Amino Alcohols from Eugenol as Potential Semisynthetic Insecticides: Chemical, Biological, and Computational Insights
Abstract
1. Introduction
2. Results and Discussion
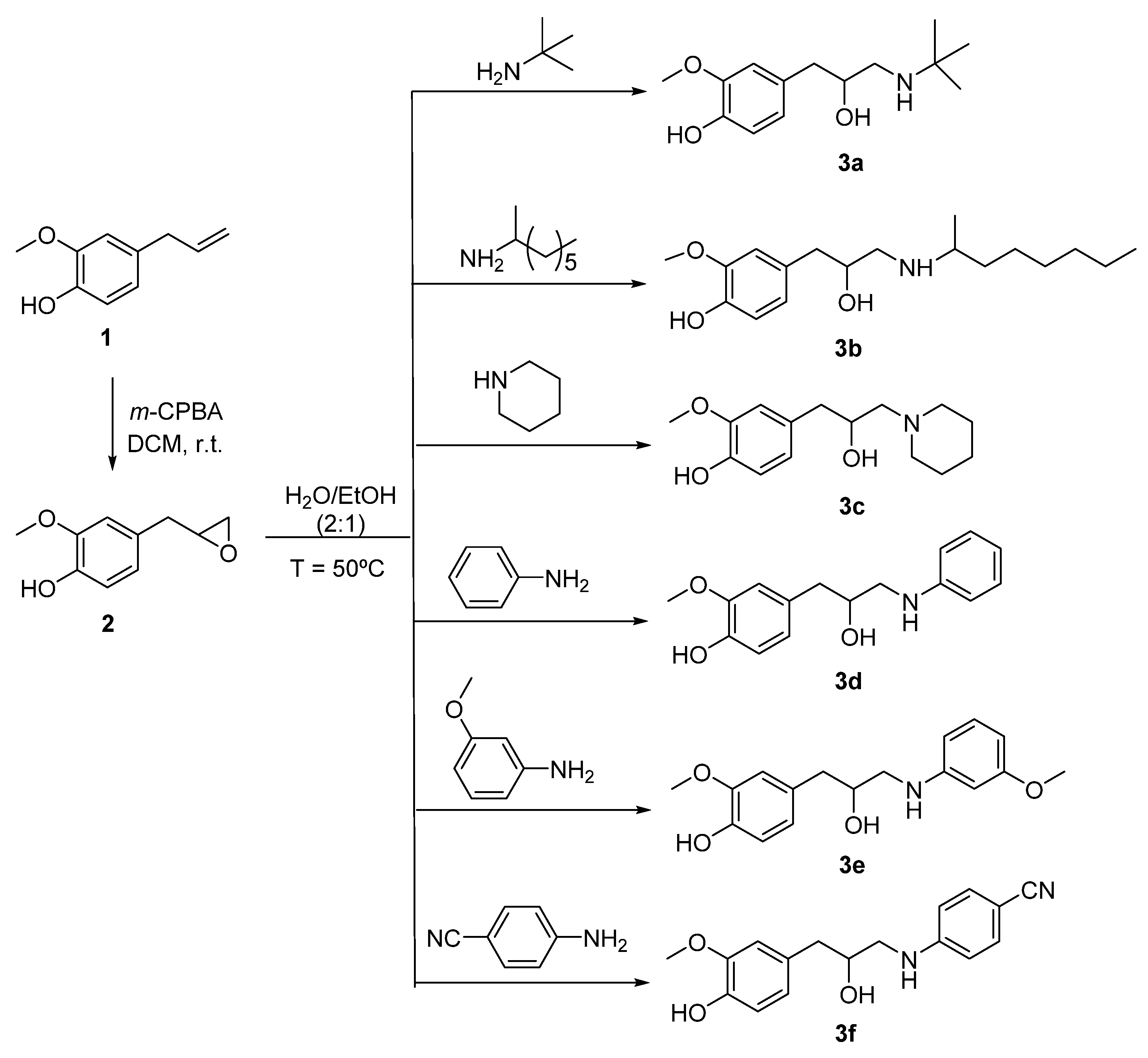
2.1. Synthesis
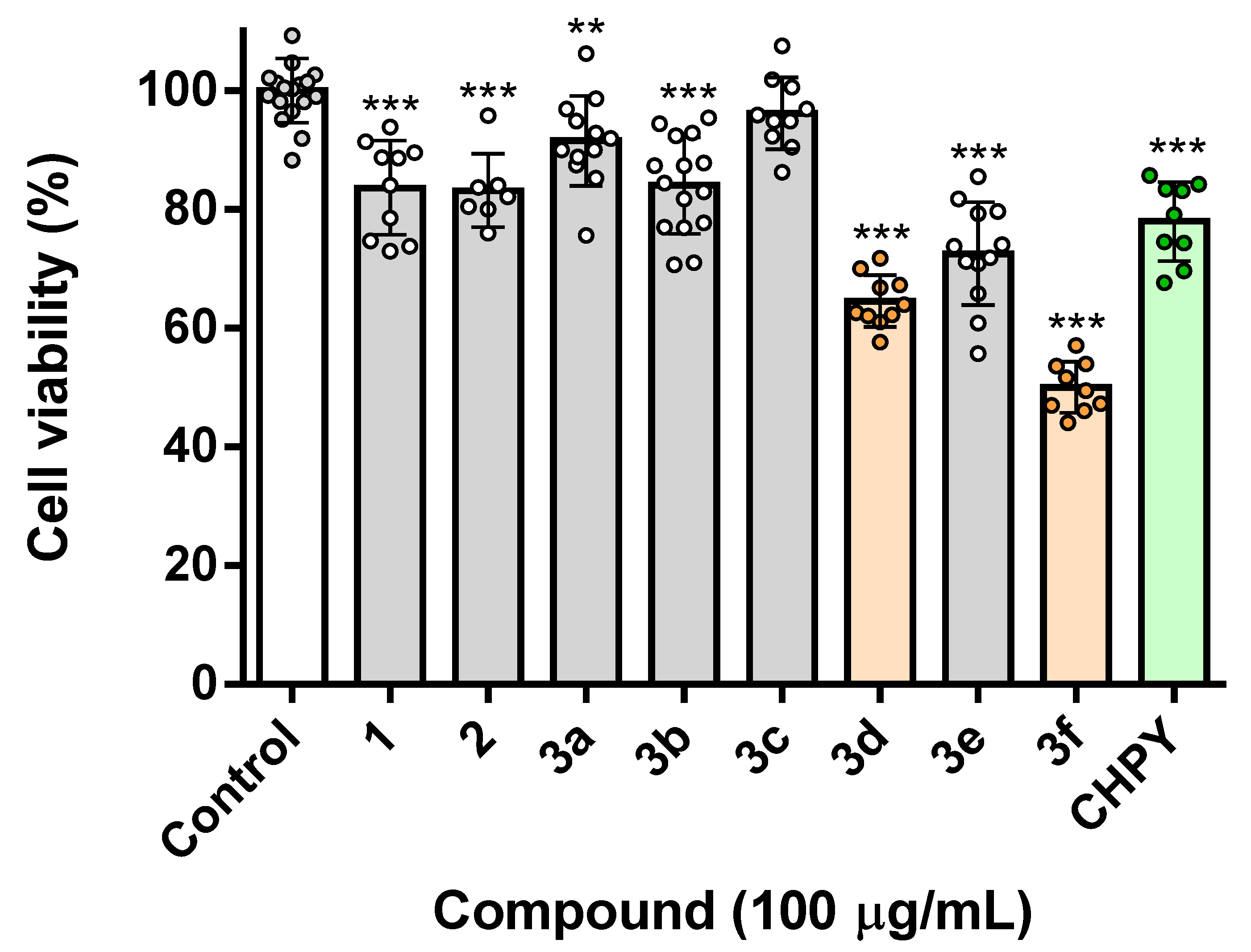
2.2. Toxicity Assessment in Insect Cells
2.3. Amino Alcohols 3d and 3f Activate Caspase-like Proteases in the Sf9 Cells
2.4. Amino Alcohols 3d and 3f Are More Toxic to Insect Cells Than Human Cells
2.5. Inverted Virtual Screening Results
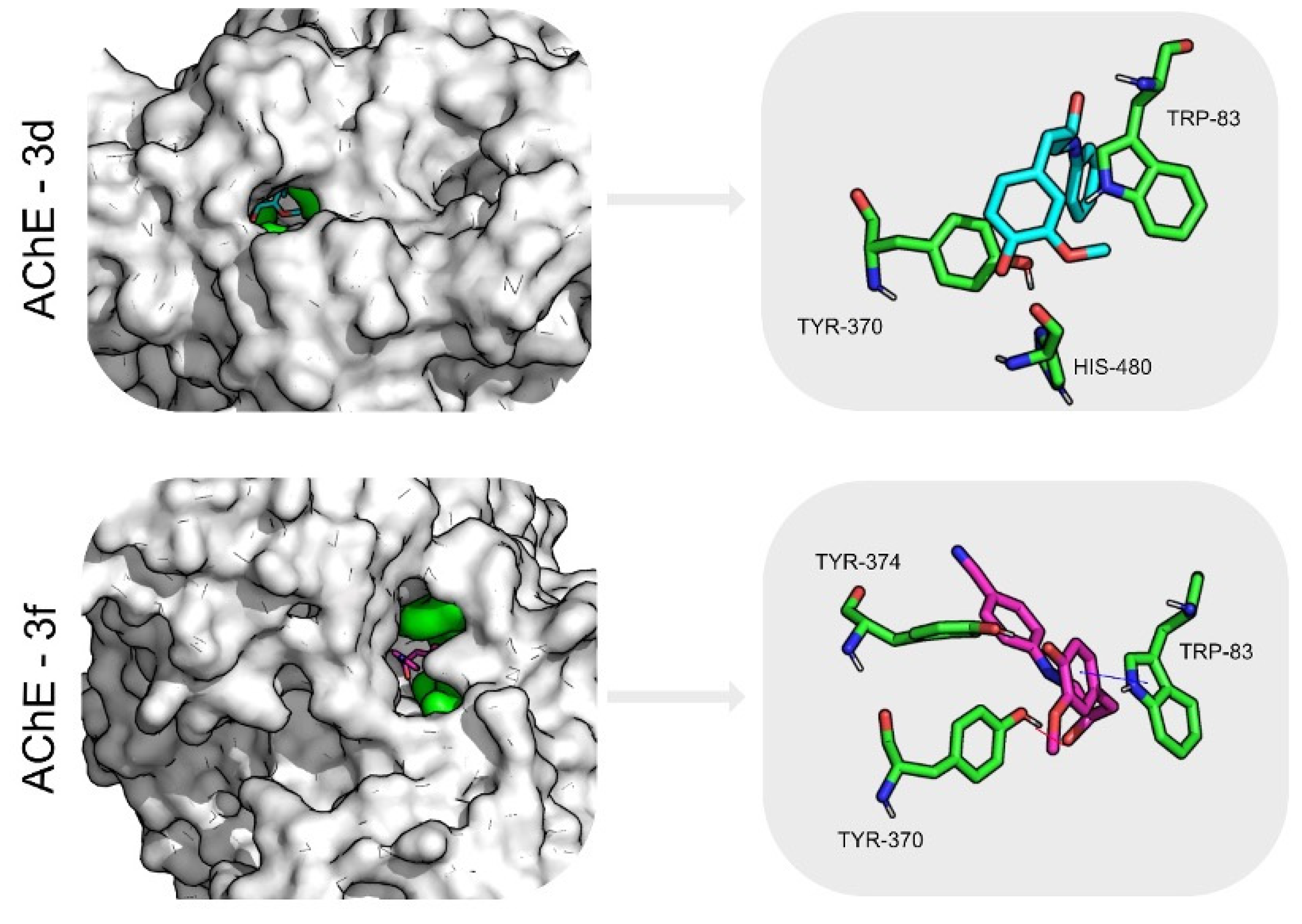
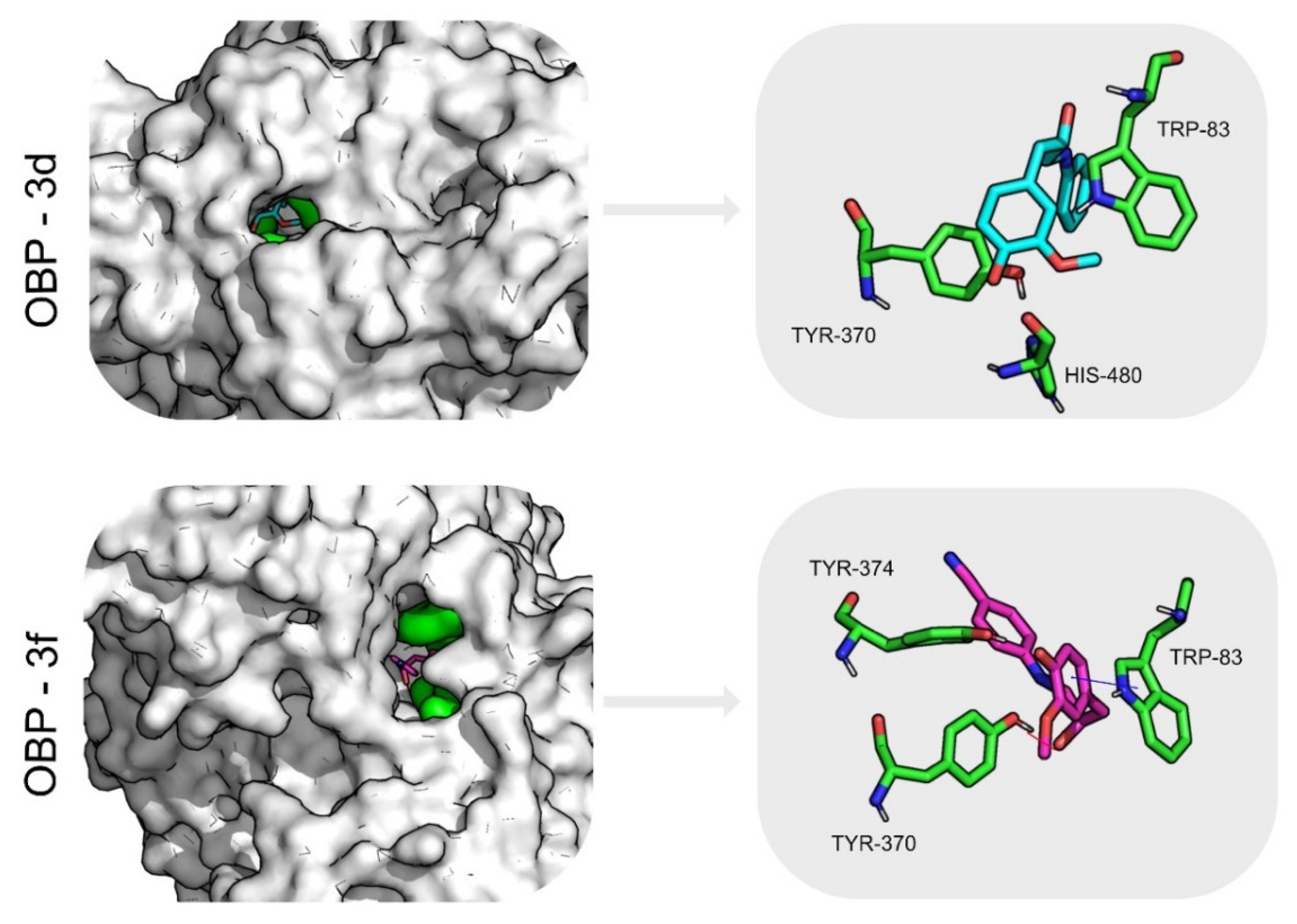
2.6. Molecular Dynamics Simulations and Free Energy Calculations Results
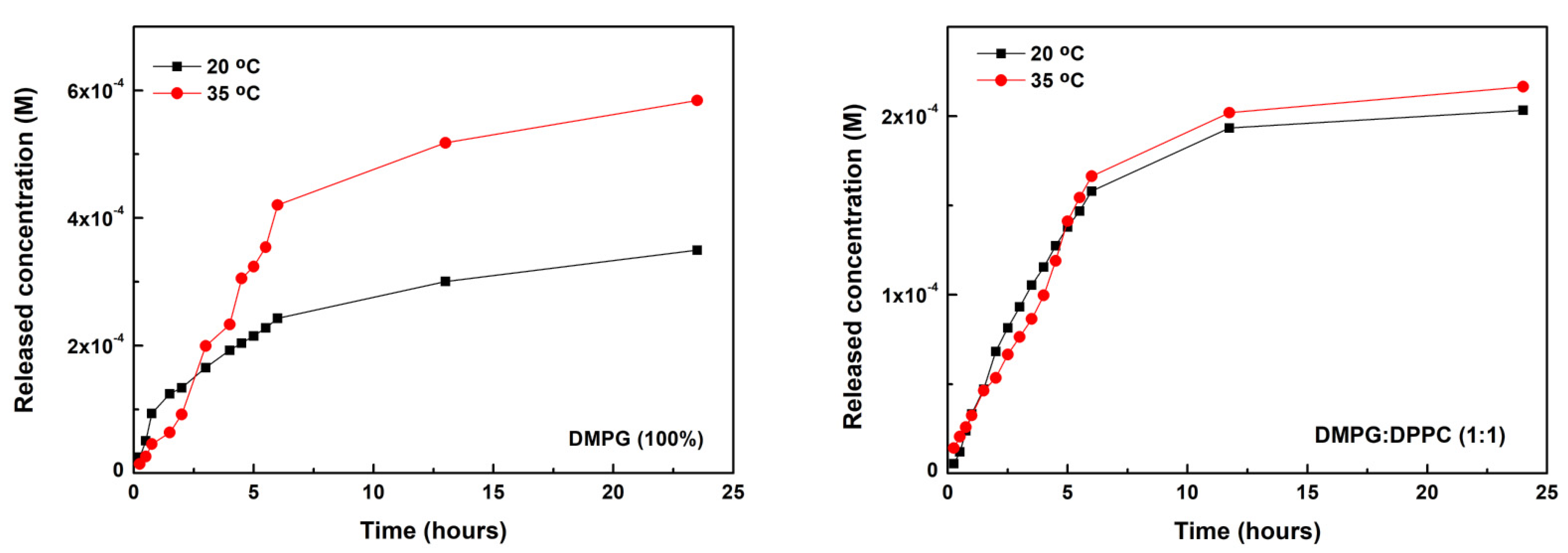
2.7. Nanoencapsulation and Release Assays
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Analytical Instruments
3.3. Synthesis of 2-Methoxy-4-(oxiran-2-ylmethyl)phenol 2
3.4. Synthesis of Amino Alcohols 3a–f
3.4.1. Synthesis of 4-(3-(Tert-butylamino)-2-hydroxypropyl)-2-methoxyphenol 3a
3.4.2. Synthesis of 4-(2-Hydroxy-3-(octan-2-ylamino)propyl)-2-methoxyphenol 3b
3.4.3. Synthesis of 4-(2-Hydroxy-3-(piperidin-1-yl)propyl)-2-methoxyphenol 3c
3.4.4. Synthesis of 4-(2-Hydroxy-3-(phenylamino)propyl)-2-methoxyphenol 3d
3.4.5. Synthesis of 4-(2-Hydroxy-3-((3-methoxyphenyl)amino)propyl)-2-methoxyphenol 3e
3.4.6. Synthesis of 4-(2-Hydroxy-3-(4-hydroxy-3methoxyphenyl)propyl)amino)benzonitrile 3f
3.5. Cell Culture
3.6. Viability Assessment
3.7. LDH Assay
3.8. Caspase-like Activity
3.9. Statistical Analysis
3.10. Molecular Docking and Inverted Virtual Screening Studies
3.11. Molecular Dynamics Simulations and Free Energy Calculations
3.12. Nanoencapsulation Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Pavela, R.; Maggi, F.; Iannarelli, R.; Benelli, G. Plant extracts for developing mosquito larvicides: From laboratory to the field, with insights on the modes of action. Acta Trop. 2019, 193, 236–271. [Google Scholar] [CrossRef]
- Salman, M.; Abbas, R.Z.; Israr, M.; Abbas, A.; Mehmood, K.; Khan, M.K.; Sindhu, Z.D.; Hussaind, R.; Saleemie, M.K.; Shaha, S. Repellent and acaricidal activity of essential oils and their components against Rhipicephalus ticks in cattle. Vet. Parasitol. 2020, 283, 109178. [Google Scholar] [CrossRef] [PubMed]
