Abstract
This review reports recent knowledge on the role of ingredients (barley, hop and yeasts), including genetic factors, on the final yield of phenolic compounds in beer, and how these molecules generally affect resulting beer attributes, focusing mainly on new attempts at the enrichment of beer phenols, with fruits or cereals other than barley. An entire section is dedicated to health-related effects, analyzing the degree up to which studies, investigating phenols-related health effects of beer, have appropriately considered the contribution of alcohol (pure or spirits) intake. For such purpose, we searched Scopus.com for any kind of experimental model (in vitro, animal, human observational or intervention) using beer and considering phenols. Overall, data reported so far support the existence of the somehow additive or synergistic effects of phenols and ethanol present in beer. However, findings are inconclusive and thus deserve further animal and human studies.
1. Introduction
Beer is a natural drink and historical evidences indicate a common use since ancient times also for medical and religious purposes [1]. Antique recipes prove widespread production back to 5000 years ago [2]. Beer is actually the most consumed alcoholic beverage in the EU and annual per capita consumption (L/year) has sharply increased in the Czech Republic (141 L), US (50–80 L) and France (33 L) [3]. Such a level of consumption has led some research to focus on the nutritional appropriateness of beer, merely considering health aspects like, for example, the intake of minerals [4] or the ability to prevent dysbiosis [5], properties also present in other beverages. Unfortunately, like wine, beer naturally contains ethanol, a well-known toxic and carcinogenic molecule [6].
Nonetheless, characteristic of beer is the high content in phenolic compounds, which are the focus of this review. The consumption of polyphenol-rich foods, like beer, is a well-accepted factor involved in the prevention of oxidative stress-associated diseases [7]. Traditionally, beer is obtained from as little as four basic ingredients: barley, hop, yeast and water. The first two ingredients naturally contain phenolics, however during beer production, these molecules undergo chemical modifications and new molecules are formed, influencing both the yield and final characteristics of a beer. Aroma, flavors, taste, astringency, body and fullness are the result of the metabolic activity of microbes on raw materials, and scientific evidences suggesting that they are all influenced by phenol content are summarized here. Moreover, this review focusses more deeply on most recent advances on the role of phenolic compounds on affecting human health status, considering how seriously researchers have tackled the effects of alcohol.
2. Main and Minor Beer Phenols
The polyphenolic composition of beers is considered as one of the quality indicators of beer processing and marketing [8]. In fact, the type and quantity of phenols influence taste, aroma and color, but also colloidal and foam stability, shortening beer’s shelf-life taste (see Section 4, “Phenols and beer attributes”). Several different groups of phenolic compounds have been reported in beer, the main ones being phenolic acids and tannins, and flavones and flavonols [9]. Because of its high concentration, also thanks to high producing yeasts (see Section 5, “The role of barley, yeast and hop genetics on beer phenols”), the simple phenolic alcohol tyrosol is one of the main phenols looked at in beer, present also in alcohol-free beers [10]. Concentration is so high in certain beers, reaching that of red wine [11], that authors have hypothesized that tyrosol could represent an indirect source, through biotransformation, of the more biologically active hydroxytyrosol [12] (see Section 6, “Phenols-related health effects of beer consumption”). In alcoholic beers, both phenols possibly protect yeast from the stress generated by high levels of ethanol, a phenomenon that has been demonstrated for wine’s resveratrol [13], indicating that phenols not only undergo changes during brewing, but they also direct it. Accordingly, non-alcoholic beers normally have lower phenolic content [14], supporting the existence of a correlation between phenols and alcohol concentrations. Among minor phenols, those derived from barley, for example alkylresorcinols, are a group of phenolic lipids for which in vitro antioxidant and antigenotoxic [15] and in vivo diet-induced obesity-suppressing [16] activities have been reported. Even if contribution to alkylresorcinols dietary intake appears not significant, higher amounts were reported in stout beer [11]. Other quantitatively minor phenols derived from hop, for example, xanthohumol and other prenylated flavonoids, contribute significantly to beer flavor and aromas and have antibacterial, anti-inflammatory and antioxidant properties, and phytoestrogen activity [17,18]. Prenylflavonoids are of particular interest for beer as, on the one hand, no other food sources other than hop are known and, on the other hand, they are present regardless of the fermentation method, ale or lager, even if higher concentrations were found in stout and India Pale Ale styles [11].
Despite the fact that prenylated flavonoids can last for 10 years in beer stored at room temperature [19], monophenols and flavonoids show a temperature- and time-dependent decay in beer [20,21]. This phenomenon was initially studied using radioactive isotopes that revealed that almost 65% of molecules belonging to the tannin fraction go through oxidation [22]. Later, other evidences supported the role of oxidation in the time-dependent decay of phenols in beer, also demonstrating the role of the intrinsic haze-forming ability of some phenols [23]. Meanwhile, acetaldehyde was also involved in haze formation, because of its ability to polymerize polyphenols and compromise beer’s flavanols level [24]. A resolutive approach to this problem could come from the implementation of dry-conservation. It was recently reported that production of microparticles from beer through high-temperature (up to 180 °C) spray-drying, used for the development of functional food with a specific heath objective, yielded a well-accepted beverage, in terms of appearance, taste and color, that kept, up to the entire period of dry-conservation (180 days), the initial amount of total phenols (measured using the Folin–Ciocalteu method) [25]. Even if no qualitative indication of phenols was reported, the study supports the validity of spray-drying in the production of non-alcoholic, high-phenols, beer-flavored beverages (see Section 9, “Phenols in non-alcoholic and isotonic beers”).
3. Phenols’ Fate during Malting and Brewing
As mentioned in the introduction, beer content in phenols depends on the type of barley and hops used for production. Even if hops contain a huge amount of phenols (up to 4% of dry matter) compared to barley (up to 0.1%), on average, four fifths of beer’s phenols come from malt or other mashed cereals, because of their significantly higher starting amount [26]. Phenols undergo both quantitative and qualitative changes during seed germination and brewing processes [27] (Figure 1). The germination of barley seed, i.e., malting, has been studied deeply and is preceded by seed hydration (steeping), during which phenolic content decreases due to leaching, and followed by seed-drying (kilning), during which the improved crumbliness of the grain enhances the enzymatic release of bound phenolic acids. Kilning can be performed at different temperatures, for example in special malts brewing in order to bring desirable flavors and colors [28]. At temperatures lower than 80 °C, kilning normally induces an increase in the amount of water-soluble total phenolic compounds [29], thanks to a Maillard-enzymatic release of phenols in the matrix [30] and to increased friability and extraction from the grain [31]. According to Leitao and colleagues, total phenolic content of barley (whose antioxidant contribution is mostly for ferulic and sinapic acids) increases four-fold during the transition to malt. Even if final yields depend on the malting procedures, the amount of phenolic compounds present in malt is inversely correlated with the degree of steeping and positively influenced by the germination temperature [32]. More recently, Koren and coworkers reported a 3- to 5-fold increase in the amount of total polyphenols during malting in six barley varieties, independently from the initial amounts [33].
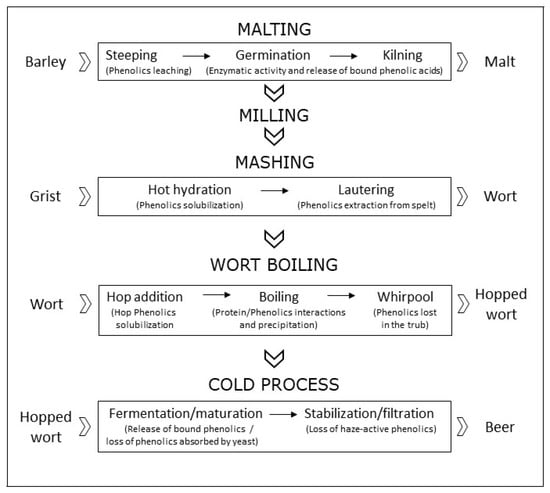
Figure 1.
Phenolic compounds’ fate during the phases of malting and brewing processes: in the phase of mashing, after an initial decrease, total phenolics amount increases 3- to 5-fold; afterwards, phenolics continue to increase throughout mashing and during hop addition, but dramatically decrease during wort boiling, whirpool, fermentation, maturation, stabilization and filtration, so that, during the entire brewing process, about 60% of the malt phenolic content is lost.
The amount of polyphenols reached in malt then significantly falls during brewing steps, depending on the protocol adopted, with a higher decrease for malt milled in wet conditions [34]. Enzymatic and non-enzymatic solubilization of phenols take place during the first step of mashing (hot hydration), and both are influenced by temperature and time, as well as the separation of wort, during which extraction of phenolic-rich spelt material occurs [9]. A successive increase of total phenolic compounds occurs in the wort separation (lautering) due to the extraction from spelt materials. Brewing is fundamentally ascribable to the metabolic activity of a fermentable carbohydrate source in the absence of oxygen, yielding alcohol and carbon dioxide. Fermentation is normally performed at fixed temperature but can be pushed at higher or lower temperatures. Hops, which were formerly included in the brewing process mainly for their preserving properties, are then added and wort boiling is started. Hops addition actually has several advantages, improving not only the bitter taste and astringency but giving protection to beer brewing yeasts, thanks to its antibacterial activity, against Gram-positive bacteria, and lowering pH to 4–4.2 [35]. During boiling, hop polyphenols are released and polymerization reactions with proteins occur, yielding precipitated complexes, responsible for the formation of chill haze, that are then lost in the successive whirpool process and during the final filtration and stabilization. Final processes are critical for polyphenols and include fermentation, warm rest, chill-lagering filtration and clarification [36]. During brewing, around 60% of the malt phenolic content is lost. Decay affects all phenolic compounds, excepting p-hydroxybenzoic acid and sinapic acid, whose concentration increases by even four-fold [31]. However, different brewing processes can deeply influence total phenolic compounds, for example bock beers are normally three times richer than dealcoholized beer, with intermediate and decreasing quantities for abbey, ale, wheat, pilsner and lager beers [36]. Recent data also indicate that beer’s content in phenols is associated with the production scale. In fact, the lesser characterized craft beers (unpasteurized and unfiltered) [37], whose production scale is limited by law in several countries (200,000 hL/year in Italy), exhibit higher total phenolic compounds’ values compared to large-scale beers [38], mainly thanks to the lack of filtration. Finally, the phenolic content of beer is affected negatively by higher temperature pasteurization treatments [39].
4. Phenols and Beer Attributes
The ability of phenols to influence beer taste has been well known since the early 1960s, when the so-called “sunlight flavor” was ascribed mainly to humulone and lupulone addition after beer fermentation [40]. Phenols’ ability to interfere with aroma, instead, was noticed around forty years ago, thanks to a S. cerevisiae “killer strain” producing a clove-like aroma [41]. Later, a study clarified that presence of the main phenolic flavors relies on yeasts capability to decarboxylate or reduce phenolic acids: 4-vinylguaiacol and 4-vinylphenol from S. cerevisiae and 4-ethylguaiacol and 4-ethylphenol from Brettanomyces sp. [42]. More recent data indicate that the ability of phenols to selectively characterize beer’s flavors relies on their chemical transformations. For example, thermal decarboxylation of ferulic acid to 4-vinyl guaiacol, occurring during wort boiling and during fermentation, induces a three-orders-of-magnitude increase in its flavor threshold [43]. Unfortunately, some metabolic reactions have side effects, like that involving cinnamic acid and yielding the toxicologically relevant styrene [44]. Moreover, higher concentrations of monophenol can turn spicy or vanilla-like sweet flavor notes to unpleasant medicinal-like flavors [45]. A recent deep analysis of the association between metabolites and sensory characteristics using two-way orthogonal partial least squares indicates that isoferulic acid affects beer’s fruity sensory attributes [46], suggesting the possibility to predict to some extent the formation of specific flavors.
With respect to aroma, phenols’ protecting properties were found almost 25 years ago: phenols were found to prevent the formation of off-flavors, before and during malting, and the phenomenon was ascribed to their antioxidant activity in barley and malt [47]. More recently, some specific monophenols that confer the typical aroma of some popular beers were identified [48] and recently reviewed [49]. Worthy of interest are Czech beers whose distribution of individual phenolic compounds, that has been brought back to the origin of raw materials and the technology used for processing, is so unique that they have been proposed for authenticity analysis [50,51]. With respect to color, after high-affinity selective removing of tannins, Dadic and Van Gheluwe observed a severe discoloration of beer, demonstrating for the first time the correlation between phenols and beer color [52]. The involvement of monoflavanols’ oxidation on beer color was further demonstrated by the recovery of oxidized molecules in polyethylene terephthalate bottle-stored beer [20]. More recently, several works have clearly demonstrated the relationship between phenols and beer color, both in small- and large-scale brewed beers [38].
