Adsorbing Volatile Organic Chemicals by Soluble Triazine-Based Dendrimers under Ambient Conditions with the Adsorption Capacity of Pyridine up to 946.2 mg/g
Abstract
:1. Introduction
2. Experimental Section
2.1. General
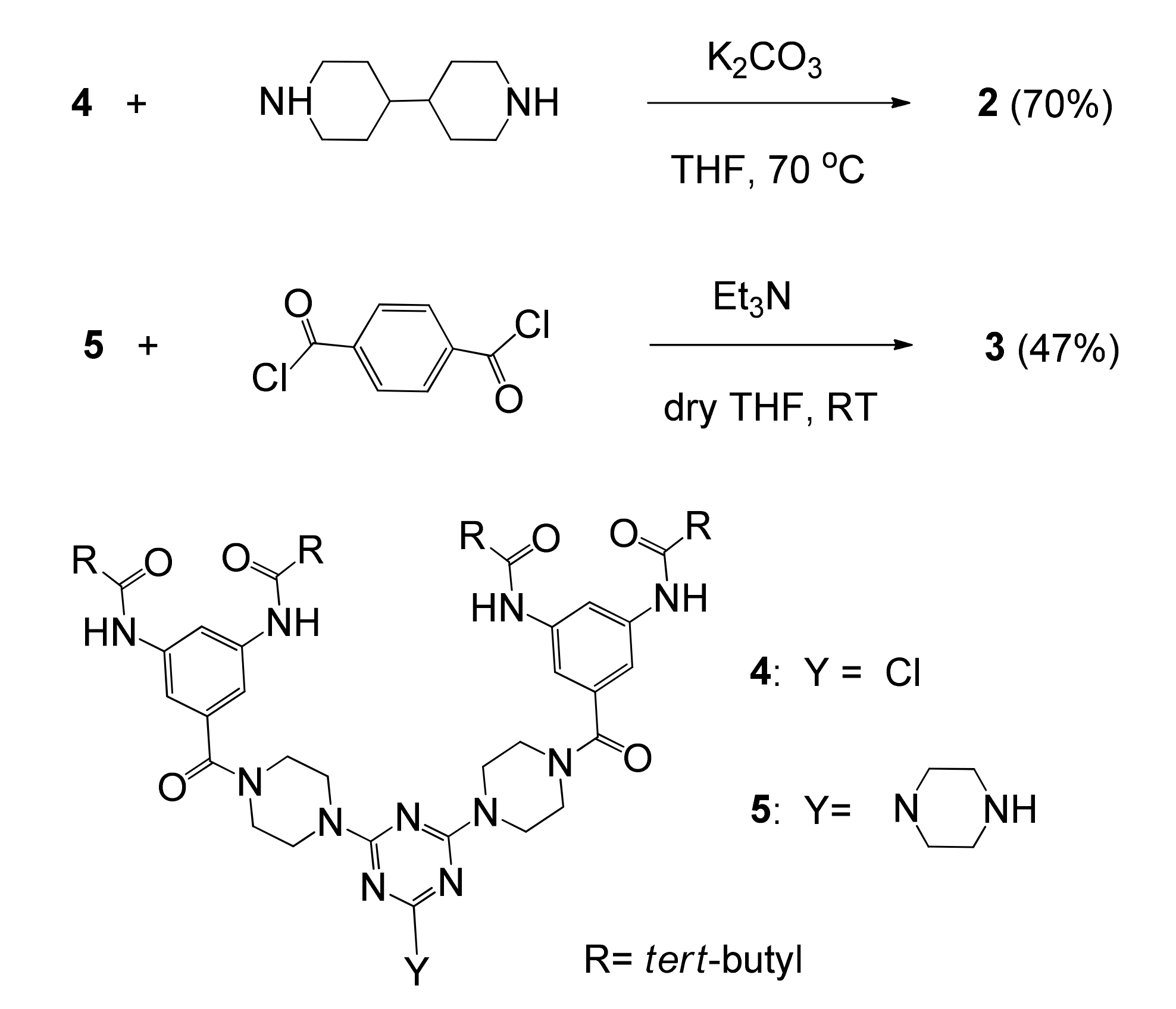
2.2. Preparation of Dendrimer 2
2.3. Preparation of Dendrimer 3
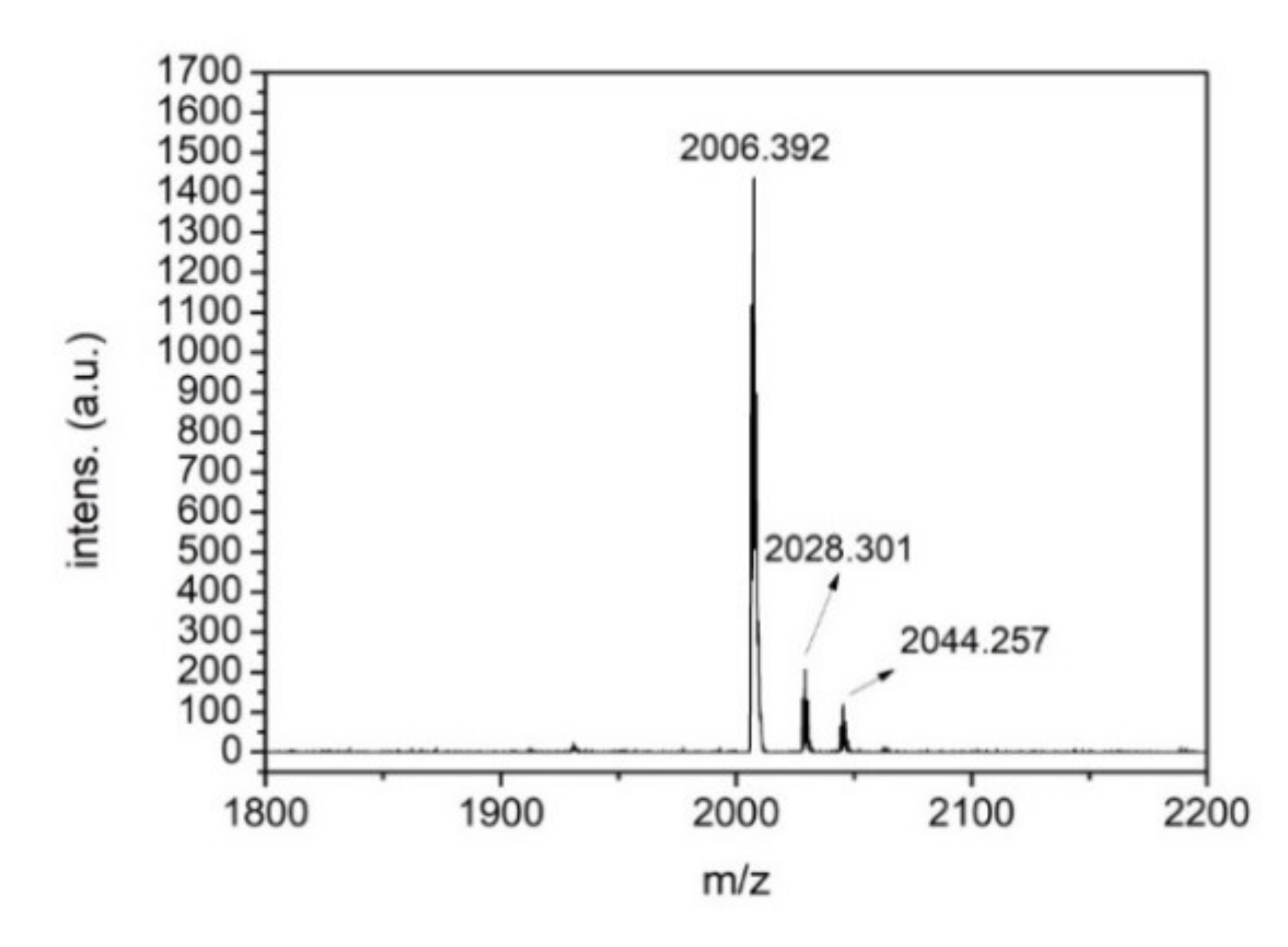
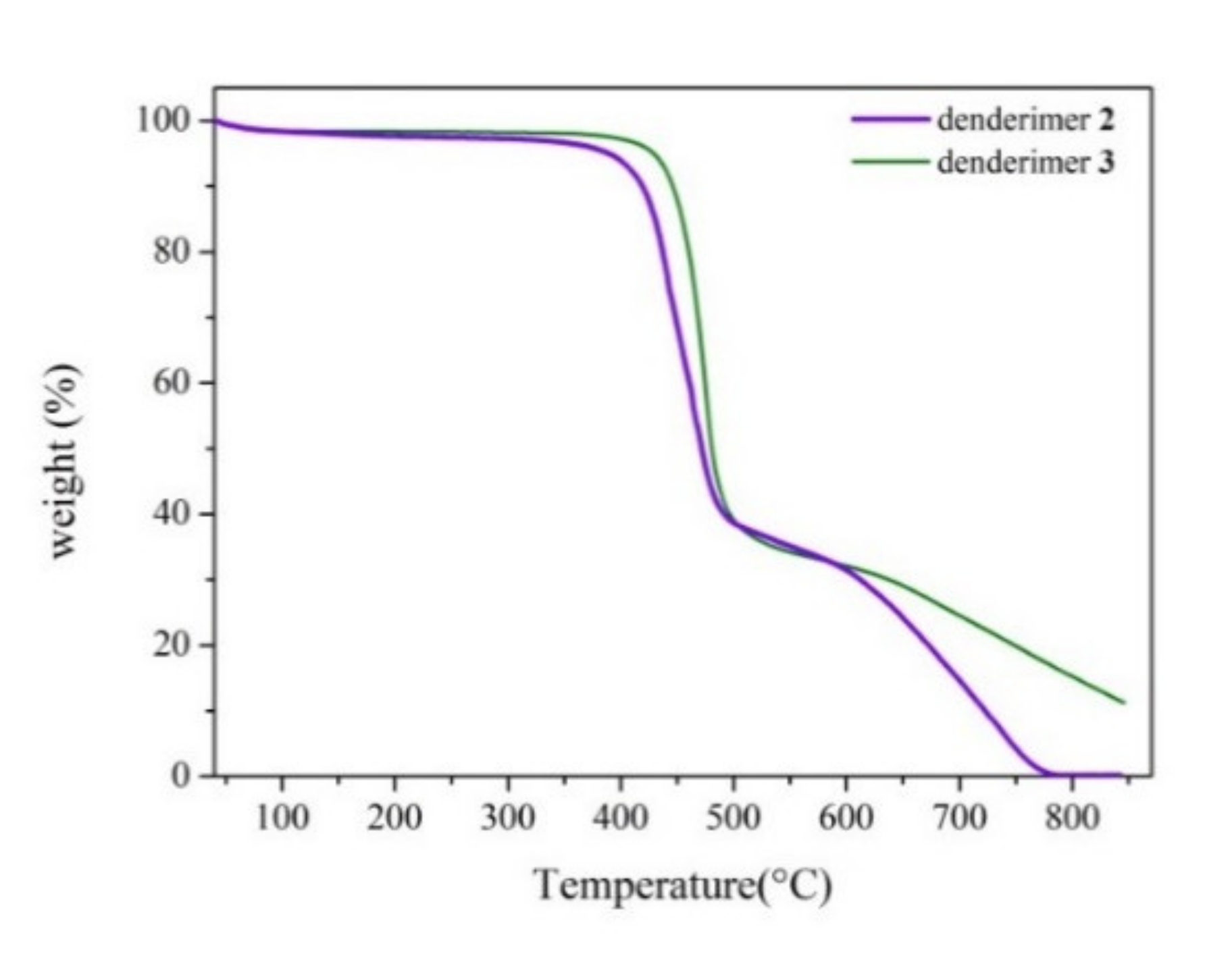
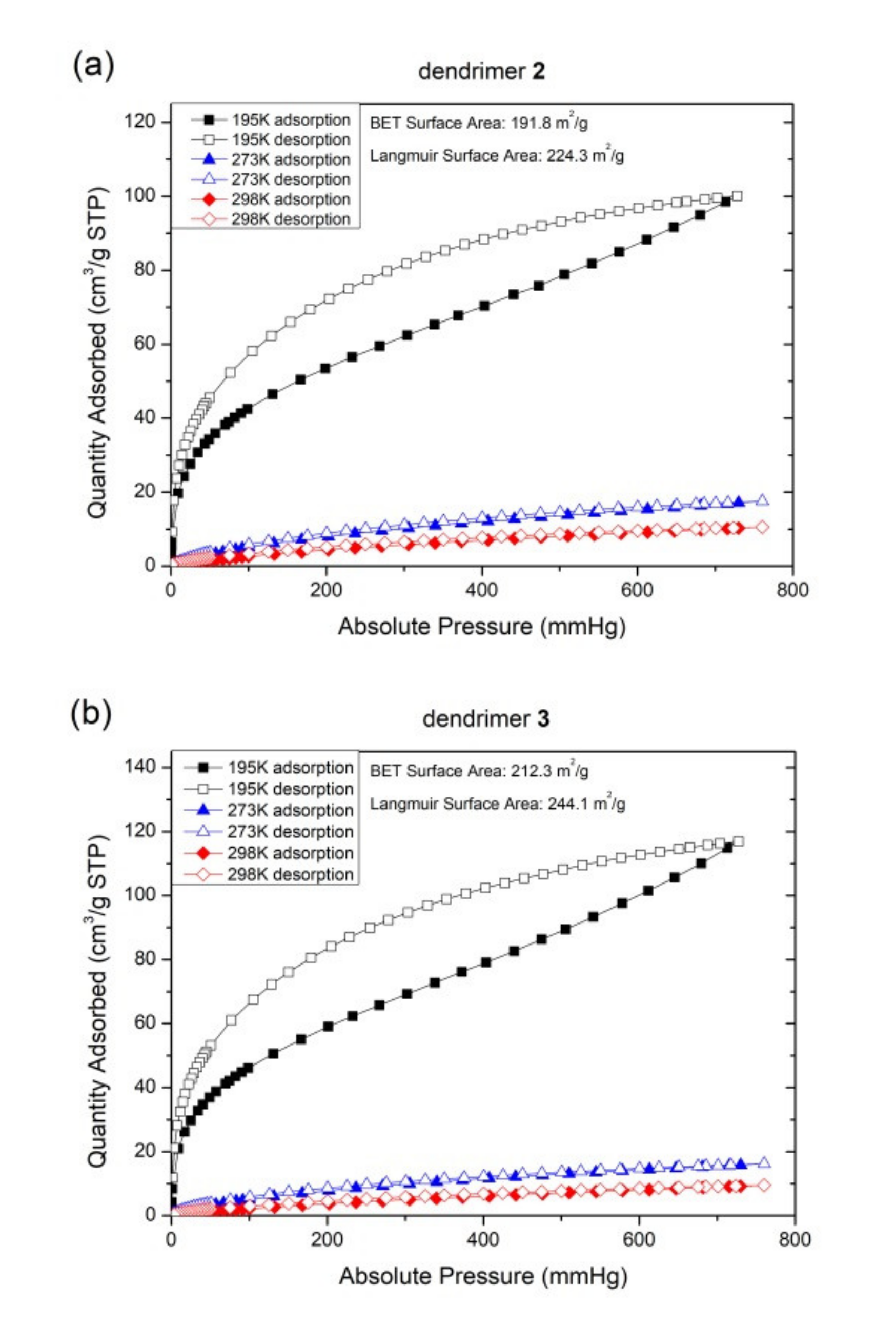
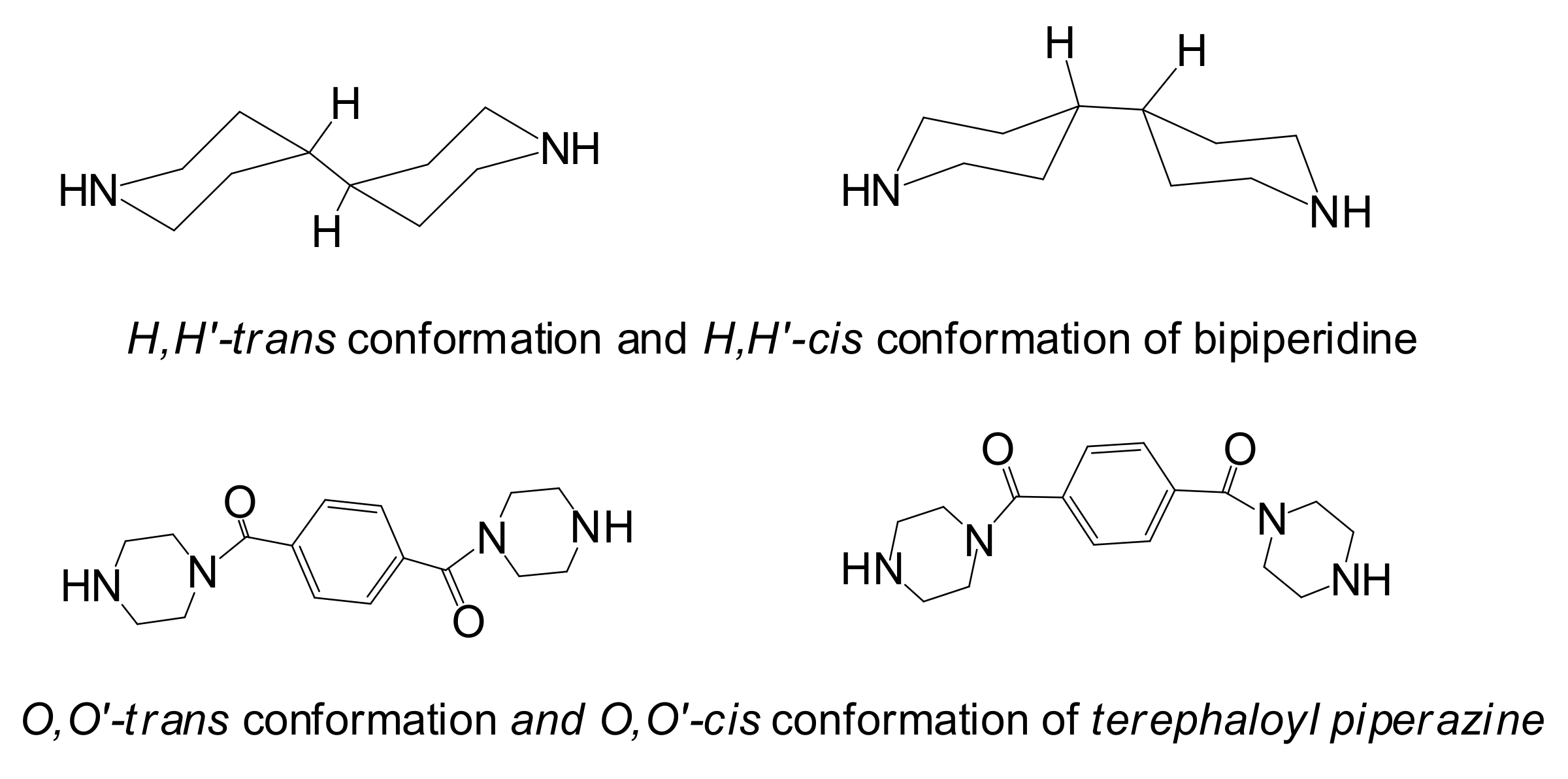
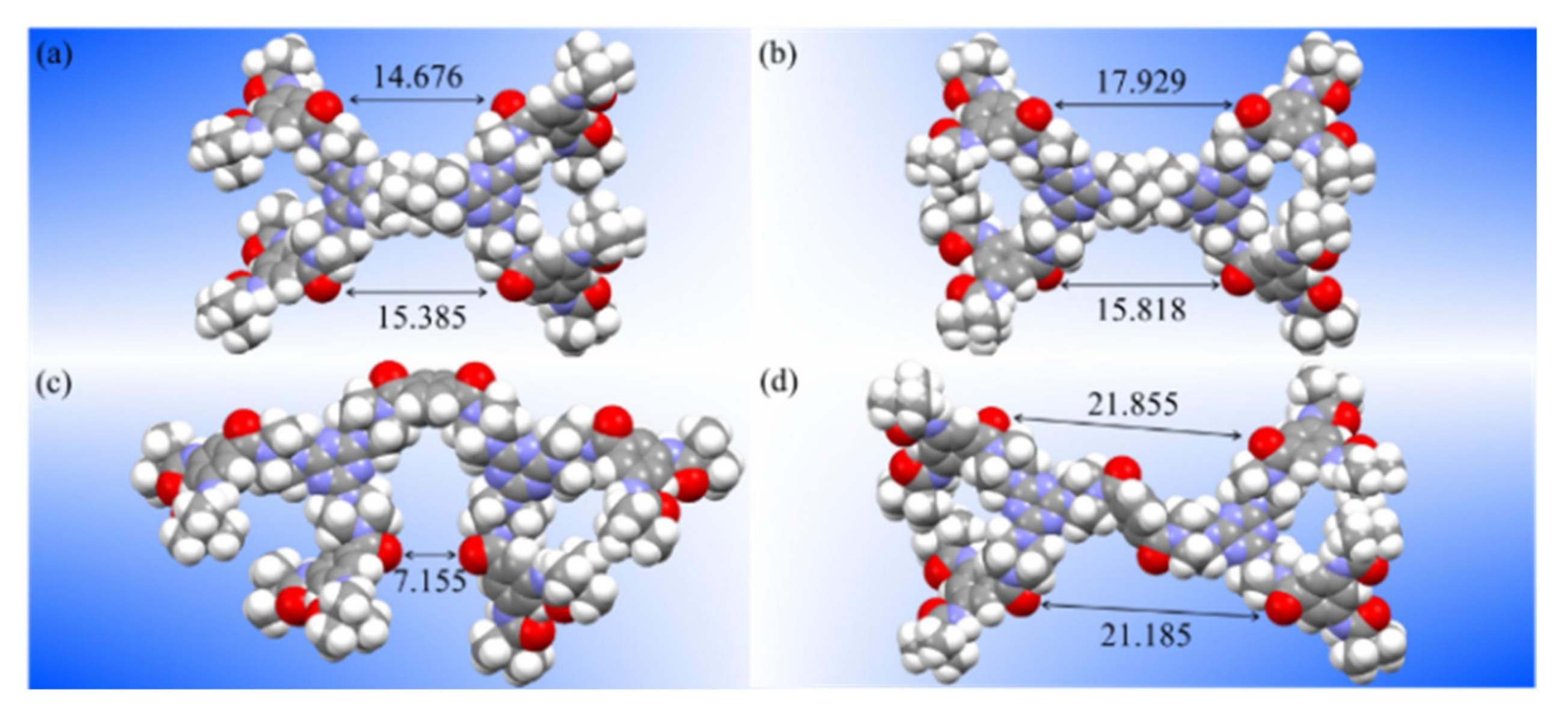
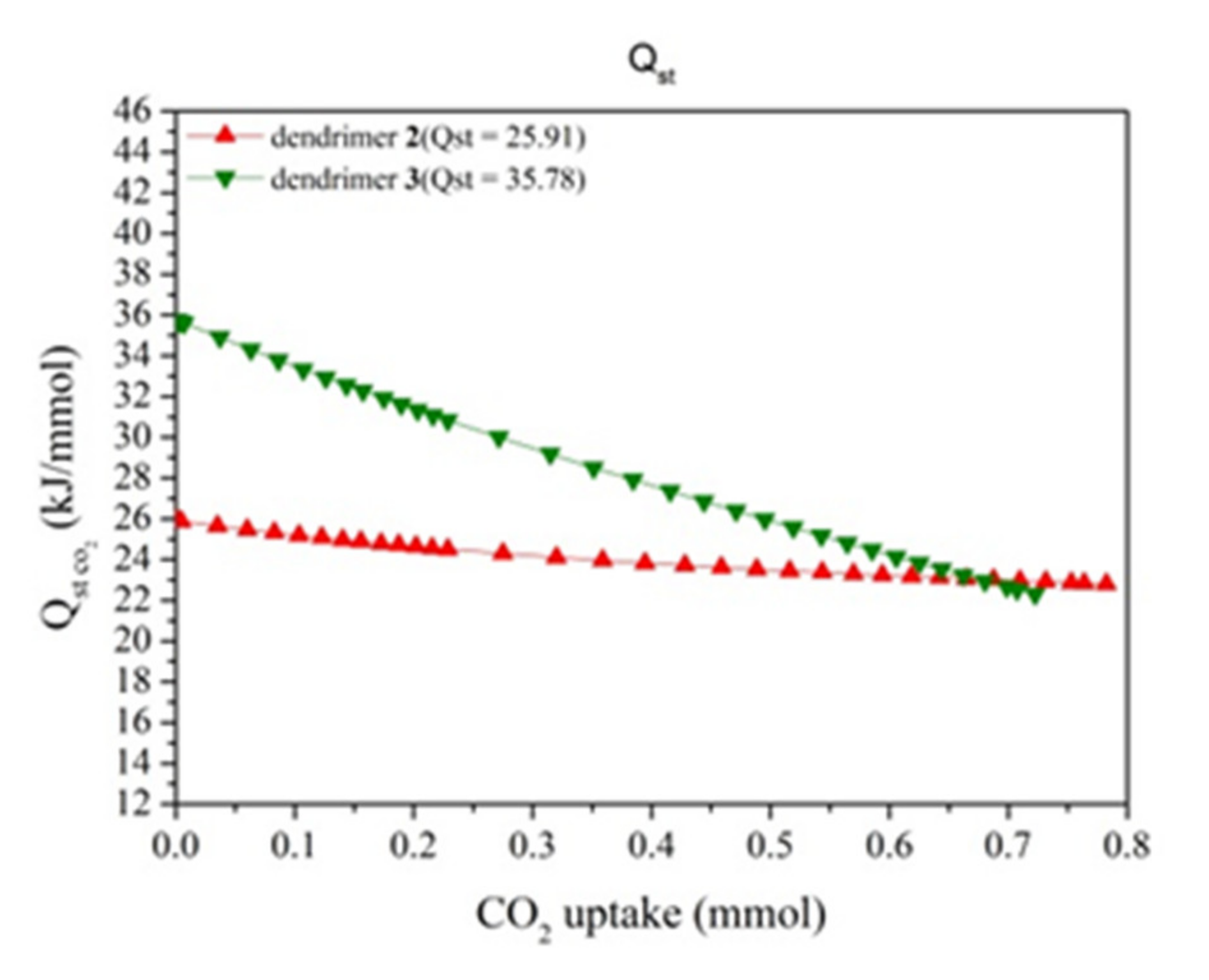
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Klett, C.; Duten, X.; Tieng, S.; Touchard, S.; Jestin, P.; Hassouni, K.; Vega-González, A. Acetaldehyde removal using an atmospheric non-thermal plasma combined with a packed bed: Role of the adsorption process. J. Hazard. Mater. 2014, 279, 356–364. [Google Scholar] [CrossRef]
- Gałęzowska, G.; Chraniuk, M.; Wolska, L. In vitro assays as a tool for determination of VOCs toxic effect on respiratory system: A critical review. Trends Anal. Chem. 2016, 77, 14–22. [Google Scholar] [CrossRef]
- McDonald, B.C.; de Gouw, J.A.; Gilman, J.B.; Jathar, S.H.; Akherati, A.; Cappa, C.D.; Jimenez, J.L.; Lee-Taylor, J.; Hayes, P.L.; McKeen, S.A.; et al. Volatile chemical products emerging as largest petrochemical source of urban organic emissions. Science 2018, 359, 760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, B.; Lei, C.; Wei, C.; Zeng, G. Chlorinated volatile organic compounds (Cl-VOCs) in environment — sources, potential human health impacts, and current remediation technologies. Environ. Int. 2014, 71, 118–138. [Google Scholar] [CrossRef]
