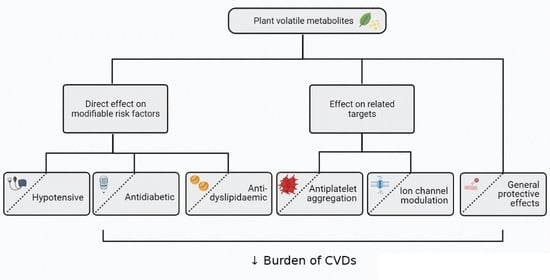
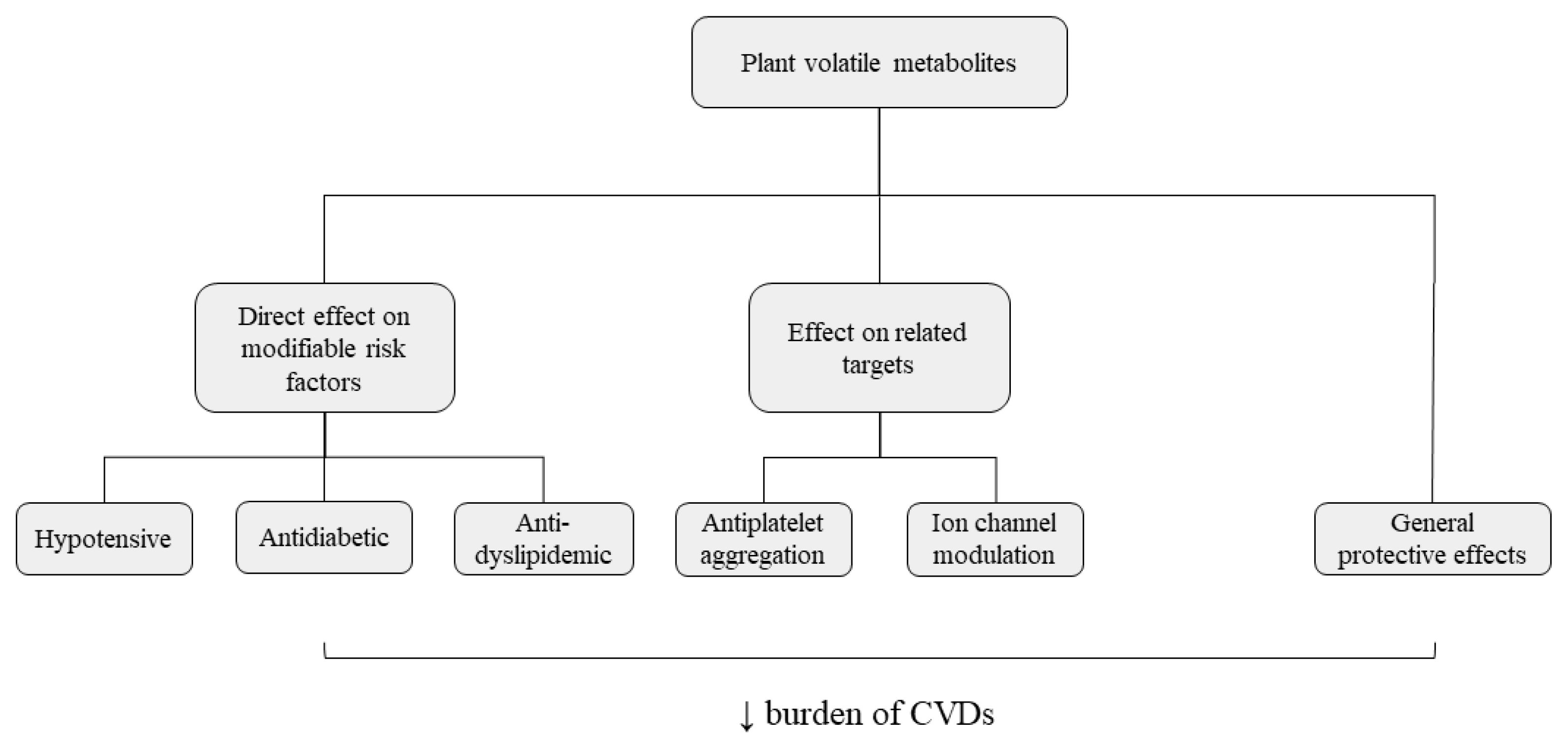
The Role of Essential Oils and Their Main Compounds in the Management of Cardiovascular Disease Risk Factors
Abstract
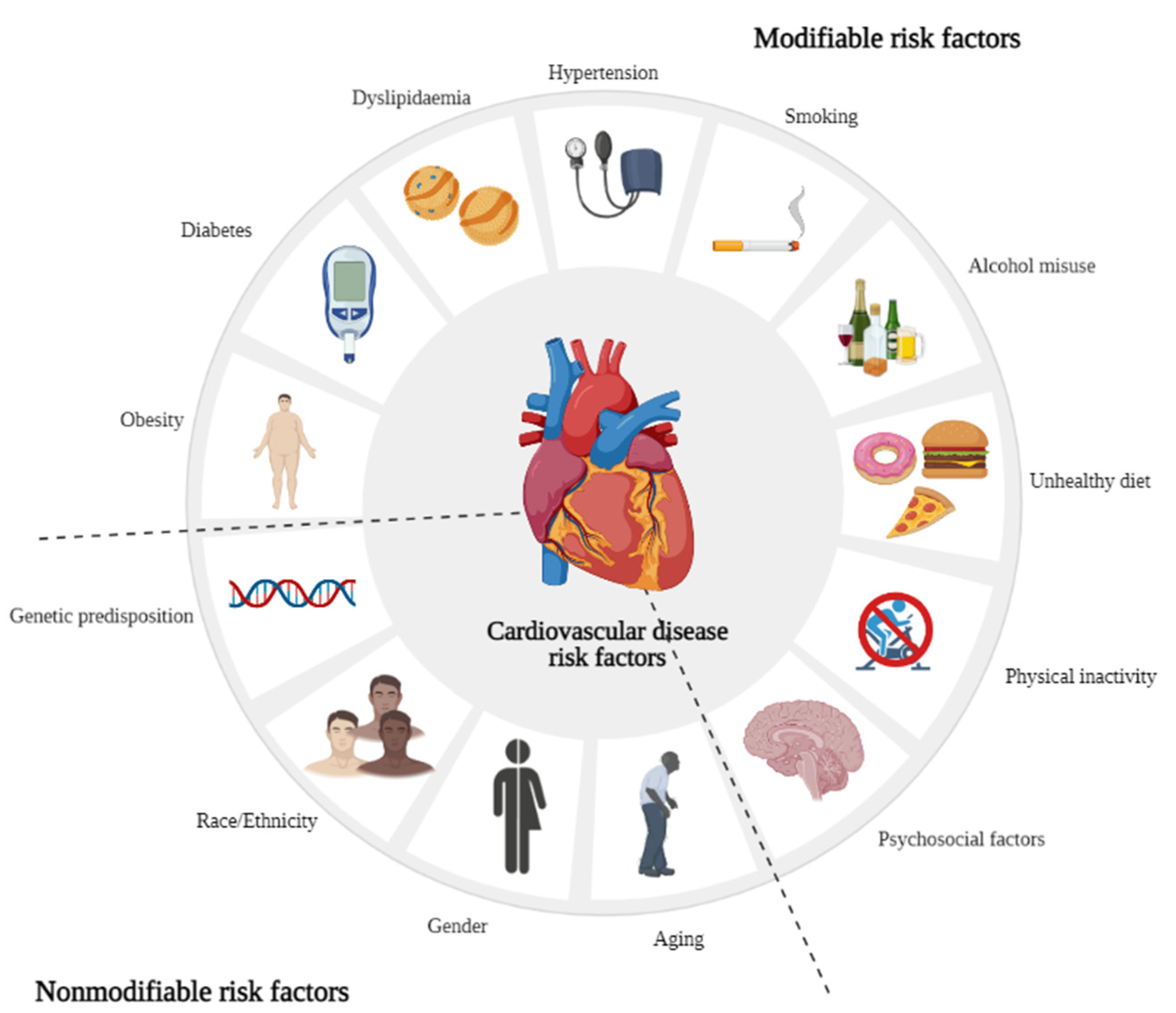
1. Introduction
2. The Potential of Essential Oils and Their Compounds in the Management of Cardiovascular Diseases Risk Factors and Related Targets
2.1. Hypertension
2.1.1. General Considerations
2.1.2. Hypotensive Essential Oils
2.1.3. Composition–Activity Relation
2.2. Diabetes and Dyslipidemia
2.2.1. General Considerations
2.2.2. Antidiabetic and Anti-Dyslipidemic Essential Oils
2.2.3. Composition–Activity Relation
2.3. Related Beneficial Effects of Essential Oils
2.3.1. Antiplatelet Effect
General Considerations
Essential Oils with Antiplatelet Effects
Composition–Activity Relation
2.3.2. Ion Channel Modulator Effect
General Considerations
Essential Oils with Ion Channel Modulation Capacity
Composition–Activity Relation
2.3.3. Other Beneficial Cardiovascular Effects
Composition–Activity Relation
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Cardiovascular Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 15 April 2021).
- North, B.J.; Sinclair, D.A. The intersection between aging and cardiovascular disease. Circ. Res. 2012, 110, 1097–1108. [Google Scholar] [CrossRef] [PubMed]
- Buttar, H.S.; Li, T.; Ravi, N. Prevention of cardiovascular diseases: Role of exercise, dietary interventions, obesity and smoking cessation. Exp. Clin. Cardiol. 2005, 10, 229–249. [Google Scholar] [PubMed]
- Leigh, J.A.; Alvarez, M.; Rodriguez, C.J. Ethnic minorities and coronary heart disease: An update and future directions. Curr. Atheroscler. Rep. 2016, 18, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mackay, J.; Mensah, G.A. Risk factors. In The Atlas of Heart Disease and Stroke; World Health Organization: Geneva, Switzerland, 2002; pp. 24–25. [Google Scholar]
- World Health Organization. Noncommunicable Diseases: Campaign for Action—Meeting the NCD Targets; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Timmis, A.; Townsend, N.; Gale, C.P.; Torbica, A.; Lettino, M.; Petersen, S.E.; Mossialos, E.A.; Maggioni, A.P.; Kazakiewicz, D.; May, H.T.; et al. European Society of Cardiology: Cardiovascular disease statistics 2019. Eur. Heart J. 2020, 41, 12–85. [Google Scholar] [CrossRef]
- Yusuf, S.; Hawken, S.; Ôunpuu, S.; Dans, T.; Avezum, A.; Lanas, F.; McQueen, M.; Budaj, A.; Pais, P.; Varigos, J.; et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet 2004, 364, 937–952. [Google Scholar] [CrossRef]
- Baroletti, S.; Dell’Orfano, H. Medication adherence in cardiovascular disease. Circulation 2010, 121, 1455–1458. [Google Scholar] [CrossRef]
- Cordell, G. Changing strategies in natural products chemistry. Phytochemistry 1995, 40, 1585–1612. [Google Scholar] [CrossRef]
- Wachtel-Galor, S.; Benzie, I.F.F. Herbal medicine: An introduction to its history, usage, regulation, current trends, and research needs. In Herbal Medicine: Biomolecular and Clinical Aspects; Wachtel-Galor, S., Benzie, I.F.F., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011; pp. 1–10. ISBN 9781439807132. [Google Scholar]
- Tuso, P.; Stoll, S.R.; Li, W.W. A plant-based diet, atherogenesis, and coronary artery disease prevention. Perm. J. 2015, 19, 62–67. [Google Scholar] [CrossRef]
- European Medicines Agency Herbal Medicines. Available online: https://www.ema.europa.eu/en/medicines/field_ema_web_categories%253aname_field/herbal/field_ema_herb_outcome/european-union-herbal-monograph-254/search_api_aggregation_ema_therapeutic_area_name/circulatorydisorders (accessed on 15 April 2021).
- Jenke-Kodama, H.; Müller, R.; Dittmann, E. Evolutionary mechanisms underlying secondary metabolite diversity. Prog. Drug Res. 2008, 65, 121–140. [Google Scholar] [CrossRef]
- Hartmann, T. From waste products to ecochemicals: Fifty years research of plant secondary metabolism. Phytochemistry 2007, 68, 2831–2846. [Google Scholar] [CrossRef] [PubMed]
- ISO 9235 Aromatic Natural Raw Materials-Vocabulary 2013. Available online: https://www.iso.org/obp/ui/#iso:std:iso:9235:ed-2:v1:en (accessed on 15 April 2021).