Barley seeds’ phenolic acids, flavonoids and proanthocyanidins influence quality indexes like viscosity, diastatic power and nitrogen content [53], and have an impact on beer turbidity [54], taste, bitterness and aroma [55]. With regard to hop, which was antiquely added in beer especially for its pleasant aroma and bitterness, brewing trials indicate that hop phenols can selectively reduce flavor deterioration during storage [56], specifically the sunstruck off-flavor that is formed in beer upon light exposure [57]. More recent data indicate a temporal effect. In fact, later addition of hop, just before the end of wort boiling, significantly increases phenolic content [58]. Astringency, bitterness and fullness, which are affected by the boiling time [39], have been linked to different hop phenols fractions [59,60].
5. The Role of Barley, Yeast and Hop Genetics on Beer Phenols
The yield in phenols of a beer necessarily depends on the genetic background of its raw ingredients, and differences were reported in barley grain [61], hop [62] and yeast [63]. Unfortunately, domestication of barley and hop has reduced phenols’ diversity. Nevertheless, total polyphenol content could be linked to specific quantitative trait loci in barley [64] and some specific combinations of phenols in barley can still be attributed to different genotypes. For example, the ratio between barley’s main phenolic acids, ferulic acid and p-coumaric acid, is genetically determined and combinations can also influence key agronomic traits, such as hull adherence and grain color [65], through functionally related genes [53]. Studies combining genetics and environment on wild barley cultivars, that show a wider genetic diversity in agronomic traits and abiotic stress tolerance, identified some genes involved in phenol accumulation in barley seeds. Such studies are of special relevance as they can give a picture of the loss of genetic variation due to domestication and provide information for the set-up of breeding applications for phenols-related beer improvement. For example, a network analysis of gene expression and secondary metabolites, induced by the well-known stressor drought [66] in developing grains from several different Tibetan wild barley cultivars, recently allowed the identification of genes whose manipulation is believed to help the development of cultivars with specific contents of phenolic compounds [67]. Less data is available for a role of the genetic background on hop phenols. For example, a significant cultivar-dependent role has been recently reported for 2-phenylethyl glucoside [68], but the relevance on final quantities recoverable in beer is still lacking.
The ability of yeasts to adapt to different chemical (sugar, nitrogen) and physical (temperature, pH, oxygen, sulfur dioxide) properties resides in the great genetic diversity that has been exploited by the beer industry, i.e., for the development of strains with distinct flavor profiles. The production of different metabolites, like volatile phenols, is the direct consequence of human influence through wine and beer production. A first evidence testifying the role of the genetic background of yeasts in beer phenols came from the observation, at the beginning of the twentieth century, of volatile “ethereal substances” in English stock ales, during fermentation by Brettanomyces [69]. Brettanomyces bruxellensis, the first microorganism to be patented for beer production, was also involved in the spoilage of draught beer [70] and in the clove off-flavor (the ethylphenol 4-vinylguaiacol) [71] but, after being reported together with Lactobacillus vini as a contaminant in several ethanol-producing plants [72], was finally isolated from a number of fermented beverages and food, from cider to olives [73]. Spoilage depends on a still not fully identified gene pathway that involves two phenylacrylic acid decarboxylase (PAD) enzymes [74]. Ethylphenols production has been related to strain-dependent PAD amino acid sequence variability [75]. Thanks to their ability to convert ferulic acid to 4-vinylguaiacol, yeasts are believed to have a stronger impact on phenols than thermal processing steps [76]. Yeasts also have a fundamental impact in barrel beer ageing. Barrel-aged beers are sensorially enriched beers obtained by storage of already fermented beers in wood casks or by fermentation of beer’s wort directly in wood barrels. Such processes mainly occur because of the spontaneous growth of microbes present in breweries’ atmosphere and in barrels [77]. During this fermentative incubation, a bi-directional exchange of different molecules occurs from wood and beer: some beer’s molecules are retained by the wood while others are released from wood to the beverage. Dekkera bruxellensis, another spoilage-related microbe in wine, is considered the main contributor to the aroma of aged beers, through its ability to convert hydroxycinnamic acids to volatile phenols, and has several advantages, from high ethanol yield to low pH tolerance [78]. Its spontaneous growth is accompanied by some enzymatic activities that transform wort composition and yield the final chemical and sensory profiles of aged beer.
Aiming at finding optimal conditions for accelerating wort transformations, research is focused at finding optimal chemical conditions to produce beers with specific and preferred bacterial metabolites, normally avoiding those from non-Saccharomyces species, in multi-starter cultures. For such purpose, Coelho and coworkers recently found that low glucose or high ethanol conditions favor the yield of D. bruxellensis-related metabolites over S. cerevisiae ones [79]. Ethanol-resistance and increased dominance towards other S. cerevisiae strains were also reported on mixed starter fermentations for the high polyphenols-producing S. cerevisiae var. boulardii strain [80]. A recent deep genomes/phenomes analysis involving 157 industrial S. cerevisiae strains [81] reported that production of 4-vinylguaiacol relies on specific genetic variants able to ferment maltotriose [81]. More recently, next-generation sequencing allowed the identification of a Brettanomyces strain void of phenolic off-flavors, limiting economic losses during production [82], a problem that was bypassed in S. cerevisiae by the selection of strains with inactivated alleles and/or functional copies [83]. Worth mentioning is a recent work that, seeking to explain different adaptive abilities, profiled microsatellite markers and ploidy-states of 1488 isolates coming from niches dispersed all over the world [84].
6. Phenols-Related Health Effects of Beer Consumption
While the serious damages of high alcohol intake are known, the effects of moderate consumption of alcoholic beverages are still a source of heated debate. Moderate beer consumption is believed to be associated with protective cardiovascular function and reduction in the development of neurodegenerative disease. Moreover, there is no evidence that moderate beer consumption can stimulate cancer. Nevertheless, alcohol consumption can become a problem for people at high risk of developing alcohol-related cancer or for those affected by cardiomyopathy, cardiac arrhythmia, depression, liver and pancreatic diseases, and is not recommended for children, adolescents, pregnant women and frail people at risk of alcoholism [85]. Anyway, beer, like wine, contains the already mentioned substances with indubitable protective capacities, not merely anti-inflammatory and antioxidant, as demonstrated by huge in vitro work on single substances [86]. However, the ambitious objective in studying the effects of beer consumption on human health is to analyze it in toto and, in order to understand the single contribution of phenols and alcohol, parallel experiments with similar doses of an equivalent non-alcoholic beer and of alcohol alone are essential. For example, Karatzi and coworkers [87] reported that both non-alcoholic and alcoholic beers improved some arterial biomarkers (reduced aortic stiffness and increased pulse pressure amplification), but the effects were also similar in a parallel vodka intervention, containing the same amount of ethanol as the alcoholic beer. However, as some other effects (wave reflections reduction) were higher in the alcoholic beer intervention compared to alcohol alone (vodka), and the endothelial function was significantly improved only after beer consumption, the authors concluded that the non-alcoholic and the alcoholic fractions of beer could have additive or synergistic effects [87].
We thus thought to analyze the fraction of similar publications that considered, in the search of the health effects of beer containing phenols, also the effects of the presence of alcohol. For such purpose, we used in Scopus.com the search string TITLE-ABS-KEY (beer AND (phenol OR polyphenol OR flavonoid) AND (observational OR administration OR consumption OR drinking OR prospective OR intervention OR crossover OR trial)) AND (LIMIT-TO (DOCTYPE, “ar”)). The search was performed on October 2020 and returned 161 documents, including 31 reviews (even if they were already excluded by the string search), 7 not pertinent articles, 9 studies merely evaluating phenols’ population intakes, 51 chemical-only reports (papers reporting chemical analyses of phenols of commercial or improved beers) and 22 reports using only single phenols in in vitro or in vivo models. For the remaining 41 (minus one not available even by the authors themselves [88]), experimental models, parameters tested and main findings are summarized in the next section and sorted chronologically by the most recent, in Tables 1–3, about in vitro and animal models, human intervention and human observational, highlighting the use of alcohol alone (spirits, eventually vodka or gin), as well as non-alcoholic beer.
6.1. In Vitro and Animal Experiments
As demonstrated by in vitro cancer cell models (Table 1), several cancer types are sensitive to the antiproliferative action of some beer components, including ethanol. For example, epithelial cells’ viability was reduced in a similar way by beer or an equivalent amount of ethanol [89]. Unfortunately, the authors did not test an alcohol-free beer. Using single molecules or a matrix containing all beer components, Machado and coworkers showed that phenols’ activities are synergic [90]. Unfortunately, in this case, ethanol was not tested. Similarly, a total extract obtained from dark beer conferred higher protection to rat C6 glioma and human SH-SY5Y neuroblastoma cells against an oxidant stressor challenge (hydrogen peroxide) compared total extracts obtained from non-alcoholic and lager beers [91]. Again, neither the phenolic compounds of beers nor an alcoholic reconstituted extract were tested.

Table 1.
In vitro and animal studies.
Wistar rats were used in several experiments with beer. One publication reported that both administration of alcoholic (4%) or lyophilized beer for 4 weeks had low, but statistically significant, beneficial effects on plasma lipidemic and antioxidant markers (total cholesterol, low-density lipoprotein (LDL) cholesterol, triglycerides and lipid peroxides), however alcohol alone was not tested and the authors themselves concluded that minimal effects observed could rely on relatively low alcoholic content of beer [100]. Next, using only a polyphenol-free beer, the same group concluded that lipid effects had to be ascribed to beer proteins, as long as effects were absent in rats fed with polyphenol-free wine [99]. In rats with skin incision-induced wound healing, feeding for 4 weeks with alcoholic beer prevented alcohol-induced markers of inflammation, oxidative stress and angiogenesis [97]. Notably, when beer was enriched with 10 mg of xanthohumol, effects were even more ameliorated. Similar results were obtained using animals with streptozotocin-induced diabetes [96]. On the same streptozotocin-induced diabetes model, hepatic glucolipid metabolism, lipogenic enzymes and glucose transporter 2 levels were tested after 5 weeks of administration of xanthohumol-enriched alcoholic beer for 5 weeks [95]. Interestingly, beer prevented all the streptozotocin-induced liver catabolic state alterations tested (fibrosis, apoptosis, glycogen depletion, GLUT2 upregulation, lipogenesis reduction) and the effect was not observed in rats fed with normal beer. The authors also tested the effect of ethanol alone but, in none of these last three works were an alcohol-free beer, nor xanthohumol alone, tested, thus it is impossible to distinguish neither the effect of beer components nor of the polyphenol itself. Furthermore, in vitro and in vivo work on xanthohumol metabolites (isoxanthohumol and 8-prenylnaringenin) previously indicated opposite effects on angiogenesis and inflammation processes (pro-angiogenetic for 8-prenylnaringenin and anti-angiogenic and anti-inflammatory for the other two) [101]. Nevertheless, a xanthohumol-fortified alcoholic beer was used again to demonstrate attenuated pharmacologically induced pulmonary vascular remodeling and improved cardiac function [93]. Also, in this case, even if effects were absent in rats fed only with ethanol, no rats were tested with an alcohol-free beer. It is noteworthy that the authors could identify the involvement of extracellular signal-regulated kinase1/2, phosphatidylinositol 3-kinase/protein kinase B and VEGF receptor 2 in the protective properties of beer towards pulmonary arterial hypertension [93]. In a prepubertal rat model, beer with 10% alcohol significantly decreased, after 4 weeks, the levels of sex hormones, compared to ethanol- or water-fed rats [92]. Again, even if authors concluded that beer inhibited the ethanol-induced increase of cleaved caspase-3 in Leydig cells, a non-alcoholic beer was not tested. In addition to the works recovered using the Scopus.com search string and mentioned in Table 1, worthy of mention are experiments showing that alcoholic-free beer can decrease the aminooxyacetic acid-induced GABA accumulation in hypertensive animals [98], and prevent brain inflammation and neurodegenerative effects induced by aluminum nitrate [94]. However, while as expected hops administration alone had a beer-overlapping positive effects to some extent, so did silicon administration, reinforcing the need for an appropriate set-up of experimental models.
