Abstract
The formation of amide bonds represents one of the most fundamental processes in organic synthesis. Transition-metal-catalyzed activation of acyclic twisted amides has emerged as an increasingly powerful platform in synthesis. Herein, we report the transamidation of N-activated twisted amides by selective N–C(O) cleavage mediated by air- and moisture-stable half-sandwich Ni(II)–NHC (NHC = N-heterocyclic carbenes) complexes. We demonstrate that the readily available cyclopentadienyl complex, [CpNi(IPr)Cl] (IPr = 1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene), promotes highly selective transamidation of the N–C(O) bond in twisted N-Boc amides with non-nucleophilic anilines. The reaction provides access to secondary anilides via the non-conventional amide bond-forming pathway. Furthermore, the amidation of activated phenolic and unactivated methyl esters mediated by [CpNi(IPr)Cl] is reported. This study sets the stage for the broad utilization of well-defined, air- and moisture-stable Ni(II)–NHC complexes in catalytic amide bond-forming protocols by unconventional C(acyl)–N and C(acyl)–O bond cleavage reactions.
1. Introduction
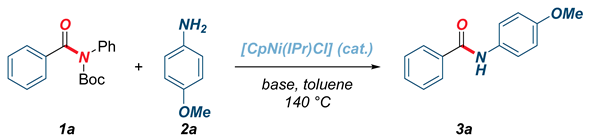
The amide bond represents one of the most fundamental and important functional groups in organic synthesis [1,2,3]. It is estimated that amide bonds are the common structural motif in more than 75% of new pharmaceuticals, while new methods for the formation of amide bonds have been intensively investigated [4,5]. In this context, transamidation reactions represent a highly attractive, unconventional method for the synthesis of amide bonds by transforming a more reactive amide bond into a new, more thermodynamically stable amide counterpart [6,7,8,9,10]. In recent years, the selective activation of C(acyl)–N amide bonds has been achieved by the controlled metal insertion into the resonance activated bonds in twisted amides (i.e., non-planar amides) [11,12,13]. This general approach circumvents the low reactivity of amides resulting from nN→π*C=O conjugation (resonance of 15–20 kcal/mol in planar amides), while providing a powerful platform for organic synthesis [14,15]. Transamidation reactions of twisted amide N–C(O) bonds have been achieved using well-defined Pd(II)–NHC catalysts as well as by using air-sensitive Ni(cod)2 in combination with NHC ligands [16,17,18,19,20,21]. These reactions provide a variety of novel methods for the synthesis of ubiquitous amide bonds and have been extended to catalytic amidation reactions of activated phenolic and unactivated methyl esters by O–C(O) cleavage [22,23,24,25]. In continuation of our studies on activation of amide bonds and organometallic catalysis, in this Special Issue of Editorial Board members of the Organometallic Section of Molecules, we report transamidation of N-activated amides by selective N–C(O) cleavage mediated by air- and moisture-stable half-sandwich Ni(II)–NHC (NHC = N-heterocyclic carbenes) complexes [26,27,28,29,30,31,32,33]. Most importantly, we demonstrate that readily available cyclopentadienyl complex extensively developed by Chetcuti, namely [CpNi(IPr)Cl] (IPr = 1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene) [34,35,36,37,38,39,40,41,42,43], promotes highly selective transamidation of the N–C(O) bond in twisted N-Boc amides with non-nucleophilic anilines (Figure 1). The reaction provides access to secondary anilides via the non-conventional amide bond-forming pathway. Furthermore, the amidation of activated phenolic and unactivated methyl esters mediated by [CpNi(IPr)Cl] is reported. This study sets the stage for the broad utilization of well-defined, air- and moisture-stable Ni(II)–NHC complexes in catalytic amide bond-forming protocols by unconventional C(acyl)–N and C(acyl)–O bond cleavage reactions.
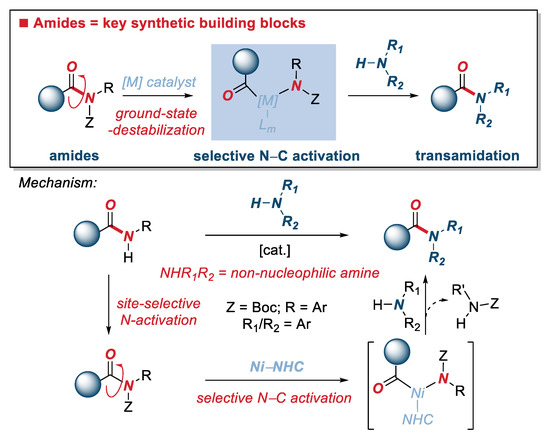
Figure 1.