- Council of Europe. European Pharmacopoeia, 7th ed.; Directorate for the Quality of Medicines & HealthCare of the Council of Europe: Strasbourg, France, 2010; ISBN 978-92-871-6700-2. [Google Scholar]
- Hüsnü, K.; Başer, C.; Demirci, F. Chemistry of essential oils. In Flavours and Fragrances; Berger, R.G., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 43–86. ISBN 978-3-540-49339-6. [Google Scholar]
- Whelton, P.K. Primary prevention of hypertension: Clinical and public health advisory from the national high blood pressure education program. JAMA 2002, 288, 1882. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, Y.; Iwashima, Y. Higher blood pressure as a risk factor for diseases other than stroke and ischemic heart disease. Hypertension 2015, 66, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Taddei, S.; Bruno, R.M.; Masi, S.; Solini, A. Epidemiology and pathophysiology of hypertension. In ESC CardioMed; Williams, B., Ed.; Oxford University Press: Oxford, UK, 2018; pp. 2377–2388. [Google Scholar]
- Kannel, W. Risk stratification in hypertension: New insights from the Framingham study. Am. J. Hypertens. 2000, 13, S3–S10. [Google Scholar] [CrossRef]
- Egan, B.M.; Stevens-Fabry, S. Prehypertension—Prevalence, health risks, and management strategies. Nat. Rev. Cardiol. 2015, 12, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Trinder, Y. Common and less common adverse effects of antihypertensives: A general practitioner’s perspective. S. Afr. Fam. Pract. 2012, 54, S31–S32. [Google Scholar] [CrossRef]
- Giles, T.D.; Sander, G.E.; Nossaman, B.D.; Kadowitz, P.J. Impaired vasodilation in the pathogenesis of hypertension: Focus on nitric oxide, endothelial-derived hyperpolarizing factors, and prostaglandins. J. Clin. Hypertens. 2012, 14, 198–205. [Google Scholar] [CrossRef]
- Touyz, R.M.; Alves-Lopes, R.; Rios, F.J.; Camargo, L.L.; Anagnostopoulou, A.; Arner, A.; Montezano, A.C. Vascular smooth muscle contraction in hypertension. Cardiovasc. Res. 2018, 114, 529–539. [Google Scholar] [CrossRef]
- Han, C.; Qi, J.; Gao, S.; Li, C.; Ma, Y.; Wang, J.; Bai, Y.; Zheng, X. Vasodilation effect of volatile oil from Allium macrostemon Bunge are mediated by PKA/NO pathway and its constituent dimethyl disulfide in isolated rat pulmonary arterials. Fitoterapia 2017, 120, 52–57. [Google Scholar] [CrossRef]
- Santos, B.A.; Roman-Campos, D.; Carvalho, M.S.; Miranda, F.M.F.; Carneiro, D.C.; Cavalcante, P.H.; Cândido, E.A.F.; Filho, L.X.; Cruz, J.S.; Gondim, A.N.S. Cardiodepressive effect elicited by the essential oil of Alpinia speciosa is related to L-type Ca2+ current blockade. Phytomedicine 2011, 18, 539–543. [Google Scholar] [CrossRef]
- Pinto, N.V.; Assreuy, A.M.S.; Coelho-de-Souza, A.N.; Ceccatto, V.M.; Magalhães, P.J.C.; Lahlou, S.; Leal-Cardoso, J.H. Endothelium-dependent vasorelaxant effects of the essential oil from aerial parts of Alpinia zerumbet and its main constituent 1,8-cineole in rats. Phytomedicine 2009, 16, 1151–1155. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Hu, H.S.; Shen, X.C. Endothelium-dependent vasodilatation effects of the essential oil from Fructus Alpiniae Zerumbet (EOFAZ) on rat thoracic aortic rings in vitro. Phytomedicine 2013, 20, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Interaminense, L.F.L.; Dos Ramos-Alves, F.E.; de Siqueira, R.J.B.; Xavier, F.E.; Duarte, G.P.; Magalhães, P.J.C.; Maia, J.G.S.; Sousa, P.J.D.C.; Lahlou, S. Vasorelaxant effects of 1-nitro-2-phenylethane, the main constituent of the essential oil of Aniba canelilla, in superior mesenteric arteries from spontaneously hypertensive rats. Eur. J. Pharm. Sci. 2013, 48, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Lahlou, S.; Magalhães, P.J.C.; de Siqueira, R.J.B.; Figueiredo, A.F.; Interaminense, L.F.L.; Maia, J.G.S.; da Sousa, P.J.C. Cardiovascular effects of the essential oil of Aniba canelilla bark in normotensive rats. J. Cardiovasc. Pharmacol. 2005, 46, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Dib, I.; Fauconnier, M.L.; Sindic, M.; Belmekki, F.; Assaidi, A.; Berrabah, M.; Mekhfi, H.; Aziz, M.; Legssyer, A.; Bnouham, M.; et al. Chemical composition, vasorelaxant, antioxidant and antiplatelet effects of essential oil of Artemisia campestris L. from Oriental Morocco. BMC Complement. Altern. Med. 2017, 17, 82. [Google Scholar] [CrossRef] [PubMed]
- Spadaro, F.; Costa, R.; Circosta, C.; Occhiuto, F. Volatile composition and biological activity of key lime Citrus aurantifolia essential oil. Nat. Prod. Commun. 2012, 7, 1523–1526. [Google Scholar] [CrossRef] [PubMed]
- Kang, P.; Ryu, K.H.; Lee, J.M.; Kim, H.K.; Seol, G.H. Endothelium- and smooth muscle-dependent vasodilator effects of Citrus aurantium L. var. amara: Focus on Ca2+ modulation. Biomed. Pharmacother. 2016, 82, 467–471. [Google Scholar] [CrossRef]
- Kang, P.; Suh, S.H.; Min, S.S.; Seol, G.H. The essential oil of Citrus bergamia Risso induces vasorelaxation of the mouse aorta by activating K+ channels and inhibiting Ca2+ influx. J. Pharm. Pharmacol. 2013, 65, 745–749. [Google Scholar] [CrossRef] [PubMed]
- De França-Neto, A.; Cardoso-Teixeira, A.C.; Medeiros, T.C.; do Quinto-Farias, M.S.; de Sampaio, C.M.S.; Coelho-de-Souza, A.N.; Lahlou, S.; Leal-Cardoso, J.H. Essential oil of Croton argyrophylloides: Toxicological aspects and vasorelaxant activity in rats. Nat. Prod. Commun. 2012, 7, 1397–1400. [Google Scholar] [CrossRef] [PubMed]
- Lahlou, S.; Leal-Cardoso, J.H.; Magalhães, P.J. Essential oil of Croton nepetaefolius decreases blood pressure through an action upon vascular smooth muscle: Studies in DOCA-salt hypertensive rats. Planta Med. 2000, 66, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Lahlou, S.; Leal-Cardoso, J.H.; Magalhães, P.J.C.; Coelho-de-Souza, A.N.; Duarte, G.P. Cardiovascular effects of the essential oil of Croton nepetaefolius in rats: Role of the autonomic nervous system. Planta Med. 1999, 65, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, P.J.C.; Lahlou, S.; Jucá, D.M.; Coelho-de-Souza, L.N.; da Frota, P.T.T.; da Costa, A.M.G.; Leal-Cardoso, J.H. Vasorelaxation induced by the essential oil of Croton nepetaefolius and its constituents in rat aorta are partially mediated by the endothelium. Fundam. Clin. Pharmacol. 2008, 22, 169–177. [Google Scholar] [CrossRef]
- Martinsen, A.; Baccelli, C.; Navarro, I.; Abad, A.; Quetin-Leclercq, J.; Morel, N. Vascular activity of a natural diterpene isolated from Croton zambesicus and of a structurally similar synthetic trachylobane. Vascul. Pharmacol. 2010, 52, 63–69. [Google Scholar] [CrossRef]
- De Siqueira, R.J.B.; Leal-Cardoso, J.; Couture, R.; Lahlou, S. Role of capsaicin-sensitive sensory nerves in mediation of the cardiovascular effects of the essential oil of Croton zehntneri leaves in anaesthetized rats. Clin. Exp. Pharmacol. Physiol. 2006, 33, 238–247. [Google Scholar] [CrossRef] [PubMed]
- De Menezes, I.A.C.; Moreira, I.J.A.; de Paula, J.W.A.; Blank, A.F.; Antoniolli, A.R.; Quintans-Júnior, L.J.; Santos, M.R.V. Cardiovascular effects induced by Cymbopogon winterianus essential oil in rats: Involvement of calcium channels and vagal pathway. J. Pharm. Pharmacol. 2010, 62, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, H.; Sharifi, M.; Esmailidehaj, M.; Rezvani, M.E.; Hafizibarjin, Z. Vasodilatory effect of asafoetida essential oil on rat aorta rings: The role of nitric oxide, prostacyclin, and calcium channels. Phytomedicine 2017, 36, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Tognolini, M.; Ballabeni, V.; Bertoni, S.; Bruni, R.; Impicciatore, M.; Barocelli, E. Protective effect of Foeniculum vulgare essential oil and anethole in an experimental model of thrombosis. Pharmacol. Res. 2007, 56, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.R.V.; Carvalho, A.A.; Medeiros, I.A.; Alves, P.B.; Marchioro, M.; Antoniolli, A.R. Cardiovascular effects of Hyptis fruticosa essential oil in rats. Fitoterapia 2007, 78, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.S.; Menezes, P.M.N.; de Sá, P.G.S.; de Oliveira, A.L.S.; Souza, E.A.A.; da Almeida, J.R.G.S.; de Lima, J.T.; Uetanabaro, A.P.T.; dos Silva, T.R.S.; Peralta, E.D.; et al. Chemical composition and pharmacological properties of the essential oils obtained seasonally from Lippia thymoides. Pharm. Biol. 2016, 54, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Nunes Guedes, D.; Silva, D.F.; Barbosa-Filho, J.M.; Almeida De Medeiros, I. Endothelium-dependent hypotensive and vasorelaxant effects of the essential oil from aerial parts of Mentha x villosa in rats. Phytomedicine 2004, 11, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Lahlou, S.; Lima Carneiro-Leão, R.F.; Leal-Cardoso, J.H. Cardiovascular effects of the essential oil of Mentha x villosa in DOCA-salt-hypertensive rats. Phytomedicine 2002, 9, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Cherkaoui-Tangi, K.; Israili, Z.H.; Lyoussi, B. Vasorelaxant effect of essential oil isolated from Nigella sativa L. seeds in rat aorta: Proposed mechanism. Pak. J. Pharm. Sci. 2016, 29, 1–8. [Google Scholar]
- Interaminense, L.F.L.; Jucá, D.M.; Magalhães, P.J.C.; Leal-Cardoso, J.H.; Duarte, G.P.; Lahlou, S. Pharmacological evidence of calcium-channel blockade by essential oil of Ocimum gratissimum and its main constituent, eugenol, in isolated aortic rings from DOCA-salt hypertensive rats. Fundam. Clin. Pharmacol. 2007, 21, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Pires, A.F.; Madeira, S.V.F.; Soares, P.M.G.; Montenegro, C.M.; Souza, E.P.; Resende, A.C.; Soares de Moura, R.; Assreuy, A.M.S.; Criddle, D.N. The role of endothelium in the vasorelaxant effects of the essential oil of Ocimum gratissimum in aorta and mesenteric vascular bed of rats. Can. J. Physiol. Pharmacol. 2012, 90, 1380–1385. [Google Scholar] [CrossRef]