- Mossa, A.-T.H. Green pesticides: Essential oils as biopesticides in insect-pest management. J. Environ. Sci. Technol. 2016, 9, 354–378. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Upadhyay, S.; Bhuiyan, M.; Bhattacharya, P.R. A review on prospects of essential oils as biopesticide in insect-pest management. J. Pharm. Phytother. 2009, 1, 52–63. [Google Scholar]
- Cortés-Rojas, D.F.; Fernandes de Souza, C.R.; Pereira Oliveira, W. Clove (Syzygium aromaticum): A precious spice. Asian Pac. J. Trop. Biomed. 2014, 4, 90–96. [Google Scholar] [CrossRef]
- Evambe, G.; Canales, L.; Banik, B.K. Antimicrobial activity of eugenol derivatives. Heterocycl. Lett. 2011, 1, 2231–3087. [Google Scholar]
- Sun, W.-J.; Lv, W.-J.; Li, L.-N. Eugenol confers resistance to tomato yellow leaf curl virus (TYLCV) by regulating the expression of SlPer1 in tomato plants. New Biotechnol. 2016, 33, 345–354. [Google Scholar] [CrossRef]
- Fonseca, D.V.; Salgado, P.R.R.; Neto, H.C.A.; Golzio, A.M.F.O.; Filho, M.R.D.C.; Melo, C.G.F.; Leite, F.C.; Piuvezam, M.R.; Pordeus, L.C.M.; Filho, J.M.B. Ortho-eugenol exhibits anti-nociceptive and anti-inflammatory activities. Int. Immunopharmacol. 2016, 38, 402–408. [Google Scholar] [CrossRef]
- Awasthi, P.K.; Dixit, S.C.; Dixit, N.; Sinha, A.K. Eugenol derivatives as future potential drugs. Drugs 2008, 1, 215–220. [Google Scholar]
- Enan, E.E. Molecular and pharmacological analysis of an octopamine receptor from American cockroach and fruit fly in response to plant essential oils. Arch. Insect Biochem. Physiol. 2005, 59, 161–171. [Google Scholar] [CrossRef]
- Silva, F.F.M.; Monte, F.J.Q.; Lemos, T.L.G.; Nascimento, P.G.G.; Costa, A.K.M.; Paiva, L.M.M. Eugenol derivatives: Synthesis, characterization, and evaluation of antibacterial and antioxidant activities. Chem. Cent. J. 2018, 12, 34. [Google Scholar] [CrossRef]
- Corey, E.J.; Zhang, F.-Y. re- and si-Face-selective nitroaldol reactions catalyzed by a rigid chiral quaternary ammonium salt: A highly stereoselective synthesis of the HIV protease inhibitor amprenavir (Vertex 478). Angew. Chem. Int. Ed. 1999, 38, 1931–1934. [Google Scholar] [CrossRef]
- Ager, D.J.; Prakash, I.; Schaad, D.R. 1,2-Amino alcohols and their heterocyclic derivatives as chiral auxiliaries in asymmetric synthesis. Chem. Rev. 1996, 96, 835–876. [Google Scholar] [CrossRef]