Transamidation of N-activated amides by selective N–C(O) cleavage.
2. Results
Although we have identified well-defined Pd(II)–NHC complexes for transamidation reactions of activated amides and esters [18,19,20,21], we have been investigating air- and moisture-stable Ni(II)–NHCs based on naturally more abundant Ni as 3d transition metal [26,27,28]. We were attracted to the well-defined, air- and moisture-stable, half-sandwich, cyclopentadienyl [CpNi(IPr)Cl] complex (Figure 2) owing to its ready availability, ease of handling and the potential to prepare more reactive cyclopentadienyl Ni(II)–NHC analogues [29,30,31,32,33]. Notably, [CpNi(IPr)Cl] has emerged as a highly attractive catalyst for several classes of cross-coupling reactions [29,30,31,32,33]; however, transamidations and amidation reactions using this well-defined catalyst have been elusive.
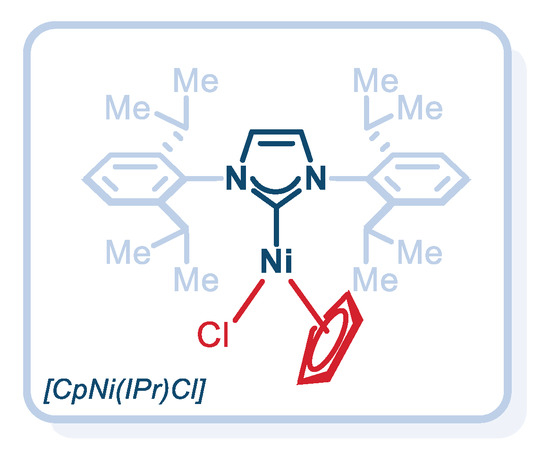
Figure 2.
Structure of the air- and moisture-stable, well-defined, half-sandwich, cyclopentadienyl [CpNi(IPr)Cl] complex.
We initiated our studies by evaluating the reaction conditions for the [CpNi(IPr)Cl]-catalyzed transamidation of N-Boc activated amide 1a with 4-methoxyaniline 2a (Table 1). Of note, twisted N-Boc amides are readily prepared from the corresponding secondary amides by N-chemoselective tert-butoxycarbonylation. The N-carbamate activation permits for decreasing amidic resonance (RE, resonance energy, 7.2 kcal/mol), while providing a thermodynamic pathway for transamidation by rendering the leaving group non-nucleophilic [14,15]. After optimization, we have identified conditions for the transamidation in quantitative yield using [CpNi(IPr)Cl] (10 mol%) as a catalyst in the presence of K2CO3 as a base in toluene at 140 °C (Table 1, entry 1). We found that K3PO4 is also an effective base under these conditions (Table 1, entry 2). Furthermore, decreasing the catalyst loading to [CpNi(IPr)Cl] (5 mol%) resulted in lower conversions (Table 1, entries 3–4). Importantly, control reactions in the absence of the [CpNi(IPr)Cl] catalyst resulted in the recovery of the starting material, thus demonstrating that the catalyst is required for the reaction (Table 1, entries 5–6). Several other optimization conditions are worth noting (not shown): (1) lowering the reaction temperature resulted in significantly lower conversion (110 °C, 26%); (2) reactions at low catalyst loading resulted in low conversion (1 mol%, 13%).

Table 1.
Optimization of the transamidation of amide 1a using [CpNi(IPr)Cl].1
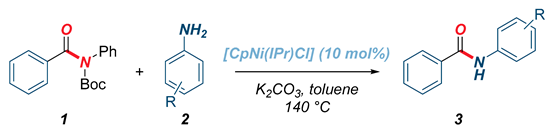
With the optimized conditions in hand, the scope of the transamidation reaction catalyzed by the well-defined [CpNi(IPr)Cl] complex was examined with respect to the aniline component (Table 2). As shown, the reaction performed well using electron-donating (3a), para-substituted (3b), ortho-sterically hindered (3c), meta-substituted (3d), and electron-withdrawing (3e–f) anilines. It is worthwhile to note that the reaction efficiency decreased using electron-deficient nucleophiles. Furthermore, di-ortho-substituted anilines were unproductive substrates in the reaction, indicating excessive steric hindrance.