6.2. Role of Alcohol on Phenols’ Metabolism and Beer Antioxidant and Anti-Inflammatory Properties, and on Cardiovascular-Related Effects
Phenolic acids’ absorption, previously reported both in low-alcohol [102] and alcoholic beer [103], is impaired by ethanol removal from beer [104]. The opposite effect of alcohol has been reported for tyrosol metabolization to hydroxytyrosol following beer consumption, as mentioned above. In particular, the administration of a single dose of 250 mL of blonde beer was associated to higher urinary recovery of tyrosol, whilst an identical dose of alcohol-free beer yielded higher urinary recovery of hydroxytyrosol [12]. However, as alcohol consumption proportionally increases hydroxytyrosol excretion through dopamine metabolism [105], hydroxytyrosol bioavailability is hardly attributable only to beer phenols.
Among first beer intervention studies (Table 2), there is an almost-perfectly set-up randomized acute administration of either 4.5% alcoholic beer (n = 14), or dealcoholized beer or 4.5% water solution of ethanol (n = 7), for the evaluation of the contribution of beer’s alcohol [104]. Results demonstrated that a significant increase in plasma antioxidant capacity (TRAP) could be obtained only following alcoholic beer administration. Unfortunately, no crossover intervention was performed, and the effects were studied only in a temporally limited manner. In another similar, but a crossover, acute intervention of beer or wine (or vodka for the evaluation of the contribution of alcohol) inhibition of oxidative stress induced (by 100% normobaric O2 breathing) was tested [106]. Analysis of stiffness 3 h after administration showed that only wine prevented oxygen-induced oxidative stress, possibly because of the higher content of polyphenols compared to beer i.e., 2.6 g/L vs. 0.4 g/L gallic acid equivalents (GAE) [106]. No one can say if such a low phenols amount in an equivalent alcohol-free beer could have produced the effects observed with wine. Daily supplementation of breastfeeding mothers (n = 30) with 660 mL of non-alcoholic beer was associated with an improvement of mothers’ plasma and breastmilk antioxidant capacities, assessed 30 days postpartum, compared to control non-supplemented mothers [107]. For obvious reasons, an alcoholic beer was not tested. Administration of alcohol-free beer (500 mL) for 45 days to postmenopausal women (n = 29) was associated with a reduction of several indicators of early protein oxidation, especially reducing cholesterol levels in subjects with higher than 240 mg/dL [108], supporting the usefulness of long-term alcohol-free beer consumption in fighting low-grade chronic inflammation and preventing metabolic disorders. As an alcoholic beer was not tested, one might speculate that alcohol can abolish the beneficial effect. However, previous work that used a crossover intervention trial (healthy drinkers, n = 27) to switch consumption of beers with similar phenolic content (310–330 mg/L) for 4 weeks, from low (0.9%) to high (4.9%) alcohol and vice versa, indicates that while the switch to low-alcohol did not change in vitro LDL oxidizability, the opposite switch did [109]. On the other hand, only non-alcoholic beer daily consumption for one week (17 healthy females, 330 mL) was associated to an increase in the urinary antioxidant capacity, as measured by Trolox equivalents [110], contradicting the results of the study reported at the beginning of this paragraph.

Table 2.
Intervention studies (n, subjects’ number; y, age (years)).
One observational study (1604 subjects of the IMMIDIET (Dietary Habit Profile in European Communities with Different Risk of Myocardial Infarction: the Impact of Migration as a Model of Gene-Environment Interaction) study, 26–65 years, see Table 3) supports a somewhat interfering property of alcohol on non-alcoholic components of beer. In fact, adjustment of beer intake for alcohol content broke the association between beer consumption and higher plasma and red blood cell omega 3 fatty acids [119]. In the overweight or class 1 obese healthy subjects, the daily consumption of alcoholic beer (but not of alcoholic-free beer with similar amount of total phenols) for four weeks raised HDL levels in subjects with low LDL-lipid profile and facilitated cholesterol efflux from macrophages, without affecting body mass index (BMI), liver and kidney functions, potentially reducing the risk of vessels occlusion by cholesterol deposition [120]. As the consumption of alcohol alone was not tested, it is not possible to exclude that the effects could be at least partially ascribable to alcohol. Similarly, in a crossover study of 28 daily healthy nonsmoking normotensive men consuming alcoholic beer (1125 mL; 41 g alcohol) for 4 weeks, an increase of the awake systolic blood pressure and the asleep heart rate was reported, however the effects were identical in men consuming red wine containing the same amount of alcohol [118], and an alcohol-free beer was not tested. Similarly, analysis of stiffness, 3 h after administration of alcoholic beer or vodka, showed that both protected against oxygen-induced increase in arterial stiffness, making the authors conclude that the observation was probably due to a central vasodilatatory effect of alcohol itself [106]. Again, Gorinstein and coworkers found that alcoholic beer consumption (330 mL daily, containing 510 mg of polyphenols and 20 g of alcohol for 30 days) ameliorated markers of coronary atherosclerosis of hypercholesterolemic in non-drinker males (n = 42, 43–71 years) during recovery from coronary bypass surgery [117]. Unfortunately, the control group of the randomized single-blind trial had only water “with minerals of beer”, making it impossible to ascribe effects to either phenols or to alcohol. In a double-blind intervention of healthy male runners (n = 277), daily consumption of non-alcoholic beer, for 3 weeks before and 2 weeks after a marathon, reduced interleukin-6 immediately after the race, total blood leukocyte counts immediately and 24 h after the race and post-marathon incidence of upper respiratory tract illness [116]. However, like for breastfeeding mothers mentioned above, alcoholic beer was not tested, we guess for similar obvious reasons. Also, other observational studies (Table 3) suffer from this limitation. For example, a significant inverse association between beer consumption (and not for coffee, nuts, tea, olive oil and red or white wine) and hypertension was found by means of food frequency questionnaires submitted to 2044 adults [121], however neither the consumption of alcohol-free beer nor the contribution of pure alcoholic beverages were evaluated.

Table 3.
Observational studies (n, subjects’ number; y, age (years)).
An open, randomized, crossover, finely set-up controlled intervention trial of 33 high-cardiovascular risk males drinking daily, for 4 weeks, a non-alcoholic beer (containing 1243 mg of total polyphenols) or an alcoholic beer (containing 1209 mg of total polyphenol and 30 g of ethanol), was repeatedly used (apparently with the same composition of subjects) by a group of Spanish researchers during the last 6 years, to investigate the possible synergistic effects of beer polyphenols and alcohol, using as control an administration of gin (containing 30 g of ethanol). Firstly, in an attempt to use urinary isoxanthohumol as a marker of beer consumption, a similar amount of the metabolite was recovered following non-alcoholic or alcoholic beer consumption, and no excretion was found following gin administration [115]. Notably, group differences in a female sub-population were found, but only an alcoholic beer was tested. Next, they looked for circulating endothelial progenitor cells (EPC) and reported that non-alcoholic beer consumption increased the number of circulating EPCs by 5 units, while in the alcoholic beer group, the increase was 8-fold. However, even if observations were not statistically significant, alcohol alone (gin) induced a 5-fold decrease in the number of circulating EPCs [114], suggesting the existence of some influencing, maybe genetic, factors. Then, they reported that only non-alcoholic beer consumption reduced leukocyte adhesion molecules and inflammatory biomarkers (decreased homocysteine and increased serum folic acid) [113], suggesting a possible antagonistic effect between alcohol and the non-alcoholic fraction of beer. Importantly, the alcoholic beer improved other plasma lipid and inflammation markers (high-density lipoprotein cholesterol, apolipoproteins A1 and A2, and adiponectin) and decreased fibrinogen and interleukin 5, but the effects were ascribed to alcohol as identical effects were observed following administration of a gin dose containing the same amount of alcohol (30 g). Finally, the group of Spanish researchers applied liquid chromatography-coupled Linear Trap Quadropole-Orbitrap mass spectrometry to discover the urinary metabolites produced in the intervention study. Increased urine excretion of hop α-acids and fermentation products were found following beer consumption with respect to the gin administration, but differences were slight and not completely reported [112].
6.3. Role of Alcohol on Phenols-Related Effects of Beer on Cancer
A case-control association study (over 14 years) of child acute lymphoblastic leukemia (n = 491 + 491) found an inverse relation with maternal moderate consumption (self-reported) of beer (and wine, but not spirits), making authors suggest a protective effect of flavonoids [130]. However, a positive relation was reported also for fathers, which is difficult to explain and minimizes the observation’s reliability. In a similar matched case-control study of drinking/smoking habits (over 10 years) of leukoplakia patients (n = 187 + 187; 40–65 years), while a role of regular wine consumption was associated with a decreased probability of disease occurrence (compared to that of spirit drinking that was associated to increased risk), no significant effect for moderate beer drinking was found [126]. The authors concluded that weaker effects of beer were probably due to the different composition in substances synergistically or antagonistically, i.e., polyphenols, interacting with ethanol [126]. Nonetheless, using a 20-country wide one-year (2002) evaluation of alcoholic beverages consumption and total deaths for oral cancer, the same authors estimated a lower risk for beer (and wine) consumers compared to heavy alcohol consumption from spirits [128]. Similarly, the consumption of beer (nor liquor) could not be associated with prostate cancer risk, in a population case-control study taking into account the self-reported alcohol consumption (n = 753 + 703; 40–64 years), even if the same authors reported a reduced relative risk associated with increasing level of red wine consumption [129]. More recently, lack of association with prostate cancer was reported for beer consumption (but also for wine and liquor) in a bigger prospective study (n = 84,170; 45–69 years) [124].
6.4. Role of Alcohol on Phenols-Related Effects of Beer on the Microbiota
According to a relationship between microbiota, host genes and diet [131], recent work investigated the possibility that alcohol-free beer, acting at the level of gut microbiota, could prevent the metabolic syndrome (MS). In fact, occurrence of MS can be promoted by gut microbiota dysbiosis, through low-grade inflammation and alteration of lipid metabolism. Gut microbiota dysbiosis can in turn be induced by alteration of the relative abundance of bacterial families [132]. Thus, a daily administration for one month of 355 mL of non-alcoholic beer was found associated to a decrease in fasting blood serum glucose and an increase in functional β-cells only, and the effect was not observed with an alcoholic beer containing a similar amount of phenolic compounds [111]. Moreover, the authors observed an enrichment of the microbiota diversity, also with the alcoholic beer, but only alcohol-free beer consumption was associated to a specific microbiota diversity with healthier function, suggesting that alcohol inhibited the positive effects of beer. As β-diversity was observed only after 30 days of treatment, the authors hypothesized that the effect on gut microbiota could depend on polyphenols and phenolic acids [111]. Similar results were obtained in an observational study, especially for higher butyric acid concentration in consumers versus non-consumers of beer [122], but no estimation of phenols intake was performed, nor were consumption of alcohol-free beer nor spirits-only drinkers recorded. On the other hand, another observational study on the microbiota of 916 UK female twins found association only for wine drinkers but not for beer (nor all other alcohols) [123], but also in this case, the consumption of alcohol-free beer was not considered. Figure 2 summarizes gut microbiota changes after beer consumption.
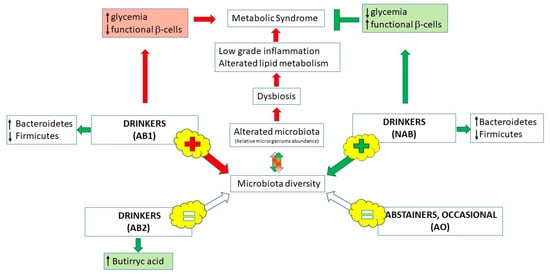
Figure 2.
Schematic representation of relationship between beer, gut microbiota and metabolic syndrome. Phenolic compounds contained in non-alcoholic beer have a positive effect on the microbiota dysbiosis, one of the main causes of metabolic syndrome, but the effect is prevented by alcohol presence. Non-alcoholic beer consumption also determines a positive modification of some parameters typical of metabolic syndrome such as glycemia and the β-cells’ function (AB1, drinkers of 355 mL/day of alcoholic beer; NAB, drinkers of 355 mL/day of non-alcoholic beer [111]). On the other hand, moderate beer consumption can increase the production of butyric acid, a fundamental molecule produced by the microbiota and useful for its healthy implications (AB2, drinkers of 200–600 mL/day; AO, abstainers or occasional consumers of <1.5 alcohol g/day [122]).
6.5. Role of Alcohol on Other Phenols-Related Effects of Beer
In an observational follow-up prospective study (34 years) of the association between alcoholic beverage consumption (using repeated surveys) and dementia (n = 1462 women, 38–60 years), beer consumption was associated to reduced dementia risk compared to subjects consuming only spirits [125]. Unfortunately, the consumption of alcohol-free beer was not taken into account. Moreover, in a case-control study of the association, in postmenopausal women (n = 35,816; 55–69 years), between specific self-reported drinking/smoking habits (over 20 years) and diabetes, a reduction of risk was observed for moderate consumption of either beer, red or white wines, but also for liquor, making the authors disprove the hypothesis that flavonoids could protect from diabetes onset [127].