- O’Brien, P. Sharpless asymmetric aminohydroxylation: Scope, limitations, and use in synthesis. Angew. Chem. Int. Ed. Engl. 1999, 38, 326–329. [Google Scholar] [CrossRef]
- Li, G.; Chang, H.-T.; Sharpless, K.B. Catalytic asymmetric aminohydroxylation (AA) of olefins. Angew. Chem. Int. Ed. Engl. 1996, 35, 451–454. [Google Scholar] [CrossRef]
- Manda, B.R.; Prasad, A.N.; Thatikonda, N.R.; Lacerda, V., Jr.; Barbosa, L.R.; Santos, H.; Romão, W.; Pavan, F.R.; Ribeiro, C.M.; dos Santos, E.A.; et al. Synthesis, antibacterial and antitubercular evaluation of cardanol and glycerol based β-amino alcohol derivatives. J. Braz. Chem. Soc. 2018, 29, 639–648. [Google Scholar] [CrossRef]
- Shi, C.; Ren, C.; Zhang, E.; Jin, H.; Yu, X.; Wang, S. Synthesis of β-amino alcohols using the tandem reduction and ring-opening reaction of nitroarenes and epoxides. Tetrahedron 2016, 72, 3839–3843. [Google Scholar] [CrossRef]
- Ullmann, N.; Caggiano, S.; Cutrera, R. Salbutamol and around. Ital. J. Pediatr. 2015, 41, A74. [Google Scholar] [CrossRef]
- Ang, W.; Ye, W.; Sang, Z.; Liu, Y.; Yang, T.; Deng, Y.; Luo, Y.; Wei, Y. Discovery of novel bis-oxazolidinone compounds as potential potent and selective antitubercular agents. Bioorg. Med. Chem. Lett. 2014, 24, 1496–1501. [Google Scholar] [CrossRef]
- Cioc, R.C.; van der Niet, D.J.H.; Janssen, E.; Dr Ruijter, E.; Prof Dr Orru, R.V.A. One-pot synthesis of N-substituted β-amino alcohols from aldehydes and isocyanides. Chem. Eur. J. 2015, 21, 7808–7813. [Google Scholar] [CrossRef]
- Lopes, A.I.F.; Monteiro, M.; Araújo, A.R.L.; Rodrigues, A.R.O.; Castanheira, E.M.S.; Pereira, D.M.; Olim, P.; Fortes, A.G.; Gonçalves, M.S.T. Cytotoxic plant extracts towards insect cells: Bioactivity and nanoencapsulation studies for application as biopesticides. Molecules 2020, 25, 5855. [Google Scholar] [CrossRef]
- Fernandes, M.J.G.; Pereira, R.B.; Pereira, D.M.; Fortes, A.G.; Castanheira, E.M.S.; Gonçalves, M.S.T. New eugenol derivatives with enhanced insecticidal activity. Int. J. Mol. Sci. 2020, 21, 9257. [Google Scholar] [CrossRef]
- Silvius, J.R. Thermotropic phase transitions of pure lipids in model membranes and their modifications by membrane proteins. Lipid-Protein Interact. 1982, 2, 239–281. [Google Scholar]