Table 2.
Scope of anilines in the transamidation of amide 1a using [CpNi(IPr)Cl].1
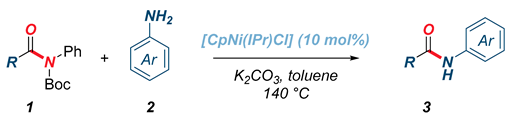
Next, the scope of the reaction with respect to the amide group was evaluated (Table 3). As shown, primary and secondary alkyl amides (3g–h), electron-rich (3i–j) as well as electron-deficient (3k) aromatic amides underwent efficient transamidation under Ni–NHC catalysis. Furthermore, cinnamyl amide was found to be a suitable reaction partner for the transamidation (3l). Similar to the scope of anilines, steric hindrance on the amide component was not tolerated.

Table 3.
Scope of amides in transamidation with aniline 2a using [CpNi(IPr)Cl].1
In consideration of the promising reactivity of twisted N-Boc amides using well-defined cyclopentadienyl half-sandwich [CpNi(IPr)Cl], we further explored amidation reactions of activated phenolic esters and unactivated methyl esters (Scheme 1 and Scheme 2). We were pleased to find that the amidation of phenyl benzoate proceeded in quantitative yield using K3PO4 as a base under otherwise the same reaction conditions as those used for the transamidation of amides (Scheme 1). Importantly, control reactions in the absence of the catalyst unambiguously verified that [CpNi(IPr)Cl] is required for the reaction. Interestingly, we also found that amidation of unactivated methyl benzoate proceeded in 67% yield, while a substantial enhancement of reactivity (94% yield) was observed by increasing the reaction temperature to 160 °C (Scheme 2). As expected, no reaction was observed in the absence of [CpNi(IPr)Cl] (<2%, not detected).
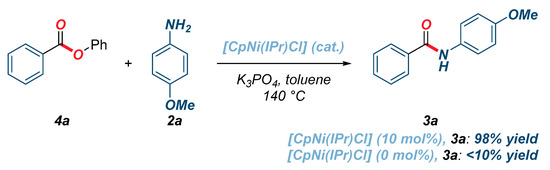
Scheme 1.
Amidation of activated phenolic ester using [CpNi(IPr)Cl].
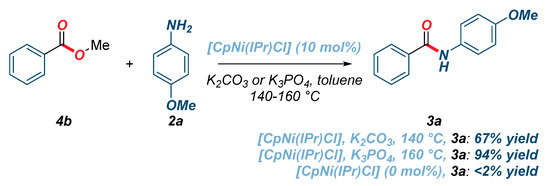
Scheme 2.
Amidation of unactivated methyl ester using [CpNi(IPr)Cl].
To gain preliminary insight into the reaction profile, kinetic studies were performed (Figure 3). As shown, the reaction reached 60% conversion after 3 h, while 77% conversion was observed after 6 h. The induction period was not observed in the kinetic profiling studies. We tentatively propose that the mechanism involves oxidative addition of the N–C bond to nickel. Other nickel sources, such as NiCp2 or NiCl2, catalyze the reaction albeit in lower yields. Studies on the mechanism and the expansion of the substrate scope are ongoing and will be reported in due course.
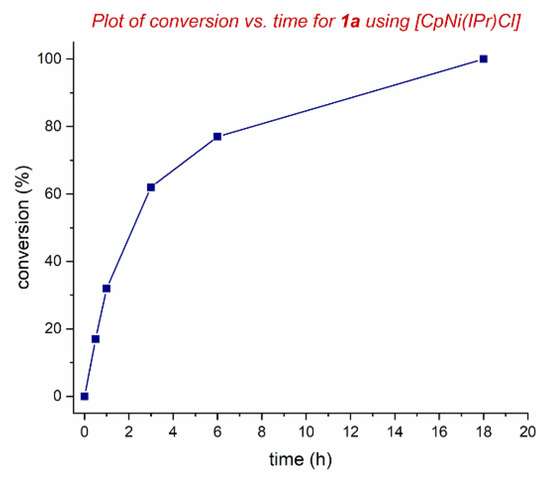
Figure 3.