- Bari, M.A.; Kindzierski, W.B. Ambient volatile organic compounds (VOCs) in Calgary, Alberta: Sources and screening health risk assessment. Sci. Total Environ. 2018, 631-632, 627–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varela-Gandía, F.J.; Berenguer-Murcia, Á.; Lozano-Castelló, D.; Cazorla-Amorós, D.; Sellick, D.R.; Taylor, S.H. Total oxidation of naphthalene using palladium nanoparticles supported on BETA, ZSM-5, SAPO-5 and alumina powders. Appl. Catal. 2013, 129, 98–105. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Yu, Y.; Shao, Q.; Long, C. Porous polymeric resin for adsorbing low concentration of VOCs: Unveiling adsorption mechanism and effect of VOCs’ molecular properties. Sep. Purif. Technol. 2019, 228, 115755. [Google Scholar] [CrossRef]
- Wang, H.; Nie, L.; Li, J.; Wang, Y.; Wang, G.; Wang, J.; Hao, Z. Characterization and assessment of volatile organic compounds (VOCs) emissions from typical industries. Chin. Sci. Bull. 2013, 58, 724–730. [Google Scholar] [CrossRef] [Green Version]
- He, C.; Cheng, J.; Zhang, X.; Douthwaite, M.; Pattisson, S.; Hao, Z. Recent Advances in the Catalytic Oxidation of Volatile Organic Compounds: A Review Based on Pollutant Sorts and Sources. Chem. Rev. 2019, 119, 4471–4568. [Google Scholar] [CrossRef]