- Modjinou, T.; Versace, D.-L.; Abbad-Andaloussi, S.; Langlois, V.; Renard, E. Antibacterial and antioxidant photoinitiated epoxy co-networks of resorcinol and eugenol derivatives. Mater. Today Commun. 2017, 12, 19–28. [Google Scholar] [CrossRef]
- Azizi, N.; Saidi, M.R. Highly chemoselective addition of amines to epoxides in water. Org. Lett. 2015, 7, 3649–3651. [Google Scholar] [CrossRef]
- Fink, S.L.; Cookson, B.T. Apoptosis, pyroptosis, and necrosis: Mechanistic description of dead and dying eukaryotic cells. Infect. Immun. 2005, 73, 1907–1916. [Google Scholar] [CrossRef] [PubMed]
- Lang, G.-J.; Yan, Z.K.; Zhang, C.-X. Can acetylcholinesterase serve as a target for developing more selective insecticides? Curr. Drug Targets. 2012, 13, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, S.; Lagarde, A.; Iovinella, I.; Legrand, P.; Tegoni, M.; Pelosi, P.; Cambillau, C. Crystal structure of Apis mellifera OBP14, a C-minus odorant-binding protein, and its complexes with odorant molecules. Insect Biochem. Mol. Bio. 2012, 42, 41–50. [Google Scholar] [CrossRef]
- González-González, A.; Palma-Millanao, R.; Yáñez, O.; Rojas, M.; Mutis, A.; Venthur, H.; Quiroz, A.; Ramírez, C.C. Virtual screening of plant volatile compounds reveals a high affinity of Hylamorpha elegans (Coleoptera: Scarabaeidae) odorant-binding proteins for sesquiterpenes from Its native host. J. Insect Sci. 2016, 16, 30. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.S.; Xiao, S.; Carlson, J.R. The diverse small proteins called odorant-binding proteins. Open Biol. 2018, 8, 180208. [Google Scholar] [CrossRef]
- Pelosi, P.; Iovinella, I.; Zhu, J.; Wang, G.; Dani, F.R. Beyond chemoreception: Diverse tasks of soluble olfactory proteins in insects. Biol. Rev. 2018, 93, 184–200. [Google Scholar] [CrossRef]
- Pelosi, P.; Mastrogiacomo, R.; Iovinella, I.; Tuccori, E.; Persaud, K.C. Structure and biotechnological applications of odorant-binding proteins. Appl. Microbiol. Biotechnol. 2014, 98, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Raval, N.; Maheshwari, R.; Kalyane, D.; Youngren-Ortiz, S.R.; Chougule, M.B.; Tekade, R.K. Importance of physicochemical characterization of nanoparticles in pharmaceutical product development. In Advances in Pharmaceutical Product Development and Research: Basic Fundamentals of Drug Delivery; Chapter 10; Academic Press: Cambridge, MA, USA, 2019; pp. 369–400. [Google Scholar]