Kinetic profile of 1a. Conditions: 1a, 4-MeO-C6H4-NH2 (2.0 equiv), [CpNi(IPr)Cl] (10 mol%), K2CO3 (3.0 equiv), toluene (0.25 M), 140 °C, 0–18 h.
3. Materials and Methods
General Information. General methods have been published [18].
General Procedure for [CpNi(IPr)Cl] Catalyzed Transamidation. In a typical procedure, an oven-dried vial was charged with a N-Boc amide or ester substrate (neat, 1.0 equiv), aniline (2.0 equiv), K2CO3 (3.0 equiv), [CpNi(IPr)Cl] (10 mol%), placed under a positive pressure of argon, and subjected to three evacuation/backfilling cycles under high vacuum. Toluene (0.25 M) was added at room temperature, the reaction was placed in a preheated oil bath at 140 °C, and stirred at 140 °C. After the indicated time, the reaction was cooled down, diluted with CH2Cl2 (10 mL), filtered, and concentrated. The sample was analyzed by 1H-NMR (CDCl3, 500 MHz) and GC-MS to obtain conversion, selectivity and yield using internal standard and comparison with authentic samples. All yields have been determined by 1H-NMR spectroscopy (CDCl3, 500 MHz).
Representative Isolation Procedure for [CpNi(IPr)Cl] Catalyzed Transamidation. An oven-dried vial was charged with tert-butyl benzoyl(phenyl)carbamate (neat, 29.7 mg, 1.0 equiv), 4-methoxyaniline (24.6 mg, 2.0 equiv), K2CO3 (41.6 mg, 3.0 equiv), [CpNi(IPr)Cl] (10 mol%, 5.6 mg), placed under a positive pressure of argon, and subjected to three evacuation/backfilling cycles under high vacuum. Toluene (0.25 M) was added at room temperature, the reaction mixture was placed in a preheated oil bath at 140 °C, and stirred for 18 h at 140 °C. After the indicated time, the reaction was cooled down, diluted with CH2Cl2 (10 mL), filtered, and concentrated. A sample was analyzed by 1H-NMR (CDCl3, 500 MHz) and GC-MS to obtain conversion, yield and selectivity using internal standard and comparison with authentic samples. Purification by chromatography on silica gel (hexanes/ethyl acetate) afforded the title product. Yield 88% (20.1 mg). N-(4-Methoxyphenyl)benzamide. White solid. 1H-NMR (500 MHz, CDCl3) δ 7.86 (d, J = 7.5 Hz, 2 H), 7.76 (s, 1 H), 7.59–7.51 (m, 3 H), 7.47 (t, J = 7.4 Hz, 2 H), 6.91 (d, J = 8.9 Hz, 2 H), 3.81 (s, 3 H). 13C NMR (125 MHz, CDCl3) δ 157.00, 135.40, 132.04, 131.35, 129.10, 127.31, 122.43, 114.61, 55.86. [CpNi(IPr)Cl] has been prepared by the previously reported procedure [1].
4. Conclusions
In summary, we have reported on the transamidation reactions of N-activated amides by selective N–C(O) cleavage mediated by the well-defined, air- and moisture-stable half-sandwich [CpNi(IPr)Cl] complex. This class of Ni(II)–NHC cyclopentadienyl complexes has gained significant attention in organometallic catalysis owing to the beneficial properties of this class of catalysts; however, transamidation reactions of amides and amidation reactions of esters mediated by these complexes have been elusive. The present study demonstrates that highly selective transamidation of the N–C(O) bond in twisted N-Boc amides as well as activated phenolic and unactivated methyl esters with non-nucleophilic anilines under [CpNi(IPr)Cl] catalysis is feasible, thus providing an unconventional and unified method for the synthesis of secondary anilides by C(acyl)–N and C(acyl)–O bond cleavage reactions. It should be mentioned that the twisted amide starting materials are prepared from 2° amides by N-chemoselective tert-butoxycarbonylation [14], which provides a two-step transamidation method that could potentially be applied in late-stage derivatization of pharmaceuticals and natural products. The unique versatility of [CpNi(IPr)Cl] sets the stage for the broad application of Ni(II)–NHC cyclopentadienyl complexes in amide bond-forming reactions by N–C(O)/O–C(O) cleavage. Future studies will focus on the development of new classes of [CpNi(NHC)X] complexes for selective transformations of amide and ester bonds by N–C(O)/O–C(O) activation.
