (470 g) was suspended in water and partitioned successively with n-hexane (2 × 1.5 L), CH2Cl2 (2 × 1.5 L), and EtOAc (2 × 1.5 L). The CH2Cl2-soluble fraction (13 g) was separated by MPLC with Lichroprep RP-18 column and eluted with MeOH-H2O gradient system (10:90 to 100:0) to give eleven fractions (DCC1-DCC11). DCC1 (1.2 g) was separated on a silica gel column and eluted with CH2Cl2-MeOH gradient (from 100:0 to 0:100, 400 mL for each step) to obtain seven fractions (DCC1-1-DCC1-7) by MPLC. DCC1-4 (90 mg) was further purified by preparative HPLC (Waters system, YMC J’sphere ODS-H80, 150 × 20 mm i.d., MeCN-H2O, 30:70 to 60:40, flow rate 6 mL/min) to yield compound 1 (tR = 20.1 min, 15 mg). DCC7 (1.0 g) was subjected to silica gel column chromatography and eluted with CH2Cl2-MeOH (from 100:0 to 0:100, 400 mL for each step) to give seven fractions (DCC7-1-DCC7-7) by MPLC. DCC7-4 (90 mg) was further purified by preparative HPLC (MeCN-H2O, 35:65 to 65:35) to yield compound 2 (tR = 21.4 min, 17 mg). DCC9 (2.2 g) was separated on a silica gel column and eluted with CH2Cl2-MeOH gradient (from 100:0 to 0:100, 400 mL for each step) to obtain nine fractions (DCC9-1-DCC9-9) by MPLC. DCC9-7 (130 mg) was further purified by preparative HPLC (MeCN-H2O, 30:70 to 60:40) to afford compounds 3 (tR = 18.9 min, 6 mg) and 5 (tR = 23.1 min, 4 mg). DCC9-9 (90 mg) was further purified by preparative HPLC (MeCN-H2O, 30:70 to 60:40) to afford compound 4 (tR = 21.9 min, 5 mg).
3.5. Characterization of Compounds 1–5
Dianthiamide A (1, cyclo-(Gly1-l-Phe2-l-Leu3-l-trans-Proa4-l-cis-Prob5-l-Ile6-l-Asn7)), Yellow amorphous powder; [α]25D -41.2 (c 0.05, MeOH); UV (MeOH) λmax (log ε) 203 (3.65) nm; ECD (MeOH) λmax (Δε) 201 (−10.9), 210 (−6.5), 219 (−7.8) nm; IR υmax (film) 3330, 2944, 1657, 1530, 1454 cm−1; 1H NMR (700 MHz, DMSO-d6) and 13C NMR (175 MHz, DMSO-d6), see Table 1; ESI–MS m/z 739 [M + H]+; HRESI–TOF–MS m/z 739.4144 [M + H]+ (calcd for C37H55N8O8, 739.4137).
Dianthiamide B (2, cyclo-(Gly1-l-Leu2-l-trans-Pro3-l-Phe4-l-Asp5-l-Ile6)), Yellow amorphous powder; [α]25D -20.0 (c 0.05, MeOH); UV (MeOH) λmax (log ε) 202 (3.70) nm; ECD (MeOH) λmax (Δε) 201 (−3.2), 205(−1.5), 217 (−4.2) nm; IR υmax (film) 3312, 2972, 1644, 1530, 1448 cm−1; 1H NMR (700 MHz, DMSO-d6) and 13C NMR (175 MHz, DMSO-d6), see Table 2; ESI–MS m/z 647 [M + Na]+; HRESI–TOF–MS m/z 647.3161 [M + Na]+ (calcd for C32H44N6NaO7, 647.3163).
