Abstract
Lilac aldehydes are considered as principal olfactory molecules of lilac flowers. We have designed, prepared, and evaluated a set of racemic seco-analogues of such natural products. The synthesis employs commercially available α-chloroketones as substrates that are transformed in four steps to target compounds. Their qualitative olfactory analysis revealed that the opening of the tetrahydrofuran ring leads to a vanishing of original flowery scent with the emergence of spicy aroma accompanied by green notes, and/or fruity aspects of novel seco-analogues. These results suggest the important osmophoric role of THF moiety for the generation of the typical flowery aroma associated with lilac aldehydes.
1. Introduction
Lilac aldehydes 1 (Figure 1) were first isolated in 1974 from an oil [] of lilac flowers (Syringa vulgaris L., Oleaceae). Being considered as important phytovolatiles, structure elucidation and olfactometric analysis of all stereoisomers was performed []. Consequently, the biosynthesis of lilac aldehydes was proposed and investigated []. Since then, these naturally occurring monoterpenes were found in flowers of many other species, including kiwifruit [], the White Campion [], and the Lesser Butterfly orchid []. Thus, fragrant lilac aldehydes represent an attractive olfactory target for insect pollinators. It is known that these flower-scenting molecules are much sought after by (nocturnal) moth species [,,], butterflies [,], and even mosquitos [,,]. In this context, lilac aldehydes are being developed as chemical markers of the botanical origin of honeys [,,,]. Interestingly, lilac aldehydes were recently found as odour-active compounds in oysters, and thus, could be potentially used as freshness indicators for this delicacy []. For perfumery purposes, synthetically prepared lilac aldehydes are used almost exclusively. Interestingly, the major naturally occurring (5′S)-stereoisomers 1a–d have a lower odour threshold by 1–2 orders of magnitude in comparison to lilac aldehydes 1e–h with (5′R)- absolute configuration [] (Figure 1).
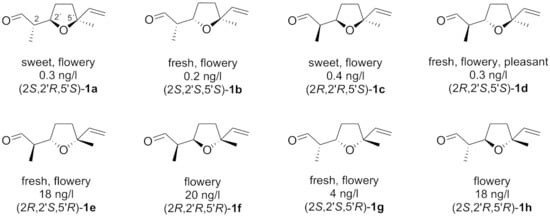
Figure 1.
All possible stereoisomers of lilac aldehydes 1a–1h with their respective odour thresholds.
Up to date, numerous syntheses of racemic [,,,,,,,] and enantiomerically pure lilac aldehydes 1 are known [,,,]. Recently, we have published the first comprehensive study investigating the importance of respective substituents on the genuine flowery odour of lilac aldehydes. We have found [] that the addition of methyl group to C-2 stereogenic centre of lilac aldehydes 1 significantly shifts the original flowery odour to a rather herbal scent of their unnatural homologues 2. In addition, various functional groups have only minimal effect on the scent variations (Figure 2).
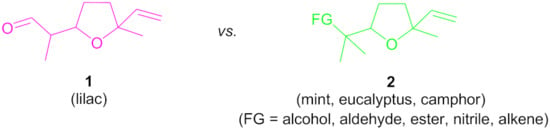
Figure 2.
Comparison of olfactory profiles of lilac aldehydes 1 vs. methylated homologues 2.
As a continuation of our research, we decided to investigate the influence of tetrahydrofuran ring and/or alkene moiety on the olfactory properties of C-2 methylated homologues of lilac aldehydes 2. Thus, we have devised a new set of (racemic) seco-analogues 3–7 featuring a higher conformational freedom and/or variable substitution pattern of the C=C bond (Figure 3).
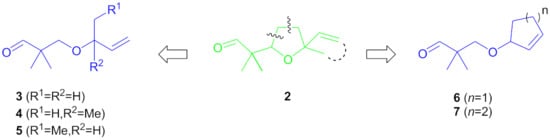
Figure 3.
Structural design of racemic seco-analogues 3–7 of C-2 methylated lilac aldehydes 2.
2. Results and Discussion
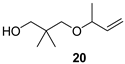
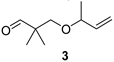
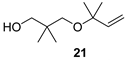
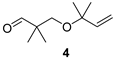
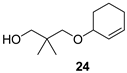
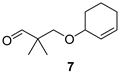
The preparation of novel seco-analogues 3–7 features a common synthetic strategy that starts with an acid-catalysed ketalisation of commercially available α-chloroketones 8–11 with neopentyl glycol followed by elimination of intermediary chloroketals 12–15 under basic conditions []. Unlike the chlorocyclopentane ketal 14, the elimination of chlorocyclohexane ketal 15 provided an inseparable mixture of desired cyclohexene ketal 19 and its regioisomer 25 in a ratio of 5:1 (based on the integration of respective olefinic signals in 1H NMR spectrum). Thus, the obtained alkenyl ketals 16–19 were reductively opened either by diisobutyl aluminium hydride [] or methyl magnesium bromide [] to furnish the corresponding alcohols 20–24. In case of DIBAL reduction of mixture of ketals 19 + 25, only 19 was opened while 25 remained untouched under the reaction conditions. This enabled the efficient FLC separation of unreacted ketal 25 from desired alcohol 24. Eventually, the final Dess–Martin oxidation [] of pure alcohols 20–24 provided target aldehydes 3–7 in overall yields of 2–21% over four steps (Scheme 1).
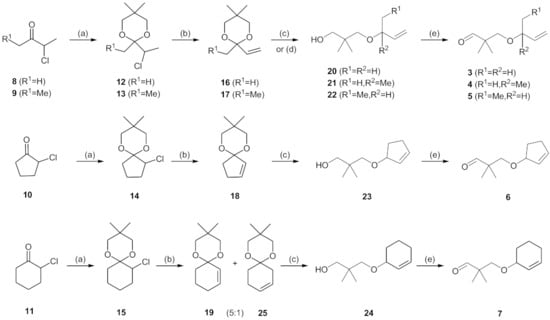
Scheme 1.
Preparation of seco-analogues 3–7. Reagents and conditions: (a) cat. p-TsOH, 2,2-dimethyl-1,3-propanediol, cyclohexane, reflux (Dean–Stark), 5–27 h, crude 12 (90%), FLC or vacuum distillation, 13 (51%), 14 (82%), 15 (87%); (b) KOH (11 equiv), 1,2-ethanediol, 160 °C, 7–23 h, FLC or vacuum distillation, 16 (60%), 17 (71%), 18 (60%), 19 + 25 (5:1, 83%); (c) DIBAL (3–5 equiv), THF/toluene, 50 °C, 2–4 d, FLC or vacuum distillation, 20 (61%), 22 (15%), 23 (18%), 24 (48%); (d) MeMgBr (10 equiv), toluene/Et2O, 80 °C, 4 h, MPLC, 21 (23%); (e) Dess–Martin periodinane (1.10–1.25 equiv), NaHCO3 (0.27 equiv), DCM, RT, 2–6 h, FLC or PTLC, 3 (38%), 4 (39%), 5 (46%), 6 (22%), 7 (60%).