Author Contributions
J.B. and M.M.R. conducted experimental work and analyzed the data. M.S. supervised the project and wrote the paper. All authors contributed to the experiment design and reaction development. All authors have read and agreed to the published version of the manuscript.
Funding
Rutgers University and the NSF (CAREER CHE-1650766) are acknowledged for support. The 500 MHz spectrometer was supported by the NSF-MRI grant (CHE-1229030).
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Greenberg, A.; Breneman, C.M.; Liebman, J.F. The Amide Linkage: Structural Significance in Chemistry, Biochemistry, and Materials Science; Wiley: New York, NY, USA, 2000. [Google Scholar]
- Hughes, B. Amino Acids, Peptides and Proteins in Organic Chemistry; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]
- Pattabiraman, V.R.; Bode, J.W. Rethinking amide bond synthesis. Nature 2011, 480, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Roughley, S.D.; Jordan, A.M. The Medicinal Chemist’s Toolbox: An Analysis of Reactions Used in the Pursuit of Drug Candidates. J. Med. Chem. 2011, 54, 3451–3479. [Google Scholar] [CrossRef] [PubMed]
- Bryan, M.C.; Dunn, P.J.; Entwistle, D.; Gallou, F.; Koenig, S.G.; Hayler, J.D.; Hickey, M.R.; Hughes, S.; Kopach, M.E.; Moine, G.; et al. Key Green Chemistry research areas from a pharmaceutical manufacturers’ perspective revisited. Green Chem. 2018, 20, 5082–5103. [Google Scholar] [CrossRef]
- Loomis, W.D.; Stumpf, P.K. Transamination and Transamidation. In Nitrogen Metabolism; Allen, E.K., Allen, O.N., Böttger, I., Caspersson, T., Dillemann, G., Engel, H., Fischer, H., Guggenheim, M., Haas, P., Haurowitz, F., et al., Eds.; Springer: Berlin, Germany, 1958. [Google Scholar]
- De Figueiredo, R.M.; Suppo, J.S.; Campagne, J.M. Nonclassical Routes for Amide Bond Formation. Chem. Rev. 2016, 116, 12029–12122. [Google Scholar] [CrossRef]
- Ojeda-Porras, A.; Gamba-Sanchez, D. Recent Developments in Amide Synthesis Using Nonactivated Starting Materials. J. Org. Chem. 2016, 81, 11548–11555. [Google Scholar] [CrossRef]
- Acosta-Guzmán, P.; Mateus-Gómez, A.; Gamba-Sánchez, D. Direct Transamidation Reactions: Mechanism and Recent Advances. Molecules 2018, 23, 2382. [Google Scholar] [CrossRef]
- Li, G.; Szostak, M. Non-Classical Amide Bond Formation: Transamidation and Amidation of Activated Amides and Esters by Selective N–C/O–C Cleavage. Synthesis 2020, 52, 2579–2599. [Google Scholar] [CrossRef]
- Li, G.; Ma, S.; Szostak, M. Amide Bond Activation: The Power of Resonance. Trends Chem. 2020, 2, 914–928. [Google Scholar] [CrossRef]
- Takise, R.; Muto, K.; Yamaguchi, J. Cross-coupling of aromatic esters and amides. Chem. Soc. Rev. 2017, 46, 5864–5888. [Google Scholar] [CrossRef]
- Dander, J.E.; Garg, N.K. Breaking Amides using Nickel Catalysis. ACS Catal. 2017, 7, 1413–1423. [Google Scholar] [CrossRef]