Dianthiamide C (3, cyclo-(Gly1-l-Leu2-l-Ser3-l-trans-Pro4-l-Phe5-l-Ile6-l-Leu7)), Yellow amorphous powder; [α]25D -45.2 (c 0.05, MeOH); UV (MeOH) λmax (log ε) 204 (3.24) nm; ECD (MeOH) λmax (Δε) 207 (+1.4), 226 (−8.6) nm; IR υmax (film) 3311, 2944, 1644, 1530, 1462 cm−1; 1H NMR (700 MHz, DMSO-d6) and 13C NMR (175 MHz, DMSO-d6), see Table 3; ESI–MS m/z 750 [M + Na]+; HRESI–TOF–MS m/z 750.4165 [M + Na]+ (calcd for C37H57N7NaO8, 750.4160).
Dianthiamide D (4, cyclo-(Gly1-l-Ala2-l-Vala3-l-Ile4-l-trans-Proa5-l-Valb6-l-Valc7-l-trans-Prob8)), Yellow amorphous powder; [α]25D -37.8 (c 0.05, MeOH); UV (MeOH) λmax (log ε) 210 (3.44) nm; ECD (MeOH) λmax (Δε) 201 (+9.1), 219 (−7.1) nm; IR υmax (film) 3312, 2943, 1644, 1548, 1454 cm−1; 1H NMR (700 MHz, DMSO-d6) and 13C NMR (175 MHz, DMSO-d6), see Table 4; ESI–MS m/z 755 [M + Na]+; HRESI–TOF–MS m/z 755.4448 [M + Na]+ (calcd for C36H60N8NaO8, 755.4426).
Dianthiamide E (5, cyclo-(Gly1-l-Asn2-l-trans-Pro3-l-Leu4-l-Thr5-l-Ile6-l-Phe7)), Yellow amorphous powder; [α]25D -41.2 (c 0.05, MeOH); UV (MeOH) λmax (log ε) 203 (3.47) nm; ECD (MeOH) λmax (Δε) 201 (−5.8) nm; IR υmax (film) 3309, 2942, 1741, 1644, 1548 cm−1; 1H NMR (700 MHz, DMSO-d6) and 13C NMR (175 MHz, DMSO-d6), see Table 5; ESI–MS m/z 743 [M + H]+; HRESI–TOF–MS m/z 743.4097 [M + H]+ (calcd for C36H55N8O9, 743.4086).
3.6. Absolute Configuration of Amino Acids in 1–5 Using Marfey’s Method
Compounds 1–5 (0.5 mg) were hydrolyzed in 1 mL of 6 N HCl at 105 °C for 12 h. After cooling to room temperature, the hydrolysate was evaporated to dryness and redissolved in 200 μL of water and 1 M NaHCO3 (20 μL). A solution of N-α-(2,4-dinitro-5-fluorophenyl)-l-alaninamide (l-FDAA, Marfey’s reagent, Sigma, 100 μL, 1%) in acetone was added to each reaction vial. The reaction mixture was heated at 37 °C for 1 h, quenched by adding 1 N HCl (20 μL), and then dissolved in CH3CN (800 μL). A volume of 5 μL of the FDAA derivatives were analyzed by LC/MS (YMC UltraHT Pro C18, S-2 μm, 12 nm, 50 × 2.0 mm, flow rate: 0.2 mL/min) at RT, and monitored by UV absorption at 340 nm. Aqueous CH3CN containing 0.1% TFA was used as the mobile phase in a gradient mode (10–50% CH3CN for 0–40 min). From each standard, 50 mM aqueous solution of d- or l-amino acid (Ala, Asp, Phe, Ile, allo-Ile, Leu, Asn, Pro, Ser, Thr, allo-Thr, and Val) were taken, and 1 M NaHCO3 (20 μL) and a solution of N-α-(2,4-dinitro-5-fluorophenyl)-l-alaninamide (l-FDAA, Marfey’s reagent, Sigma, 100 μL, 1%) in acetone was added. The reaction mixture was heated at 37 oC for 1 h, quenched by adding 1 N HCl (20 μL), and then dissolved in CH3CN (800 μL). A volume of 5 μL of the FDAA derivatives were analyzed by LC/MS (YMC UltraHT Pro C18, S-2 μm, 12 nm, 50 × 2.0 mm, flow rate: 0.2 mL/min) at RT and monitored by UV absorption at 340 nm. Aqueous CH3CN containing 0.1% TFA was used as the mobile phase in a gradient mode (10–50% CH3CN for 0–40 min). The following retention times (min) were observed for the l-FDAA derivatives of the standards, respectively: 19.5 (l-Ala) and 22.7 (d-Ala), 15.0 (l-Asp) and 18.1 (d-Asp), 29.7 (l-Phe) and 33.4 (d-Phe), 29.5 (l-Leu) and 34.4 (d-Leu), 30.3 (l-Ile), 31.0(l-allo-Ile) and 35.2 (d-Ile), 20.8 (l-Pro) and 22.1 (d-Pro), 13.6 (l-Ser) and 14.5 (d-Ser), 14.7 (l-Thr), 15.2 (l-allo-Thr) and 18.2 (d-Thr), and 25.4 (l-Val) and 29.6 (d-Val).