With seco-analogues of lilac aldehydes 3–7 in our hands, we have undertaken their qualitative sensory evaluation. Since lilac alcohols also contribute to the complex scent of lilac flowers, we have also included their acyclic analogues 20–24 in the olfactory screening. The results are summarised in Table 1. Evidently, the opening of the tetrahydrofuran ring leads to a vanishing of flowery scent typical for lilac aldehydes 1, suggesting the important olfactophoric role of THF moiety. Thus, ring-opened analogues 3–5 bearing an acyclic alkene exhibit a rather common spicy, sharp aroma with green/herbal notes. The scent of their congeners featuring a cyclic alkene is further shifted towards sweet, fruity aspects for 6 and herbal, green notes for 7. Interestingly and for comparison, while the respective alcohols 20, 22, 23, and 24 commonly exhibit sweet(ish) aroma with oily/fatty and turpentine-like facets, alcohol 21 features a pleasant balsamic aroma with minty-eucalypty notes. Finally, the odour intensity for target compounds was rather weak to moderate with only short aroma persistence in a range of tens of minutes.

Table 1.
Olfactory properties of compounds 3–7, 20–24 prepared via Scheme 1.
3. Materials and Methods
3.1. Materials and Methods
Chemicals and reagents were purchased from commercial sources (Alfa Aesar, Sigma–Aldrich) and were used without further purification. Anhydrous solvents were prepared by distillation and standing over activated 4Å molecular sieves under argon atmosphere. Hexanes refer to a mixture of C-6 alkanes (b.p. 60–80 °C). Yields refer to chromatographically and spectroscopically (1H NMR) homogeneous material, unless otherwise stated. Reactions were monitored by thin layer chromatography (TLC) carried out on aluminium sheets pre-coated with silica gel 60 F254 (Merck). Visualisation was performed using shortwave UV light followed by dipping TLC plates in either basic solution of KMnO4, acidic solution of vanillin or acidic solution of ceric ammonium nitrate followed by heating with a heat gun. Preparative thin layer chromatography (PTLC) was carried out on glass plates (20 × 20 cm) coated with PLC silica gel 60 (layer thickness 1 mm) with concentrating zone (20 × 4 cm). Flash column chromatography (FLC) was performed using Silica Gel 60 (particle size 40–63 μm). Medium-pressure liquid chromatography (MPLC) was performed on Büchi Sepacor System equipped with Pump Module C-615 and Fraction Collector C-660 using silica gel (particle size 15–40 μm). NMR spectra were recorded in CDCl3 on a Varian INOVA 300 (300 MHz for 1H, 75 MHz for 13C nuclei), Varian 400-MR (399.9 MHz for 1H, 100.6 MHz for 13C nuclei) or Varian VNMRS 600 (600 MHz for 1H, 151 MHz for 13C nuclei) NMR spectrometer and were correctly shifted using residual non-deuterated solvent as an internal reference (CHCl3: δH = 7.26 ppm, δC = 77.16 ppm (central peak of a 1:1:1 triplet). Chemical shifts (δ) are quoted in ppm. Liquid chromatography-mass spectrometry (LC-MS) analyses were performed on Agilent 1200 Series instrument equipped with a multimode MS detector using the MM ESI/APCI ionisation method (column Zorbax Eclipse XDB-18, 150 × 4.6 mm, particle size 5 μm, eluent water with 0.1% HCO2H/CH3CN, 70:30, flow 1.5 mL/min). High-resolution mass spectra (HR-MS) were recorded on a Thermo Scientific Orbitrap Velos mass spectrometer with a heated electrospray ionisation (HESI) source in positive and/or negative mode. FTIR spectra were obtained on a Nicolet 5700 spectrometer (Thermo Electron) equipped with a Smart Orbit (diamond crystal ATR) accessory using the reflectance technique (4000–400 cm–1). The sensory analysis was performed by authors in a clean and odourless environment at 22 °C by using testing strips of odourless absorbent paper. This was wetted with the tested compound and the paper strip was smelled at certain intervals, while the scent was recorded.
3.2. Synthetic Procedures and Analytical Data
3.2.1. General Experiment for the Preparation of Chloroketals (12–15)
A stirred solution of chloroketone 8–11, 2,2-dimethyl-1,3-propanediol (1.0 or 1.2 equiv) and p-TsOH·H2O (0.023 equiv) in cyclohexane (c = 0.7 M) was refluxed in a round bottom flask equipped with a Dean–Stark apparatus. After the indicated time, the mixture was cooled to RT and solvent was evaporated in vacuo (bath temperature 50–55 °C, pressure 30–50 mbar) and the resulting oil dissolved in Et2O. The ethereal solution was sequentially washed with sat. aq. NaHCO3 soln., water and brine, dried (MgSO4) and concentrated in vacuo (35 °C, 30 mbar). The obtained crude product was further used as such or purified either by bulb-to-bulb vacuum distillation (Kugelrohr) or FLC to furnish a corresponding chloroketal 12–15. The structural characterization (1H-NMR, 13C-NMR spectra) of the new compound are presented in Supplementary Materials.
2-(1-Chloroethyl)-2,5,5-trimethyl-[1,3]dioxane (12)
Prepared accordingly to Ref. []: commercial 3-chlorobutanone 8 (5.86 mL, 56 mmol), 2,2-dimethyl-1,3-propanediol (5.87 g, 56 mmol), p-TsOH·H2O (246 mg, 1.3 mmol), cyclohexane (81 mL), reflux (100 °C), 17 h, concentrated in vacuo (50 °C, 30 mbar), Et2O (61 mL), NaHCO3 (37 mL), water (37 mL), brine (37 mL), crude chloroketal 12 (9.79 g, 90%) as yellow oil; Rf (hexanes/AcOEt 5:1) 0.74; δH (300 MHz, CDCl3) 4.14 (q, J = 6.8 Hz, 1H, CHCl), 3.61 (dd, J = 11.5, 8.9 Hz, 2H, OCH2), 3.52–3.40 (m, 2H, OCH2), 1.53 (d, J = 6.8 Hz, 3H, Me), 1.46 (s, 3H, Me), 1.08 (s, 3H, Me2), 0.85 (s, 3H, Me2). NMR spectrum fully corresponds to the literature data []; m/z (ESI) 157.2 (100, M-Cl+), 158.0 (9%), 175.2 (76, M-Cl+H2O+), 176.0 (8%), tR = 2.34 min.
2-(1-Chloroethyl)-2-ethyl-5,5-dimethyl-[1,3]dioxane (13)
2-Chloropentan-3-one 9 (2.73 g, 23 mmol, prepared accordingly to Ref. []), 2,2-dimethyl-1,3-propanediol (2.36 g, 23 mmol), p-TsOH·H2O (99 mg, 0.52 mmol), cyclohexane (32.6 mL), reflux (130 °C), 27 h, concentrated in vacuo (50 °C, 45 mbar), Et2O (40 mL), NaHCO3 (30 mL), water (30 mL), brine (30 mL), FLC (176 g SiO2, Hex: Et2O 12:1, 650 mL), pure chloroketal 13 (2.41 g, 51%) as colourless oil; Rf (hexanes/AcOEt 6:1) 0.67; νmax (ATR) 2956, 2869, 1469, 1396, 1186, 1142, 1126, 1093, 1081, 1038, 1016, 957, 916 cm−1; δH (600 MHz, CDCl3) 4.35 (q, J = 6.8 Hz, 1H, CHCl), 3.59 (d, J = 11.5 Hz, 1H, OCH2), 3.55 (d, J = 11.5 Hz, 1H, OCH2), 3.49 (d, J = 11.5 Hz, 1H, OCH2), 3.48 (d, J = 11.5 Hz, 1H, OCH2), 1.96 (m, 2H, CH2), 1.55 (d, J = 6.8 Hz, 3H, Me), 1.04 (s, 3H, Me2), 0.93 (t, J = 7.5 Hz, 3H, Me), 0.90 (s, 3H, Me2); δC (151 MHz, CDCl3) 100.1 (OCq), 70.2 (2 × OCH2), 56.5 (CHCl), 29.6 (Cq), 22.9 (Me2), 22.5 (Me2), 21.2 (CH2), 18.7 (Me), 7.0 (Me); m/z (ESI) 189.2 (100, M-Cl+H2O+), 190.0 (10%), 171.2 (95, M-Cl+), 172.2 (8%), tR = 2.96 min; HR-MS (HESI): M+, found 170.1305. C10H18O2 requires 170.1301.