7. Fruit-Based Enrichment of Beer Phenols
Beer is considered a promising beverage in the context of functional foods, which are food items with, in theory, health benefits, due to the enrichment with specific ingredients or bioactive compounds. Beer has high market opportunities because of an already high acceptancy of new organoleptic characteristics, due to widespread and previous diffusion of craft beers. Several ingredients have been added such as wheat, corn, rice and fruits. The phenolic profiles of several commercialized beers enriched with ingredients have already been reported and reviewed [8,133]. Studies agree that fruits’ refermentation and maturation within beer production is associated to a significant increase of flavors and bioactive compounds supporting benefits of fruit contribution to beer’s consumer acceptance. Both qualitative and quantitative increases in phenols have been reported in beers enriched with whole fruits during fermentation and works mainly focused on the role of the technological processes applied. However, rarely did a study report more than one fruit supplement. An exception is a recent report that compared individual phenols amounts in commercial beers enriched with cherry, raspberry, peach, apricot, grape, plum, orange or apple, and respective contribution to the antioxidant activity [134]. Importantly, this work demonstrates that fruit beers may be enriched with bioactive compounds (catechin, rutin, myricetin, quercetin and resveratrol) that are undetectable in conventional beers at identical extraction conditions and indicates enrichment with peels to be very promising because of the highest amount polyphenols and flavonoids content and antioxidant activity. Notably, resveratrol was found in beers enriched with all fruits except one (plum), with the highest level being measured in grape beer [134].
Other recent beer-added ingredients are quince fruit, mango, sweet potato and olive leaves. Because of organoleptic characteristics, quince fruit is specifically appreciated as a processed food. Many studies have shown that quince fruit lends itself as an affordable and good source of phenolic acids and flavonoids; in particular, in vitro assays have shown that phenols are the main compounds responsible for fruit’s hydrophilic antioxidant activity [135]. Quince fruit phenols have been extensively studied [136] and recent data indicate that the addition of different quince cultivars, with different sensory attributes or antioxidant content, can selectively modulate the final content in specific phenols and related sensory descriptors attributes [137]. The addition of quince increased the total polyphenol content, the total hydroxicinnamic acids, concentration of main volatile compounds related with fruity sensory descriptors, and led to higher intensities of floral and fruity sensory attributes [137]. The addition of mango fruit, naturally reach in phenols [138], yielded beers with higher polyphenol content and aroma than traditional beer, especially if the fruit was homogenized before addition, on the condition that no thermal treatment was performed [139].
The addition of dried flakes of sweet potato, naturally rich in phenols [140], before beer brewing increased both total phenols (about 10%) and flavonoids (about 20%) content without changing physicochemical and sensory parameters of beer, which also benefited from an important increase in β-carotene [141].
Also, dried olives or resulting extracts, that contain not only common phenols, but also the olive tree family-exclusive secoiridoids [142], were added to beer, and the resulting beer had positive flavor and aroma, but low colloidal stability and showed increasing haze formation during storage due to very high polyphenols content [143]. Similar increase in colloidal haze was reported also for beers with added omija fruits, questioning the validity of increasing the phenolic content of beers too much [144]. Beers enriched with lignans from wood chips or extracts displayed excessive bitter taste and unusual resin aroma, indicating the need for technological approaches to avoid significant changes to the characteristics of beer. A possible solution could come from the use of hot water as a unique solvent, already applied for the removal of resins from wood chips or lignan extracts from the knots of spruce trees (Picea abies), a strategy that yielded beer with as much as 100 mg/L of lignans.
8. Cereal-Based Enrichment of Beer Phenols
Apart from barley, other malted cereals have been used since antiquity for the development of fermented beverages, in a somehow geographical way, for example, rice in India [145], millet in Nigeria [146], sorghum in South Africa [147] and Corn in Mexico [148]. Regarding the latter, a pulque-fermented drink known as “Sendechó” was antiquely prepared by the Mazahuas population in the Valley of Mexico, using chili and pigmented corn varieties with high content of phenolic compounds, mainly anthocyanins [149], which are completely absent in barley beer. In the attempt of developing a beer with traditional ingredients, pulque was substituted with hop and brewer’s yeast in an ale fermentation process performed with guajillo chili and blue corn malt. The result was a beer with total polyphenols concentration up to 560 mg GAE/L and of total anthocyanins up to 19.4 mg cyaniding-3-glucoside/L [150]. More recently, the same laboratory obtained blue or red corn malt blended beers with even higher total phenols amount (up to 849.5 mg GAE/L) and identified anthocyanins responsible for the final color yield of red and blue corn beers (pelargonidin-3-glucoside and cyanidin-3-glucoside) [151]. Authors also identified, in corn beers both previously reported and unreported, volatile phenols conferring desirable aromas to beers. Such results are promising with respect to previous reports of lower content of phenols in corn-added beers [152]. Nevertheless, to our knowledge, no consumer acceptability of such beers has been evaluated, and this aspect is crucial especially for the high content of phenols that can contribute to high spicy perception and for astringency of anthocyanins [151]. Among other gluten-free beers, those obtained from oat [153], sorghum [147], teff [154], millet [146], buckwheat [155] and quinoa [156] are in theory valid alternatives in terms of phenols considering the grain natural content, even if very little is available on the phenolic content of such beers, i.e., only a sum of aromatic alcohols was reported for millet [157]. A noteworthy emerging exception is represented by indigenous beer-like fermented beverages “ikigage” [158], “burukutu” and “pito” [159], drinks for which 4-vinylphenols quantities have been reported. Another exception is that of rice-based alcoholic beverages of Assam, India, with total polyphenol content up to 631.33 mg GAE/L [160].
One frequent issue of non-barley cereals is the low diastatic power, that traps phenols, making necessary the combination with other cereals or the addition of exogenous enzymes [161]. Addition to the mashing process of recombinant ferulic acid esterase [162] was recently proven as a valid remedy also for the low amount of the desirable phenol 4-vinylguaiacol (derived from ferulic acid by enzymic decarboxylation), a common issue of top-fermented wheat beer [163]. More recently, the strategy was further implemented by producing yeasts expressing bacterial ferulic acid decarboxylase [164].
9. Phenols in Non-Alcoholic and Isotonic Beers
Driving laws and a healthier lifestyle have increased the popularity of non-alcoholic beers. In order to not exceed the limit of 0.5% (v/v) alcohol or to produce beer with a limited alcohol content, two approaches are exploited. The first one consists in limiting the fermentation process, and hence the alcohol production, using low-alcohol yeasts or producing a wort with low degrees Plato and low diastatic power in order to obtain more dextrins than fermentable sugars. The alternative approach involves physical methods to remove the alcohol at the end of brewing, for example by vacuum evaporation or reverse osmosis treatments. Unfortunately, limiting the fermentation process can bring about inadequate conversion of wort to beer and, on the other hand, physical methods for alcohol removal can deteriorate beer composition [165]. Osmotic distillation using a membrane contactor was recently shown to be able to maintain the total phenols content in a low-alcohol top-fermented beer [166]. On the other hand, using the fermentation interruption approach, De Fusco and coworkers recently obtained a low-alcohol isotonic beer with an amount of total phenolic compounds similar to that of Pilsen beer and sport drinks [167]. Isotonic beers are an improvement on low-alcohol beers with similar rehydration potential of sports drinks [168] (beverages with specific osmolality and carbohydrate content [169]) and with the advantage of containing bioactive molecules. Notably, De Fusco and coworkers found that fermentation interruption did not significantly affect total phenols level [167]. Nevertheless, experiments are needed to test the shelf-life of low-alcohol isotonic beers and specifically to test if phenols’ antimicrobial activity is adequate in such low-alcoholic and carbohydrate-containing beverages [170].
10. Future Directions and Conclusions
Here, we attempted to review the more recent findings on beer phenols and their role in human health. Particular attention was dedicated to the role of genetic factors and to the enrichment with phenolic compounds by cereals different from barley or fruits naturally rich in phenols. In this respect, it would be interesting to investigate to what extent fruit addition also increases the alcoholic content, which has health and consumer acceptance consequences that are not negligible. One other interesting question regards the huge amount of debris produced by beer production, especially in terms of phenolic compounds (1% in by-product spent grain [171]) that could be recycled for beer enrichment itself. Recent reports indicate that the recovery of phenols can be improved using the fungi Rhizopus oligosporus as a fermenting organism [172].
Even if the Scopus.com search string we used is arbitrary and may not entirely represent the research on health effects of beer ascribable to the presence of phenolic compounds, less than of 25% (40 out of 161) of entries we retrieved were reports on the in vivo (human or animal) or in vitro effects of phenolic compounds within in toto beer. Most of the research is focused on evaluating the effects of single phenolic compounds of beer, which, however, can give rise to partial conclusions that need further experiments performed in physiological conditions. For example, as beer contains a mean amount of xanthohumol around 0.2 mg/L, what is the rationale for supplementing volunteers with an enriched drink containing a daily dose of 12 mg of xanthohumol [173], corresponding to the amount found in 60 L of beer? Indeed, several techniques have been used, starting from almost 20 years ago and featuring a patented addition of an enriched hop product [174], in order to increase the amount of this compound in beer up to 10 mg/L [175,176,177]. However, even if studies with enriched beers are helpful for assessing the metabolic fate of phenolic compounds, in order to correctly evaluate healthy effects of beer consumption, researchers should consider, besides the side effects of alcohol (where ethically possible), also those possibly due to yet uncharacterized molecules, i.e., those resulting from the addition of the enriched hop product, and those due to a non-physiological consumption of a single flavonoid for which pro-apoptotic effects are already known [178]. In fact, the only health claims authorized for phenolics by the European Food Safety Authority regard, at the moment, olive oil hydroxytyrosol and cocoa flavanols, with high daily amounts (5 and 200 mg, respectively) that can, however, be easily consumed in the context of a balanced diet [179,180]. Among retrieved reports, only six investigated the effects of phenols in the presence and absence of alcohol, thus also considering the effects of alcohol alone. Actually, four publications belong to the same Spanish research group [112,113,114,115], and thus probably refer to the same and unique small population of 33 high-cardiovascular-risk males.
In conclusion, studies applying a parallel administration of non-alcoholic beer or/and alcohol alone, in both animal and human intervention studies, support the existence of somehow interfering effects of phenols and ethanol. However, in order to better highlight additive or synergistic effects, further correctly set-up human interventional crossover or observational, or at least animal, studies are required. From this point of view, even insect models could deserve more attention. In fact, using D. melanogaster fed with a total beer extract, Merinas-Amo and colleagues were able to demonstrate the synergic interaction between different molecules contained in beer [181].
Author Contributions
Scopus.com investigation, R.A.; writing—review and editing, R.A., G.P. and S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Homan, M.M. Beer and Its Drinkers: An Ancient Near Eastern Love Story. Near East. Archaeol. 2004, 67, 84–95. [Google Scholar] [CrossRef]
- Wang, J.; Liu, L.; Ball, T.; Yu, L.; Li, Y.; Xing, F. Revealing a 5,000-y-old beer recipe in China. Proc. Natl. Acad. Sci. USA 2016, 201601465. [Google Scholar] [CrossRef]
- Brewers. Contribution made by Beer to the European Economy. Available online: https://brewersofeurope.org/uploads/mycms-files/documents/publications/2020/contribution-made-by-beer-to-EU-economy-2020.pdf (accessed on 1 October 2020).