- Ballabeni, V.; Tognolini, M.; Bertoni, S.; Bruni, R.; Guerrini, A.; Rueda, G.M.; Barocelli, E. Antiplatelet and antithrombotic activities of essential oil from wild Ocotea quixos (Lam.) Kosterm. (Lauraceae) calices from Amazonian Ecuador. Pharmacol. Res. 2007, 55, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.L.; Marques, A.M.; Sudo, R.T.; Kaplan, M.A.C.; Zapata-Sudo, G. Vasodilator activity of the essential oil from aerial parts of Pectis brevipedunculata and its main constituent citral in rat aorta. Molecules 2013, 18, 3072. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, H.M.; Khan, T.; Wahid, F.; Khan, R.; Shah, A.J. Chemical composition and vascular and intestinal smooth muscle relaxant effects of the essential oil from Psidium guajava fruit. Pharm. Biol. 2016, 54, 2679–2684. [Google Scholar] [CrossRef] [PubMed]
- Shiva Kumar, A.; Jeyaprakash, K.; Chellappan, D.R.; Murugan, R. Vasorelaxant and cardiovascular properties of the essential oil of Pogostemon elsholtzioides. J. Ethnopharmacol. 2017, 199, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, H.M.; Khan, T.; Wahid, F.; Khan, R.; Shah, A.J. Chemical composition and vasorelaxant and antispasmodic effects of essential oil from Rosa indica L. petals. Evid. Based Complement. Altern. Med. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bigliani, M.C.; Rossetti, V.; Grondona, E.; Lo Presti, S.; Paglini, P.M.; Rivero, V.; Zunino, M.P.; Ponce, A.A. Chemical compositions and properties of Schinus areira L. essential oil on airway inflammation and cardiovascular system of mice and rabbits. Food Chem. Toxicol. 2012, 50, 2282–2288. [Google Scholar] [CrossRef] [PubMed]
- Zadeh, G.S.; Panahi, N. Endothelium-independent vasorelaxant activity of Trachyspermum ammi essential oil on rat aorta. Clin. Exp. Hypertens. 2017, 39, 133–138. [Google Scholar] [CrossRef]
- De Correia, A.C.; Ferreira, T.F.; Martins, I.R.R.; Macêdo, C.L.; de Monteiro, F.; Costa, V.C.O.; Tavares, J.F.; Silva, M.S.; Paredes-Gamero, E.J.; Buri, M.V.; et al. Essential oil from the leaves of Xylopia langsdorfiana (Annonaceae) as a possible spasmolytic agent. Nat. Prod. Res. 2015, 29, 980–984. [Google Scholar] [CrossRef]
- Lahlou, S.; Galindo, C.A.B.; Leal-Cardoso, J.H.; Fonteles, M.C.; Duarte, G.P. Cardiovascular effects of the essential oil of Alpinia zerumbet leaves and its main constituent, terpinen-4-ol, in rats: Role of the autonomic nervous system. Planta Med. 2002, 68, 1097–1102. [Google Scholar] [CrossRef] [PubMed]
- Lahlou, S.; Interaminense, L.F.L.; Leal-Cardoso, J.H.; Duarte, G.P. Antihypertensive effects of the essential oil of Alpinia zerumbet and its main constituent, terpinen-4-ol, in DOCA-salt hypertensive conscious rats. Fundam. Clin. Pharmacol. 2003, 17, 323–330. [Google Scholar] [CrossRef]
- Da Cunha, G.H.; de Moraes, M.O.; Fechine, F.V.; Frota Bezerra, F.A.; Silveira, E.R.; Canuto, K.M.; de Moraes, M.E.A. Vasorelaxant and antihypertensive effects of methanolic fraction of the essential oil of Alpinia zerumbet. Vascul. Pharmacol. 2013, 58, 337–345. [Google Scholar] [CrossRef]
- De Siqueira, R.J.; Rodrigues, K.M.S.; da Silva, M.T.B.; Correia Junior, C.A.B.; Duarte, G.P.; Magalhães, P.J.C.; dos Santos, A.A.; Maia, J.G.S.; da Cunha, P.J.S.; Lahlou, S. Linalool-rich rosewood oil induces vago-vagal bradycardic and depressor reflex in rats. Phyther. Res. 2014, 28, 42–48. [Google Scholar] [CrossRef]
- De Siqueira, R.J.B.; Magalhães, P.J.C.; Leal-Cardoso, J.H.; Duarte, G.P.; Lahlou, S. Cardiovascular effects of the essential oil of Croton zehntneri leaves and its main constituents, anethole and estragole, in normotensive conscious rats. Life Sci. 2006, 78, 2365–2372. [Google Scholar] [CrossRef] [PubMed]
- De Siqueira, R.J.B.; Duarte, G.P.; Magalhães, P.J.C.; Lahlou, S. Cardiovascular effects of the essential oil of Croton zehntneri leaves in DOCA-salt hypertensive, conscious rats. Nat. Prod. Commun. 2013, 8, 1167–1170. [Google Scholar] [CrossRef]
- Alves-Santos, T.R.; de Siqueira, R.J.B.; Duarte, G.P.; Lahlou, S. Cardiovascular effects of the essential oil of Croton argyrophylloides in normotensive rats: Role of the autonomic nervous system. Evid. Based Complement. Altern. Med. 2016, 2016, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lahlou, S.; Carneiro-Leão, R.F.L.; Leal-Cardoso, J.H.; Toscano, C.F. Cardiovascular effects of the essential oil Mentha x villosa and its main constituent, piperitenone oxide, in normotensive anaesthetised rats: Role of the autonomic nervous system. Planta Med. 2001, 67, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Lahlou, S.; Magalhães, P.J.C.; Carneiro-Leão, R.F.L.; Leal-Cardoso, J.H. Involvement of nitric oxide in the mediation of the hypotensive action of the essential oil of Mentha x villosa in normotensive conscious rats. Planta Med. 2002, 68, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Matos, F.J.D.A.; Machado, M.I.L.; Craveiro, A.A.; Alencar, J.W.; Barbosa, J.M.; da Cunha, E.V.L.; Hiruma, C.A. Essential oil of Mentha x villosa Huds. from Northeastern Brazil. J. Essent. Oil Res. 1999, 11, 41–44. [Google Scholar] [CrossRef]
- Interaminense, L.F.L.; Leal-Cardoso, J.H.; Magalhães, P.J.C.; Duarte, G.P.; Lahlou, S. Enhanced hypotensive effects of the essential oil of Ocimum gratissimum leaves and its main constituent, eugenol, in DOCA-salt hypertensive conscious rats. Planta Med. 2005, 71, 376–378. [Google Scholar] [CrossRef] [PubMed]
- Lahlou, S.; Interaminense Lde, F.; Leal-Cardoso, J.H.; Morais, S.M.; Duarte, G.P. Cardiovascular effects of the essential oil of Ocimum gratissimum leaves in rats: Role of the autonomic nervous system. Clin. Exp. Pharmacol. Physiol. 2004, 31, 219–225. [Google Scholar] [CrossRef]
- Jung, Y.J. Effects of Aromatherapy on Blood Bressure, Heart Rate Variability, and Serum Catecholamines in the Pre-Hypertension Middle Aged Women. Ph.D. Thesis, The Catholic University of Korea, Seoul, Korea, 2007. [Google Scholar]
- Hwang, J.H. The effects of the inhalation method using essential oils on blood pressure and stress responses of clients with essential hypertension. Taehan. Kanho. Hakhoe. Chi. 2006, 36, 1123–1134. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.H. A clinical study on the effects of the aromatherapy for hypertension. J. Orient Neuropsychiatry 2002, 13, 3–18. [Google Scholar]
- Kim, I.H.; Kim, C.; Seong, K.; Hur, M.H.; Lim, H.M.; Lee, M.S. Essential oil inhalation on blood pressure and salivary cortisol levels in prehypertensive and hypertensive subjects. Evid. Based Complement. Altern. Med. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Story, G.M.; Cruz-Orengo, L. Feel the burn. Am. Sci. 2007, 95, 326. [Google Scholar] [CrossRef]
- Kopincová, J.; Púzserová, A.; Bernátová, I. L-NAME in the cardiovascular system–nitric oxide synthase activator? Pharmacol. Reports 2012, 64, 511–520. [Google Scholar] [CrossRef]
- Mcdonald, T.F.; Pelzer, S.; Trautwein, W.; Pelzer, D.J. Regulation and modulation of calcium channels in cardiac, skeletal, and smooth muscle cells. Physiol. Rev. 1994, 74, 365–507. [Google Scholar] [CrossRef]
- Ferreira, S.H.; Moncada, S.; Vane, J.R. Indomethacin and aspirin abolish prostaglandin release from the spleen. Nat. New Biol. 1971, 231, 237–239. [Google Scholar] [CrossRef]
- Bassolé, I.H.N.; Juliani, H.R. Essential oils in combination and their antimicrobial properties. Molecules 2012, 17, 3989. [Google Scholar] [CrossRef] [PubMed]
- Menezes, I.A.C.; Barreto, C.M.N.; Antoniolli, A.R.; Santos, M.R.V.; de Sousa, D.P. Hypotensive activity of terpenes found in essential oils. Z. Naturforsch. C. 2010, 65, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Anjos, P.J.C.; Lima, A.O.; Cunha, P.S.; De Sousa, D.P.; Onofre, A.S.C.; Ribeiro, T.P.; Medeiros, I.A.; Antoniolli, Â.R.; Quintans-Júnior, L.J.; Santos, M.R.V. Cardiovascular effects induced by linalool in normotensive and hypertensive rats. Z. Naturforsch. Sect. C J. Biosci. 2013, 68 C, 181–190. [Google Scholar] [CrossRef]
- Bastos, J.F.A.; Moreira, Í.J.A.; Ribeiro, T.P.; Medeiros, I.A.; Antoniolli, A.R.; De Sousa, D.P.; Santos, M.R. V Hypotensive and vasorelaxant effects of citronellol, a monoterpene alcohol, in rats. Basic Clin. Pharmacol. Toxicol. 2010, 106, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, T.P.; Porto, D.L.; Menezes, C.P.; Antunes, A.A.; Silva, D.F.; De Souza, D.P.; Nakao, L.S.; Braga, V.A.; Medeiros, I.A. Unraveling the cardiovascular effects induced by α-terpineol: A role for the NO-cGMP pathway. Clin. Exp. Pharmacol. Physiol. 2010, 37, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.; Shabir, H.; Basir, S.F.; Khan, L.A. Inhibition of As(III) and Hg(II) caused aortic hypercontraction by eugenol, linalool and carvone. J. Smooth Muscle Res. 2014, 50, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Baccelli, C.; Martinsen, A.; Morel, N.; Quetin-Leclercq, J. Vasorelaxant activity of essential oils from Croton zambesicus and some of their constituents. Planta Med. 2010, 76, 1506–1511. [Google Scholar] [CrossRef] [PubMed]
- De Menezes-Filho, J.E.R.; Gondim, A.N.S.; Cruz, J.S.; de Souza, A.A.; Dos Santos, J.N.A.; Conde-Garcia, E.A.; de Sousa, D.P.; Santos, M.S.; de Oliveira, E.D.; de Vasconcelos, C.M.L. Geraniol blocks calcium and potassium channels in the mammalian myocardium: Useful effects to treat arrhythmias. Basic Clin. Pharmacol. Toxicol. 2014, 115, 534–544. [Google Scholar] [CrossRef]
- Guedes, D.N.; Silva, D.F.; Barbosa-Filho, J.M.; Medeiros, I.A. Muscarinic agonist properties involved in the hypotensive and vasorelaxant responses of rotundifolone in rats. Planta Med. 2002, 68, 700–704. [Google Scholar] [CrossRef]
- Lahlou, S.; Figueiredo, A.F.; Magalhães, P.J.C.; Leal-Cardoso, J.H. Cardiovascular effects of 1,8-cineole, a terpenoid oxide present in many plant essential oils, in normotensive rats. Can. J. Physiol. Pharmacol. 2002, 80, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.C.M.S.; Damiani, C.E.N.; Moreira, C.M.; Stefanon, I.; Vassallo, D.V. Eucalyptol, an essential oil, reduces contractile activity in rat cardiac muscle. Braz. J. Med. Biol. Res. Rev. Bras. Pesqui. Med. Biol. 2005, 38, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Guedes, D.N.; Silva, D.F.; Barbosa-Filho, J.M.; Medeiros, I.A. Calcium antagonism and the vasorelaxation of the rat aorta induced by rotundifolone. Braz. J. Med. Biol. Res. 2004, 37, 1881–1887. [Google Scholar] [CrossRef] [PubMed]