1-Chloro-8,8-dimethyl-6,10-dioxaspiro [4.5] decane (14)
Commercial 2-chlorocyclopentanone 10 (755 µL, 7.5 mmol), 2,2-dimethyl-1,3-propanediol (943 mg, 9.1 mmol), p-TsOH·H2O (33 mg, 0.17 mmol), cyclohexane (11 mL), reflux (128 °C), 4.5 h, concentrated in vacuo (50 °C, 50 mbar), Et2O (15 mL), NaHCO3 (6 mL), water (6 mL), brine (6 mL), FLC (41 g SiO2, Hex: AcOEt 6:1, 270 mL), pure chloroketal 14 (1.27 g, 82%) as colourless oil; Rf (hexanes/AcOEt 5:1) 0.67; νmax (ATR) 2954, 2867, 1472, 1396, 1337, 1207, 1144, 1112, 1076, 1016, 837 cm−1; δH (300 MHz, CDCl3) 4.39–4.34 (m, 1H, CHCl), 3.55–3.45 (m, 4H, 2 × OCH2), 2.29–1.60 (m, 6H, 3xCH2), 1.00 (s, 3H, Me2), 0.98 (s, 3H, Me2); δC (151 MHz, CDCl3) 107.6 (OCq), 72.3 (OCH2), 72.1 (OCH2), 60.7 (CHCl), 32.9 (CH2), 31.1 (CH2), 30.0 (Cq), 22.4 (Me2), 22.3 (Me2), 18.6 (CH2); HR-MS (HESI): M+, found 204.0913. C10H17ClO2 requires 204.0912.
7-Chloro-3,3-dimethyl-1,5-dioxaspiro[5.5]undecane (15)
Commercial 2-chlorocyclohexanone 11 (860 µL, 7.5 mmol), 2,2-dimethyl-1,3-propanediol (942 mg, 9.0 mmol), p-TsOH·H2O (33 mg, 0.17 mmol), cyclohexane (10.9 mL), reflux (160 °C), 10 h, concentrated in vacuo (55 °C, 50 mbar), Et2O (20 mL), NaHCO3 (5 mL), water (5 mL), brine (5 mL), bulb-to-bulb distillation (175–180 °C, 27 mbar), pure chloroketal 15 (1.43 g, 87%) as yellow oil.; Rf (hexanes/AcOEt 5:1) 0.67; νmax (ATR) 2942, 2864, 1472, 1446, 1395, 1364, 1215, 1167, 1112, 1111, 1096, 1016, 965, 804 cm−1; δH (600 MHz, CDCl3) 4.23–4.18 (m, 1H, CHCl), 3.62 (d, J = 11.4 Hz, 1H, OCH2), 3.58 (d, J = 11.5 Hz, 1H, OCH2), 3.52 (dd, J = 11.4, 1.8 Hz, 1H, OCH2), 3.45 (dd, J = 11.4, 1.8 Hz, 1H, OCH2), 2.33–2.23, 2.07–2.00, 1.96–1.88, 1.76–1.68 (4xm, 4 × 1H, 2 × CH2), 1.57–1.37 (m, 4H, 2 × CH2), 1.12 (s, 3H, Me2), 0.87 (s, 3H, Me2). 1H NMR spectrum fully corresponds the literature data []; δC (151 MHz, CDCl3) 97.2 (OCq), 70.3 (OCH2), 69.8 (OCH2), 61.6 (CHCl), 32.0 (Cq, CH2), 30.0 (CH2), 27.4 (CH2), 22.8 (Me2), 22.4 (Me2), 21.6 (CH2); HR-MS (HESI): M+, found 218.1071. C11H19ClO2 requires 218.1068.
3.2.2. General Experiment for the Preparation of Alkene Ketals (16–19, 25)
To a stirred solution of potassium hydroxide (11 eq.) in ethylene glycol was added to chloroketal 12–15 at 120 °C and the reaction mixture was heated at 160 °C for 7–23 h. After cooling to RT, water was added and extracted with Et2O. The organic phase was washed with water and brine, dried (MgSO4) and concentrated in vacuo (35 °C, 30–200 mbar). The crude product was purified either by bulb-to-bulb vacuum distillation (Kugelrohr) or FLC to furnish a corresponding ketal 16–19, 25.
2,5,5-Trimethyl-2-vinyl-[1,3]dioxane (16)
KOH (31.72 g, 0.57 mol), ethylene glycol (68 mL), chloroketal 12 (9.76 g, 51 mmol), 23 h, water (340 mL), Et2O (3 × 150 mL), water (110 mL), brine (110 mL), concentrated in vacuo (35 °C, 30 mbar), bulb-to-bulb vacuum distillation (80–95 °C, 27 mbar), ketal 16 (4.76 g, 60%) as colourless oil; Rf (hexanes/AcOEt 5:1) 0.72; δH (300 MHz, CDCl3) 5.76 (dd, J = 17.8, 10.8 Hz, 1H, CH=CH2), 5.38 (dd, J = 13.6, 1.5 Hz, 1H, CH=CH2), 5.33 (dd, J = 6.6, 1.5 Hz, 1H, CH=CH2), 3.58 (d, J = 10.6 Hz, 2H, OCH2), 3.32 (dt, J = 11.1, 1.2 Hz, 2H, OCH2), 1.40 (s, 3H, Me), 1.17 (s, 3H, Me2), 0.70 (s, 3H, Me2). NMR spectrum is in accordance with the literature data [].
2-Ethyl-5,5-dimethyl-2-vinyl-[1,3]dioxane (17)
KOH (7.46 g, 0.13 mol), ethylene glycol (16 mL), chloroketal 13 (2.46 g, 12 mmol), 21 h, water (200 mL), Et2O (3 × 80 mL), water (80 mL), brine (80 mL), concentrated in vacuo (35 °C, 200 mbar), FLC (112 g SiO2, DCM, 400 mL), ketal 17 (1.44 g, 71%) as colourless oil; Rf (DCM) 0.54; νmax (ATR) 2952, 2870, 1471, 1403, 1395, 1363, 1179, 1154, 1085, 955, 914 cm−1; δH (600 MHz, CDCl3) 5.66 (dd, J = 17.7, 11.0 Hz, 1H, CH=CH2), 5.40 (dd, J = 11.0, 1.6 Hz, 1H, CH=CH2), 5.33 (dd, J = 17.8, 1.6 Hz, 1H, CH=CH2), 3.61 (d, J = 11.0 Hz, 2H, OCH2), 3.32 (d, J = 11.2 Hz, 2H, OCH2), 1.65 (q, J = 7.5 Hz, 2H, CH2), 1.17 (s, 3H, Me2), 0.92 (t, J = 7.5 Hz, 3H, Me), 0.69 (s, 3H, Me2); δC (151 MHz, CDCl3) 137.4 (CH=CH2), 119.0 (CH=CH2), 100.5 (OCq), 71.5 (2 × OCH2), 34.5 (CH2), 30.2 (Cq), 22.8 (Me2), 22.0 (Me2), 7.1 (Me); m/z (ESI) 171.2 (82, M+H+), 172.2 (8%), tR = 2.89 min; HR-MS (HESI): M+, found 170.1310. C10H18O2 requires 170.1301.