- Osorio-Paz, I.; Brunauer, R.; Alavez, S. Beer and its non-alcoholic compounds in health and disease. Crit. Rev. Food Sci. Nutr. 2019, 1–14. [Google Scholar] [CrossRef]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef]
- Palmer, K. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 98: Painting, Firefighting and Shiftwork. International Agency for Research on Cancer. Occup. Med. (Chic. Ill.) 2011, 61, 521–522. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary Intake and Bioavailability of Polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef]
- Mitić, S.S.; Paunović, D.Đ.; Pavlović, A.N.; Tošić, S.B.; Stojković, M.B.; Mitić, M.N. Phenolic Profiles and Total Antioxidant Capacity of Marketed Beers in Serbia. Int. J. Food Prop. 2014, 17, 908–922. [Google Scholar] [CrossRef]
- Wannenmacher, J.; Gastl, M.; Becker, T. Phenolic Substances in Beer: Structural Diversity, Reactive Potential and Relevance for Brewing Process and Beer Quality. Compr. Rev. Food Sci. Food Saf. 2018, 17, 953–988. [Google Scholar] [CrossRef] [PubMed]
- Bartolomé, B.; Peña-Neira, A.; Gómez-Cordovés, C. Phenolics and related substances in alcohol-free beers. Eur. Food Res. Technol. 2000, 210, 419–423. [Google Scholar] [CrossRef]
- Boronat, A.; Soldevila-Domenech, N.; Rodríguez-Morató, J.; Martínez-Huélamo, M.; Lamuela-Raventós, R.M.; de la Torre, R. Beer Phenolic Composition of Simple Phenols, Prenylated Flavonoids and Alkylresorcinols. Molecules 2020, 25, 2582. [Google Scholar] [CrossRef]
- Soldevila-Domenech, N.; Boronat, A.; Mateus, J.; Diaz-Pellicer, P.; Matilla, I.; Pérez-Otero, M.; Aldea-Perona, A.; De La Torre, R. Generation of the antioxidant hydroxytyrosol from tyrosol present in beer and red wine in a randomized clinical trial. Nutrients 2019. [Google Scholar] [CrossRef]
- Gharwalova, L.; Sigler, K.; Dolezalova, J.; Masak, J.; Rezanka, T.; Kolouchova, I. Resveratrol suppresses ethanol stress in winery and bottom brewery yeast by affecting superoxide dismutase, lipid peroxidation and fatty acid profile. World J. Microbiol. Biotechnol. 2017, 33, 205. [Google Scholar] [CrossRef]
- Moura-Nunes, N.; Brito, T.C.; Da Fonseca, N.D.; De Aguiar, P.F.; Monteiro, M.; Perrone, D.; Torres, A.G. Phenolic compounds of Brazilian beers from different types and styles and application of chemometrics for modeling antioxidant capacity. Food Chem. 2016. [Google Scholar] [CrossRef]
- Parikka, K.; Rowland, I.R.; Welch, R.W.; Wähälä, K. In vitro antioxidant activity and antigenotoxicity of 5-n-alkylresorcinols. J. Agric. Food Chem. 2006. [Google Scholar] [CrossRef]
- Oishi, K.; Yamamoto, S.; Itoh, N.; Nakao, R.; Yasumoto, Y.; Tanaka, K.; Kikuchi, Y.; Fukudome, S.-I.; Okita, K.; Takano-Ishikawa, Y. Wheat alkylresorcinols suppress high-fat, high-sucrose diet-induced obesity and glucose intolerance by increasing insulin sensitivity and cholesterol excretion in male mice. J. Nutr. 2015. [Google Scholar] [CrossRef]
- Chen, X.; Mukwaya, E.; Wong, M.-S.; Zhang, Y. A systematic review on biological activities of prenylated flavonoids. Pharm. Biol. 2014, 52, 655–660. [Google Scholar] [CrossRef]
- Štulíková, K.; Karabín, M.; Nešpor, J.; Dostálek, P. Therapeutic Perspectives of 8-Prenylnaringenin, a Potent Phytoestrogen from Hops. Molecules 2018, 23, 660. [Google Scholar] [CrossRef]
- Intelmann, D.; Haseleu, G.; Dunkel, A.; Lagemann, A.; Stephan, A.; Hofmann, T. Comprehensive sensomics analysis of hop-derived bitter compounds during storage of beer. J. Agric. Food Chem. 2011. [Google Scholar] [CrossRef]
- Callemien, D.; Collin, S. Involvement of flavanoids in beer color instability during storage. J. Agric. Food Chem. 2007. [Google Scholar] [CrossRef]
- Heuberger, A.L.; Broeckling, C.D.; Lewis, M.R.; Salazar, L.; Bouckaert, P.; Prenni, J.E. Metabolomic profiling of beer reveals effect of temperature on non-volatile small molecules during short-term storage. Food Chem. 2012. [Google Scholar] [CrossRef]
- Owades, J.L.; Jakovac, J. Study of Beer Oxidation with O18. Proc. Annu. Meet. Am. Soc. Brew. Chem. 1966, 24, 180–183. [Google Scholar] [CrossRef]
- Asano, K.; Ohtsu, K.; Shinagawa, K.; Hashimoto, N. Affinity of proanthocyanidins and their oxidation products for haze-forming proteins of beerand the formation of chill haze. Agric. Biol. Chem. 1984. [Google Scholar] [CrossRef]
- Delcour, J.A.; Dondeyne, P.; Trousdale, E.K.; Singleton, V.L. The reactions between polyphenols and aldehydes and the influence of acetaldehyde on haze formation in beer. J. Inst. Brew. 1982. [Google Scholar] [CrossRef]
- Maia, P.D.D.S.; dos Santos Baião, D.; da Silva, V.P.F.; Lemos Miguel, M.A.; Quirino Lacerda, E.C.; de Araújo Calado, V.M.; da Silva Carneiro, C.; Finotelli, P.V.; Pierucci, A.P.T.R. Microencapsulation of a craft beer, nutritional composition, antioxidant stability, and drink acceptance. LWT 2020, 133, 110104. [Google Scholar] [CrossRef]
- Callemien, D.; Collin, S. Use of RP-HPLC-ESI(–)-MS/MS to Differentiate Various Proanthocyanidin Isomers in Lager Beer Extracts. J. Am. Soc. Brew. Chem. 2008, 66, 109–115. [Google Scholar] [CrossRef]
- Corso, M.; Perreau, F.; Mouille, G.; Lepiniec, L. Specialized phenolic compounds in seeds: Structures, functions, and regulations. Plant. Sci. 2020, 296, 110471. [Google Scholar] [CrossRef]
- Gretenhart, K.E. Specialty malts. Tech. Q. Master Brew. Assoc. Am. 1997, 34, 102–106. [Google Scholar]
- Lu, J.; Zhao, H.; Chen, J.; Fan, W.; Dong, J.; Kong, W.; Sun, J.; Cao, Y.; Cai, G. Evolution of phenolic compounds and antioxidant activity during malting. J. Agric. Food Chem. 2007. [Google Scholar] [CrossRef]
- Maillard, M.-N.; Berset, C. Evolution of Antioxidant Activity during Kilning: Role of Insoluble Bound Phenolic Acids of Barley and Malt. J. Agric. Food Chem. 1995, 43, 1789–1793. [Google Scholar] [CrossRef]
- Leitao, C.; Marchioni, E.; Bergaentzlé, M.; Zhao, M.; Didierjean, L.; Miesch, L.; Holder, E.; Miesch, M.; Ennahar, S. Fate of polyphenols and antioxidant activity of barley throughout malting and brewing. J. Cereal Sci. 2012. [Google Scholar] [CrossRef]
- Munoz-Insa, A.; Gastl, M.; Becker, T. Influence of malting on the protein composition of triticale (× Triticosecale Wittmack) “trigold.”. Cereal Chem. 2016. [Google Scholar] [CrossRef]
- Koren, D.; Kun, S.; Hegyesné Vecseri, B.; Kun-Farkas, G. Study of antioxidant activity during the malting and brewing process. J. Food Sci. Technol. 2019. [Google Scholar] [CrossRef]
- Szwajgier, D. Dry and wet milling of malt. a preliminary study comparing fermentable sugar, total protein, total phenolics and the ferulic acid content in non-hopped worts. J. Inst. Brew. 2011. [Google Scholar] [CrossRef]
- Defernez, M.; Foxall, R.J.; O’Malley, C.J.; Montague, G.; Ring, S.M.; Kemsley, E.K. Modelling beer fermentation variability. J. Food Eng. 2007, 83, 167–172. [Google Scholar] [CrossRef]
- Piazzon, A.; Forte, M.; Nardini, M. Characterization of phenolics content and antioxidant activity of different beer types. J. Agric. Food Chem. 2010. [Google Scholar] [CrossRef]
- Cheiran, K.P.; Raimundo, V.P.; Manfroi, V.; Anzanello, M.J.; Kahmann, A.; Rodrigues, E.; Frazzon, J. Simultaneous identification of low-molecular weight phenolic and nitrogen compounds in craft beers by HPLC-ESI-MS/MS. Food Chem. 2019. [Google Scholar] [CrossRef]
- Bertuzzi, T.; Mulazzi, A.; Rastelli, S.; Donadini, G.; Rossi, F.; Spigno, G. Targeted healthy compounds in small and large-scale brewed beers. Food Chem. 2020. [Google Scholar] [CrossRef]
- Zhao, H. Effects of Processing Stages on the Profile of Phenolic Compounds in Beer. In Processing and Impact on Active Components in Food; Academic Press: Cambridge, MA, USA, 2015; ISBN 9780124047099. [Google Scholar]
- Obata, Y.; Koshika, M. Studies on the Sunlight Flavor of Beer. Bull. Agric. Chem. Soc. Jpn. 1960, 24, 644–646. [Google Scholar] [CrossRef]
- Taylor, R.; Kirsop, B.H. Occurrence of a killer strain of Saccharomyces cerevisiae in a batch fermentation plant. J. Inst. Brew. 1979, 85, 325. [Google Scholar] [CrossRef]
- Vanbeneden, N.; Van Roey, T.; Willems, F.; Delvaux, F.; Delvaux, F.R. Release of phenolic flavour precursors during wort production: Influence of process parameters and grist composition on ferulic acid release during brewing. Food Chem. 2008. [Google Scholar] [CrossRef]
- Vanbeneden, N.; Gils, F.; Delvaux, F.; Delvaux, F.R. Formation of 4-vinyl and 4-ethyl derivatives from hydroxycinnamic acids: Occurrence of volatile phenolic flavour compounds in beer and distribution of Pad1-activity among brewing yeasts. Food Chem. 2008. [Google Scholar] [CrossRef]
- Schwarz, K.J.; Stübner, R.; Methner, F.J. Formation of styrene dependent on fermentation management during wheat beer production. Food Chem. 2012. [Google Scholar] [CrossRef] [PubMed]
- Sterckx, F.L.; Missiaen, J.; Saison, D.; Delvaux, F.R. Contribution of monophenols to beer flavour based on flavour thresholds, interactions and recombination experiments. Food Chem. 2011. [Google Scholar] [CrossRef] [PubMed]
- Bettenhausen, H.M.; Barr, L.; Broeckling, C.D.; Chaparro, J.M.; Holbrook, C.; Sedin, D.; Heuberger, A.L. Influence of malt source on beer chemistry, flavor, and flavor stability. Food Res. Int. 2018. [Google Scholar] [CrossRef]
- Maillard, M.N.; Soum, M.H.; Boivin, P.; Berset, C. Antioxidant activity of barley and malt: Relationship with phenolic content. LWT Food Sci. Technol. 1996. [Google Scholar] [CrossRef]
- Langos, D.; Granvogl, M.; Schieberle, P. Characterization of the key aroma compounds in two Bavarian wheat beers by means of the sensomics approach. J. Agric. Food Chem. 2013. [Google Scholar] [CrossRef]
- Lentz, M. The impact of simple phenolic compounds on beer aroma and flavor. Fermentation 2018, 4, 20. [Google Scholar] [CrossRef]
- Marova, I.; Parilova, K.; Friedl, Z.; Obruca, S.; Duronova, K. Analysis of phenolic compounds in lager beers of different origin: A contribution to potential determination of the authenticity of Czech beer. Chromatographia 2011. [Google Scholar] [CrossRef]
- Olšovská, J.; Čejka, P.; Sigler, K.; Hönigová, V. The phenomenon of Czech beer: A review. Czech. J. Food Sci. 2014, 32, 309–319. [Google Scholar] [CrossRef]
- Dadic, M.; Van Gheluwe, G.E.A. Role of polyphenols and non-volatiles in beer quality. Master Brew. Assoc. Am. Tech. Q. 1973, 10, 69–73. [Google Scholar]
