Abstract
This work describes the utility of pyrazole-4-carbaldehyde 1 as starting material for the synthesis of a novel potent series of 5α-reductase and aromatase inhibitors derived from 1,2,3-triazole derivative. Condensation of 1 with active methylene and different amino pyrazoles produced the respective Schiff bases 2–4, 8 and 9. On the other hand, 1 was reacted with ethyl cyanoacetate and thiourea in one-pot reaction to afford the pyrazolo-6- thioxopyridin-2-[3H]-one (10). Moreover, α–β unsaturated chalcone derivative 11 was prepared via the reaction of compound 1 with P-methoxy acetophenone, which in turn reacted with each of ethyl cyanoacetate, malononitrile, hydrazine hydrate, and thiosemicarbazide to afford the corresponding pyridine and pyrazole derivatives 13, 14, 17, and 20. The structure of newly synthesized compounds was characterized by analytical and spectroscopic data (IR, MS and NMR). All new compounds were evaluated against 5α-reductase and aromatase inhibitors and the results showed that many of these compounds inhibit 5α-reductase and aromatase activity; compound 13 was found to be the highest potency among the tested samples comparing with the reference drugs.
1. Introduction
The pharmaceutical importance of 1,2,3-triazole ring is due to its efficacy as c-Met kinase inhibitors [1], aromatase inhibitors [2], 5α-reductase inhibitors [3], virostatic [4], antiproliferative agents [5,6,7], GABA-antagonists [8], antimicrobial agents [9,10], anti-bacterial agents [11], novel inhibitors of human immunodeficiency virus type1 protease [12], glycosidase inhibitors, antimalarial agents [13], antihistaminic agents [14], nucleosides [15], as synthetic intermediates for some antibiotic compounds [16], and also for treatment of Alzheimer’s disease [17].
On the other hand, pyrazoline derivatives are a very important class of heterocyclic compounds characterized by biological activities as antiviral [18], anticancer [19], antimicrobial agent [20], 5α-reductase, and aromatase inhibitors [21,22].
We are incorporated in a research group aimed to produce biologically active heterocyclic compounds using available chemicals [5,6,23,24,25,26,27,28,29,30,31]. Our growing interest in this manuscript is incorporation between 1,2,3-triazole ring and pyrazoline ring in a new heterocyclic compound 1 [5]. The starting compound 1 will react with appropriate active methylene to give compounds 2–4, while, a newly substituted pyrazole derivative 5–9 will be formed when compounds 2–4 react with hydrazine hydrate and different amino pyrazole. Chalcone derivative 11 is prepared via the reaction substituted pyrazole 1 with P-methoxy acetophenone, which in turn reacts with each of ethyl cyanoacetate, malononitrile hydrazine hydrate, and thiosemicarbazide to give the corresponding pyridine and pyrazole derivatives 13, 14, 17, and 20, respectively. The newly synthesized compounds were evaluated against 5-α reductase and aromatase inhibitors to study their activities as anti- hormone-dependent cancer compounds.
2. Results and Discussion
2.1. Chemistry
It has been found that the reaction of pyrazole (3-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazole-4-carbaldehyde (1) with some selected active methylene (namely, malononitrile, acetyl acetone and diethyl malonate) with the presence of triethylamine in ethanol gave the respective new 3-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)methylene) derivatives 2–4. The structures of compounds 2–4 were confirmed on the basis of their microanalytical and spectral data. For compound 2-((3-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)methylene) malononitrile (2), taken as a representative example, its IR spectrum (KBr/cm−1) revealed a strong sharp absorption band at v 2222 cm−1 corresponding to C≡N group and two absorption bands at v 1620 cm−1 and 1640 cm−1due to C=C and C=N. Its 1H-NMR spectrum (DMSO-d6, δ ppm.) showed three signals at δ = 2.52, 6.37 and 8.34 ppm. due to -CH3 and -CH pyrazole and CH-olefinic of Schiff base protons, respectively. The aromatic protons (10H) appeared as a multiplet at δ = 7.33–7.94 ppm. Moreover, the structure was also supported by its mass spectrum (m/z 378) [M+], which agrees with its molecular formula C22H15N7.