8,8-Dimethyl-6,10-dioxaspiro[4.5]dec-1-ene (18)
KOH (3.88 g, 69 mmol), ethylene glycol (8.3 mL), chloroketal 14 (1.27 g, 6.2 mmol), 7 h, water (90 mL), Et2O (3 × 50 mL), water (50 mL), brine (50 mL), concentrated in vacuo (35 °C, 30 mbar), bulb-to-bulb vacuum distillation (125–130 °C, 28 mbar), ketal 18 (0.62 g, 60%) as yellow oil; Rf (hexanes/AcOEt 6:1) 0.51; νmax (ATR) 2952, 2855, 1473, 1395, 1363, 1203, 1141, 1129, 1088, 1048, 1036, 955, 908, 894 cm−1; δH (600 MHz, CDCl3) 6.16 (dt, J = 5.8, 2.2 Hz, 1H, CH=CH2), 6.10 (dt, J = 5.9, 2.4 Hz, 1H, CH=CH2), 3.59 (d, J = 11.3 Hz, 2H, OCH2), 3.53 (d, J = 11.3 Hz, 2H, OCH2), 2.42 (dt, J = 7.5, 6.7, 2.3 Hz, 2H, CH2), 2.14–2.11 (m, 2H, CH2), 1.01 (s, 3H, Me2), 0.98 (s, 3H, Me2); δC (151 MHz, CDCl3) 136.9 (CH=CH2), 128.9 (CH=CH2), 111.9 (OCq), 72.5 (2 × OCH2), 33.4 (CH2), 30.0 (Cq), 29.6 (CH2), 22.6 (Me2), 22.4 (Me2); HR-MS (HESI): M+, found 168.1144. C10H16O2 requires 168.1145.
3,3-Dimethyl-1,5-dioxaspiro[5.5]undec-7-ene (19) and 3,3-Dimethyl-1,5-dioxaspiro[5.5]undec-8-ene (25)
KOH (2.86 g, 51 mmol), ethylene glycol (6.1 mL), chloroketal 15 (1.0 g, 4.6 mmol), 18.5 h, water (60 mL), Et2O (3 × 20 mL), water (20 mL), brine (20 mL), concentrated in vacuo (35 °C, 30 mbar), bulb-to-bulb vacuum distillation (145–155 °C, 27 mbar) to give a mixture of ketals 19:25 in ratio 5:1 determined by 1H NMR spectroscopy (0.69 g, 83%) as colourless oil; Rf (hexanes/AcOEt 5:1) 0.59.
Ketal (19): δH (600 MHz, CDCl3) 6.10 (dt, J = 10.4, 2.1 Hz, 1H, CH=CH2), 5.92 (dt, J = 10.3, 3.6 Hz, 1H, CH=CH2), 3.62 (d, J = 11.4 Hz, 2H, OCH2), 3.51 (d, J = 11.4 Hz, 2H, OCH2), 2.07–2.03 (m, 2H, CH2), 1.93–1.90 (m, 2H, CH2), 1.75–1.70 (m, 2H, CH2), 1.02 (s, 3H, Me2), 0.95 (s, 3H, Me2); δC (151 MHz, CDCl3) 132.1 (CH=CH2), 125.2 (CH=CH2), 95.1 (OCq), 70.4 (2 × OCH2), 32.0 (CH2), 30.1 (Cq), 25.6 (CH2), 22.8 (Me2), 22.6 (Me2), 19.3 (CH2); m/z (ESI) 183.0 (53, M+H+), 184.0 (7%), tR = 2.44 min.
Ketal (25): νmax (ATR) 3027, 2952, 2861, 1470, 1361, 1269, 1251, 1105, 1036, 1015, 845 cm−1; δH (600 MHz, CDCl3) 5.70–5.66 (m, 1H, CH=CH2), 5.58–5.54 (m, 1H, CH=CH2), 3.59 (d, J = 11.5 Hz, 2H, OCH2), 3.49 (d, J = 11.5 Hz, 2H, OCH2), 2.39–2.35 (m, 2H, CH2), 2.16–2.11 (m, 2H, CH2), 1.96 (t, J = 6.5 Hz, 2H, CH2), 1.03 (s, 3H, Me2), 0.92 (s, 3H, Me2); δC (151 MHz, CDCl3) 126.4 (CH=CH2), 123.4 (CH=CH2), 97.0 (OCq), 70.2 (2 × OCH2), 34.8 (CH2), 30.2 (Cq), 27.3 (CH2), 23.5 (CH2), 22.8 (Me2), 22.5 (Me2); HR-MS (HESI): M+, found 183.1316. C11H18O2 requires 182.1301.
3.2.3. Preparation of Alcohol (21)
2,2-Dimethyl-3-(2-methyl-but-3-en-2-yloxy)propan-1-ol (21)
To a stirred solution of ketal 16 (0.3 g, 1.9 mmol) in anhydrous toluene (32 mL) was added 3M solution of MeMgBr in diethyl ether (6.4 mL, 19.2 mmol, 10 equiv) drop-wise at 0 °C and the mixture was heated at 80 °C for 4 h under Ar. After cooling the mixture to 0 °C, the reaction was carefully quenched with sat. aq. NH4Cl soln. (25 mL) and water (10 mL). The layers were separated and the aqueous phase was extracted with Et2O (3 × 15 mL). The combined organic layers were washed with brine (30 mL) and dried over Na2SO4. The solvents were evaporated in vacuo (75 °C, 29 mbar) and crude material was purified by MPLC(16 g SiO2, flow 50 mL/min, fraction 5 mL, gradient elution hexanes/AcOEt 90:10 → 50:50) to furnish alcohol 21 (75 mg, 23%) as colourless oil; Rf (hexanes/AcOEt 5:1) 0.37; νmax (ATR) 3433 (OH), 2976, 2870, 1475, 1415, 1360, 1150, 1076, 1044, 999, 920, 875 cm−1; δH (400 MHz, CDCl3) 5.68 (dd, J = 17.8, 10.6 Hz, 1H, CH=CH2), 5.13 (dd, J = 4.4, 1.1 Hz, 1H, CH=CH2), 5.10 (dd, J = 2.6, 1.1 Hz, 1H, CH=CH2), 3.42 (d, J = 5.0 Hz, 2H, CH2), 3.17 (brs, 3H, CH2, OH), 1.25 (s, 6H, Me2), 0.89 (s, 6H, Me2); δC (101 MHz, CDCl3) 143.5 (CH=CH2), 114.1 (CH=CH2), 75.2 (OCq), 72.7 (CH2), 72.6 (CH2), 35.5 (Cq), 25.6 (Me2), 22.0 (Me2); HR-MS (HESI): M+, found 172.1466. C10H20O2 requires 172.1463.
3.2.4. General Experiment for the Preparation of Alcohols (20, 22, 23)
To a stirred solution of ketal 16–18, anhydrous THF was added dropwise to a solution of DIBAL in toluene (1.0 M, 3–5 equiv) at 0 °C over 5–10 min under Ar. The mixture was warmed to 50 °C and stirred for 2–4.5 d. The cooled mixture (ice) was quenched with sat. aq. soln. of Rochelle salt and water, Et2O was added and stirred for 2–5 h at RT. The layers were separated and the aqueous phase was extracted with Et2O. The combined organic layers were washed with brine and dried over MgSO4. The solvents were evaporated in vacuo (73 °C, 30 mbar). The crude product was purified either by bulb-to-bulb vacuum distillation (Kugelrohr) or FLC to furnish a corresponding alcohol 20, 22, 23.