- Kraus, M.; Trommler, U.; Holzer, F.; Kopinke, F.-D.; Roland, U. Competing adsorption of toluene and water on various zeolites. Chem. Eng. J. 2018, 351, 356–363. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, L.; Zhang, X.; Chen, Y. Preparation of zeolitic imidazolate framework-8/graphene oxide composites with enhanced VOCs adsorption capacity. Microporous Mesoporous Mater. 2016, 225, 488–493. [Google Scholar] [CrossRef]
- Yu, W.; Yuan, P.; Liu, D.; Deng, L.; Yuan, W.; Tao, B.; Cheng, H.; Chen, F. Facile preparation of hierarchically porous diatomite/MFI-type zeolite composites and their performance of benzene adsorption: The effects of NaOH etching pretreatment. J. Hazard. Mater. 2015, 285, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Mihara, Y.; Tanaka, S.; Watanabe, K.; Terui, N. Nitrobenzene-adsorption capacity of carbon materials released during the combustion of woody biomass. J. Hazard. Mater. 2010, 174, 776–781. [Google Scholar] [CrossRef]
- Mohammed, J.; Nasri, N.S.; Ahmad Zaini, M.A.; Hamza, U.D.; Ani, F.N. Adsorption of benzene and toluene onto KOH activated coconut shell based carbon treated with NH3. Int. Biodeterior. Biodegrad. 2015, 102, 245–255. [Google Scholar] [CrossRef]
- Jahandar Lashaki, M.; Atkinson, J.D.; Hashisho, Z.; Phillips, J.H.; Anderson, J.E.; Nichols, M. The role of beaded activated carbon’s pore size distribution on heel formation during cyclic adsorption/desorption of organic vapors. J. Hazard. Mater. 