- Maia-Joca, R.P.M.; Joca, H.C.; Ribeiro, F.J.P.; Do Nascimento, R.V.; Silva-Alves, K.S.; Cruz, J.S.; Coelho-De-Souza, A.N.; Leal-Cardoso, J.H. Investigation of terpinen-4-ol effects on vascular smooth muscle relaxation. Life Sci. 2014, 115, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Dantas, B.P.V.; Alves, Q.L.; de Assis, K.S.; Ribeiro, T.P.; de Almeida, M.M.; de Vasconcelos, A.P.; de Araújo, D.A.M.; de Andrade Braga, V.; de Medeiros, I.A.; Alencar, J.L.; et al. Participation of the TRP channel in the cardiovascular effects induced by carvacrol in normotensive rat. Vascul. Pharmacol. 2015, 67–69, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Aydin, Y.; Kutlay, Ö.; Ari, S.; Duman, S.; Uzuner, K.; Aydin, S. Hypotensive effects of carvacrol on the blood pressure of normotensive rats. Planta Med. 2007, 73, 1365–1371. [Google Scholar] [CrossRef] [PubMed]
- Peixoto-Neves, D.; Silva-Alves, K.S.; Gomes, M.D.M.; Lima, F.C.; Lahlou, S.; Magalhães, P.J.C.; Ceccatto, V.M.; Coelho-De-Souza, A.N.; Leal-Cardoso, J.H. Vasorelaxant effects of the monoterpenic phenol isomers, carvacrol and thymol, on rat isolated aorta. Fundam. Clin. Pharmacol. 2010, 24, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Shabir, H.; Kundu, S.; Basir, S.F.; Khan, L.A. Modulation of Pb(II) caused aortal constriction by eugenol and carvacrol. Biol. Trace Elem. Res. 2014, 161, 116–122. [Google Scholar] [CrossRef]
- Koto, R.; Imamura, M.; Watanabe, C.; Obayashi, S.; Shiraishi, M.; Sasaki, Y.; Azuma, H. Linalyl acetate as a major ingredient of lavender essential oil relaxes the rabbit vascular smooth muscle through dephosphorylation of myosin light chain. J. Cardiovasc. Pharmacol. 2006, 48, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.D.; Melanaphy, D.; Purse, A.; Stokesberry, S.A.; Dickson, P.; Zholos, A. V Transient receptor potential melastatin 8 channel involvement in the regulation of vascular tone. Am. J. Physiol. Hear. Circ. Physiol. 2009, 296, H1868–H1877. [Google Scholar] [CrossRef]
- Cheang, W.S.; Lam, M.Y.; Wong, W.T.; Tian, X.Y.; Lau, C.W.; Zhu, Z.; Yao, X.; Huang, Y. Menthol relaxes rat aortae, mesenteric and coronary arteries by inhibiting calcium influx. Eur. J. Pharmacol. 2013, 702, 79–84. [Google Scholar] [CrossRef]
- De Siqueira, R.J.B.; Freire, W.B.S.; Vasconcelos-Silva, A.A.; Fonseca-Magalhães, P.A.; Lima, F.J.B.; Brito, T.S.; Mourão, L.T.C.; Ribeiro, R.A.; Lahlou, S.; Magalhães, P.J.C.; et al. In vitro characterization of the pharmacological effects induced by (-)-α-bisabolol in rat smooth muscle preparations. Can. J. Physiol. Pharmacol. 2012, 90, 23–35. [Google Scholar] [CrossRef] [PubMed]
- De Siqueira, R.J.B.; Ribeiro-Filho, H.V.; Freire, R.S.; Cosker, F.; Freire, W.B.S.; Vasconcelos-Silva, A.A.; Soares, M.A.; Lahlou, S.; Magalhães, P.J.C. (-)-α-Bisabolol inhibits preferentially electromechanical coupling on rat isolated arteries. Vascul. Pharmacol. 2014, 63, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Soares, P.M.G.; Lima, R.F.; de Freitas Pires, A.; Souza, E.P.; Assreuy, A.M.S.; Criddle, D.N. Effects of anethole and structural analogues on the contractility of rat isolated aorta: Involvement of voltage-dependent Ca2+-channels. Life Sci. 2007, 81, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Lahlou, S.; Interaminense, L.F.; Magalhaes, P.J.; Leal-Cardoso, J.H.; Duarte, G.P. Cardiovascular effects of eugenol, a phenolic compound present in many plant essential oils, in normotensive rats. J. Cardiovasc. Pharmacol. 2004, 43, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Peixoto-Neves, D.; Wang, Q.; Leal-Cardoso, J.H.; Rossoni, L.V.; Jaggar, J.H. Eugenol dilates mesenteric arteries and reduces systemic BP by activating endothelial cell TRPV4 channels. Br. J. Pharmacol. 2015, 172, 3484–3494. [Google Scholar] [CrossRef] [PubMed]
- Criddle, D.N.; Madeira, S.V.; Soares de Moura, R. Endothelium-dependent and -independent vasodilator effects of eugenol in the rat mesenteric vascular bed. J. Pharm. Pharmacol. 2003, 55, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Damiani, C.E.; Moreira, C.M.; Zhang, H.T.; Creazzo, T.L.; Vassallo, D.V. Effects of eugenol, an essential oil, on the mechanical and electrical activities of cardiac muscle. J. Cardiovasc. Pharmacol. 2004, 44, 688–695. [Google Scholar] [CrossRef]
- Damiani, C.E.N.; Rossoni, L.V.; Vassallo, D.V. Vasorelaxant effects of eugenol on rat thoracic aorta. Vascul. Pharmacol. 2003, 40, 59–66. [Google Scholar] [CrossRef]
- Raffai, G.; Kim, B.; Park, S.; Khang, G.; Lee, D.; Vanhoutte, P.M. Cinnamaldehyde and cinnamaldehyde-containing micelles induce relaxation of isolated porcine coronary arteries: Role of nitric oxide and calcium. Int. J. Nanomed. 2014, 9, 2557–2566. [Google Scholar] [CrossRef]
- Yanaga, A.; Goto, H.; Nakagawa, T.; Hikiami, H.; Shibahara, N.; Shimada, Y. Cinnamaldehyde induces endothelium-dependent and -independent vasorelaxant action on isolated rat aorta. Biol. Pharm. Bull. 2006, 29, 2415–2418. [Google Scholar] [CrossRef]
- Vasconcelos-Silva, A.A.; de Lima, F.J.B.; de Brito, T.S.; Lahlou, S.; Magalhães, P.J.C. Vasorelaxation induced by methyl cinnamate, the major constituent of the essential oil of Ocimum micranthum, in rat isolated aorta. Clin. Exp. Pharmacol. Physiol. 2014, 41, 755–762. [Google Scholar] [CrossRef]
- El Tantawy, M.E.; El Sakhawy, F.S.; El Sohly, M.A.; Ross, S.A. Chemical composition and biological activity of the essential oil of the fruit of Taxodium distichum L. rich growing in Egypt. J. Essent. Oil Res. 1999, 11, 386–392. [Google Scholar] [CrossRef]
- Reiner, Z.; Catapano, A.L.; De Backer, G.; Graham, I.; Taskinen, M.-R.; Wiklund, O.; Agewall, S.; Alegria, E.; Chapman, M.J.; Durrington, P.; et al. ESC/EAS Guidelines for the management of dyslipidaemias: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur. Heart J. 2011, 32, 1769–1818. [Google Scholar] [CrossRef]
- Ng, C.-Y.; Leong, X.-F.; Masbah, N.; Adam, S.K.; Kamisah, Y.; Jaarin, K. Heated vegetable oils and cardiovascular disease risk factors. Vascul. Pharmacol. 2014, 61, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Duncan, M.S.; Vasan, R.S.; Xanthakis, V. Trajectories of blood lipid concentrations over the adult life course and risk of cardiovascular disease and all-cause mortality: Observations from the Framingham study over 35 years. J. Am. Heart Assoc. 2019, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Perk, J.; De Backer, G.; Gohlke, H.; Graham, I.; Reiner, Z.; Verschuren, M.; Albus, C.; Benlian, P.; Boysen, G.; Cifkova, R.; et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2012, 33, 1635–1701. [Google Scholar] [CrossRef] [PubMed]
- Kromhout, D.; Menotti, A.; Kesteloot, H.; Sans, S. Prevention of coronary heart disease by diet and lifestyle: Evidence from prospective cross-cultural, cohort, and intervention studies. Circulation 2002, 105, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of different regimens to lower blood pressure on major cardiovascular events in older and younger adults: Meta-analysis of randomised trials. BMJ 2008, 336, 1121–1123. [CrossRef] [PubMed]
- Law, M.R.; Morris, J.K.; Wald, N.J. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: Meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ 2009, 338, b1665. [Google Scholar] [CrossRef]
- Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy of cholesterol-lowering therapy in 18 686 people with diabetes in 14 randomised trials of statins: A meta-analysis. Lancet 2008, 371, 117–125. [Google Scholar] [CrossRef]
- Brugts, J.J.; Yetgin, T.; Hoeks, S.E.; Gotto, A.M.; Shepherd, J.; Westendorp, R.G.J.; de Craen, A.J.M.; Knopp, R.H.; Nakamura, H.; Ridker, P.; et al. The benefits of statins in people without established cardiovascular disease but with cardiovascular risk factors: Meta-analysis of randomised controlled trials. BMJ 2009, 338, b2376. [Google Scholar] [CrossRef] [PubMed]
- Rydén, L.; Grant, P.J.; Anker, S.D.; Berne, C.; Cosentino, F.; Danchin, N.; Deaton, C.; Escaned, J.; Hammes, H.P.; Huikuri, H.; et al. ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2013, 34, 3035–3087. [Google Scholar] [CrossRef] [PubMed]
- Racette, S.B.; Lin, X.; Lefevre, M.; Spearie, C.A.; Most, M.M.; Ma, L.; Ostlund, R.E., Jr. Dose effects of dietary phytosterols on cholesterol metabolism: A controlled feeding study. Am. J. Clin. Nutr. 2010, 91, 32–38. [Google Scholar] [CrossRef]
- Reid, I.R.; Birstow, S.M.; Bolland, M.J. Calcium and cardiovascular disease. Endocrinol. Metab. 2017, 32, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Marks, A.R. Calcium and the heart: A question of life and death. J. Clin. Investig. 2003, 111, 597–600. [Google Scholar] [CrossRef] [PubMed]
- The Emerging Risk Factors Collaboration. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet 2010, 375, 2215–2222. [Google Scholar] [CrossRef]
- Singh, G.M.; Danaei, G.; Farzadfar, F.; Stevens, G.A.; Woodward, M.; Wormser, D.; Kaptoge, S.; Whitlock, G.; Qiao, Q.; Lewington, S.; et al. The age-specific quantitative effects of metabolic risk factors on cardiovascular diseases and diabetes: A pooled analysis. PLoS ONE 2013, 8, e65174. [Google Scholar] [CrossRef]
- Haffner, S.M.; Lehto, S.; Rönnemaa, T.; Pyörälä, K.; Laakso, M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N. Engl. J. Med. 1998, 339, 229–234. [Google Scholar] [CrossRef]
- Sobczak, I.S.A.; Blindauer, A.C.; Stewart, J.A. Changes in plasma free fatty acids associated with type-2 diabetes. Nutrients 2019, 11, 2022. [Google Scholar] [CrossRef]