- Cai, S.; Han, Z.; Huang, Y.; Chen, Z.-H.; Zhang, G.; Dai, F. Genetic Diversity of Individual Phenolic Acids in Barley and Their Correlation with Barley Malt Quality. J. Agric. Food Chem. 2015, 63, 7051–7057. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Huang, Y.; Hu, H.; Dai, F.; Zhang, G. Identification of QTLs associated with haze active proteins in barley. Euphytica 2015. [Google Scholar] [CrossRef]
- Han, Z.; Zhang, J.; Cai, S.; Chen, X.; Quan, X.; Zhang, G. Association mapping for total polyphenol content, total flavonoid content and antioxidant activity in barley. BMC Genomics 2018. [Google Scholar] [CrossRef] [PubMed]
- Mikyška, A.; Hrabák, M.; Hašková, D.; Šrogl, J. The role of malt and hop polyphenols in beer quality, flavour and haze stability. J. Inst. Brew. 2002. [Google Scholar] [CrossRef]
- Munoz-Insa, A.; Gastl, M.; Becker, T. Use of Polyphenol-Rich Hop Products to Reduce Sunstruck Flavor in Beer. J. Am. Soc. Brew. Chem. 2015, 73, 228–235. [Google Scholar] [CrossRef]
- Wannenmacher, J.; Cotterchio, C.; Schlumberger, M.; Reuber, V.; Gastl, M.; Becker, T. Technological influence on sensory stability and antioxidant activity of beers measured by ORAC and FRAP. J. Sci. Food Agric. 2019. [Google Scholar] [CrossRef]
- Goiris, K.; Jaskula-Goiris, B.; Syryn, E.; Van Opstaele, F.; De Rouck, G.; Aerts, G.; De Cooman, L. The flavoring potential of hop polyphenols in beer. J. Am. Soc. Brew. Chem. 2014. [Google Scholar] [CrossRef]
- Bober, A.; Liashenko, M.; Protsenko, L.; Slobodyanyuk, N.; Matseiko, L.; Yashchuk, N.; Gunko, S.; Mushtruk, M. Biochemical composition of the hops and quality of the finished beer. Potravin. Slovak J. Food Sci. 2020. [Google Scholar] [CrossRef]
- Griffiths, D.W.; Welch, R.W. Genotypic and environmental variation in the total phenol and flavanol contents of barley grain. J. Sci. Food Agric. 1982. [Google Scholar] [CrossRef]
- Forster, A.; Beck, B.; Schmidt, R.; Jansen, C.; Mellenthin, A. On the composition of low molecular polyphenols in different varieties of hops and from two growing areas. Monatsschr. Brauwiss. 2002, 55, 98–108. [Google Scholar]
- Thurston, P.A.; Tubb, R.S. Screening yeast strains for the ability to produce phenolic off-flavours: A simple method for determining phenols in wort and beer. J. Inst. Brew. 1981. [Google Scholar] [CrossRef]
- Mohammadi, M.; Endelman, J.B.; Nair, S.; Chao, S.; Jones, S.S.; Muehlbauer, G.J.; Ullrich, S.E.; Baik, B.-K.; Wise, M.L.; Smith, K.P. Association mapping of grain hardness, polyphenol oxidase, total phenolics, amylose content, and β-glucan in US barley breeding germplasm. Mol. Breed. 2014, 34, 1229–1243. [Google Scholar] [CrossRef]
- Holtekjølen, A.K.; Kinitz, C.; Knutsen, S.H. Flavanol and Bound Phenolic Acid Contents in Different Barley Varieties. J. Agric. Food Chem. 2006, 54, 2253–2260. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.M.; Cao, F.; Han, Y.; Nadira, U.A.; Zhang, G.; Wu, F. Differential changes in grain ultrastructure, amylase, protein and amino acid profiles between Tibetan wild and cultivated barleys under drought and salinity alone and combined stress. Food Chem. 2013, 141, 2743–2750. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Ahsan, M.; Adil, M.F.; Chen, X.; Nazir, M.M.; Shamsi, I.H.; Zeng, F.; Zhang, G. Identification of the gene network modules highly associated with the synthesis of phenolics compounds in barley by transcriptome and metabolome analysis. Food Chem. 2020, 323, 126862. [Google Scholar] [CrossRef] [PubMed]
- Morcol, T.B.; Negrin, A.; Matthews, P.D.; Kennelly, E.J. Hop (Humulus lupulus L.) terroir has large effect on a glycosylated green leaf volatile but not on other aroma glycosides. Food Chem. 2020. [Google Scholar] [CrossRef] [PubMed]
- Claussen, N.H. On a Method for the Application of Hansen’s Pure Yeast System in the Manufacturing of Well-Conditioned English Stock Beers. J. Inst. Brew. 1904, 10, 308–331. [Google Scholar] [CrossRef]
- Wiles, A.E. Studies of some yeasts causing spoilage of draught beer. J. Inst. Brew. 1950. [Google Scholar] [CrossRef]
- Chatonnet, P.; Dubourdie, D.; Boidron, J.-N.; Pons, M. The origin of ethylphenols in wines. J. Sci. Food Agric. 1992. [Google Scholar] [CrossRef]
- Passoth, V.; Blomqvist, J.; Schnürer, J. Dekkera bruxellensis and Lactobacillus vini form a stable ethanol-producing consortium in a commercial alcohol production process. Appl. Environ. Microbiol. 2007, 73, 4354–4356. [Google Scholar] [CrossRef]
- Steensels, J.; Daenen, L.; Malcorps, P.; Derdelinckx, G.; Verachtert, H.; Verstrepen, K.J. Brettanomyces yeasts—From spoilage organisms to valuable contributors to industrial fermentations. Int. J. Food Microbiol. 2015, 206, 24–38. [Google Scholar] [CrossRef]
- González, C.; Godoy, L.; Ganga, M.A. Identification of a second PAD1 in Brettanomyces bruxellensis LAMAP2480. Antonie Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2017. [Google Scholar] [CrossRef]
- Lentz, M.; Harris, C. Analysis of Growth Inhibition and Metabolism of Hydroxycinnamic Acids by Brewing and Spoilage Strains of Brettanomyces Yeast. Foods 2015. [Google Scholar] [CrossRef]
- Coghe, S.; Benoot, K.; Delvaux, F.; Vanderhaegen, B.; Delvaux, F.R. Ferulic Acid Release and 4-Vinylguaiacol Formation during Brewing and Fermentation: Indications for Feruloyl Esterase Activity in Saccharomyces cerevisiae. J. Agric. Food Chem. 2004. [Google Scholar] [CrossRef]
- Spitaels, F.; Wieme, A.D.; Janssens, M.; Aerts, M.; Daniel, H.-M.; Van Landschoot, A.; De Vuyst, L.; Vandamme, P. The Microbial Diversity of Traditional Spontaneously Fermented Lambic Beer. PLoS ONE 2014, 9, e95384. [Google Scholar] [CrossRef]
- Serra Colomer, M.; Funch, B.; Forster, J. The raise of Brettanomyces yeast species for beer production. Curr. Opin. Biotechnol. 2019, 56, 30–35. [Google Scholar] [CrossRef]
- Coelho, E.; Azevedo, M.; Teixeira, J.A.; Tavares, T.; Oliveira, J.M.; Domingues, L. Evaluation of multi-starter S. cerevisiae/ D. bruxellensis cultures for mimicking and accelerating transformations occurring during barrel ageing of beer. Food Chem. 2020, 323, 126826. [Google Scholar] [CrossRef]
- Capece, A.; Romaniello, R.; Pietrafesa, A.; Siesto, G.; Pietrafesa, R.; Zambuto, M.; Romano, P. Use of Saccharomyces cerevisiae var. boulardii in co-fermentations with S. cerevisiae for the production of craft beers with potential healthy value-added. Int. J. Food Microbiol. 2018. [Google Scholar] [CrossRef]
- Gallone, B.; Steensels, J.; Prahl, T.; Soriaga, L.; Saels, V.; Herrera-Malaver, B.; Merlevede, A.; Roncoroni, M.; Voordeckers, K.; Miraglia, L.; et al. Domestication and Divergence of Saccharomyces cerevisiae Beer Yeasts. Cell 2016, 166, 1397–1410.e16. [Google Scholar] [CrossRef]
- Colomer, M.S.; Chailyan, A.; Fennessy, R.T.; Olsson, K.F.; Johnsen, L.; Solodovnikova, N.; Forster, J. Assessing Population Diversity of Brettanomyces Yeast Species and Identification of Strains for Brewing Applications. Front. Microbiol. 2020, 11, 637. [Google Scholar] [CrossRef]
- Langdon, Q.K.; Peris, D.; Baker, E.P.; Opulente, D.A.; Nguyen, H.-V.; Bond, U.; Gonçalves, P.; Sampaio, J.P.; Libkind, D.; Hittinger, C.T. Fermentation innovation through complex hybridization of wild and domesticated yeasts. Nat. Ecol. Evol. 2019, 3, 1576–1586. [Google Scholar] [CrossRef] [PubMed]
- Avramova, M.; Cibrario, A.; Peltier, E.; Coton, M.; Coton, E.; Schacherer, J.; Spano, G.; Capozzi, V.; Blaiotta, G.; Salin, F.; et al. Brettanomyces bruxellensis population survey reveals a diploid-triploid complex structured according to substrate of isolation and geographical distribution. Sci. Rep. 2018, 8, 4136. [Google Scholar] [CrossRef] [PubMed]
- De Gaetano, G.; Costanzo, S.; Di Castelnuovo, A.; Badimon, L.; Bejko, D.; Alkerwi, A.; Chiva-Blanch, G.; Estruch, R.; La Vecchia, C.; Panico, S.; et al. Effects of moderate beer consumption on health and disease: A consensus document. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 443–467. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Karatzi, K.; Rontoyanni, V.G.; Protogerou, A.D.; Georgoulia, A.; Xenos, K.; Chrysou, J.; Sfikakis, P.P.; Sidossis, L.S. Acute effects of beer on endothelial function and hemodynamics: Asingle-blind, crossover study in healthy volunteers. Nutrition 2013. [Google Scholar] [CrossRef] [PubMed]
- De Miranda, R.C.; Paiva, E.S.; Suter Correia Cadena, S.M.; Brandt, A.P.; Vilela, R.M. Polyphenol-rich foods alleviate pain and ameliorate quality of life in fibromyalgic women. Int. J. Vitam. Nutr. Res. 2017. [Google Scholar] [CrossRef]
- Loguercio, C.; Tuccillo, C.; Federico, A.; Fogliano, V.; Del Vecchio Blanco, C.; Romano, M. Alcoholic beverages and gastric epithelial cell viability: Effect on oxidative stress-induced damage. J. Physiol. Pharmacol. 2009, 60, 87–92. [Google Scholar]
- Machado, J.C.; Faria, M.A.; Melo, A.; Ferreira, I.M.P.L.V.O. Antiproliferative effect of beer and hop compounds against human colorectal adenocarcinome Caco-2 cells. J. Funct. Foods 2017. [Google Scholar] [CrossRef]
- Alonso-Andrés, P.; Martín, M.; Albasanz, J.É.L. Modulation of adenosine receptors and antioxidative effect of beer extracts in in vitro models. Nutrients 2019, 11, 1258. [Google Scholar] [CrossRef]
- Oczkowski, M.; Rembiszewska, A.; Dziendzikowska, K.; Wolińska-Witort, E.; Kołota, A.; Malik, A.; Stachoń, M.; Lachowicz, K.; Gromadzka-Ostrowska, J. Beer consumption negatively regulates hormonal reproductive status and reduces apoptosis in Leydig cells in peripubertal rats. Alcohol 2019. [Google Scholar] [CrossRef]
- Silva, A.F.; Faria-Costa, G.; Sousa-Nunes, F.; Santos, M.F.; Ferreira-Pinto, M.J.; Duarte, D.; Rodrigues, I.; Guimarães, J.T.; Leite-Moreira, A.; Moreira-Gonçalves, D.; et al. Anti-remodeling effects of xanthohumol-fortified beer in pulmonary arterial hypertension mediated by ERK and AKT inhibition. Nutrients 2019, 11, 583. [Google Scholar] [CrossRef] [PubMed]
- Merino, P.; Santos-López, J.A.; Mateos, C.J.; Meseguer, I.; Garcimartín, A.; Bastida, S.; Sánchez-Muniz, F.J.; Benedí, J.; González-Muñoz, M.J. Can nonalcoholic beer, silicon and hops reduce the brain damage and behavioral changes induced by aluminum nitrate in young male Wistar rats? Food Chem. Toxicol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Lima-Fontes, M.; Costa, R.; Rodrigues, I.; Soares, R. Xanthohumol Restores Hepatic Glucolipid Metabolism Balance in Type 1 Diabetic Wistar Rats. J. Agric. Food Chem. 2017. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.; Negrão, R.; Valente, I.; Castela, A.; Duarte, D.; Guardão, L.; Magalhães, P.J.; Rodrigues, J.A.; Guimarães, J.T.; Gomes, P.; et al. Xanthohumol modulates inflammation, oxidative stress, and angiogenesis in type 1 diabetic rat skin wound healing. J. Nat. Prod. 2013. [Google Scholar] [CrossRef] [PubMed]