3.7. Cytotoxicity Assay
Human non-small cell lung cancer A549 and human stomach adenocarcinoma MKN-28 cells were purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA). A549 and MKN-28 cells were cultured as monolayers in RPMI1640 medium containing 10% fetal bovine serum (FBS), 1% penicillin/streptomycin at 37 °C in a humidified atmosphere of 5% CO2. Growth-inhibitory effect of the isolated compounds on A549 cells and MKN-28 cells was evaluated using MTT assay [28]. Briefly, 5 × 103 cells of A549 cells or MKN-28 cells were seeded in each well of a 96-well plate, respectively, and incubated for 24 h. A549 cells or MKN-28 cells were then treated with various concentrations of compounds 1–5. The concentration range of the compound tested for the evaluation of the cytotoxic activity was 5–200 µM. After incubation of 48 h, MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) solution was added to each well and the plate was incubated for 4 h. The medium in each well was replaced with dimethyl sulfoxide (DMSO) to dissolve blue formazan crystals. The absorbance at 540 nm was measured using microplate reader (Molecular devices; SpectraMax, CA, USA). All data processing and IC50 values were analyzed using GraphPad Prism v.5 (GraphPad Software, La Jolla, CA, USA). Docetaxel was used as a positive control with an IC50 value of 0.08 μM on A549 cells [29].
4. Conclusions
We report the isolation and structure determination of five undescribed orbitides, dianthiamides A–E, from the whole plants of D. chinensis. The previously reported orbitides isolated from the genus Dianthus tend to have five to six amino acid residues [4,5,6,7,8,9]. All orbitides in this study, however, are characteristic of the presence of six to eight amino acids, while they are featured by containing at least one proline residue. All isolates were tested for their cytotoxic activity, and dianthiamide A (1) exhibited weak activity against the A549 cell line. Furthermore, from a chemotaxonomical point of view, it is noteworthy that this finding expands the orbitides diversity in the genus Dianthus.
Supplementary Materials
The following are available online, Figures S1–S50: 1H, 13C-NMR, COSY, HSQC, HMBC, ROESY, ECD, HRESI–MS, ESI–MS/MS spectra, and Marfey’s analysis data of new compounds 1–5.
Author Contributions
J.W.L., J.G.K., J.S.H., Y.B.C., and Y.J.L. performed the experiments; D.H.S. and J.T.H. helped with data analysis; D.L. and M.K.L. helped with structure elucidation. J.W.L. and B.Y.H. designed the study and revised the manuscript; All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MIST) (No. 2020R1A2C1008406 and the Medical Research Center Program 2017R1A5A2015541).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data is contained within the article or Supplementary Material.