- Maack, C.; Lehrke, M.; Backs, J.; Heinzel, F.R.; Hulot, J.-S.; Marx, N.; Paulus, W.J.; Rossignol, P.; Taegtmeyer, H.; Bauersachs, J.; et al. Heart failure and diabetes: Metabolic alterations and therapeutic interventions: A state-of-the-art review from the Translational Research Committee of the Heart Failure Association–European Society of Cardiology. Eur. Heart J. 2018, 39, 4243–4254. [Google Scholar] [CrossRef]
- Lee, M.-H.; Chen, Y.-Y.; Tsai, J.-W.; Wang, S.-C.; Watanabe, T.; Tsai, Y.-C. Inhibitory effect of β-asarone, a component of Acorus calamus essential oil, on inhibition of adipogenesis in 3T3-L1 cells. Food Chem. 2011, 126, 1–7. [Google Scholar] [CrossRef]
- Shen, X.-C.; Tao, L.; Li, W.-K.; Zhang, Y.-Y.; Luo, H.; Xia, Y.-Y. Evidence-based antioxidant activity of the essential oil from Fructus, A. zerumbet on cultured human umbilical vein endothelial cells’ injury induced by ox-LDL. BMC Complement. Altern. Med. 2012, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Zeng, Y.; Xu, Y.; Zhang, Y.; Jiang, Y.; Tao, L.; Shen, X. The endothelial protective properties of essential oil from Fructus Alpiniae zerumbet via the Akt/NOS-NO signaling pathway in vitro. Planta Med. 2014, 80, 1628–1634. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Lee, H.-J.; Jeong, S.-J.; Lee, M.-H.; Kim, S.-H. Essential oil of Pinus koraiensis leaves exerts antihyperlipidemic effects via up-regulation of low-density lipoprotein receptor and inhibition of acyl-coenzyme A: Cholesterol acyltransferase. Phyther. Res. 2012, 26, 1314–1319. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.J.; Park, K.W.; Kim, K.H.; Kim, C.-T.; Baek, J.P.; Bang, K.-H.; Choi, Y.-M.; Lee, S.-J. Asian plantain (Plantago asiatica) essential oils suppress 3-hydroxy-3-methyl-glutaryl-co-enzyme A reductase expression in vitro and in vivo and show hypocholesterolaemic properties in mice. Br. J. Nutr. 2008, 99, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Belhadj, S.; Hentati, O.; Hammami, M.; Ben Hadj, A.; Boudawara, T.; Dammak, M.; Zouari, S.; El Feki, A.F. Metabolic impairments and tissue disorders in alloxan-induced diabetic rats are alleviated by Salvia officinalis L. essential oil. Biomed. Pharmacother. 2018, 108, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.F.; Azevedo, M.F.; Araujo, R.; Fernandes-Ferreira, M.; Pereira-Wilson, C. Metformin-like effect of Salvia officinalis (common sage): Is it useful in diabetes prevention? Br. J. Nutr. 2006, 96, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Vasudeva, N.; Sharma, S. GC-MS analysis and screening of antidiabetic, antioxidant and hypolipidemic potential of Cinnamomum tamala oil in streptozotocin induced diabetes mellitus in rats. Cardiovasc. Diabetol. 2012, 11, 1–11. [Google Scholar] [CrossRef]
- Singh, V.; Jain, M.; Misra, A.; Khanna, V.; Rana, M.; Prakash, P.; Malasoni, R.; Dwivedi, A.K.; Dikshit, M.; Barthwal, M.K. Curcuma oil ameliorates hyperlipidaemia and associated deleterious effects in golden Syrian hamsters. Br. J. Nutr. 2013, 110, 437–446. [Google Scholar] [CrossRef]
- El-Soud, N.A.; El-Laithy, N.; El-Saeed, G.; Wahby, M.S.; Khalil, M.; Morsy, F.; Shaffie, N. Antidiabetic activities of Foeniculum vulgare Mill. essential oil in streptozotocin-induced diabetic rats. Maced. J. Med. Sci. 2011, 4, 139–146. [Google Scholar] [CrossRef]
- Al-Okbi, S.Y.; Hussein, A.M.S.; Elbakry, H.F.H.; Fouda, K.A.; Mahmoud, K.F.; Hassan, M.E. Health benefits of fennel, rosemary volatile oils and their nano-forms in dyslipidemic rat model. Pak. J. Biol. Sci. 2018, 21, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Al-Okbi, S.Y.; Mohamed, D.A.; Hamed, T.E.; Edris, A.E. Protective effect of clove oil and eugenol microemulsions on fatty liver and dyslipidemia as components of metabolic syndrome. J. Med. Food 2014, 17, 764–771. [Google Scholar] [CrossRef]
- Keihan, G.S.; Gharib, M.H.; Momeni, A.; Hemati, Z.; Sedighin, R. A comparison between the effect of Cuminum cyminum and vitamin E on the level of leptin, paraoxonase 1, HbA1c and oxidized LDL in diabetic patients. Int. J. Mol. Cell. Med. 2016, 5, 229–235. [Google Scholar] [CrossRef]
- Jafari, S.; Sattari, R.; Ghavamzadeh, S. Evaluation the effect of 50 and 100 mg doses of Cuminum cyminum essential oil on glycemic indices, insulin resistance and serum inflammatory factors on patients with diabetes type II: A double-blind randomized placebo-controlled clinical trial. J. Tradit. Complement. Med. 2017, 7, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, S.; Pari, L. Role of thymol on hyperglycemia and hyperlipidemia in high fat diet-induced type 2 diabetic C57BL/6J mice. Eur. J. Pharmacol. 2015, 761, 279–287. [Google Scholar] [CrossRef]
- Ezhumalai, M.; Ashokkumar, N.; Pugalendi, K.V. Combination of carvacrol and rosiglitazone ameliorates high fat diet induced changes in lipids and inflammatory markers in C57BL/6J mice. Biochimie 2015, 110, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Galle, M.; Kladniew, B.R.; Castro, M.A.; Villegas, S.M.; Lacunza, E.; Polo, M.; De Bravo, M.G.; Crespo, R. Modulation by geraniol of gene expression involved in lipid metabolism leading to a reduction of serum-cholesterol and triglyceride levels. Phytomedicine 2015, 22, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Jayachandran, M.; Chandrasekaran, B.; Namasivayam, N. Effect of geraniol, a plant derived monoterpene on lipids and lipid metabolizing enzymes in experimental hyperlipidemic hamsters. Mol. Cell. Biochem. 2015, 398, 39–53. [Google Scholar] [CrossRef]
- Vallianou, I.; Peroulis, N.; Pantazis, P.; Hadzopoulou-Cladaras, M. Camphene, a plant-derived monoterpene, reduces plasma cholesterol and triglycerides in hyperlipidemic rats independently of HMG-CoA reductase activity. PLoS ONE 2011, 6, e20516. [Google Scholar] [CrossRef]
- Naderi, G.A.; Asgary, S.; Ani, M.; Sarraf-Zadegan, N.; Safari, M.R. Effect of some volatile oils on the affinity of intact and oxidized low-density lipoproteins for adrenal cell surface receptors. Mol. Cell. Biochem. 2004, 267, 59–66. [Google Scholar] [CrossRef]
- Basha, R.H.; Sankaranarayanan, C. β-Caryophyllene, a natural sesquiterpene lactone attenuates hyperglycemia mediated oxidative and inflammatory stress in experimental diabetic rats. Chem. Biol. Interact. 2016, 245, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Kumawat, V.S.; Kaur, G. Insulinotropic and antidiabetic effects of β-caryophyllene with L-arginine in type 2 diabetic rats. J. Food Biochem. 2020, 44. [Google Scholar] [CrossRef]
- Basha, R.H.; Sankaranarayanan, C. Protective role of β-caryophyllene, a sesquiterpene lactone on plasma and tissue glycoprotein components in streptozotocin-induced hyperglycemic rats. J. Acute Med. 2015, 5, 9–14. [Google Scholar] [CrossRef]
- Youssef, D.A.; El-Fayoumi, H.M.; Mahmoud, M.F. Beta-caryophyllene protects against diet-induced dyslipidemia and vascular inflammation in rats: Involvement of CB2 and PPAR-γ receptors. Chem. Biol. Interact. 2019, 297, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Youssef, D.A.; El-Fayoumi, H.M.; Mahmoud, M.F. Beta-caryophyllene alleviates diet-induced neurobehavioral changes in rats: The role of CB2 and PPAR-γ receptors. Biomed. Pharmacother. 2019, 110, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Basha, R.H.; Sankaranarayanan, C. β-Caryophyllene, a natural sesquiterpene, modulates carbohydrate metabolism in streptozotocin-induced diabetic rats. Acta Histochem. 2014, 116, 1469–1479. [Google Scholar] [CrossRef] [PubMed]
- Suijun, W.; Zhen, Y.; Ying, G.; Yanfang, W. A role for trans-caryophyllene in the moderation of insulin secretion. Biochem. Biophys. Res. Commun. 2014, 444, 451–454. [Google Scholar] [CrossRef]
- Baddar, N.W.A.-H.; Aburjai, T.A.; Taha, M.O.; Disi, A.M. Thujone corrects cholesterol and triglyceride profiles in diabetic rat model. Nat. Prod. Res. 2011, 25, 1180–1184. [Google Scholar] [CrossRef] [PubMed]
- Alkhateeb, H.; Al-Duais, M.; Qnais, E.; Trad, B.; Matalgah, L. Plasma glucose-lowering effect of thujone and its molecular mechanisms of action in streptozotocin-induced diabetic rats. Pharmacol. Online 2018, 1, 196–208. [Google Scholar]
- Alkhateeb, H.H. Thujone improves glucose homeostasis in streptozotocin-induced diabetic rats through activation of Akt/glycogen synthase kinase-3β signaling pathway. J. Exp. Integr. Med. 2015, 5, 30–35. [Google Scholar] [CrossRef]
- Chellian, R.; Pandy, V.; Mohamed, Z. Pharmacology and toxicology of α- and β-asarone: A review of preclinical evidence. Phytomedicine 2017, 32, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Thakare, M.M.; Surana, S.J. β-Asarone modulate adipokines and attenuates high fat diet-induced metabolic abnormalities in Wistar rats. Pharmacol. Res. 2016, 103, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Poplawski, J.; Lozowicka, B.; Dubis, A.T.; Lachowska, B.; Witkowski, S.; Siluk, D.; Petrusewicz, J.; Kaliszan, R.; Cybulski, J.; Strzalkowska, M.; et al. Synthesis and hypolipidemic and antiplatelet activities of α-asarone isomers in humans (in vitro), mice (in vivo), and rats (in vivo). J. Med. Chem. 2000, 43, 3671–3676. [Google Scholar] [CrossRef] [PubMed]
- Venkadeswaran, K.; Thomas, P.A.; Geraldine, P. An experimental evaluation of the anti-atherogenic potential of the plant, Piper betle, and its active constitutent, eugenol, in rats fed an atherogenic diet. Biomed. Pharmacother. 2016, 80, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Venkadeswaran, K.; Muralidharan, A.R.; Annadurai, T.; Ruban, V.V.; Sundararajan, M.; Anandhi, R.; Thomas, P.A.; Geraldine, P. Antihypercholesterolemic and antioxidative potential of an extract of the plant, Piper betle, and its active constituent, eugenol, in Triton WR-1339-induced hypercholesterolemia in experimental rats. Evid. Based. Complement. Alternat. Med. 2014, 2014, 478973. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Pu, C.; Zhou, P.; Wang, P.; Liang, D.; Wang, Q.; Hu, Y.; Li, B.; Hao, X. Cinnamaldehyde prevents endothelial dysfunction induced by high glucose by activating Nrf2. Cell. Physiol. Biochem. 2015, 36, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, S.; Holmes, A.; Loscalzo, J. Platelets and cardiovascular disease. Eur. J. Cardiovasc. Nurs. 2002, 1, 273–288. [Google Scholar] [CrossRef]