3-(But-3-en-2-yloxy)-2,2-dimethylpropan-1-ol (20)
Ketal 16 (0.5 g, 3.2 mmol), THF (16 mL), 1M DIBAL in toluene (17.4 mL, 17.4 mmol, 5.5 equiv, 7 min), 50 °C, 44 h, sat. aq. Rochelle salt soln. (25 mL), water (5 mL), Et2O (150 mL), 5 h, Et2O (2 × 25 mL), brine (50 mL), bulb-to-bulb vacuum distillation (135–145 °C, 31 mbar), alcohol 20 (307 mg, 61%) as colourless oil; Rf (hexanes/AcOEt 5:1) 0.3; νmax (ATR) 3399 (OH), 2972, 2956, 2870, 1475, 1421, 1368, 1319, 1094, 1044, 991, 921 cm−1; δH (300 MHz, CDCl3) 5.70 (ddd, J = 17.3, 10.3, 7.1 Hz, 1H, CH=CH2), 5.17 (ddd, J = 14.6, 1.6, 1.0 Hz, 1H, CH=CH2), 5.12 (ddd, J = 7.6, 1.6, 1.0 Hz, 1H, CH=CH2), 3.82–3.71 (m, 1H, CH), 3.44 (s, 2H, CH2), 3.34 (d, J = 8.9 Hz, 1H, CH2), 3.16 (d, J = 8.8 Hz, 1H, CH2), 2.91 (brs, 1H, OH), 1.23 (d, J = 6.4 Hz, 3H, Me), 0.91 (s, 6H, Me2); δC (151 MHz, CDCl3) 140.0 (CH=CH2), 115.9 (CH=CH2), 78.2 (CH2), 77.8 (CH), 72.4 (CH2), 35.9 (Cq), 21.9 (Me2), 21.2 (Me); HR-MS (HESI): M+, found 158.1303. C9H18O2 requires 158.1301.
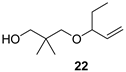
2,2-Dimethyl-3-(pent-1-en-3-yloxy)propan-1-ol (22)
Ketal 17 (0.8 g, 4.7 mmol), THF (23.5 mL), 1M DIBAL in toluene (25.9 mL, 25.9 mmol, 5.5 equiv, 5 min), 50 °C, 4.5 d, sat. aq. Rochelle salt soln. (42 mL), water (30 mL), Et2O (80 mL), 2 h, Et2O (2 × 40 mL), brine (80 mL), FLC (14.6 g SiO2, Hex/AcOEt 7:1, 180 mL), alcohol 22 (122 mg, 15%) as colourless oil; Rf (hexanes/AcOEt 5:1) 0.43; νmax (ATR) 3411 (OH), 2961, 2873, 1475, 1422, 1322, 1091, 1045, 993, 921 cm−1; δH (600 MHz, CDCl3) 5.64 (ddd, J = 17.2, 10.5, 7.5 Hz, 1H, CH=CH2), 5.20–5.14 (m, 2H, CH=CH2), 3.50 (dd, J = 13.6, 6.7 Hz, 1H, CH), 3.44 (d, J = 5.2 Hz, 2H, OCH2), 3.38 (d, J = 8.8 Hz, 1H, OCH2), 3.11 (d, J = 8.8 Hz, 1H, OCH2), 2.91 (t, J = 5.6 Hz, 1H, OH), 1.63–1.47 (m, 2H, CH2), 0.93 (s, 3H, Me), 0.90 (s, 3H, Me), 0.90 (t, J = 7.5 Hz, 3H, Me); δC (151 MHz, CDCl3) 138.6 (CH=CH2), 116.9 (CH=CH2), 83.5 (OCH), 78.4 (OCH2), 72.4 (OCH2), 36.0 (Cq), 28.3 (CH2), 21.9 (Me2), 9.6 (Me); HR-MS (HESI): M+, found 172.1463. C10H20O2 requires 172.1458.
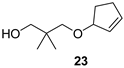
3-(Cyclopent-2-enyloxy)-2,2-dimethylpropanol (23)
Ketal 18 (596 mg, 3.5 mmol), THF (17.7 mL), 1M DIBAL in toluene (10.6 mL, 10.6 mmol, 3 equiv, 10 min), 50 °C, 50 h, sat. aq. Rochelle salt soln. (13 mL), water (20 mL), Et2O (60 mL), 2 h, Et2O (2 × 30 mL), brine (60 mL), FLC (27.9 g SiO2, gradient elution Hex/AcOEt 8:1 (360 mL) → 1:1 (100 mL)), alcohol 23 (107 mg, 18%) as colourless oil; Rf (hexanes/AcOEt 5:1) 0.29; νmax (ATR) 3419 (OH), 2953, 2854, 1474, 1359, 1116, 1082, 1041 cm−1; δH (600 MHz, CDCl3) 6.02–5.99 (m, 1H, CH=CH), 5.82–5.79 (m, 1H, CH=CH), 4.53–4.50 (m, 1H, CH), 3.43 (s, 2H, OCH2), 3.32 (d, J = 8.7 Hz, 1H, OCH2), 3.29 (d, J = 8.7 Hz, 1H, OCH2), 2.91 (brs, 1H, OH), 2.50–2.43 (m, 1H, CH2), 2.28–2.22 (m, 1H, CH2), 2.16–2.09 (m, 1H, CH2), 1.77–1.70 (m, 1H, CH2), 0.91 (s, 6H, Me2); δC (151 MHz, CDCl3) 135.9 (CH=CH), 130.4 (CH=CH), 85.4 (CH), 78.1 (OCH2), 72.4 (OCH2), 35.9 (Cq), 31.0 (CH2), 29.6 (CH2), 22.0 (Me2); m/z (ESI) 169.2 (100, M-H+), 170.2 (10, M+), tR = 1.96 min; HR-MS (HESI): M+, found 170.1312. C10H18O2 requires 170.1301.
3.2.5. Preparation of Alcohol (24)
To a cooled mixture of ketals 19 + 25 (5:1, 754 mg, 4.1 mmol) in anhydrous THF (20.7 mL) 1M solution of DIBAL in toluene (18.6 mL, 18.6 mmol, 4.5 equiv) was added dropwise at 0 °C over 5 min under Ar. The mixture was warmed to 50 °C and stirred for 88 h. The cooled mixture (ice) was quenched with sat. aq. soln. of Rochelle salt (30 mL) and water (15 mL), diluted with Et2O (70 mL) and stirred for 2.5 h at RT. The layers were separated and the aqueous phase was extracted with Et2O (2 × 35 mL). The combined organic layers were washed with brine (70 mL) and dried over MgSO4. The solvents were evaporated in vacuo (73 °C, 30 mbar). The crude material was purified by FLC (31 g SiO2, gradient elution DCM/iPrOH 70:1 (213 mL) → 35:1 (36 mL)) to furnish desired alcohol 24 (303 mg, 48%) and unreacted ketal 25 (75 mg) as colourless oils.