Acknowledgments
The authors wish to thank the Korea Basic Science Institute for the NMR spec.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Oshima, Y.; Ohsawa, T.; Oikawa, K.; Konno, C.; Hikino, H. Structures of dianosides A and B, analgesic principles of Dianthus superbus var. longicalycinus herbs. Planta Med. 1984, 50, 40–43. [Google Scholar] [CrossRef]
- Oshima, Y.; Ohsawa, T.; Hikino, H. Structure of dianosides C, D, E and F, triterpenoid saponins of Dianthus superbus var. longicalycinus herb. Planta Med. 1984, 50, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Oshima, Y.; Ohsawa, T.; Hikino, H. Structures of dianosides G, H and I, triterpenoid saponins of Dianthus superbus var. longicalycinus herbs. Planta Med. 1984, 50, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Tan, N.H.; Zhou, J.; Wu, H.M. Cyclopeptides from Dianthus superbus. Phytochemistry 1998, 49, 1453–1456. [Google Scholar] [CrossRef]
- Hsieh, P.W.; Chang, F.R.; Wu, C.C.; Wu, K.Y.; Li, C.M.; Chen, S.L.; Wu, Y.C. New cytotoxic cyclic peptides and dianthramide from Dianthus superbus. J. Nat. Prod. 2004, 67, 1522–1527. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, P.W.; Chang, F.R.; Wu, C.C.; Li, C.M.; Wu, K.Y.; Chen, S.L.; Yen, H.F.; Wu, Y.C. Longicalycinin A, a new cytotoxic cyclic peptide from Dianthus superbus var. longicalycinus (MAXIM.) WILL. Chem. Pharm. Bull. 2005, 53, 336–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.; Zhang, F.M.; Yang, Y.B.; Yang, X.Q.; Cao, Q.E.; Ding, Z.T. A new cyclopeptide from Dianthus caryophyllus. Chin. Chem. Lett. 2008, 19, 193–195. [Google Scholar] [CrossRef]
- Tong, Y.; Luo, J.G.; Wang, R.; Wang, X.B.; Kong, L.Y. New cyclic peptides with osteoblastic proliferative activity from Dianthus superbus. Bioorg. Med. Chem. Lett. 2012, 22, 1908–1911. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Huang, M.; Wang, Z.; Zheng, Y.; Zeng, G.; He, W.; Tan, N. Cyclopentapeptides from Dianthus chinensis. J. Pept. Sci. 2015, 21, 550–553. [Google Scholar] [CrossRef] [PubMed]
- Ponchet, M.; Martin-Tanguy, J.; Marais, A.; Poupet, A. Dianthramides A and B, two N-benzoylanthranilic acid derivatives from elicited tissues of Dianthus caryophyllus. Phytochemistry 1984, 23, 1901–1903. [Google Scholar] [CrossRef]
- Ponchet, M.; Favre-Bonvin, J.; Hauteville, M.; Ricci, P. Dianthramides (N-benzoyl and N-paracoumarylanthranilic acid derivatives) from elicited tissues of Dianthus caryophyllus. Phytochemistry 1988, 27, 725–730. [Google Scholar] [CrossRef]
- Luo, J.G.; Chen, X.; Kong, L.Y. Three new triterpenoid saponins from Dianthus superbus. Chem. Pharm. Bull. 2011, 59, 518–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakano, T.; Sugimoto, S.; Matsunami, K.; Otsuka, H. Dianthosaponins A-F, triterpene saponins, flavonoid glycoside, aromatic amide glucoside and γ-pyrone glucoside from Dianthus japonicus. Chem. Pharm. Bull. 2011, 59, 1141–1148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terahara, N.; Yamaguchi, M.A. 1H NMR spectral analysis of the malylated anthocyanins from Dianthus. Phytochemistry 1986, 25, 2906–2907. [Google Scholar] [CrossRef]
- Shimizu, M.; Hayashi, T.; Shimizu, K.; Morita, N. A pyran-type glycoside from Dianthus superbus var. longicalycinus. Phytochemistry 1982, 21, 245–247. [Google Scholar] [CrossRef]