- Carobbio, A.; Thiele, J.; Passamonti, F.; Rumi, E.; Ruggeri, M.; Rodeghiero, F.; Randi, M.L.; Bertozzi, I.; Vannucchi, A.M.; Antonioli, E.; et al. Risk factors for arterial and venous thrombosis in WHO-defined essential thrombocythemia: An international study of 891 patients. Blood 2011, 117, 5857–5859. [Google Scholar] [CrossRef] [PubMed]
- Gawaz, M. Role of platelets in coronary thrombosis and reperfusion of ischemic myocardium. Cardiovasc. Res. 2004, 61, 498–511. [Google Scholar] [CrossRef]
- Schanze, N.; Bode, C.; Duerschmied, D. Platelet contributions to myocardial ischemia/reperfusion injury. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- Sangk; Uhl, K.; Shuldiner, A.R.; Klein, T.E.; Altman, R.B. Platelet aggregation pathway. Pharmacogenet. Genomics 2011, 21, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Tognolini, M.; Barocelli, E.; Ballabeni, V.; Bruni, R.; Bianchi, A.; Chiavarini, M.; Impicciatore, M. Comparative screening of plant essential oils: Phenylpropanoid moiety as basic core for antiplatelet activity. Life Sci. 2006, 78, 1419–1432. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.O.W.; Zhao, X.; Ye, X.; Zhang, C.; Hao, J.; He, J.; Zhu, X.; Xu, H.; Yang, X. Effect of essential oil of Syringa pinnatifolia Hemsl. var. alashanensis on ischemia of myocardium, hypoxia and platelet aggregation. J. Ethnopharmacol. 2010, 131, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.C.; Uang, B.J.; Tsai, C.Y.; Wu, H.L.; Lin, B.R.; Lee, C.S.; Chen, Y.J.; Chang, C.H.; Tsai, Y.L.; Kao, C.J.; et al. Hydroxychavicol, a novel betel leaf component, inhibits platelet aggregation by suppression of cyclooxygenase, thromboxane production and calcium mobilization. Br. J. Pharmacol. 2007, 152, 73–82. [Google Scholar] [CrossRef] [PubMed]
- You, J.H.; Kang, P.; Min, S.S.; Seol, G.H. Bergamot essential oil differentially modulates intracellular Ca2+ Levels in vascular endothelial and smooth muscle cells. J. Cardiovasc. Pharmacol. 2013, 61, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Maiwulanjiang, M.; Bi, C.W.C.; Lee, P.S.C.; Xin, G.; Miernisha, A.; Lau, K.M.; Xiong, A.; Li, N.; Dong, T.T.X.; Aisa, H.A.; et al. The volatile oil of Nardostachyos Radix et Rhizoma induces endothelial nitric oxide synthase activity in HUVEC cells. PLoS ONE 2015, 10, e0116761. [Google Scholar] [CrossRef] [PubMed]
- Magyar, J.; Szentandrássy, N.; Bányász, T.; Fülöp, L.; Varró, A.; Nánási, P.P. Effects of terpenoid phenol derivatives on calcium current in canine and human ventricular cardiomyocytes. Eur. J. Pharmacol. 2004, 487, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Magyar, J.; Szentandrássy, N.; Bányász, T.; Fülöp, L.; Varró, A.; Nánási, P.P. Effects of thymol on calcium and potassium currents in canine and human ventricular cardiomyocytes. Br. J. Pharmacol. 2002, 136, 330–338. [Google Scholar] [CrossRef]
- Szentandrássy, N.; Szigeti, G.; Szegedi, C.; Sárközi, S.; Magyar, J.; Bányász, T.; Csernoch, L.; Kovács, L.; Nánási, P.P.; Jóna, I. Effect of thymol on calcium handling in mammalian ventricular myocardium. Life Sci. 2004, 74, 909–921. [Google Scholar] [CrossRef] [PubMed]
- Earley, S.; Gonzales, A.L.; Garcia, Z.I. A dietary agonist of transient receptor potential cation channel V3 elicits endothelium-dependent vasodilation. Mol. Pharmacol. 2010, 77, 612–620. [Google Scholar] [CrossRef]
- Sensch, O.; Vierling, W.; Brandt, W.; Reiter, M. Effects of inhibition of calcium and potassium currents in guinea-pig cardiac contraction: Comparison of β-caryophyllene oxide, eugenol, and nifedipine. Br. J. Pharmacol. 2000, 131, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Collazo, J.; Alonso-Carbajo, L.; López-Medina, A.I.; Alpizar, Y.A.; Tajada, S.; Nilius, B.; Voets, T.; López-López, J.R.; Talavera, K.; Pérez-García, M.T.; et al. Cinnamaldehyde inhibits L-type calcium channels in mouse ventricular cardiomyocytes and vascular smooth muscle cells. Pflugers Arch. 2014, 466, 2089–2099. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, N.R.F.; Leal-Cardoso, J.H.; Lessa, L.M.A.; Roriz-Filho, J.S.; Cunha, K.M.A.; Fonteles, M.C. Terpinen-4-ol: Mechanisms of relaxation on rabbit duodenum. J. Pharm. Pharmacol. 2005, 57, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Astudillo, A.; Hong, E.; Bye, R.; Navarrete, A. Antispasmodic activity of extracts and compounds of Acalypha phleoides Cav. Phytother. Res. 2004, 18, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Danesi, F.; Elementi, S.; Neri, R.; Maranesi, M.; D’Antuono, L.F.; Bordoni, A. Effect of cultivar on the protection of cardiomyocytes from oxidative stress by essential oils and aqueous extracts of basil (Ocimum basilicum L.). J. Agric. Food Chem. 2008, 56, 9911–9917. [Google Scholar] [CrossRef] [PubMed]
- Szűcs, G.; Murlasits, Z.; Török, S.; Kocsis, G.F.; Pálóczi, J.; Görbe, A.; Csont, T.; Csonka, C.; Ferdinandy, P. Cardioprotection by farnesol: Role of the mevalonate pathway. Cardiovasc. Drugs Ther. 2013, 27, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.P.; Sudjarwo, G.W.; Jung, S.H.; Lee, D.; Lee, D.Y.; Lee, G.B.; Baek, S.; Kim, D.Y.; Lee, H.M.; Kim, B.; et al. Carvacrol inhibits atherosclerotic neointima formation by downregulating reactive oxygen species production in vascular smooth muscle cells. Atherosclerosis 2015, 240, 367–373. [Google Scholar] [CrossRef]
- Liu, F.; Huang, Z.-Z.; Sun, Y.-H.; Li, T.; Yang, D.-H.; Xu, G.; Su, Y.-Y.; Zhang, T. Four main active ingredients derived from a Traditional Chinese Medicine Guanxin Shutong capsule cause cardioprotection during myocardial ischemia injury calcium overload suppression. Phyther. Res. 2017, 31, 507–515. [Google Scholar] [CrossRef]
- Fouad, A.A.; Yacoubi, M.T. Mechanisms underlying the protective effect of eugenol in rats with acute doxorubicin cardiotoxicity. Arch. Pharm. Res. 2011, 34, 821–828. [Google Scholar] [CrossRef]
- Mnafgui, K.; Hajji, R.; Derbali, F.; Gammoudi, A.; Khabbabi, G.; Ellefi, H.; Allouche, N.; Kadri, A.; Gharsallah, N. Anti-inflammatory, antithrombotic and cardiac remodeling preventive effects of eugenol in isoproterenol-induced myocardial infarction in Wistar rat. Cardiovasc. Toxicol. 2016, 16, 336–344. [Google Scholar] [CrossRef]
- Song, F.; Li, H.; Sun, J.; Wang, S. Protective effects of cinnamic acid and cinnamic aldehyde on isoproterenol-induced acute myocardial ischemia in rats. J. Ethnopharmacol. 2013, 150, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wu, Q.-Q.; Liu, Y.; Hu, Z.-F.; Bian, Z.-Y.; Tang, Q.-Z. Cinnamaldehyde attenuates pressure overload-induced cardiac hypertrophy. Int. J. Clin. Exp. Pathol. 2015, 8, 14345–14354. [Google Scholar]
- Zhao, H.; Zhang, M.; Zhou, F.; Cao, W.; Bi, L.; Xie, Y.; Yang, Q.; Wang, S. Cinnamaldehyde ameliorates LPS-induced cardiac dysfunction via TLR4-NOX4 pathway: The regulation of autophagy and ROS production. J. Mol. Cell. Cardiol. 2016, 101, 11–24. [Google Scholar] [CrossRef]
- Shi, H.-X.; Yang, J.; Yang, T.; Xue, Y.-L.; Liu, J.; Li, Y.-J.; Zhang, D.-D.; Xu, J.-W.; Bian, K. Alpha-asarone protects endothelial cells from injury by angiotensin II. Evid. Based Complement. Altern. Med. 2014, 2014, 1–7. [Google Scholar] [CrossRef] [PubMed]
| Plant Species (Family) | Essential Oils Major Compounds | Study Model | Effect | Reference |
|---|---|---|---|---|
| In Vitro Studies | ||||
| Allium macrostemon Bunge (Amaryllidaceae) | Dimethyl trisulfide (34.93%), dimethyl disulfide (11.61%) | Isolated rat pulmonary arteries | Relaxation | [27] |
| Alpinia speciosa K. Schum (Zingiberaceae) | Terpinen-4-ol (38%), 1,8-cineole (18%), γ-terpinene (12%) | Rat left atria | ↓ Force of contraction in a dose-dependent manner (IC50 = 292.2 µg/mL); ↓ sinus rhythm (IC50 = 595.4 µg/mL) | [28] |
| Alpinia zerumbet K. Schum (Zingiberaceae) | 1,8-Cineole (33.3%), terpinen-4-ol (19.4%), p-cymene (11.4%) | Endothelium-intact rat aortic rings pre-contracted with Phe | Incomplete relaxation | [29] |
| β-Phellandrene (16.4%), β-pinene (15.1%), 1,8-cineole (11%) | Rat aortic rings pre-contracted with norepinephrine and KCl | Inhibited contraction | [30] | |
| Aniba canelilla (H.B.K.) Mez (Lauraceae) | EO without chemical characterization | Mesenteric arteries isolated from SHR | Relaxation on arteries contracted by K+ (IC50 = 294.19 µg/mL) or Phe (IC50 = 11.07 µg/mL); ↓ contractions evoked by phorbol butyrate and Phe in Ca2+-free medium; ↓ contractions induced by CaCl2 or BaCl2 in Ca2+-free and high K+ medium | [31] |
| K+-induced contractions in rat aortic rings | IC50 = 64.5 µg/mL | [32] | ||
| Artemisia campestris L. (Asteraceae) | Spathulenol (10.2%), β-eudesmol (4.05%), p-cymene (3.83%) | Endothelium-intact rat aortic rings contracted with Phe | Contraction | [33] |
| Citrusaurantifolia (Christm) Swingle (Rutaceae) | Limonene (58.4%), β-pinene (15.4%) | Isolated rabbit aortic rings cultured in high K+ medium | Relaxation by activating Ca2+ channels | [34] |
| Citrus aurantium L. var. amara (Rutaceae) | Linalool (23.2%), β-pinene (9.6%), limonene (8.54%) | Endothelium-intact rat aortic rings pre-contracted with prostaglandin F2α | Vasorelaxation | [35] |
| Citrus bergamia Risso (Rutaceae) | D-Limonene (43.5%), linalyl acetate (25.5%) | Mouse aortic rings endothelium-intact and -denuded | Inhibited contraction elicited by PGF2α | [36] |
| Croton argyrophylloides Muell. Arg. (Euphorbiaceae) | Spathulenol (26.7%), caryophyllene oxide (13.1%), β-elemene (12.2%) | Endothelium-intact rat aortic rings and mesenteric arteries pre-contracted with Phe | Vasorelaxation on aortic rings (IC50 = 141.1 µg/mL) and mesenteric arteries (IC50 = 46.1 µg/mL) | [37] |
| Croton nepetaefolius Baill. (Euphorbiaceae) | 1,8-Cineole (25.4%), bicyclogermacrene (11.1%) | Aortic rings isolated from DOCA-salt-hypertensive rats | ↓ Contractions elicited by Phe | [38] |
| RAT mesenteric vascular bed preparations | ↓ Loss of flow caused by KCl | [39] | ||