2016, 315, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Niknaddaf, S.; Atkinson, J.D.; Gholidoust, A.; Fayaz, M.; Awad, R.; Hashisho, Z.; Phillips, J.H.; Anderson, J.E.; Nichols, M. Influence of Purge Gas Flow and Heating Rates on Volatile Organic Compound Decomposition during Regeneration of an Activated Carbon Fiber Cloth. Ind. Eng. Chem. Res. 2020, 59, 3521–3530. [Google Scholar] [CrossRef]
- Liu, S.; Chen, J.; Peng, Y.; Hu, F.; Li, K.; Song, H.; Li, X.; Zhang, Y.; Li, J. Studies on toluene adsorption performance and hydrophobic property in phenyl functionalized KIT-6. Chem. Eng. J. 2018, 334, 191–197. [Google Scholar] [CrossRef]
- Kuang, W.; Liu, Y.-N.; Huang, J. Phenol-modified hyper-cross-linked resins with almost all micro/mesopores and their adsorption to aniline. J. Colloid Interface Sci. 2017, 487, 31–37. [Google Scholar] [CrossRef]
- Gan, Y.; Chen, G.; Sang, Y.; Zhou, F.; Man, R.; Huang, J. Oxygen-rich hyper-cross-linked polymers with hierarchical porosity for aniline adsorption. Chem. Eng. J. 2019, 368, 29–36. [Google Scholar] [CrossRef]
- Xia, M.; Jin, C.; Kong, X.; Jiang, M.; Lei, D.; Lei, X. Green removal of pyridine from water via adsolubilization with lignosulfonate intercalated layered double hydroxide. Adsorpt. Sci. Technol. 2018, 36, 982–998. [Google Scholar] [CrossRef]
- Vellingiri, K.; Kumar, P.; Deep, A.; Kim, K.-H. Metal-organic frameworks for the adsorption of gaseous toluene under ambient temperature and pressure. Chem. Eng. J. 2017, 307, 1116–1126. [Google Scholar] [CrossRef]