- Negrão, R.; Costa, R.; Duarte, D.; Gomes, T.T.; Coelho, P.; Guimarães, J.T.; Guardão, L.; Azevedo, I.; Soares, R. Xanthohumol-supplemented beer modulates angiogenesis and inflammation in a skin wound healing model. Involvement of local adipocytes. J. Cell. Biochem. 2012. [Google Scholar] [CrossRef]
- Jastrzebski, Z.; Gorinstein, S.; Czyzewska-Szafran, H.; Leontowicz, H.; Leontowicz, M.; Trakhtenberg, S.; Remiszewska, M. The effect of short-term lyophilized beer consumption on established hypertension in rats. Food Chem. Toxicol. 2007. [Google Scholar] [CrossRef]
- Gorinstein, S.; Caspi, A.; Pawelzik, E.; Deldago-Licon, E.; Libman, I.; Trakhtenberg, S.; Weisz, M.; Martin-Belloso, O. Proteins of beer affect lipid levels in rats. Nutr. Res. 2001. [Google Scholar] [CrossRef]
- Gorinstein, S.; Zemser, M.; Weisz, M.; Halevy, S.; Martin-Belloso, O.; Trakhtenberg, S. The influence of alcohol-containing and alcohol-free beverages on lipid levels and lipid peroxides in serum of rats. J. Nutr. Biochem. 1998. [Google Scholar] [CrossRef]
- Negrão, R.; Costa, R.; Duarte, D.; Taveira Gomes, T.; Mendanha, M.; Moura, L.; Vasques, L.; Azevedo, I.; Soares, R. Angiogenesis and inflammation signaling are targets of beer polyphenols on vascular cells. J. Cell. Biochem. 2010. [Google Scholar] [CrossRef]
- Bourne, L.; Paganga, G.; Baxter, D.; Hughes, P.; Rice-Evans, C. Absorption of ferulic acid from low-alcohol beer. Free Radic. Res. 2000. [Google Scholar] [CrossRef]
- Nardini, M.; Natella, F.; Scaccini, C.; Ghiselli, A. Phenolic acids from beer are absorbed and extensively metabolized in humans. J. Nutr. Biochem. 2006. [Google Scholar] [CrossRef] [PubMed]
- Ghiselli, A.; Natella, F.; Guidi, A.; Montanari, L.; Fantozzi, P.; Scaccini, C. Beer increases plasma antioxidant capacity in humans. J. Nutr. Biochem. 2000. [Google Scholar] [CrossRef]
- Pérez-Mañá, C.; Farré, M.; Pastor, A.; Fonseca, F.; Torrens, M.; Menoyo, E.; Pujadas, M.; Frias, S.; Langohr, K.; de la Torre, R. Non-linear formation of EtG and FAEEs after controlled administration of low to moderate doses of ethanol. Alcohol Alcohol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Krnic, M.; Modun, D.; Budimir, D.; Gunjaca, G.; Jajic, I.; Vukovic, J.; Salamunic, I.; Sutlovic, D.; Kozina, B.; Boban, M. Comparison of acute effects of red wine, beer and vodka against hyperoxia-induced oxidative stress and increase in arterial stiffness in healthy humans. Atherosclerosis 2011. [Google Scholar] [CrossRef] [PubMed]
- Codoñer-Franch, P.; Hernández-Aguilar, M.T.; Navarro-Ruiz, A.; López-Jaén, A.B.; Borja-Herrero, C.; Valls-Bellés, V. Diet supplementation during early lactation with non-alcoholic beer increases the antioxidant properties of breastmilk and decreases the oxidative damage in breastfeeding mothers. Breastfeed. Med. 2013. [Google Scholar] [CrossRef] [PubMed]
- Martínez Alvarez, J.R.; Bellés, V.V.; López-Jaén, A.B.; Marín, A.V.; Codoñer-Franch, P. Effects of alcohol-free beer on lipid profile and parameters of oxidative stress and inflammation in elderly women. Nutrition 2009. [Google Scholar] [CrossRef] [PubMed]
- Croft, K.D.; Puddey, I.B.; Rakic, V.; Abu-Amsha, R.; Dimmitt, S.B.; Beilin, L.J. Oxidative susceptibility of low-density lipoproteins—Influence of regular alcohol use. Alcohol. Clin. Exp. Res. 1996. [Google Scholar] [CrossRef]
- Franco, L.; Bravo, R.; Galan, C.; Sanchez, C.; Rodriguez, A.B.; Barrigs, C.; Cubero Juánez, J. Effects of beer, Hops (Humulus lupulus) on total antioxidant capacity in plasma of stressed subjects. Cell Membr. Free Radic. Res. 2013, 5, 232–235. [Google Scholar]
- Hernández-Quiroz, F.; Nirmalkar, K.; Villalobos-Flores, L.E.; Murugesan, S.; Cruz-Narváez, Y.; Rico-Arzate, E.; Hoyo-Vadillo, C.; Chavez-Carbajal, A.; Pizano-Zárate, M.L.; García-Mena, J. Influence of moderate beer consumption on human gut microbiota and its impact on fasting glucose and β-cell function. Alcohol 2020. [Google Scholar] [CrossRef]
- Quifer-Rada, P.; Chiva-Blanch, G.; Jáuregui, O.; Estruch, R.; Lamuela-Raventós, R.M. A discovery-driven approach to elucidate urinary metabolome changes after a regular and moderate consumption of beer and nonalcoholic beer in subjects at high cardiovascular risk. Mol. Nutr. Food Res. 2017. [Google Scholar] [CrossRef]
- Chiva-Blanch, G.; Magraner, E.; Condines, X.; Valderas-Martínez, P.; Roth, I.; Arranz, S.; Casas, R.; Navarro, M.; Hervas, A.; Sisó, A.; et al. Effects of alcohol and polyphenols from beer on atherosclerotic biomarkers in high cardiovascular risk men: A randomized feeding trial. Nutr. Metab. Cardiovasc. Dis. 2015. [Google Scholar] [CrossRef] [PubMed]
- Chiva-Blanch, G.; Condines, X.; Magraner, E.; Roth, I.; Valderas-Martínez, P.; Arranz, S.; Casas, R.; Martínez-Huélamo, M.; Vallverdú-Queralt, A.; Quifer-Rada, P.; et al. The non-alcoholic fraction of beer increases stromal cell derived factor 1 and the number of circulating endothelial progenitor cells in high cardiovascular risk subjects: A randomized clinical trial. Atherosclerosis 2014. [Google Scholar] [CrossRef] [PubMed]
- Quifer-Rada, P.; Martínez-Huélamo, M.; Chiva-Blanch, G.; Jáuregui, O.; Estruch, R.; Lamuela-Raventós, R.M. Urinary isoxanthohumol is a specific and accurate biomarker of beer consumption. J. Nutr. 2014. [Google Scholar] [CrossRef] [PubMed]
- Scherr, J.; Nieman, D.C.; Schuster, T.; Habermann, J.; Rank, M.; Braun, S.; Pressler, A.; Wolfarth, B.; Halle, M. Nonalcoholic beer reduces inflammation and incidence of respiratory tract illness. Med. Sci. Sports Exerc. 2012. [Google Scholar] [CrossRef]
- Gorinstein, S.; Caspi, A.; Libman, I.; Leontowicz, H.; Leontowicz, M.; Tashma, Z.; Katrich, E.; Jastrzebski, Z.; Trakhtenberg, S. Bioactivity of beer and its influence on human metabolism. Int. J. Food Sci. Nutr. 2007. [Google Scholar] [CrossRef]
- Zilkens, R.R.; Burke, V.; Hodgson, J.M.; Barden, A.; Beilin, L.J.; Puddey, I.B. Red wine and beer elevate blood pressure in normotensive men. Hypertension 2005. [Google Scholar] [CrossRef]
- Di Giuseppe, R.; De Lorgeril, M.; Salen, P.; Laporte, F.; Di Castelnuovo, A.; Krogh, V.; Siani, A.; Arnout, J.; Cappuccio, F.P.; Van Dongen, M.; et al. Alcohol consumption and n-3 polyunsaturated fatty acids in healthy men and women from 3 European populations. Am. J. Clin. Nutr. 2009. [Google Scholar] [CrossRef]
- Padro, T.; Muñoz-García, N.; Vilahur, G.; Chagas, P.; Deyà, A.; Antonijoan, R.M.; Badimon, L. Moderate beer intake and cardiovascular health in overweight individuals. Nutrients 2018. [Google Scholar] [CrossRef]
- Godos, J.; Sinatra, D.; Blanco, I.; Mulè, S.; La Verde, M.; Marranzano, M. Association between dietary phenolic acids and hypertension in a mediterranean cohort. Nutrients 2017, 9, 1069. [Google Scholar] [CrossRef]
- González-Zancada, N.; Redondo-Useros, N.; Díaz, L.E.; Gómez-Martínez, S.; Marcos, A.; Nova, E. Association of Moderate Beer Consumption with the Gut Microbiota and SCFA of Healthy Adults. Molecules 2020, 25, 4772. [Google Scholar] [CrossRef]
- Le Roy, C.I.; Wells, P.M.; Si, J.; Raes, J.; Bell, J.T.; Spector, T.D. Red Wine Consumption Associated With Increased Gut Microbiota α-Diversity in 3 Independent Cohorts. Gastroenterology 2020. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.; Haque, R.; Van Den Eeden, S.K.; Caan, B.J.; Poon, K.Y.T.; Quinn, V.P. Red wine consumption and risk of prostate cancer: The California men’s health study. Int. J. Cancer 2010. [Google Scholar] [CrossRef]
- Mehlig, K.; Skoog, I.; Guo, X.; Schütze, M.; Gustafson, D.; Waern, M.; Östling, S.; Björkelund, C.; Lissner, L. Alcoholic beverages and incidence of dementia: 34-Year follow-up of the prospective population study of women in Göteborg. Am. J. Epidemiol. 2008. [Google Scholar] [CrossRef]
- Petti, S.; Scully, C. Association between different alcoholic beverages and leukoplakia among non- to moderate-drinking adults: A matched case-control study. Eur. J. Cancer 2006. [Google Scholar] [CrossRef]
- Nettleton, J.A.; Harnack, L.J.; Scrafford, C.G.; Mink, P.J.; Barraj, L.M.; Jacobs, D.R. Dietary flavonoids and flavonoid-rich foods are not associated with risk of type 2 diabetes in postmenopausal women. J. Nutr. 2006. [Google Scholar] [CrossRef]
- Petti, S.; Scully, C. Oral cancer: The association between nation-based alcohol-drinking profiles and oral cancer mortality. Oral Oncol. 2005. [Google Scholar] [CrossRef]
- Schoonen, W.M.; Salinas, C.A.; Kiemeney, L.A.L.M.; Stanford, J.L. Alcohol consumption and risk of prostate cancer in middle-aged men. Int. J. Cancer 2005. [Google Scholar] [CrossRef]
- Infante-Rivard, C.; Krajinovic, M.; Labuda, D.; Sinnett, D. Childhood acute lymphoblastic leukemia associated with parental alcohol consumption and polymorphisms of carcinogen-metabolizing genes. Epidemiology 2002. [Google Scholar] [CrossRef]
- Ussar, S.; Griffin, N.W.; Bezy, O.; Fujisaka, S.; Vienberg, S.; Softic, S.; Deng, L.; Bry, L.; Gordon, J.I.; Kahn, C.R. Interactions between gut microbiota, host genetics and diet modulate the predisposition to obesity and metabolic syndrome. Cell Metab. 2015. [Google Scholar] [CrossRef]
- Chávez-Carbajal, A.; Nirmalkar, K.; Pérez-Lizaur, A.; Hernández-Quiroz, F.; Ramírez-Del-Alto, S.; García-Mena, J.; Hernández-Guerrero, C. Gut microbiota and predicted metabolic pathways in a sample of Mexican women affected by obesity and obesity plus metabolic syndrome. Int. J. Mol. Sci. 2019, 20, 438. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, W.; Lu, J.; Zhao, M. Phenolic profiles and antioxidant activities of commercial beers. Food Chem. 2010. [Google Scholar] [CrossRef]
- Nardini, M.; Garaguso, I. Characterization of bioactive compounds and antioxidant activity of fruit beers. Food Chem. 2020, 305, 125437. [Google Scholar] [CrossRef] [PubMed]
- Legua, P.; Serrano, M.; Melgarejo, P.; Valero, D.; Martínez, J.J.; Martínez, R.; Hernández, F. Quality parameters, biocompounds and antioxidant activity in fruits of nine quince (Cydonia oblonga Miller) accessions. Sci. Hortic. (Amsterdam) 2013, 154, 61–65. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Bielicki, P. Polyphenolic composition, antioxidant activity, and polyphenol oxidase (PPO) activity of quince (cydonia oblonga miller) varieties. J. Agric. Food Chem. 2013. [Google Scholar] [CrossRef]