| 1,8-Cineole (25.4%) | Endothelium-intact rat aortic rings | ↓ Contractions evoked by KCl (IC50 = 26.7 µg/mL) | [40] | |
| Croton zambesicus Müll. Arg. (Euphorbiaceae) | ent-Trachyloban-3-one (1.4–28.0%), caryophyllene oxide (2.9–25.9%), longifolene (0.4–26.4%) | Endothelium-intact rat aortic rings | Vasorelaxant activity (IC50 = 5.6–11.8 µg/mL) | [41] |
| Croton zehntneri Pax et Hoffm. (Euphorbiaceae) | Estragole (46%), trans-anethole (42.1%) | Endothelium-intact rat aortic preparations | ↑ Phe-induced contractions (10 and 30 µg/mL); ↓ Phe-induced contractions (300–1000 µg/mL) | [42] |
| Cymbopogon winterianus Jowitt (Poaceae) | Geraniol (40.1%), citronellal (27.4%), citronellol (10.5%) | Rat mesenteric arteries contracted with KCl | Vasorelaxation on rings with (Emax = 125%) and without (Emax = 117%) endothelium; vasorelaxation in endothelium-denuded rings precontracted with KCl (Emax = 121%) | [43] |
| Ferula asafoetida L. (Apiaceae) | Di-(2-methyl-1,3-oxathiolanyl)methane (22.43%), trans-propenyl sec butyl disulfide (14.59%), 2-ethyltetrahydro- thiophene (10.61%), trans, trans-dibenzylideneacetone (10.07%) | K+-induced contractions in rat aortic rings | Relaxation on rings in the presence (IC50 = 1.6 µL/L) and absence (IC50 = 19.2 µL/L) of endothelium | [44] |
| Foeniculum vulgare Mill. (Apiaceae) | trans-Anethole (75.8%), Estragole (4.6%) | Phe-induced contractions in rat aortic rings | ↓ Contractions on endothelium intact (IC50 = 108 μg/mL) and denuded (IC50 = 147 μg/mL) aortic rings | [45] |
| K+-induced contractions in rat aortic rings | ↓ Contractions on endothelium intact (IC50 = 64 μg/mL) and denuded (IC50 = 52 μg/mL) aortic rings | |||
| Hyptis fruticosa Salzm. ex Benth (Lamiaceae) | α-Pinene, caryophyllene, 1,8-cineole | Endothelium-intact and -denuded rings from rat mesenteric artery pre-contracted with Phe | Relaxation (Emax = 64% and 122%, respectively); ↓ contractions induced by CaCl2 (Emax = 12% and 81%, respectively) | [46] |
| Lippia thymoides Mart. & Schauer (Verbenaceae) | β-Caryophyllene (26.3–17.2%) | Endothelium-intact and endothelium-denuded rat rings | Relaxation on endothelium-intact (IC50 = 305–544 µg/mL) and endothelium-denuded (IC50 = 150–283 µg/mL) rings | [47] |
| Mentha x villosa Huds. (Lamiaceae) | Piperitenone oxide (95.9%) | Isolated rat atrial preparations; Rat aortic rings | Dose-dependent negative chronotropic (IC50 = 229 µg/mL) and ionotropic (IC50 = 120 µg/mL) effects; Relaxation on aortic rings contracted with Phe- (IC50 = 255 µg/mL), PGF2α-induced (IC50 = 174 µg/mL) and KCl (IC50 = 165 µg/mL) | [48] |
| Isolated rat aortic rings contracted by KCl | Relaxation (IC50 = 61 µg/mL and 109 µg/mL for endothelium-intact and denuded rings, respectively) | [49] | ||
| Nigella sativa L. (Ranunculaceae) | EO without chemical characterization | Intact rat aortic rings precontracted with noradrenaline and high K+ | Vasorelaxation | [50] |
| Ocimum gratissimum L. (Lamiaceae) | Eugenol (43.7%) | Endothelium-intact rat aortic preparations | Vasorelaxation; ↓ Ca2+-induced contractions in Ca2+-free medium | [51] |
| Eugenol (52.1%) | Endothelium-intact rat aortic rings | ↓ Phe-induced contraction | [52] | |
| Rat mesenteric vascular beds | ↓ Noradrenaline-induced perfusion pressure | |||
| Ocotea quixos (Lam.) Kosterm. (Lauraceae) | trans-Cinnamaldehyde (27.8%), Methyl cinnamate (21.6%) | Rat aortic rings | ↓ Phe-induced contractions on endothelium-intact (IC50 = 86 µg/mL) and endothelium-denuded (IC50 = 110 µg/mL) rings | [53] |
| Pectis brevipedunculata (Gardner) Sch. Bip. (Asteraceae) | Neral (32.7%), geranial (49.2%) | Phe-contracted rat aortic rings | Vasorelaxation on endothelium-intact (IC50 = 0.044%) and endothelium-denuded (IC50 = 0.093%) rings | [54] |
| Psidium guajava L. (Myrtaceae) | Butanoic acid methyl ester, 3-methyl glutaric anhydride, 1-butanol | Rat aortic rings | Vasorelaxation in aortic rings precontracted with Phe (EC50 = 6.23 mg/mL) and high K+ (EC50 = 5.52 mg/mL) | [55] |
| Pogostemon elsholtzioides Benth. (Lamiaceae) | Curzene (46.1%) | Rat aortic rings pre-contracted with Phe | Relaxation | [56] |
| Rosa indica L. (Rosaceae) | Methyl santonilate, butanoic acid, 2-methyl-5-oxo-1-cyclopentene-1-yl ester | Rat aortic rings | Vasorelaxation in aortic rings precontracted with high K+ (EC50 = 5.80 mg/mL) and Phe (EC50 = 7.39 mg/mL) | [57] |
| Schinus areira L. (Anacardiaceae) | α-Pinene (13.8%), limonene (12.8%), camphene (12.6%), β-caryophyllene (11.9%) | Ex vivo model of rabbit hearts | Inhibited the cardiac contractility induced by norepinephrine | [58] |
| Trachyspermum ammi (L.) Sprague (Apiaceae) | Thymol (38.1%), limonene (33.3%), p-cymene (23.1%) | Rat aortic rings | ↓ Contractions of aortic rings induced by Phe (IC50 = 54.4 µg/mL), KCl (IC50 = 49 µg/mL) in the presence (IC50 = 46.6 µg/mL) and absence (IC50 = 45.2 µg/mL) of endothelium | [59] |
| Xylopia langsdorfiana A. St.-Hil. and Tul. (Annonaceae) | Germacrene D (22.9%), trans-β-guaiene (22.6%), β-caryophyllene (15.7%) | Isolated rat aortic rings contracted with Phe | Weak inhibition of contractions | [60] |
| In Vivo Studies | ||||
| Alpinia zerumbet K. Schum (Zingiberaceae) | Terpinen-4-ol (28.1%), 1,8-cineole (15.1%), γ-terpinene (13.7%) | Anesthetized and conscious rats | Hypotension | [61] |
| Uninephrectomized normotensive rats | Hypotension | [62] | ||
| DOCA-salt hypertensive rats | ↓ MAP | |||
| Terpinene-4-ol (57.35%), 1,8-cineole (27.81%) | L-NAME-induced hypertensive rats | ↓ MAP, SBP and DBP | [63] | |
| Aniba canelilla (H.B.K.) Mez (Lauraceae) | 1-Nitro-2-phenylethane (52.4%), methyl eugenol (38.6%) | Anesthetized and conscious rats | Hypotension with bradycardia | [32] |
| Aniba rosaeodora var. amazonica Ducke (Lauraceae) | (−)-Linalool (50.6%), (+)-linalool (49.4%) | Anesthetized rats | Hypotension with bradycardia | [64] |
| Cymbopogon winterianus Jowitt (Poaceae) | Geraniol (40.1%), citronellal (27.4%), citronellol (10.5%) | Conscious normotensive rats | Hypotension with tachycardia | [43] |
| Croton zehntneri Pax et Hoffm. (Euphorbiaceae) | Estragole (46%), trans-anethole (42.1%) | Conscious, normotensive rats | ↓ MAP, ↓ HR (phase I); ↑ MAP, ↓ HR (phase II) | [65] |
| Anesthetized, normotensive rats | Hypotension with bradycardia | [42] | ||
| Conscious DOCA-salt hypertensive rats | ↓ MAP, ↓ HR (phase I, 5–20 mg/kg); ↑ MAP, ↓ HR (phase II, 10, and 20 mg/kg) | [66] | ||
| Croton argyrophylloides Muell. Arg. (Euphorbiaceae) | Spathulenol (26.65%), caryophyllene oxide (13.13%), 𝛽-elemene (12.15%), 𝛽-caryophyllene (10.94%) | Conscious or anesthetized normotensive rats | Hypotension with tachycardia | [67] |
| Hyptis fruticosa Salzm., ex Benth (Lamiaceae) | α-Pinene, caryophyllene, 1,8-cineole | Non-anesthetized normotensive rats | Hypotension with tachycardia | [46] |
| Mentha x villosa Huds. (Lamiaceae) | Piperitenone oxide (95.9%) | DOCA-salt hypertensive rats | ↓ MAP without bradycardia | [49] |
| Hypotension and ↓ HR | [48] | |||
| Piperitenone oxide (62.3%), γ-muurolene (16.0%) | Anesthetized rats | Hypotension with bradycardia | [68,69] | |
| Piperitenone oxide (95.9%) | ||||
| Piperitenone oxide (55.4%), γ-muurolene (13.1%) | Normotensive conscious rats | ↓ MAP and HR | [70] | |
| Ocimum gratissimum L. (Lamiaceae) | Eugenol (43.7%), 1,8-cineole (32.7%) | Conscious DOCA-salt hypertensive rats | Hypotension | [51] |
| Hypotension with bradycardia | [71] | |||
| Uninephrectomized hypertensive rats | Hypotension with bradycardia | [71] | ||
| Anesthetized or conscious, normotensive rats | ↓ MAP, ↓ HR | [72] | ||
| Pogostemon elsholtzioides Benth. (Lamiaceae) | Curzene (46.1%) | Anesthetized rats | ↓ SBP, DBP, MAP, and HR | [56] |
| Schinus areira L. (Anacardiaceae) | α-Pinene (13.8%), limonene (12.8%), camphene (12.6%) | Non-anesthetized normotensive rats | ↓ SBP, DBP, and MAP | [58] |
| Clinical Trials | ||||
| Lavender (Lamiaceae) | EO without chemical characterization | Prehypertensive middle aged women | ↓ SBP and DBP | [73] |
| Lavender (Lamiaceae):ylang ylang (Annonaceae):bergamot (Rutaceae) (5:3:2) | EO without chemical characterization | Individuals with essential hypertension | ↓ SBP and DBP | [74] |
| Lavender (Lamiaceae) | EO without chemical characterization | Hypertensive individuals | ↓ SBP 5-, 30- and 60-min post application ↓ DBP 60-min post application | [75] |
| Lavender (Lamiaceae):marjoram (Lamiaceae) (1:1) | EO without chemical characterization | |||
| Lavender (Lamiaceae):marjoram (Lamiaceae):ylang-ylang (Annonaceae) (4:3:3) | EO without chemical characterization | |||
| Lavender (Lamiaceae):ylang-ylang (Annonaceae):marjoram (Lamiaceae):neroli (Rutaceae) (20:15:10:2) | EO without chemical characterization | Pre- and hypertensive individuals | ↓ Ambulatory BP (SBP (140.6 to 129.9 mmHg) and daytime DBP (90.5 to 83.3 mmHg) | [76] |
| Plant Species (Family) | Essential Oils Major Compounds | Study Model | Effect | References |
|---|---|---|---|---|
| In Vitro Studies | ||||
| Acorus calamus L. (Acoraceae) | β-Asarone (56.8%), eu-asarone (17.4%), cinnamaldehyde (4.7%) | MDI-induced 3T3-L1 differentiation | Prevents fat accumulation and preadipocytes differentiation into adipocytes | [131] |
| Alpinia zerumbet K. Schum (Zingiberaceae) | β-Phellandrene (16.4%), β-pinene (15.1%), 1,8-cineole (11%) | Human umbilical vessel endothelial cells (HUVECs) | ↑ Cell viability in oxLDL-induced injury in HUVECs; ↓ LDH release (328.68 vs. 555.15 U/L) and MDA levels; ↑ GSH contents and ↑ SOD, CAT, GSH-Px activity | [132] |
| Human aortic endothelial cells (HAECs) treated with oxLDL | ↑ Cell viability; ↓ LDH release; ↑ MMP; ↓ ROS production; ↑ NO production; ↑ mRNA and protein levels of Akt/p-Akt, eNOS and sGC; ↓ iNOS levels | [133] | ||
| Pinus koraiensis Siebold and Zucc (Pinaceae) | Camphene (21.1%), D-limonene (21.0%), α-pinene (16.7%) | HepG2 cells | ↑ mRNA and protein levels of LDL receptor; ↓ mRNA levels SREBP-1c, SREBP-2, HMG-CoA reductase, FAS and GPAT; ↓ activity of hACAT 1 and 2; ↓ oxidation of LDL | [134] |