- Barroso, R.P.; Perez, K.R.; Cuccovia, I.M.; Lamy, M.T. Aqueous dispersions of DMPG in low salt contain leaky vesicles. Chem. Phys. Lipids 2012, 165, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Davarani, F.H.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Papadopoulou, V.; Kosmidis, K.; Vlachou, M.; Macheras, P. On the use of the Weibull function for the discernment of drug release mechanisms. Int. J. Pharm. 2006, 309, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Chouiter, M.I.; Boulebd, H.; Pereira, D.M.; Valentão, P.; Andrade, P.B.; Belfaitah, A.; Silva, A.M. New chalcone-type compounds and 2-pyrazoline derivatives: Synthesis and caspase-dependent anticancer activity. Future Med. Chem. 2020, 12, 493–509. [Google Scholar] [CrossRef]
- Amorim, C.; Veloso, S.R.S.; Castanheira, E.M.S.; Hilliou, L.; Pereira, R.B.; Pereira, D.M.; Martins, J.Á.; Jervis, P.J.; Ferreira, P.M.T. Bolaamphiphilic bis-dehydropeptide hydrogels as potential drug release systems. Gels 2021, 7, 52. [Google Scholar] [CrossRef]
- Seeliger, D.; de Groot, B.L. Ligand docking and binding site analysis with PyMOL and Autodock/Vina. J. Comput. Aided Mol. Des. 2010, 24, 417–422. [Google Scholar] [CrossRef]
- Jones, G.; Willett, P.; Glen, R.C.; Leach, A.R.; Taylor, R. Development and validation of a genetic algorithm for flexible docking. J. Mol. Biol. 1997, 267, 727–748. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2009, 31, 455–461. [Google Scholar] [CrossRef]
- Sander, T.; Freyss, J.; von Korff, M.; Rufener, C. DataWarrior: An open-source program for chemistry aware data visualization and analysis. J. Chem. Inf. Model. 2015, 55, 460–473. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An open chemical toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general amber force field. J. Comp. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef]
- Maier, J.A.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K.E.; Simmerling, C. ff14SB: Improving the accuracy of protein side chain and backbone parameters from ff99SB. J. Chem. Theory Comput. 2015, 11, 3696–3713. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, RevisionD.02; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Wang, J.; Wang, W.; Kollman, P.A.; Case, D.A. Automatic atom type and bond type perception in molecular mechanical calculations. J. Mol. Graph. Mode. 2006, 25, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Lapaillerie, D.; Charlier, C.; Fernandes, H.S.; Sousa, S.F.; Lesbats, P.; Weigel, P.; Favereaux, A.; Guyonnet-Duperat, V.; Parissi, V. In silico, in vitro and in cellulo models for monitoring SARS-CoV-2 Spike/Human ACE2 complex, viral entry and cell fusion. Viruses 2021, 3, 365. [Google Scholar] [CrossRef]
- Martins, F.G.; Melo, A.; Sousa, S.F. Identification of new potential inhibitors of quorum sensing through a specialized multi-level computational approach. Molecules 2021, 26, 2600. [Google Scholar] [CrossRef]
- Moorthy, N.S.H.N.; Sousa, S.F.; Ramos, M.J.; Fernandes, P.A. Molecular dynamic simulations and structure-based pharmacophore development for farnesyltransferase inhibitors discovery. J. Enzym. Inhib. Med. Chem. 2016, 31, 1428–1442. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sousa, S.F.; Cerqueira, N.M.F.S.A.; Brás, N.; Fernandes, P.A.; Ramos, M.J. Enzymatic “tricks”: Carboxylate shift and sulfur shift. Int. J. Quantum Chem. 2014, 114, 1253–1256. [Google Scholar] [CrossRef]
- Sousa, S.F.; Coimbra, J.T.; Paramos, D.; Pinto, R.; Guimarães, R.S.; Teixeira, V.; Fernandes, P.A.; Ramos, M.J. Molecular dynamics analysis of a series of 22 potential farnesyltransferase substrates containing a CaaX-motif. J. Mol. Model. 2013, 19, 673–688. [Google Scholar] [CrossRef] [PubMed]
- Marcial, B.L.; Sousa, S.F.; Barbosa, I.L.; Dos Santos, H.F.; Ramos, M.J. Chemically modified tetracyclines as inhibitors of MMP-2 matrix metalloproteinase: A molecular and structural study. J. Phys. Chem. B 2012, 116, 13644–13654. [Google Scholar] [CrossRef] [PubMed]
- Sousa, S.F.; Fernandes, P.A.; Ramos, M.J. Molecular dynamics simulations on the critical states of the farnesyltransferase enzyme. Bioorg. Med. Chem. 2009, 17, 3369–3378. [Google Scholar] [CrossRef] [PubMed]
- Sousa, S.F.; Fernandes, P.A.; Ramos, M.J. Enzyme flexibility and the catalytic mechanism of farnesyltransferase: Targeting the relation. J. Phys. Chem. B 2008, 112, 8681–8691. [Google Scholar] [CrossRef]
- Wang, E.; Sun, H.; Wang, J.; Wang, Z.; Liu, H.; Zhang, J.Z.H.; Tingjun, H.T. End-point binding free energy calculation with MM/PBSA and MM/GBSA: Strategies and applications in drug design. Chem. Rev. 2019, 119, 9478–9508. [Google Scholar] [CrossRef] [PubMed]