3-(Cyclohex-2-enyloxy)-2,2-dimethylpropanol (24): Rf (DCM/iPrOH 70:1) 0.19; νmax (ATR) 3426 (OH), 3026, 2934, 2865, 1474, 1393, 1080, 1045, 947, 898, 725 cm−1; δH (300 MHz, CDCl3) 5.84–5.81 (dtd, J = 10.1, 3.5, 1.2 Hz, 1H, CH=CH), 5.74 (ddt, J = 10.1, 3.2, 2.0 Hz, 1H, CH=CH), 3.83–3.75 (m, 1H, CH), 3.44 (d, J = 3.8 Hz, 2H, OCH2), 3.38 (d, J = 8.7 Hz, 1H, OCH2), 3.33 (d, J = 8.6 Hz, 1H, OCH2), 2.99 (brs, 1H, OH), 2.10–1.45 (m, 6H, 3 × CH2), 0.92 (s, 6H, Me2); δC (151 MHz, CDCl3) 131.0 (CH=CH), 127.3 (CH=CH), 78.1 (OCH2), 73.5 (CH), 72.4 (OCH2), 36.0 (Cq), 28.2 (CH2), 25.1 (CH2), 21.9 (Me2), 19.1 (CH2); m/z (ESI) 105.2 (100%), 185.2 (58, M+H+), 186.2 (10%), tR = 3.13 min; HR-MS (HESI): M+, found 184.1445. C11H20O2 requires 184.1458.
3.2.6. General Experiment for the Preparation of Aldehydes (3–7)
To a solution of alcohol 20–24 in anhydrous DCM Dess–Martin periodinane (DMP, 1.10–1.25 equiv) and NaHCO3 (0.27 equiv) was added under Ar. The mixture was stirred for 2–6 h at RT and quenched with sat. aq. NaHCO3 soln. followed by addition of sat. aq. Na2S2O3 soln. and the stirring continued until all solids dissolved (1–4 h). The layers were separated, the aqueous layer was extracted with DCM and the combined organic layers were washed with brine and dried over MgSO4. The solvent was removed in vacuo (40 °C, 100–200 mbar) and residue was purified by either FLC or PTLC to furnish corresponding aldehyde 3–7.
3-(But-3-en-2-yloxy)-2,2-dimethylpropan-1-al (3)
Alcohol 20 (163 mg, 1.03 mmol), DCM (10.3 mL), DMP (546 mg, 1.29 mmol, 1.25 equiv), NaHCO3 (24 mg, 0.28 mmol), 3 h, sat. aq. NaHCO3 soln. (8.4 mL), sat. aq. Na2S2O3 soln. (8.4 mL), 1 h, DCM (3 × 25 mL), brine (40 mL), evaporation in vacuo (40 °C, 200 mbar), FLC (8.5 g SiO2, DCM, 150 mL), aldehyde 3 (62 mg, 38%) as colourless oil; Rf (DCM) 0.5; νmax (ATR) 2974, 2932, 2871, 1729 (C=O), 1474, 1367, 1092, 992, 925, 910 cm−1; δH (600 MHz, CDCl3) 9.55 (s, 1H, CHO), 5.68 (ddd, J = 17.3, 10.3, 7.1 Hz, 1H, CH=CH2), 5.15 (dt, J = 17.2, 1.3 Hz, 1H, CH=CH2), 5.11 (dt, J = 10.3, 1.4 Hz, 1H, CH=CH2), 3.78–3.72 (m, 1H, CH), 3.46 (d, J = 9.3 Hz, 1H, CH2), 3.29 (d, J = 9.3 Hz, 1H, CH2), 1.19 (d, J = 6.4 Hz, 3H, Me), 1.06 (s, 6H, 2 × Me); δC (151 MHz, CDCl3) 205.7 (CHO), 140.1 (CH=CH2), 115.8 (CH=CH2), 77.6 (CH), 73.4 (CH2), 47.0 (Cq), 21.0 (Me), 19.0 (Me2); m/z (ESI) 157.2 (26, M+H+), 158.2 (3%), tR = 2.48 min; HR-MS (HESI): M+, found 156.1139. C9H16O2 requires 156.1145.
2,2-Dimethyl-3-(2-methyl-but-3-en-2-yloxy)propan-1-al (4)
Alcohol 21 (81 mg, 0.47 mmol), DCM (4.7 mL), DMP (219 mg, 0.52 mmol), NaHCO3 (11 mg, 0.13 mmol), 2.5 h, sat. aq. NaHCO3 soln. (3.8 mL, sat. aq. Na2S2O3 soln. (3.8 mL), 1 h, DCM (3 × 12 mL), brine (25 mL), evaporation in vacuo (40 °C, 200 mbar), FLC (6.3 g SiO2, Hexanes/Et2O 8:1, 180 mL), aldehyde 4 (31 mg, 39%) as colourless oil; Rf (hexanes/Et2O 8:1) 0.55; νmax (ATR) 2976, 1731 (C=O), 1361, 1150, 1080, 1002, 924 cm−1; δH (400 MHz, CDCl3) 9.52 (s, 1H, CHO), 5.75 (dd, J = 17.8, 10.6 Hz, 1H, CH=CH2), 5.12 (dd, J = 4.9, 1.2 Hz, 1H, CH=CH2), 5.08 (dd, J = 2.2, 1.2 Hz, 1H, CH=CH2), 3.28 (s, 2H, CH2), 1.22 (s, 6H, 2 × Me), 1.04 (s, 6H, 2 × Me); δC (100 MHz, CDCl3) 206.1 (CHO), 143.7 (CH=CH2), 113.9 (CH=CH2), 74.9 (OCq), 67.8 (CH2), 46.7 (Cq), 25.5 (Me2), 19.0 (Me2); HR-MS (HESI): M+, found 170.1309. C10H18O2 requires 170.1301.
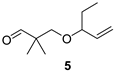
2,2-Dimethyl-3-(pent-1-en-3-yloxy)propan-1-al (5)
Alcohol 22 (107 mg, 0.62 mmol), DCM (6.2 mL), DMP (328 mg, 0.77 mmol), NaHCO3 (14 mg, 0.17 mmol), 3 h, sat. aq. NaHCO3 soln. (5 mL), sat. aq. Na2S2O3 soln. (5 mL), 1.5 h., DCM (3 × 15 mL), brine (30 mL), evaporation in vacuo (40 °C, 100 mbar), FLC (8.8 g SiO2, Hexanes/Et2O 7:1, 80 mL), aldehyde 5 (48 mg, 46%) as colourless oil; Rf (hexanes/Et2O 5:1) 0.61; νmax (ATR) 2996, 1731, 1454, 1322, 1092, 924 cm−1; δH (600 MHz, CDCl3) 9.56 (s, 1H, CHO), 5.62 (ddd, J = 17.0, 10.6, 7.5 Hz, 1H, CH=CH2), 5.17–5.13 (m, 2H, CH=CH2), 3.50 (d, J = 9.3 Hz, 1H, OCH2), 3.51–3.46 (m, 1H, CH), 3.24 (d, J = 9.3 Hz, 1H, OCH2), 1.59–1.51 (m, 1H, CH2), 1.50–1.42 (m, 1H, CH2), 1.07 (s, 6H, 2 x Me), 0.86 (t, J = 7.4 Hz, 3H, Me); δC (151 MHz, CDCl3) 205.7 (CHO), 138.8 (CH=CH2), 116.7 (CH=CH2), 83.4 (CH), 73.7 (OCH2), 47.2 (Cq), 28.2 (CH2), 19.0 (Me2), 9.6 (Me); HR-MS (HESI): M+, found 170.1305. C10H18O2 requires 170.1301.