- Meng, G.; Shi, S.; Lalancette, R.; Szostak, R.; Szostak, M. Reversible Twisting of Primary Amides via Ground State N–C(O) Destabilization: Highly Twisted Rotationally Inverted Acyclic Amides. J. Am. Chem. Soc. 2018, 140, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Ielo, L.; Pace, V.; Holzer, W.; Rahman, M.M.; Meng, G.; Szostak, R.; Szostak, M. Electrophilicity Scale of Activated Amides: 17O NMR and 15N NMR Chemical Shifts of Acyclic Twisted Amides in N−C(O) Cross-Coupling. Chem. Eur. J. 2020, 26, 16246–16250. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.L.; Yamano, M.M.; Zhou, Y.; Anthony, S.M.; Garg, N.K. A two-step approach to achieve secondary amide transamidation enabled by nickel catalysis. Nat. Commun. 2016, 7, 11554. [Google Scholar] [CrossRef] [PubMed]
- Dander, J.E.; Baker, E.L.; Garg, N.K. Nickel-catalyzed transamidation of aliphatic amide derivatives. Chem. Sci. 2017, 8, 6433–6438. [Google Scholar] [CrossRef]
- Meng, G.; Lei, P.; Szostak, M. A General Method for Two-Step Transamidation of Secondary Amides Using Commercially Available, Air- and Moisture-Stable Palladium/NHC (N-Heterocyclic Carbene) Complexes. Org. Lett. 2017, 19, 2158–2161. [Google Scholar] [CrossRef]
- Shi, S.; Szostak, M. Pd–PEPPSI: A general Pd–NHC precatalyst for Buchwald–Hartwig cross-coupling of esters and amides (transamidation) under the same reaction conditions. Chem. Commun. 2017, 53, 10584–10587. [Google Scholar] [CrossRef]
- Zhou, T.; Li, G.; Nolan, S.P.; Szostak, M. [Pd(NHC)(acac)Cl]: Well-Defined, Air-Stable, and Readily Available Precatalysts for Suzuki and Buchwald-Hartwig Cross-coupling (Transamidation) of Amides and Esters by N-C/O-C Activation. Org. Lett. 2019, 21, 3304–3309. [Google Scholar] [CrossRef]
- Li, G.; Zhou, T.; Poater, A.; Cavallo, L.; Nolan, S.P.; Szostak, M. Buchwald-Hartwig cross-coupling of amides (transamidation) by selective N–C(O) cleavage mediated by air- and moisture-stable [Pd(NHC)(allyl)Cl] precatalysts: Catalyst evaluation and mechanism. Catal. Sci. Technol. 2020, 10, 710–716. [Google Scholar] [CrossRef]
- Ben Halima, T.; Vandavasi, J.K.; Shkoor, M.; Newman, S.G. A Cross-Coupling Approach to Amide Bond Formation from Esters. ACS Catal. 2017, 7, 2176–2180. [Google Scholar] [CrossRef]
- Halima, B.T.; Masson-Makdissi, J.; Newman, S.G. Nickel-Catalyzed Amide Bond Formation from Methyl Esters. Angew. Chem. Int. Ed. 2018, 57, 12925–12929. [Google Scholar] [CrossRef]
- Zheng, Y.L.; Newman, S.G. Methyl Esters as Cross-Coupling Electrophiles: Direct Synthesis of Amide Bonds. ACS Catal. 2019, 9, 4426–4433. [Google Scholar] [CrossRef]
- Li, G.; Ji, C.L.; Hong, X.; Szostak, M. Highly Chemoselective, Transition-Metal-Free Transamidation of Unactivated Amides and Direct Amidation of Alkyl Esters by N–C/O–C Cleavage. J. Am. Chem. Soc. 2019, 141, 11161–11172. [Google Scholar] [CrossRef] [PubMed]
- Tasker, S.Z.; Standley, E.A.; Jamison, T.F. Recent advances in homogeneous nickel catalysis. Nature 2014, 509, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Ananikov, V.P. Nickel: The “Spirited Horse” of Transition Metal Catalysis. ACS Catal. 2015, 5, 1964–1971. [Google Scholar] [CrossRef]
- Diccianni, J.B.; Diao, T. Mechanisms of Nickel-Catalyzed Cross-Coupling Reactions. Trends Chem. 2019, 1, 830–844. [Google Scholar] [CrossRef]
- Danopoulos, A.A.; Simler, T.; Braunstein, P. N-Heterocyclic Carbene Complexes of Copper, Nickel, and Cobalt. Chem. Rev. 2019, 119, 3730–3961. [Google Scholar] [CrossRef]
- Henrion, M.; Ritleng, V.; Chetcuti, M.J. Nickel N-Heterocyclic Carbene-Catalyzed C–C Bond Formation: Reactions and Mechanistic Aspects. ACS Catal. 2015, 5, 1283–1302. [Google Scholar] [CrossRef]