- Jaafar-Maalej, C.; Diab, R.; Andrieu, V.; Elaissari, A.; Fessi, H. Ethanol injection method for hydrophilic and lipophilic drug-loaded liposome preparation. J. Liposome Res. 2010, 20, 228–243. [Google Scholar] [CrossRef]



| Target | PDB | PLP | ASP | ChemScore | GoldScore | Vina | Overall Ranking |
|---|---|---|---|---|---|---|---|
| Ecdysone receptor | 1R20 | 68.82 | 36.26 | 30.28 | 57.54 | −7.40 | 5 |
| 1R1K | 74.97 | 35.04 | 35.86 | 56.38 | −8.60 | ||
| Chitinase | 3WL1 | 75.87 | 50.28 | 30.745 | 63.06 | −8.05 | 4 |
| 3WQV | 73.47 | 45.81 | 31.21 | 59.7 | −8.15 | ||
| β-N-acetyl-D-hexosaminidase OfHex1 | 3NSN | 78.01 | 57.04 | 36.30 | 70.90 | −7.10 | 3 |
| 3OZP | 72.91 | 51.61 | 31.11 | 67.35 | −8.05 | ||
| N-Acetylglucosamine-1-phosphate uridyltransferase (GlmU) | 2V0K | 65.88 | 30.02 | 25.93 | 55.305 | −7.15 | 13 |
| 2VD4 | 58.64 | 28.58 | 25.97 | 46.5 | −6.20 | ||
| Acetylcholinesterase (AChE) | 1QON | 89.26 | 58.71 | 40.87 | 71.41 | −8.90 | 2 |
| 4EY6 | 79.52 | 51.20 | 39.12 | 62.63 | −8.45 | ||
| 1DX4 | 85.66 | 51.32 | 37.48 | 67.86 | −8.70 | ||
| Prophenoloxidase (PPO) | 3HHS | 77.89 | 40.86 | 31.48 | 62.17 | −6.85 | 6 |
| p-Hydroxyphenylpyruvate dioxygenase | 6ISD | 70.33 | 37.53 | 31.03 | 55.00 | −7.35 | 9 |
| Voltage-gated sodium channel | 6A95 | 68.72 | 31.27 | 28.95 | 57.86 | −6.75 | 12 |
| Octopamine receptor | 4N7C | 57.07 | 37.56 | 31.60 | 61.72 | −5.55 | 11 |
| Sterol carrier protein-2 (HaSCP-2) | 4UEI | 65.70 | 37.22 | 31.64 | 50.255 | −7.40 | 10 |
| Peptide deformylase | 5CY8 | 76.49 | 33.49 | 29.60 | 65.65 | −7.75 | 7 |
| α-Esterase-7 | 5TYJ | 68.31 | 39.23 | 33.26 | 53.04 | −6.95 | 8 |
| 5TYP | 68.54 | 42.26 | 32.22 | 55.565 | −6.95 | ||
| Odorant Binding Protein | 5V13 | 86.69 | 52.52 | 41.24 | 66.46 | −6.90 | 1 |
| 2GTE | 79.14 | 41.46 | 38.55 | 69.11 | −7.55 | ||
| 3N7H | 82.54 | 44.31 | 36.01 | 69.11 | −6.90 | ||
| 3K1E | 91.51 | 48.43 | 41.90 | 74.58 | −9.30 |
| Average RMSD of the Complex (Å) | Average RMSD of the Ligand (Å) | Ligand SASA (Å2) | Percentage of Potential Ligand SASA Buried (%) | Average Number H-bonds | ΔGbind (kcal/mol) | Main Contributors | ||
|---|---|---|---|---|---|---|---|---|
| AChE | 3d | 4.6 ± 0.6 | 1.6 ± 0.4 | 59.6 ± 16.9 | 88 | 0.2 ± 0.1 | −18.3 ± 0.1 | Trp83 (−2.4 ± 0.8) Tyr370 (−1.3 ± 0.4) His480 (−1.3 ± 0.6) |