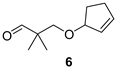
3-(Cyclopent-2-enyloxy)-2,2-dimethylpropan-1-al (6)
Alcohol 23 (87 mg, 0.51 mmol), DCM (5.1 mL), DMP (270 mg, 0.64 mmol), NaHCO3 (12 mg, 0.14 mmol), 2 h, sat. aq. NaHCO3 soln. (4.2 mL), sat. aq. Na2S2O3 soln. (4.2 mL), 2 h, DCM (3 × 13 mL), brine (30 mL), evaporation in vacuo (40 °C, 200 mbar), PTLC (Hexanes/Et2O 3:1), aldehyde 6 (19 mg, 22%) as colourless oil; Rf (hexanes/Et2O 3:1) 0.61; νmax (ATR) 2931, 2854, 1729 (C=O), 1359, 1116, 1087, 1043, 897, 731 cm−1; δH (600 MHz, CDCl3) 9.55 (s, 1H, CHO), 6.01–5.98 (m, 1H, CH=CH), 5.82–5.79 (m, 1H, CH=CH), 4.55–4.51 (m, 1H, CH), 3.44 (d, J = 9.2 Hz, 1H, OCH2), 3.42 (d, J = 9.2 Hz, 1H, OCH2), 2.48–2.41 (m, 1H, CH2), 2.28–2.21 (m, 1H, CH2), 2.14–2.07 (m, 1H, CH2), 1.75–1.68 (m, 1H, CH2), 1.07 (s, 6H, 2 × Me); δC (151 MHz, CDCl3) 205.8 (CHO), 135.7 (CH=CH), 130.5 (CH=CH), 85.4 (CH), 73.3 (OCH2), 47.0 (Cq), 31.1 (CH2), 29.4 (CH2), 19.1 (Me2); HR-MS (HESI): M+, found 168.1141. C10H16O2 requires 168.1145.
3-(Cyclohex-2-enyloxy)-2,2-dimethylpropan-1-al (7)
Alcohol 24 (150 mg, 0.81 mmol), DCM (8.1 mL), DMP (432 mg, 1.02 mmol), NaHCO3 (19 mg, 0.23 mmol), 5.5 h, sat. aq. NaHCO3 soln. (6.6 mL), sat. aq. Na2S2O3 soln. (6.6 mL), 3.5 h, DCM (3 × 20 mL), brine (30 mL), evaporation in vacuo (40 °C, 100 mbar), FLC (7.9 g SiO2, DCM, 180 mL), aldehyde 7 (89 mg, 60%) as colourless oil; Rf (DCM) 0.56; νmax (ATR) 2933, 2865, 1729 (C=O), 1474, 1395, 1084, 948, 895, 726 cm−1; δH (600 MHz, CDCl3) 9.57 (s, 1H, CHO), 5.85–5.81 (m, 1H, CH=CH), 5.73–5.69 (m, 1H, CH=CH), 3.82–3.77 (m, 1H, CH), 3.50 (d, J = 9.1 Hz, 1H, OCH2), 3.46 (d, J = 9.1 Hz, 1H, OCH2), 2.06–1.99 (m, 1H, CH2), 1.97–1.90 (m, 1H, CH2), 1.82–1.76 (m, 1H, CH2), 1.75–1.68 (m, 1H, CH2), 1.65–1.58 (m, 1H, CH2), 1.55–1.48 (m, 1H, CH2), 1.08 (s, 3H, Me), 1.07 (s, 3H, Me); δC (151 MHz, CDCl3) 205.8 (CHO), 130.8 (CH=CH), 127.5 (CH=CH), 73.6 (CH), 73.3 (OCH2), 47.1 (Cq), 28.1 (CH2), 25.2 (CH2), 19.2 (CH2), 19.1 (Me2); HR-MS (HESI): M+, found 182.1302. C11H18O2 requires 182.1301.
4. Conclusions
We have designed, synthesised, and evaluated a set of new (racemic) seco-analogues 3–7 of lilac aldehydes featuring a higher conformational freedom and/or variable substitution pattern of the alkene moiety. Thus, starting from commercially available α-chloroketones 8–11, we have prepared target aldehydes from intermediary alcohols 20–24 via the reductive ring-opening of respective ketals 16–19. The qualitative olfactory analysis of novel seco-analogues 3–7 revealed that the opening of tetrahydrofuran ring leads to a vanishing of flowery scent typical for lilac aldehydes 1, suggesting the important osmophoric role of THF moiety in these natural products. Moreover, while the ring-opened analogues 3–5 bearing an acyclic alkene exhibit a rather common spicy aroma with green notes, the scent of their congeners featuring a cyclic alkene is further shifted towards fruity aspects for 6 and herbal notes for 7. For comparison purposes, we have also included alcohols 20–24 in the olfactory screening. Interestingly, while the respective alcohols 20, 22, 23, and 24 commonly exhibit sweet aroma with fatty and turpentine-like facets, alcohol 21 features a pleasant balsamic aroma with minty-eucalypty notes. However, the aroma intensity for all compounds was weak to moderate with only short persistence over time.
Supplementary Materials
The copies of NMR spectra of new compounds are available online.
Author Contributions
Conceptualization and methodology, V.D. and P.S.; synthesis and analytics, V.D.; writing—original draft preparation, review and editing, V.D. and P.S.; supervision and project administration, P.S.; funding acquisition, P.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Grant Agency of the Slovak Republic (VEGA 1/0162/20).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Jana Doháňošová for NMR service, Sylvia Lazovská for IR measurements, and Ivan Špánik for HRMS spectra.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Wakayama, S.; Namba, S. Lilac Aldehydes. Bull. Chem. Soc. Jpn. 1974, 47, 1293–1294. [Google Scholar] [CrossRef] [Green Version]
- Kreck, M.; Mosandl, A. Synthesis, Structure Elucidation, and Olfactometric Analysis of Lilac Aldehyde and Lilac Alcohol Stereoisomers. J. Agric. Food Chem. 2003, 51, 2722–2726. [Google Scholar] [CrossRef] [PubMed]
- Kreck, M.; Püschel, S.; Wüst, M.; Mosandl, A. Biogenetic studies in Syringa vulgaris L.: Synthesis and bioconversion of deuterium-labelled precursors into lilac aldehydes and lilac alcohols. J. Agric. Food Chem. 2003, 51, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Matich, A.J.; Young, H.; Allen, J.M.; Wang, M.Y.; Fielder, S.; McNeilage, M.A.; MacRae, E.A. Actinidia arguta: Volatile compounds in fruit and flowers. Phytochemistry 2003, 63, 285–301. [Google Scholar] [CrossRef]
- Dötterl, S.; Wolfe, L.M.; Jürgens, A. Qualitative and quantitative analyses of flower scent in Silene latifolia. Phytochemistry 2005, 66, 203–213. [Google Scholar] [CrossRef]
- Plepys, D.; Ibarra, F.; Löfstedt, C. Volatiles from flowers of Platanthera bifolia (Orchidaceae) attractive to the silver Y moth, Autographa gamma (Lepidoptera: Noctuidae). Oikos 2002, 99, 69–74. [Google Scholar] [CrossRef]
- Plepys, D.; Ibarra, F.; Francke, W.; Löfstedt, C. Odour-mediated nectar foraging in the silver Y moth, Autographa gamma (Lepidoptera: Noctuidae): Behavioural and electrophysiological responses to floral volatiles. Oikos 2002, 99, 75–82. [Google Scholar] [CrossRef]
- Dötterl, S.; Jürgens, A.; Seifert, K.; Laube, T.; Weißbecker, B.; Schütz, S. Nursery pollination by a moth in Silene latifolia: The role of odours in eliciting antennal and behavioural responses. New Phytol. 2006, 169, 707–718. [Google Scholar] [CrossRef]