- Arnison, P.G.; Bibb, M.J.; Bierbaum, G.; Bowers, A.A.; Bugni, T.S.; Bulaj, G.; Camarero, J.A.; Campopiano, D.J.; Challis, G.L.; Clardy, J.; et al. Ribosomally synthesized and post-translationally modified peptide natural products: Overview and recommendations for a universal nomenclature. Nat. Prod. Rep. 2013, 30, 108–160. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, S.D.; Pinto, M.E.F.; Ferreira, D.; Bolzani, V.S. Biologically active orbitides from the Euphorbiaceae family. Planta Med. 2018, 84, 558–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houshdar Tehrani, M.H.; Gholibeikian, M.; Bamoniri, A.; Mirjalili, B.B.F. Cancer treatment by Caryophyllaceae-type cyclopeptides. Front. Endocrinol. 2021, 11, 600856. [Google Scholar] [CrossRef]
- Rubin, G.M.; Ding, Y. Recent advances in the biosynthesis of RiPPs from multicore-containing precursor peptides. J. Ind. Microbiol Biotechnol. 2020, 47, 659–674. [Google Scholar] [CrossRef]
- Gholibeikian, M.; Bamoniri, A.; HoushdarTehrani, M.H.; Fatemeh Mirjalili, B.B.; Bijanzadeh, H.R. Structure-activity relationship studies of longicalcynin A analogues, as anticancer cyclopeptides. Chem. Biol. Interact. 2020, 315, 108902. [Google Scholar] [CrossRef]
- Siemion, I.Z.; Wieland, T.; Pook, K.H. Influence of the distance of the proline carbonyl from the β and γ carbon on the 13C chemical shifts. Angew. Chem. Int. Ed. Engl. 1975, 14, 702–703. [Google Scholar] [CrossRef]
- Schmidt, G.; Grube, A.; Kock, M. Stylissamides A-D-New proline-containing cyclic heptapeptides from the marine sponge Stylissa caribica. Eur. J. Org. Chem. 2007, 24, 4103–4110. [Google Scholar] [CrossRef]
- Iwasaki, K.; Iwasaki, A.; Sumimoto, S.; Sano, T.; Hitomi, Y.; Ohno, O.; Suenaga, K. Croissamide, a proline-rich cyclic peptide with an N-prenylated tryptophan from a marine cyanobacterium Symploca sp. Tetrahedron Lett. 2018, 59, 3806–3809. [Google Scholar] [CrossRef]
- Zhan, K.X.; Jiao, W.H.; Yang, F.; Li, J.; Wang, S.P.; Li, Y.S.; Han, B.N.; Lin, H.W. Reniochalistatins A-E, cyclic peptides from the marine sponge Reniochalina stalagmitis. J. Nat. Prod. 2014, 77, 2678–2684. [Google Scholar] [CrossRef] [PubMed]
- Cândido-Bacani, P.D.M.; Figueiredo, P.D.O.; Matos, M.D.F.; Garcez, F.R.; Garcez, W.S. Cytotoxic orbitide from the latex of Croton urucurana. J. Nat. Prod. 2015, 78, 2754–2760. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Sudoh, Y.; Tsuchiya, Y.; Okuda, T.; Matsuura, N.; Motojima, A.; Oikawa, T.; Igarashi, Y. Hikiamides A-C, cyclic pentadepsipeptides from Fusarium sp. TAMA 456. J. Nat. Prod. 2015, 78, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, Y.; Yamamoto, K.; Fukuda, T.; Shojima, A.; Nakayama, J.; Carro, L.; Trujillo, M.E. Arthroamide, a cyclic depsipeptide with quorum sensing inhibitory activity from Arthrobacter sp. J. Nat. Prod. 2015, 78, 2827–2831. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Lee, J.W.; Jang, H.; Lee, H.L.; Kim, J.G.; Wu, W.; Lee, D.; Kim, E.H.; Kim, Y.; Hong, J.T.; et al. Dimeric- and trimeric sesquiterpenes from the flower of Inula japonica. Phytochemistry 2018, 155, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Jo, M.J.; Lee, Y.J.; Park, C.W.; Chung, Y.B.; Kim, J.S.; Lee, M.K.; Shin, D.H. Evaluation of the physicochemical properties, pharmacokinetics, and in vitro anticancer effects of docetaxel and osthol encapsulated in methoxy poly(ethylene glycol)-b-poly(caprolactone) polymeric micelles. Int. J. Mol. Sci. 2020, 22, 231. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).