- Shafiei, M.; Alivand, M.S.; Rashidi, A.; Samimi, A.; Mohebbi-Kalhori, D. Synthesis and adsorption performance of a modified micro-mesoporous MIL-101(Cr) for VOCs removal at ambient conditions. Chem. Eng. J. 2018, 341, 164–174. [Google Scholar] [CrossRef]
- Pirzadeh, K.; Ghoreyshi, A.A.; Rohani, S.; Rahimnejad, M. Strong Influence of Amine Grafting on MIL-101 (Cr) Metal–Organic Framework with Exceptional CO2/N2 Selectivity. Ind. Eng. Chem. Res. 2020, 59, 366–378. [Google Scholar] [CrossRef]
- Sudan, S.; Gładysiak, A.; Valizadeh, B.; Lee, J.-H.; Stylianou, K.C. Sustainable Capture of Aromatic Volatile Organic Compounds by a Pyrene-Based Metal–Organic Framework under Humid Conditions. Inorg. Chem. 2020, 59, 9029–9036. [Google Scholar] [CrossRef] [PubMed]
- INRS. Pyridine. Available online: https://www.inrs.fr/publications/bdd/fichetox/fiche.html?refINRS=FICHETOX_85 (accessed on 6 July 2021).
- Yamamoto, K.; Imaoka, T.; Tanabe, M.; Kambe, T. New Horizon of Nanoparticle and Cluster Catalysis with Dendrimers. Chem. Rev. 2020, 120, 1397–1437. [Google Scholar] [CrossRef] [PubMed]
- Neumann, P.; Dib, H.; Caminade, A.M.; Hey-Hawkins, E. Redox Control of a Dendritic Ferrocenyl-Based Homogeneous Catalyst. Angew. Chem. Int. Ed. 2015, 54, 311–314. [Google Scholar] [CrossRef]
- Chauhan, A.S. Dendrimers for Drug Delivery. Molecules 2018, 23, 938. [Google Scholar] [CrossRef] [Green Version]
- Janaszewska, A.; Lazniewska, J.; Trzepiński, P.; Marcinkowska, M.; Klajnert-Maculewicz, B. Cytotoxicity of Dendrimers. Biomolecules 2019, 9, 330. [Google Scholar] [CrossRef] [Green Version]