- Ritleng, V.; Henrion, M.; Chetcuti, M.J. Nickel N-Heterocyclic Carbene-Catalyzed C–Heteroatom Bond Formation, Reduction, and Oxidation: Reactions and Mechanistic Aspects. ACS Catal. 2016, 6, 890–906. [Google Scholar] [CrossRef]
- Banach, Ł.; Guńka, P.A.; Zachara, J.; Buchowicz, W. Half-sandwich Ni(II) complexes [Ni(Cp)(X)(NHC)]: From an underestimated discovery to a new chapter in organonickel chemistry. Coord. Chem. Rev. 2019, 389, 19–58. [Google Scholar] [CrossRef]
- Zhao, Q.; Meng, G.; Nolan, S.P.; Szostak, M. N-Heterocyclic Carbene Complexes in C–H Activation Reactions. Chem. Rev. 2020, 120, 1981–2048. [Google Scholar] [CrossRef]
- Ritleng, V.; Oertel, A.M.; Chetcuti, M.J. Half-sandwich NHC-nickel(ii) complexes as pre-catalysts for the fast Suzuki coupling of aryl halides: A comparative study. Dalton Trans. 2010, 39, 8153–8160. [Google Scholar] [CrossRef] [PubMed]
- Oertel, A.M.; Ritleng, V.; Burr, L.; Chetcuti, M.J. Synthesis and Structural Characterization of Half-Sandwich Nickel Complexes Bearing Two Different N-Heterocyclic Carbene Ligands. Organometallics 2011, 30, 6685–6691. [Google Scholar] [CrossRef]
- Oertel, A.M.; Ritleng, V.; Chetcuti, M.J. Synthesis and Catalytic Activity in Suzuki Coupling of Nickel Complexes Bearing n-Butyl- and Triethoxysilylpropyl-Substituted NHC Ligands: Toward the Heterogenization of Molecular Catalysts. Organometallics 2012, 31, 2829–2840. [Google Scholar] [CrossRef]
- Henrion, M.; Chetcuti, M.J.; Ritleng, V. From acetone metalation to the catalytic α-arylation of acyclic ketones with NHC–nickel(ii) complexes. Chem. Commun. 2014, 50, 4624–4627. [Google Scholar] [CrossRef]
- Bheeter, L.P.; Henrion, M.; Brelot, L.; Darcel, C.; Chetcuti, M.J.; Sortais, J.B.; Ritleng, V. Hydrosilylation of Aldehydes and Ketones Catalyzed by an N-Heterocyclic Carbene-Nickel Hydride Complex under Mild Conditions. Adv. Synth. Catal. 2012, 354, 2619–2624. [Google Scholar] [CrossRef]
- Bheeter, L.P.; Henrion, M.; Chetcuti, M.J.; Darcel, C.; Ritleng, V.; Sortais, J.B. Cyclopentadienyl N-heterocyclic carbene–nickel complexes as efficient pre-catalysts for the hydrosilylation of imines. Catal. Sci. Technol. 2013, 3, 3111–3116. [Google Scholar] [CrossRef]
- Oertel, A.M.; Freudenreich, J.; Gein, J.; Ritleng, V.; Veiros, L.F.; Chetcuti, M.J. Intramolecular Nitrile C–H Bond Activation in Nickel NHC Complexes: A Route to New Nickelacycles. Organometallics 2011, 30, 3400–3411. [Google Scholar] [CrossRef]
- Kelly, R.A.; Scott, N.M.; Díez-González, S.; Stevens, E.D.; Nolan, S.P. Simple Synthesis of CpNi(NHC)Cl Complexes (Cp=Cyclopentadienyl; NHC=N-Heterocyclic Carbene). Organometallics 2005, 24, 3442–3447. [Google Scholar] [CrossRef]
- Martin, A.R.; Makida, Y.; Meiries, S.; Slawin, A.M.Z.; Nolan, S.P. Enhanced Activity of [Ni(NHC)CpCl] Complexes in Arylamination Catalysis. Organometallics 2013, 32, 6265–6270. [Google Scholar] [CrossRef]
- Buchspies, J.; Rahman, M.; Szostak, M. Suzuki–Miyaura Cross-Coupling of Amides Using Well-Defined, Air- and Moisture-Stable Nickel/NHC (NHC=N-Heterocyclic Carbene) Complexes. Catalysts 2020, 10, 372. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).