The newly synthesized Schiff bases derivatives 2–4 have now been investigated as key molecules for building new fused pyrazole derivatives through the condensation reaction on their carboxylic double bond with nucleophile hydrazine hydrate, to give compounds 5–7 respectively (Scheme 1). The structures of 5–7 were established on the basis of their elemental analysis and spectral data (MS, IR, and 1H-NMR). As an example, structure 6 was supported by its mass spectrum (m/z 407) [M+], which agrees with its molecular formula C24H21N7. Its IR spectrum (KBr/cm−1) showed an absorption band at v 1600 cm−1, which corresponds to the C=C, a band at v 1620 cm−1 due to C=N, and a band at v 2862, 2924 cm−1ue to CH-aliphatic. Its 1H-NMR spectrum displayed four singlet signals at δ = 2.53, 2.75, 6.37, and 8.35 ppm. due to two -CH3, CH-pyrazole and CH-olefinic, respectively, one multiple signal at δ = 7.35–7.84 ppm. related to the 10 protons of aromatic. he other bands of compounds 5 and 7 appeared in IR, 1H-NMR spectra and molecular weight determination (MS) in the expected regions.
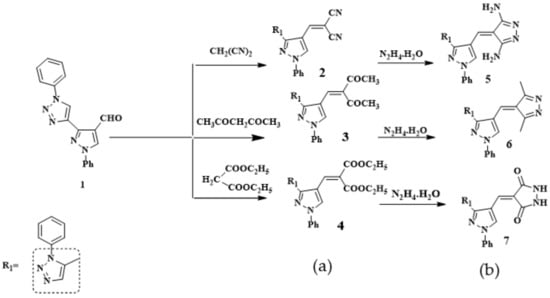
Scheme 1.
Reaction of compound 1 with (a) active methylenes; (b) the products with hydrazine hydrate.
Compound 1 was allowed to react with each of 4-cyano-3-aminopyrazole and 5-phenyl-3-aminopyrazole in boiling glacial acetic acid via Schiff-base to afford the corresponding 8 and 9 respectively in a good yield (Scheme 2). The structure of compound 8 and 9 were confirmed by different spectroscopic tools; structure 8 was supported by its mass spectrum (m/z 419) [M+], which agrees with its molecular formula C23H17N9. Its IR spectrum (KBr/cm−1) revealed a strong absorption band at v 2222 cm−1 due to C≡N and a strong absorption band at v 3105 cm−1 attributed to the NH group. 1H-NMR spectrum of compounds 8 and 9 revealed a proton at δ = 8.50 and 8.51 ppm. which was assigned to the appearance of the CH-olefinic of Schiff-base. The other bands of compounds 8 and 9 appeared in IR, 1H-NMR spectra and molecular weight determination (MS) in the expected regions.
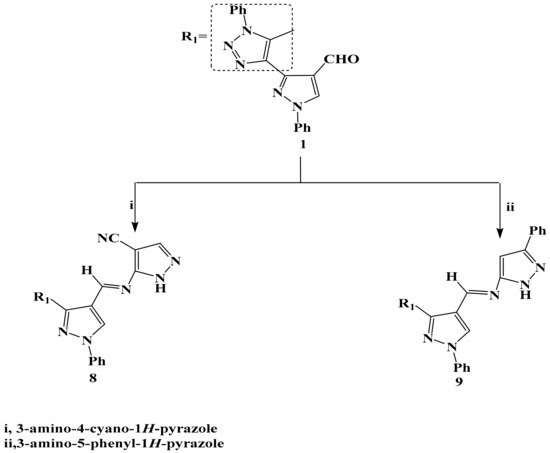
Scheme 2.
The reaction of compound 1 with amino pyrazoles.
It has now been found that one-pot reaction of compound 1 with ethyl cyanoacetate and thiourea in the presence of hydrochloric acid in refluxing ethanol afforded the corresponding derivative 10 through the mechanism illustrated in (Scheme 3). The reaction proceeded via tetrahedral mechanism, in which the N-C bond was formed before the CO bond started to break and ethanol eliminated, and consequently a lot of energy was accumulated in the reaction medium, which offset the activation energy of the reaction and a facile conversion occurred; then cyclization took place via the addition of an amino group to the nitrile group to yield the desired product 10. Structure 10 was supported by its mass spectrum (m/z 454) [M+], which agrees with its molecular formula C23H18N8OS. Its IR spectrum (KBr/cm−1) showed a strong absorption band at v 1400 cm−1, which is attributed to C=S, a strong absorption band at v 3228 cm−1 due to (NH) and a strong absorption forked band at v 3317, 3348 cm−1due to the NH2 group. Its 1H-NMR spectrum displayed five singlets at δ = 2.53, 6.31, 6.82, 8.21, and 9.50 ppm. due to CH3, CH-pyrazole, NH2group, CH-olefinic, and NH protons, respectively, and one multiple at δ = 7.35-7.84 ppm. related to the 10 protons of aromatic.