- Sherje, A.P.; Jadhav, M.; Dravyakar, B.R.; Kadam, D. Dendrimers: A versatile nanocarrier for drug delivery and targeting. Int. J. Pharm. 2018, 548, 707–720. [Google Scholar] [CrossRef]
- Lee, C.-H.; Tsai, M.-R.; Chang, Y.-T.; Lai, L.-L.; Lu, K.-L.; Cheng, K.-L. Preparation of Unconventional Dendrimers that Contain Rigid NH—Triazine Linkages and Peripheral tert-Butyl Moieties for CO2-Selective Adsorption. Chem. Eur. J. 2013, 19, 10573–10579. [Google Scholar] [CrossRef]
- Lee, C.-H.; Soldatov, D.V.; Tzeng, C.-H.; Lai, L.-L.; Lu, K.-L. Design of a Peripheral Building Block for H-Bonded Dendritic Frameworks and Analysis of the Void Space in the Bulk Dendrimers. Sci. Rep. 2017, 7, 3649. [Google Scholar] [CrossRef]
- Kecili, R.; Hussain, C.M. Chapter 4—Mechanism of Adsorption on Nanomaterials. In Nanomaterials in Chromatography; Chaudhery Mustansar, H., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 89–115. [Google Scholar]
- Sing, K.S.; Everett, D.H.; Haul, R.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. International union of pure commission on colloid and surface chemistry including catalysis* reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Yang, Z.; Wang, P.; Yan, Y.; Ran, J. Adsorption materials for volatile organic compounds (VOCs) and the key factors for VOCs adsorption process: A review. Sep. Purif. Technol. 2020, 235, 116213. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, B.; Creamer, A.E.; Cao, C.; Li, Y. Adsorption of VOCs onto engineered carbon materials: A review. J. Hazard. Mater. 2017, 338, 102–123. [Google Scholar] [CrossRef]