- Dötterl, S.; Burkhardt, D.; Weißbecker, B.; Jürgens, A.; Schütz, S.; Mosandl, A. Linalool and lilac aldehyde/alcohol in flower scents. Electrophysiological detection of lilac aldehyde stereoisomers by a moth. J. Chromatogr. A 2006, 1113, 231–238. [Google Scholar] [CrossRef]
- Andersson, S. Antennal responses to floral scents in the butterflies Inachis io, Aglais urticae (Nymphalidae), and Gonepteryx rhamni (Pieridae). Chemoecology 2003, 13, 13–20. [Google Scholar] [CrossRef]
- Andersson, S.; Dobson, H.E.M. Antennal Responses to Floral Scents in the Butterfly Heliconius melpomene. J. Chem. Ecol. 2003, 29, 2319–2330. [Google Scholar] [CrossRef] [PubMed]
- Jhumur, U.S.; Dötterl, S.; Jürgens, A. Floral Odors of Silene otites: Their Variability and Attractiveness to Mosquitoes. J. Chem. Ecol. 2008, 34, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Nyasembe, V.O.; Torto, B. Volatile phytochemicals as mosquito semiochemicals. Phytochem. Lett. 2014, 8, 196–201. [Google Scholar] [CrossRef] [Green Version]
- Lahondère, C.H.; Vinauger, C.; Okubo, R.P.; Wolff, G.H.; Chan, J.K.; Akbari, O.S.; Riffella, J.A. The olfactory basis of orchid pollination by mosquitoes. Proc. Natl. Acad. Sci. USA 2020, 117, 708–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro-Vázquez, L.; Díaz-Maroto, M.C.; Pérez-Coello, M.S. Aroma composition and new chemical markers of Spanish citrus honeys. Food Chem. 2007, 103, 601–606. [Google Scholar] [CrossRef]
- Špánik, I.; Pažitná, A.; Šiška, P.; Szolcsányi, P. The determination of botanical origin of honeys based on enantiomer distribution of chiral volatile organic compounds. Food Chem. 2014, 158, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Battesti, M.-J.; Costa, J.; Dupuy, N.; Paolini, J. Volatile components as chemical markers of the botanical origin of Corsican honeys. Flavour Fragr. J. 2018, 33, 52–62. [Google Scholar] [CrossRef] [Green Version]
- Machado, A.M.; Miguel, M.G.; Vilas-Boas, M.; Figueiredo, A.C. Honey Volatiles as a Fingerprint for Botanical Origin—A Review on their Occurrence on Monofloral Honeys. Molecules 2020, 25, 374. [Google Scholar] [CrossRef] [Green Version]
- ZZhang, J.; Liu, S.; Walton, W.C.; Wang, Y. Volatile organic compounds of Eastern oyster (Crassostrea virginica) cultured by two treatments and their changes during cold storage. Aquac. Res. 2021, 52, 1442–1452. [Google Scholar] [CrossRef]
- Naegeli, P.; Weber, G. The total synthesis of racemic davanone. Tetrahedron Lett. 1970, 12, 959–962. [Google Scholar] [CrossRef]
- Wakayama, S.; Namba, S.; Hosoi, K.; Ohno, M. The Synthesis and Absolute Configurations of Lilac Alcohols. Bull. Chem. Soc. Japan 1971, 44, 875. [Google Scholar] [CrossRef] [Green Version]
- Wakayama, S.; Namba, S.; Hosoi, K.; Ohno, M. The synthesis and the absolute configurations of lilac alcohols, new naturally occurringodorous ingredients of lilac flower. Bull. Chem. Soc. Jpn. 1973, 46, 3183–3187. [Google Scholar] [CrossRef] [Green Version]
- Weerdt, A.J.; Heide, R.; Jägers, P.; Dort, H.M. Narcissus trevithian and Narcissus geranium: Analysis and synthesis of compounds. J. Agric. Food. Chem. 1993, 41, 2063–2075. [Google Scholar]
- Katsuta, Y.; Fujita, A.; Takagi, K. Preparation of Lilac Aldehyde from 4-methyl-4-acetoxy-5-hexenal or Lilac Alcohol. Patent JP0533 9252 1993. [Google Scholar]
- Ito, N.; Azuma, M.; Wada, S.; Imano, H.; Hasebe, A. Preparation of Lilac Aldehyde. Patent JP200033 6083 2000. [Google Scholar]
- Sabitha, G.; Prasad, N.M.; Bhikshapathi, M.; Yadav, J.S. Stereospecific Total Synthesis of (+)-Davana Acid, (+)-Nordavanone and (+)-Davanone. Synthesis 2010, 5, 807–810. [Google Scholar] [CrossRef]
- Schneider, M.-A.; Dötterl, S.; Seifert, K. Diastereoselective Synthesis of a Lilac Aldehyde Isomer and Its Electrophysiological Detection by a Moth. Chem. Biodivers. 2013, 10, 1252–1259. [Google Scholar] [CrossRef]
- Nacsa, E.D.; Fielder, B.C.; Wetzler, S.P.; Srisuknimit, V.; Litz, J.P.; van Vleet, M.J.; Quach, K.; Vosburg, D.A. Direct, Biomimetic Synthesis of (+)-Artemone via a Stereoselective, Organocatalytic Cyclization. Synthesis 2015, 47, 2599–2602. [Google Scholar]
- Šiška, P.; Fodran, P.; Szolcsányi, P. Synthesis and olfactory properties of unnatural derivatives of lilac aldehydes. Tetrahedron 2014, 70, 6420–6427. [Google Scholar] [CrossRef]
- Knowles, J.P.; Whiting, A. The Effects of Ring Size and Substituents on the Rates of Acid-Catalysed Hydrolysis of Five- and Six-Membered Ring Cyclic Ketone Acetals. Eur. J. Org. Chem. 2007, 2007, 3365–3368. [Google Scholar] [CrossRef]
- Newitt, L.A.; Steel, P.G. Evidence for oxacarbenium ion intermediates in the Lewis acid promoted cleavage of spirocyclic dioxanes. J. Chem. Soc. Perkin Trans. 1997, 1, 2033–2036. [Google Scholar] [CrossRef]
- Kumar, V.S.; Floreancig, P.E. Electron Transfer Initiated Cyclizations: Cyclic Acetal Synthesis through Carbon−Carbon σ-Bond Activation. J. Am. Chem. Soc. 2001, 123, 3842–3843. [Google Scholar] [CrossRef] [PubMed]
- Bluhm, N.; Kalesse, M. Synthesis of a C20-Deoxygenated Spirangien Derivative for Target Identification. Synlett 2015, 26, 797–801. [Google Scholar]
- Lautens, M.; Colucci, J.T.; Hiebert, S.; Smith, N.D.; Bouchain, G. Total Synthesis of Ionomycin Using Ring-Opening Strategies. Org. Lett. 2002, 4, 1879–1882. [Google Scholar] [CrossRef] [PubMed]
- Mursakulov, I.G.; Guseinov, M.M.; Kasumov, N.K. Stereochemical Studies—XXVI. Conformational Equilibria of Ketals of 2-Substituted Cyclohexanones. Tetrahedron 1982, 38, 2213–2220. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).