- Zapata, P.J.; Martínez-Esplá, A.; Gironés-Vilaplana, A.; Santos-Lax, D.; Noguera-Artiaga, L.; Carbonell-Barrachina, Á.A. Phenolic, volatile, and sensory profiles of beer enriched by macerating quince fruits. LWT 2019. [Google Scholar] [CrossRef]
- Sun, J.; Li, L.; You, X.; Li, C.; Zhang, E.; Li, Z.; Chen, G.; Peng, H. Phenolics and polysaccharides in major tropical fruits: Chemical compositions, analytical methods and bioactivities. Anal. Methods 2011. [Google Scholar] [CrossRef]
- Gasinski, A.; Kawa-Rygielska, J.; Szumny, A.; Czubaszek, A.; Gasior, J.; Pietrzak, W. Volatile compounds content, physicochemical parameters, and antioxidant activity of beers with addition of mango fruit (Mangifera Indica). Molecules 2020, 25, 3033. [Google Scholar] [CrossRef]
- Lako, J.; Trenerry, V.C.; Wahlqvist, M.; Wattanapenpaiboon, N.; Sotheeswaran, S.; Premier, R. Phytochemical flavonols, carotenoids and the antioxidant properties of a wide selection of Fijian fruit, vegetables and other readily available foods. Food Chem. 2007. [Google Scholar] [CrossRef]
- Humia, B.V.; Santos, K.S.; Schneider, J.K.; Leal, I.L.; de Abreu Barreto, G.; Batista, T.; Machado, B.A.S.; Druzian, J.I.; Krause, L.C.; da Costa Mendonça, M.; et al. Physicochemical and sensory profile of Beauregard sweet potato beer. Food Chem. 2020. [Google Scholar] [CrossRef]
- Talhaoui, N.; Taamalli, A.; Gómez-Caravaca, A.M.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Phenolic compounds in olive leaves: Analytical determination, biotic and abiotic influence, and health benefits. Food Res. Int. 2015, 77, 92–108. [Google Scholar] [CrossRef]
- Guglielmotti, M.; Passaghe, P.; Buiatti, S. Use of olive (Olea europaea L.) leaves as beer ingredient, and their influence on beer chemical composition and antioxidant activity. J. Food Sci. 2020, 85, 2278–2285. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Lim, J.; Nguyen, T.T.H.; Mok, I.-K.; Piao, M.; Kim, D. Composition and biochemical properties of ale beer enriched with lignans from Schisandra chinensis Baillon (omija) fruits. Food Sci. Biotechnol. 2020, 29, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, D.J.; Barooah, M.S.; Bora, S.S.; Singaravadivel, K. Biochemical and nutritional analysis of rice beer of North East India. Indian J. Tradit. Knowl. 2014, 13, 142–148. [Google Scholar]
- Agu, R.C. Comparative study of experimental beers brewed from millet, sorghum and barley malts. Process. Biochem. 1995. [Google Scholar] [CrossRef]
- Palmer, G.H.; Etokakpan, O.U.; Igyor, M.A. Sorghum as brewing material. MIRCEN J. Appl. Microbiol. Biotechnol. 1989, 5, 265–275. [Google Scholar] [CrossRef]
- Serna-Saldivar, S.O. Maize: Foods from Maize. In Encyclopedia of Food Grains, 2nd ed.; Wrigley, C., Corke, H., Seetharaman, K., Faubion, J., Eds.; Academic Press: Oxford, UK, 2016; pp. 97–109. ISBN 978-0-12-394786-4. [Google Scholar]
- Escalante-Aburto, A.; Ramírez-Wong, B.; Torres-Chávez, P.I.; Manuel Barrón-Hoyos, J.; de Dios Figueroa-Cárdenas, J.; López-Cervantes, J. The nixtamalization process and its effect on anthocyanin content of pigmented maize, A review. Rev. Fitotec. Mex. 2013. [Google Scholar] [CrossRef]
- Flores-Calderón, A.M.D.; Luna, H.; Escalona-Buendía, H.B.; Verde-Calvo, J.R. Chemical characterization and antioxidant capacity in blue corn (Zea mays L.) malt beers. J. Inst. Brew. 2017. [Google Scholar] [CrossRef]
- Romero-Medina, A.; Estarrón-Espinosa, M.; Verde-Calvo, J.R.; Lelièvre-Desmas, M.; Escalona-Buendiá, H.B. Renewing Traditions: A Sensory and Chemical Characterisation of Mexican Pigmented Corn Beers. Foods 2020, 9, 886. [Google Scholar] [CrossRef]
- Fumi, M.D.; Galli, R.; Lambri, M.; Donadini, G.; De Faveri, D.M. Effect of full-scale brewing process on polyphenols in Italian all-malt and maize adjunct lager beers. J. Food Compos. Anal. 2011, 24, 568–573. [Google Scholar] [CrossRef]
- Klose, C.; Mauch, A.; Wunderlich, S.; Thiele, F.; Zarnkow, M.; Jacob, F.; Arendt, E.K. Brewing with 100% oat malt. J. Inst. Brew. 2011. [Google Scholar] [CrossRef]
- Di Ghionno, L.; Sileoni, V.; Marconi, O.; De Francesco, G.; Perretti, G. Comparative study on quality attributes of gluten-free beer from malted and unmalted teff [Eragrostis tef (zucc.) trotter]. LWT Food Sci. Technol. 2017. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, T.J.; Chen, J. Changes of the flavonoid and phenolic acid content and antioxidant activity of tartary buckwheat beer during the fermentation. Adv. Mater. Res. 2013, 781–784, 1619–1624. [Google Scholar] [CrossRef]
- Kordialik-Bogacka, E.; Bogdan, P.; Pielech-Przybylska, K.; Michałowska, D. Suitability of unmalted quinoa for beer production. J. Sci. Food Agric. 2018. [Google Scholar] [CrossRef] [PubMed]
- Zarnkow, M.; Faltermaier, A.; Back, W.; Gastl, M.; Arendt, E.K. Evaluation of different yeast strains on the quality of beer produced from malted proso millet (Panicum miliaceum L.). Eur. Food Res. Technol. 2010. [Google Scholar] [CrossRef]
- Lyumugabe, F.; Bajyana Songa, E.; Wathelet, J.P.; Thonart, P. Volatile compounds of the traditional sorghum beers “ikigage” brewed with Vernonia amygdalina “umubirizi.”. Cerevisia 2013. [Google Scholar] [CrossRef]
- Onyenekwe, P.C.; Erhabor, G.O.; Akande, S.A. Characterisation of aroma volatiles of indigenous alcoholic beverages: Burukutu and pito. Nat. Prod. Res. 2016. [Google Scholar] [CrossRef]
- Handique, P.; Deka, A.K.; Deka, D.C. Antioxidant Properties and Phenolic Contents of Traditional Rice-Based Alcoholic Beverages of Assam, India. Natl. Acad. Sci. Lett. 2020. [Google Scholar] [CrossRef]
- Kordialik-Bogacka, E.; Bogdan, P.; Diowksz, A. Malted and unmalted oats in brewing. J. Inst. Brew. 2014. [Google Scholar] [CrossRef]
- Wu, D.; Cai, G.; Li, X.; Li, B.; Lu, J. Cloning and expression of ferulic acid esterase gene and its effect on wort filterability. Biotechnol. Lett. 2018. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, A.; Zhang, Z.; Speers, R.A. Enhancing the levels of 4-vinylguaiacol and 4-vinylphenol in pilot-scale top-fermented wheat beers by response surface methodology. J. Inst. Brew. 2015. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, H.; Cui, Y.; Zeng, D.; Bao, X. Increasing the level of 4-vinylguaiacol in top-fermented wheat beer by secretory expression of ferulic acid decarboxylase from Bacillus pumilus in brewer’s yeast. Biotechnol. Lett. 2020. [Google Scholar] [CrossRef]
- Brányik, T.; Silva, D.P.; Baszczyňski, M.; Lehnert, R.; Almeida e Silva, J.B. A review of methods of low alcohol and alcohol-free beer production. J. Food Eng. 2012, 108, 493–506. [Google Scholar] [CrossRef]
- Liguori, L.; De Francesco, G.; Russo, P.; Perretti, G.; Albanese, D.; Di Matteo, M. Quality Attributes of Low-Alcohol Top-Fermented Beers Produced by Membrane Contactor. Food Bioprocess. Technol. 2016. [Google Scholar] [CrossRef]
- De Fusco, D.O.; Madaleno, L.L.; Del Bianchi, V.L.; da Silva Bernardo, A.; Assis, R.R.; de Almeida Teixeira, G.H. Development of low-alcohol isotonic beer by interrupted fermentation. Int. J. Food Sci. Technol. 2019. [Google Scholar] [CrossRef]
- Desbrow, B.; Murray, D.; Leveritt, M. Beer as a sports drink? Manipulating beer’s ingredients to replace lost fluid. Int. J. Sport Nutr. Exerc. Metab. 2013. [Google Scholar] [CrossRef] [PubMed]
- Maughan, R.J. The sports drink as a functional food: Formulations for successful performance. Proc. Nutr. Soc. 1998. [Google Scholar] [CrossRef]
- Menz, G.; Vriesekoop, F.; Zarei, M.; Zhu, B.; Aldred, P. The growth and survival of food-borne pathogens in sweet and fermenting brewers’ wort. Int. J. Food Microbiol. 2010. [Google Scholar] [CrossRef] [PubMed]
- Meneses, N.G.T.; Martins, S.; Teixeira, J.A.; Mussatto, S.I. Influence of extraction solvents on the recovery of antioxidant phenolic compounds from brewer’s spent grains. Sep. Purif. Technol. 2013. [Google Scholar] [CrossRef]
- Cooray, S.T.; Chen, W.N. Valorization of brewer’s spent grain using fungi solid-state fermentation to enhance nutritional value. J. Funct. Foods 2018. [Google Scholar] [CrossRef]
- Ferk, F.; Mišík, M.; Nersesyan, A.; Pichler, C.; Jäger, W.; Szekeres, T.; Marculescu, R.; Poulsen, H.E.; Henriksen, T.; Bono, R.; et al. Impact of xanthohumol (a prenylated flavonoid from hops) on DNA stability and other health-related biochemical parameters: Results of human intervention trials. Mol. Nutr. Food Res. 2016. [Google Scholar] [CrossRef]
- Wunderlich, S.; Zürcher, A.; Back, W. Enrichment of xanthohumol in the brewing process. Mol. Nutr. Food Res. 2005. [Google Scholar] [CrossRef] [PubMed]
- Karabín, M.; Jelínek, L.; Kinčl, T.; Hudcová, T.; Kotlíková, B.; Dostálek, P. New approach to the production of xanthohumol-enriched beers. J. Inst. Brew. 2013. [Google Scholar] [CrossRef]
- Machado, J.C.; Faria, M.A.; Melo, A.; Martins, Z.E.; Ferreira, I.M.P.L.V.O. Modeling of α-acids and xanthohumol extraction in dry-hopped beers. Food Chem. 2019. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, P.J.; Dostalek, P.; Cruz, J.M.; Guido, L.F.; Barros, A.A. The impact of a xanthohumol-enriched hop product on the behavior of xanthohumol and isoxanthohumol in pale and dark beers: A pilot scale approach. J. Inst. Brew. 2008. [Google Scholar] [CrossRef]
- Benelli, R.; Ven, R.; Ciarlo, M.; Carlone, S.; Barbieri, O.; Ferrari, N. The AKT/NF-κB inhibitor xanthohumol is a potent anti-lymphocytic leukemia drug overcoming chemoresistance and cell infiltration. Biochem. Pharmacol. 2012. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to polyphenols in olive and protection of LDL particles from oxidative damage (ID 1333, 1638, 1639, 1696, 2865), maintenance of normal blood HDL cholesterol concentrations (ID 1639), mainte. EFSA J. 2011, 9, 2033. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the modification of the authorisation of a health claim related to cocoa flavanols and maintenance of normal endothelium-dependent vasodilation pursuant to Article 13(5) of Regulation (EC) No 1924/2006 following a request in accordan. EFSA J. 2014, 12, 3654. [Google Scholar] [CrossRef]
- Merinas-Amo, T.; Tasset-Cuevas, I.; Díaz-Carretero, A.M.; Alonso-Moraga, Á.; Calahorro, F. In vivo and in vitro studies of the role of lyophilised blond Lager beer and some bioactive components in the modulation of degenerative processes. J. Funct. Foods 2016. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).