- Yang, K.; Sun, Q.; Xue, F.; Lin, D. Adsorption of volatile organic compounds by metal–organic frameworks MIL-101: Influence of molecular size and shape. J. Hazard. Mater. 2011, 195, 124–131. [Google Scholar] [CrossRef]
- Xu, F.; Xian, S.; Xia, Q.; Li, Y.; Li, Z. Effect of Textural Properties on the Adsorption and Desorption of Toluene on the Metal-Organic Frameworks HKUST-1 and MIL-101. Adsorp. Sci. Technol. 2013, 31, 325–339. [Google Scholar] [CrossRef]
- Qin, W.; Cao, W.; Liu, H.; Li, Z.; Li, Y. Metal-organic framework MIL-101 doped with palladium for toluene adsorption and hydrogen storage. RSC Adv. 2014, 4, 2414–2420. [Google Scholar] [CrossRef]
- Pan, H.; Ritter, J.A.; Balbuena, P.B. Examination of the Approximations Used in Determining the Isosteric Heat of Adsorption from the Clausius−Clapeyron Equation. Langmuir 1998, 14, 6323–6327. [Google Scholar] [CrossRef]
- Zukal, A.; Pawlesa, J.; Čejka, J. Isosteric heats of adsorption of carbon dioxide on zeolite MCM-22 modified by alkali metal cations. Adsorption (Boston) 2009, 15, 264–270. [Google Scholar] [CrossRef]
- Pribylov, A.A.; Murdmaa, K.O.; Solovtsova, O.V.; Knyazeva, M.K. Methane adsorption on various metal-organic frameworks and determination of the average adsorption heats at supercritical temperatures and pressures. Russ. Chem Bull. 2018, 67, 1807–1813. [Google Scholar] [CrossRef]