| Plantago asiatica L. (Plantaginaceae) | Linalool (82.5%) | HepG2 cells | ↑ LDL receptor; ↓ HMG-CoA reductase and LDL oxidation | [135] |
| Salvia officinalis L. (Lamiaceae) | α-Thujone (29%), 1,8-cineole (12%), β-caryophyllene (6.4%) | In vitro lipase and α-amylase activity inhibition | Inhibition of α-amylase (IC50 = 38 μg/mL) and lipase (IC50 = 52 μg/mL) | [136] |
| cis-Thujone (17.4%), α-humulene (13.3%), 1,8-cineole (12.7%) | Primary normal hepatocytes growing in low glucose/lactate or in high glucose conditions | ↓ Glucose production in normal hepatocytes; ↑ Glucose consumption on high glucose conditions in normal hepatocytes | [137] | |
| In Vivo Studies | ||||
| Cinnamomum tamala, (Buch.-Ham.) Nees and Eberm (Lauraceae) | Cinnamaldehyde (44.9%), trans-cinnamyl acetate (25.3%) | STZ-induced type 2 diabetes rat model | ↓ BG after 2h (280 and 239 vs. 341 mg/dL), 4h (292 and 272 vs. 332 mg/dL) and 28 days (201 and 201 vs. 410 mg/dL); ↓ BW loss (−5 and −10 g vs. −20 g); ↓ HbA1c (7.4 and 7.0 vs. 10.8% of Hb); ↑ hepatic glycogen (46 and 62 vs. 28 mg/g of tissue); ↑ insulin (9.8 and 12 vs. 7.8 µU/mL); ↓ TC (160 and 100 vs. 222 mg/dL); ↓ TG (28 and 20 vs. 40 mg/dL); ↑ HDL-C (45 and 52 vs. 36.4 mg/dL); ↓ MDA (4.0 and 3.2 vs. 5.2 nmol/dL); ↑ GSH (20 and 32 vs. 14 µmol GSH/g) | [138] |
| Curcuma longa L (Zingiberaceae) | ar-Turmerone (31.7%), β-turmerone (14.3%), α- turmerone (11.5%) | Golden Syrian hamsters consuming a high cholesterol diet | ↓ TC, LDL-C and TG; ↑HDL-C in plasma (100 and 300 mg/kg); ↓ Hepatic TC, free cholesterol and cholesteryl ester | [139] |
| Foeniculum vulgare Mill. (Apiaceae) | EO without chemical characterization | STZ-induced diabetes rat model | ↓ BG (81.97 vs. 162.5 mg/dL); ↑ GPx activity (99.60 vs. 59.72 U/g Hb) | [140] |
| Diet-induced dyslipidemia | ↓ BG (31 vs. 25% decrease); ↓ TC (81.62 vs. 97.43 mg/dL); ↑ HDL-C (40.6 vs. 37.18 mg/dL); ↓ LDL-C (11.09 vs. 21.31 mg/dL); ↓ TG (83.63 vs. 93.49 mg/dL); ↓ TNF-α (35.61 vs. 92.71 pg/mL); ↓ MDA (8.01 vs. 10.34 nmol/L); ↓ catalase (473.90 vs. 712.20 U/L); ↓ uric acid (7 vs. 7.5 mg/dL); ↓ plasma (0.36 vs. 0.38 mg/dL) and urinary (13.88 vs. 15.90 mg/dL) creatinine; ↓ urine volume (13.60 vs. 14.90 mL); ↓ creatinine clearance (0.37 vs. 0.50 mL/min); ↓ AST (35.80 vs. 44.79 U/L) and ALT (12.11 vs. 21.70 U/L) | [141] | ||
| Plantago asiatica L. (Plantaginaceae) | Linalool (82.5%) | C57BL/6 mice | ↓ TC, TG levels; ↓ mRNA and protein levels of HMG-CoA reductase; ↑ mRNA of LDL receptor | [135] |
| Salvia officinalis L. (Lamiaceae) | α-Thujone (29%), 1,8-cineole (12%), β-caryophyllene (6.4%) | Alloxan-induced diabetes model | ↓ α-Amylase activity by 47%; ↓ fasting blood glucose by 79%; ↑ hepatic glycogen by 44%; ↓ lipase by 53.3%; ↑ hepatic and renal function | [136] |
| Syzygium aromaticum (L.) Merrill and Perry [syn. Eugenia caryophyllus (Spreng.) Bullock and S. G. Harrison] (Myrtaceae) | Eugenol (75.2%) | High fructose-induced fatty liver and dyslipidemia in rats | Plasma: ↓ TC (147.7 vs. 164 mg/dL); ↓ TG (103.2 vs. 114.4 mg/dL); ↑ HDL-C (30.8 vs. 24.1 mg/dL); ↓ LDL-C (74 vs. 106.7 mg/dL); ↓ MDA (6.6 vs. 8.2 nmol/mL); ↓ TNF-α (25.5 vs. 31.9 pg/mL); ↓ ALT (72.5 vs. 85.7 U/L); ↓ AST (63.8 vs. 84.2 U/L); ↓ bilirubin (0.408 vs. 0.506 mg/dL) Liver: ↓ TF (35.4 vs. 46.0 mg/g tissue); ↓ TC (5.2 vs. 5.5 mg/g tissue); ↓ TG (8.8 vs. 9.4 mg/g tissue) ↓ body weight gain (72.6 vs. 83.1 g) | [142] |
| Clinical trials | ||||
| Cumin (Apiaceae) | EO without chemical characterization | Diabetic patients | ↓ HbA1c (7.35 vs. 9.08%); ↓ FBG (116.4 vs. 181 mg/dL); ↓ TG (158.6 vs. 288 mg/dL); ↓ leptin (20.2 vs. 33.6 μg/mL); ↓ oxLDL (90.3 vs. 102.4 U/L); ↑ paraoxonase 1 (83.3 vs. 69.3 U/L); ↑ ApoA1 (115.4 vs. 97.7 mg/dL) | [143] |
| Healthy individuals | ↓ FBG by 55.9 mg/dL vs. 5.7 mg/dL in placebo; ↓ TNF-α by 1.38 ng/mL and CRP by 1.78 pg/mL; ↑ adiponectin by 57.11 μg/L) | [144] | ||
| Plant Species (Family) | Essential Oils Major Compounds | Study Model | Effect | References |
|---|---|---|---|---|
| In Vitro Studies | ||||
| Artemisia dracunculus L. (Asteraceae) | Estragole (70.1%) | ADP-, AA-, and U46619-induced platelet aggregation in guinea pig platelet-rich plasma | Inhibited platelet aggregation in a dose-dependent manner | [172] |
| Thrombin-induced clot formation in guinea pig platelet-rich plasma | ↓ Clot retraction in a dose-dependent manner (IC50 = 126 μg/mL) | |||
| Foeniculum vulgare Mill. (Apiaceae) | trans-Anethole (75.8%), estragole (4.6%) | ADP-, AA- and U46619-, PMA- and collagen-induced platelet aggregation in guinea pig platelet-rich plasma | Inhibited platelet aggregation in a dose-dependent manner | [172] |
| Inhibited ADP (IC50 = 50 μg/mL), AA (IC50 = 4.0 μg/mL), U46619 (IC50 = 132 μg/mL), PMA (46% at 300 μg/mL) and collagen (IC50 = 4.7 μg/mL) induced platelet aggregation | [45] | |||
| Monarda didyma L. (Lamiaceae) | Geraniol (89.5%) | Guinea pig and rat plasma | ↓ AA-induced platelet aggregation (IC50 = 13 µg/mL) | [172] |
| Ocimum basilicum L. (Lamiaceae) | Linalool (49.9%) | Guinea pig and rat plasma | ↓ AA-induced platelet aggregation (IC50 = 22 µg/mL) | [172] |
| Ocotea quixos (Lam.) Kosterm. (Lauraceae) | trans-Cinnamaldehyde (27.8), methyl cinnamate (21.6%) | ADP-, AA- and U46619-, PMA- and collagen-induced platelet aggregation in guinea pig platelet-rich plasma | Inhibited platelet aggregation in a dose-dependent manner | [172] |
| Inhibited ADP (IC50 = 70 μg/mL), AA (IC50 = 47 μg/mL), U46619 (IC50 = 67 μg/mL), PMA (IC50 = 406 μg/mL) and collagen (IC50 = 163 μg/mL) induced platelet aggregation | [53] | |||
| Thrombin-induced clot formation in guinea pig platelet-rich plasma | ↓ Clot retraction in a dose-dependent manner (IC50 = 19 μg/mL) | [172] | ||
| ADP- and U46619-induced platelet aggregation in human platelet-rich plasma | ↓ ADP (IC50 = 128 μg/mL) and U46619 (IC50 = 115 μg/mL) induced aggregation | [53] | ||
| Origanum vulgaris L. (Lamiaceae) | Carvacrol (54.4%), thymol (14.3%) | Guinea pig and rat plasma | ↓ AA-induced platelet aggregation (IC50 = 1.9 µg/mL) | [172] |
| Syringa pinnatifolia var. alashanensis (Oleaceae) | α-Cadinol (19.9%), α- muurolol (18.5%) | Primary cultured rat neonatal myocytes | ↓ ADP-induced platelet aggregation | [173] |
| Thymus vulgaris L. (Lamiaceae) | p-Cymene (15.3%) | Guinea pig and rat plasma | ↓ AA-induced platelet aggregation (IC50 = 4.7 µg/mL) | [172] |
| In Vivo Studies | ||||
| Artemisia campestris L. (Asteraceae) | Spathulenol (10.2%) | Wistar rats and albino mice | ↓ Aggregation induced by thrombin (49.73% at 1 mg/mL) and ADP (48.20% at 1 mg/mL) | [33] |
| Foeniculum vulgare Mill. (Apiaceae) | trans-Anethole (75.8%), estragole (4.6%) | Acute pulmonary thromboembolism animal model | ↓ Paralysis events (70% reduction at 30 μg/mL) | [45] |
| Thrombin-induced clot formation | ↓ Clot retraction in a dose-dependent manner (IC50 = 180 μg/mL) | [45,172] | ||
| Ocotea quixos (Lam.) Kosterm. (Lauraceae) | trans-Cinnamaldehyde (27.8%), methyl cinnamate (21.6%) | Acute pulmonary thromboembolism animal model | ↓ Paralysis events (61% and 41% reduction at 100 and 30 μg/mL); ↓ death after 5 days (81% and 66% reduction at 100 µg/mL and 30 µg/mL) | [53] |
| Plant Species (Family) | Essential Oils Major Compounds | Study Model | Effect | References |
|---|---|---|---|---|
| Alpinia speciosa K. Schum (Zingiberaceae) | Terpinen-4-ol (38%), 1,8-cineole (18%) | Whole-cell clamps | ↓ Intercellular calcium (32.6% at 25 µg/mL vs. 89.3% at 250 µg/mL) | [28] |
| Citrus aurantium L. var. amara (Rutaceae) | Linalool (23.21%), β-pinene (9.59%), limonene (8.54%) | Smooth muscle cells | Relaxation caused by modulation of intracellular Ca2+ | [35] |
| Citrus bergamia Risso (Rutaceae) | Limonene (43.5%), linalyl acetate (25.2%) | Mouse endothelial and vascular smooth muscle cells | Endothelial cells: Transient increase in intracellular Ca2+ followed by a decrease; Vascular smooth muscle cells: sustained ↑ intracellular calcium | [175] |
| Nardostachys jatamansi (D.Don) DC (Caprifoliaceae) | Calarene (38%), β-maaliene (7.9%), valerena-4,1(11)-diene (6.6%) | Human umbilical vein endothelial cells | ↑ Intracellular Ca2+ | [176] |
| Plant Species (Family) | Essential Oils Major Compounds | Study Model | Effect | References |
|---|---|---|---|---|
| In Vitro Studies | ||||
| Ocimum basilicum L. (Lamiaceae) | Linalool (36–47.5%) | Primary cultures of cardiomyocytes treated with H2O2 | ↑ Cell proliferation | [185] |
| Syringa pinnatifolia Hemsl. (Oleaceae) | α-Cadinol (19.9%), τ-muurolol (18.5%) | Primary cultured rat neonatal myocytes | ↓ H2O2-induced cell death | [173] |
| In Vivo Studies | ||||
| Syringa pinnatifolia Hemsl. (Oleaceae) | α-Cadinol (19.9%), τ-muurolol (18.5%) | Wistar rats, Kunming mice | ↑ Survivability of rats under hypoxic conditions; ↓ Deviation on ST-segment; ↓ LDH, CK and TnT; ↑ SOD activity | [173] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves-Silva, J.M.; Zuzarte, M.; Girão, H.; Salgueiro, L. The Role of Essential Oils and Their Main Compounds in the Management of Cardiovascular Disease Risk Factors. Molecules 2021, 26, 3506. https://doi.org/10.3390/molecules26123506
Alves-Silva JM, Zuzarte M, Girão H, Salgueiro L. The Role of Essential Oils and Their Main Compounds in the Management of Cardiovascular Disease Risk Factors. Molecules. 2021; 26(12):3506. https://doi.org/10.3390/molecules26123506
Chicago/Turabian StyleAlves-Silva, Jorge M., Mónica Zuzarte, Henrique Girão, and Lígia Salgueiro. 2021. "The Role of Essential Oils and Their Main Compounds in the Management of Cardiovascular Disease Risk Factors" Molecules 26, no. 12: 3506. https://doi.org/10.3390/molecules26123506
APA StyleAlves-Silva, J. M., Zuzarte, M., Girão, H., & Salgueiro, L. (2021). The Role of Essential Oils and Their Main Compounds in the Management of Cardiovascular Disease Risk Factors. Molecules, 26(12), 3506. https://doi.org/10.3390/molecules26123506