| 3f | 3.1 ± 0.2 | 1.4 ± 0.2 | 36.8 ± 10.2 | 93 | 0.5 ± 0.6 | −28.2 ± 0.2 | Tyr370 (−2.4 ± 0.1) Tyr374 (−2.5 ± 0.8) Trp83 (−1.9 ± 0.4) | |
| OBP | 3d | 2.2 ± 0.3 | 1.7 ± 0.3 | 27.2 ± 10.4 | 95 | 0.2 ± 0.5 | −31.7 ± 0.2 | Leu67 (−2.5± 0.5) Trp105 (−2.1± 0.4) Ala79 (−1.7± 0.5) |
| 3f | 2.2 ± 0.2 | 2.1 ± 0.3 | 33.2 ± 8.5 | 94 | 0.9 ± 0.9 | −41.6 ± 0.2 | Met75 (−2.9 ± 0.4) Trp105 (−2.5 ± 0.4) Phe114 (−1.8 ± 0.8) | |
| Dh ± SD (nm) | PDI ± SD | ζ-Potential ± SD (mV) | |
|---|---|---|---|
| DMPG (100%) | 196 ± 10 | 0.245 ± 0.007 | −43.8 ± 1.8 |
| DMPG:DPPC (1:1) | 223 ± 17 | 0.264 ± 0.014 | −55.2 ± 2.4 |
| EE (%) | Encapsulated Concentration (M) | |
|---|---|---|
| DMPG (100%) | 65 ± 7 | 4.39 × 10−4 M |
| DMPG:DPPC (50:50) | 92 ± 1 | 6.16 × 10−4 M |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, R.B.; Pinto, N.F.S.; Fernandes, M.J.G.; Vieira, T.F.; Rodrigues, A.R.O.; Pereira, D.M.; Sousa, S.F.; Castanheira, E.M.S.; Fortes, A.G.; Gonçalves, M.S.T. Amino Alcohols from Eugenol as Potential Semisynthetic Insecticides: Chemical, Biological, and Computational Insights. Molecules 2021, 26, 6616. https://doi.org/10.3390/molecules26216616
Pereira RB, Pinto NFS, Fernandes MJG, Vieira TF, Rodrigues ARO, Pereira DM, Sousa SF, Castanheira EMS, Fortes AG, Gonçalves MST. Amino Alcohols from Eugenol as Potential Semisynthetic Insecticides: Chemical, Biological, and Computational Insights. Molecules. 2021; 26(21):6616. https://doi.org/10.3390/molecules26216616
Chicago/Turabian StylePereira, Renato B., Nuno F. S. Pinto, Maria José G. Fernandes, Tatiana F. Vieira, Ana Rita O. Rodrigues, David M. Pereira, Sérgio F. Sousa, Elisabete M. S. Castanheira, A. Gil Fortes, and M. Sameiro T. Gonçalves. 2021. "Amino Alcohols from Eugenol as Potential Semisynthetic Insecticides: Chemical, Biological, and Computational Insights" Molecules 26, no. 21: 6616. https://doi.org/10.3390/molecules26216616
APA StylePereira, R. B., Pinto, N. F. S., Fernandes, M. J. G., Vieira, T. F., Rodrigues, A. R. O., Pereira, D. M., Sousa, S. F., Castanheira, E. M. S., Fortes, A. G., & Gonçalves, M. S. T. (2021). Amino Alcohols from Eugenol as Potential Semisynthetic Insecticides: Chemical, Biological, and Computational Insights. Molecules, 26(21), 6616. https://doi.org/10.3390/molecules26216616