- Bakhtyari, A.; Mofarahi, M. A New Approach in Predicting Gas Adsorption Isotherms and Isosteric Heats Based on Two-Dimensional Equations of State. Arab. J. Sci. Eng. 2019, 44, 5513–5526. [Google Scholar] [CrossRef]

| Guest | Nitrobenzene | Pyridine | Toluene | Hexane | |
|---|---|---|---|---|---|
| Host | |||||
| 1 in DMSO-D6 | 4 | 11 | 3 | 0.6 | |
| 2 in DMSO-D6 | 3 | 7 | 2 | 0.3 | |
| 3 in CDCl3 | 4 | 24 | 5 | 0.8 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.-C.; Chien, C.-Y.; Hsu, H.-F.; Lai, L.-L. Adsorbing Volatile Organic Chemicals by Soluble Triazine-Based Dendrimers under Ambient Conditions with the Adsorption Capacity of Pyridine up to 946.2 mg/g. Molecules 2021, 26, 4862. https://doi.org/10.3390/molecules26164862
Lu Y-C, Chien C-Y, Hsu H-F, Lai L-L. Adsorbing Volatile Organic Chemicals by Soluble Triazine-Based Dendrimers under Ambient Conditions with the Adsorption Capacity of Pyridine up to 946.2 mg/g. Molecules. 2021; 26(16):4862. https://doi.org/10.3390/molecules26164862
Chicago/Turabian StyleLu, Yao-Chih, Chia-Yun Chien, Hsiu-Fu Hsu, and Long-Li Lai. 2021. "Adsorbing Volatile Organic Chemicals by Soluble Triazine-Based Dendrimers under Ambient Conditions with the Adsorption Capacity of Pyridine up to 946.2 mg/g" Molecules 26, no. 16: 4862. https://doi.org/10.3390/molecules26164862
APA StyleLu, Y.-C., Chien, C.-Y., Hsu, H.-F., & Lai, L.-L. (2021). Adsorbing Volatile Organic Chemicals by Soluble Triazine-Based Dendrimers under Ambient Conditions with the Adsorption Capacity of Pyridine up to 946.2 mg/g. Molecules, 26(16), 4862. https://doi.org/10.3390/molecules26164862