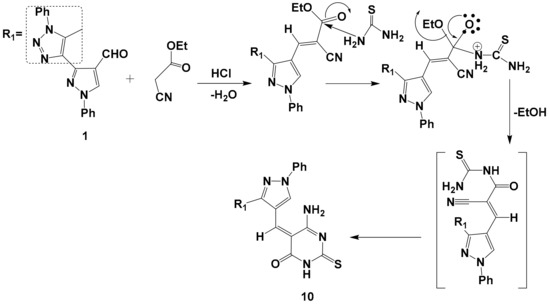
Scheme 3.
The suggested mechanism for preparation of compound 10.
Otherwise, compound number 1 reacted with P-methoxy acetophenone to give the α–β unsaturated derivative 1-(4-methoxyphenyl)-3-(3-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)prop-2-en-1-one 11 (Scheme 4).
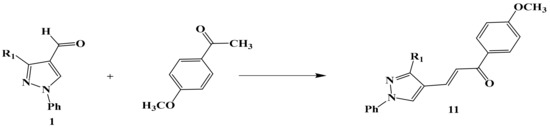
Scheme 4.
The reaction of compound 1 with p-methoxy acetophenone to form chalcone 11.
The structure of chalcone 11 was inferred from spectral data (IR, Mass, NMR), elemental analysis and chemical transformation. However, the structure of 11 was inferred chemically via its interaction with each of ethyl cyanoacetate and malononitrile to give the corresponding 13 and 14 respectively (Scheme 5). The structure of compound 13 and 14 was confirmed by spectral data (IR, Mass, NMR) and correct elemental analysis. The IR spectrum of compound 13 showed a strong absorption band at v 3066, 3133 cm−1 attributed to the NH2 group, and a strong absorption band at v 1740 cm−1attributed to the carbonyl ester and devoid of any band for the C≡N group. Its 1H-NMR exhibited a triple signal at δ = 1.02–1.21 ppm attributed to the three protons of the CH3 group of ethyl ester and as a quartet signal δ at 3.90–4.12 ppm attributed to the two protons of the CH2 group of ethyl ester, which confirmed the formation of compounds 13 and not 12.
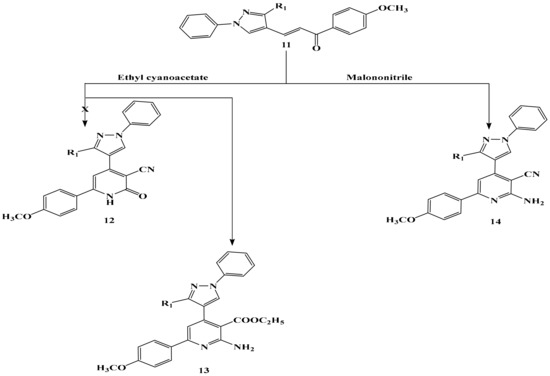
Scheme 5.
The reaction of chalcone 11 with ethyl cyanoacetate and malononitrile.
On the other hand, the IR spectrum of compound 14 showed a strong sharp absorption band at 2212 cm−1 represented the C≡N which indicated the formation of compound 14. he other bands of compounds 13 and 14 appeared in IR, 1H-NMR spectra molecular weight determination (MS) in the expected regions.
When the chalcone 11 was allowed to react with hydrazine hydrate it yielded the corresponding substituted pyrazole 17 in a good yield through the reasonable mechanism discussed in (Scheme 6). The reaction was based through hydrazone derivative followed by ring closure. The structure of compound 17 was confirmed by spectral data (IR, Mass, NMR), elemental analysis and chemical transformation. The bands of compounds 17 appeared in IR, 1H-NMR spectra molecular weight determination (MS) in the expected regions.
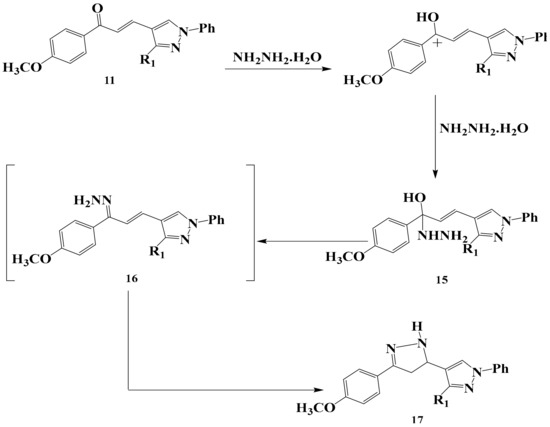
Scheme 6.
The reaction mechanism of chalcone 11 with hydrazine hydrate to form pyrazole derivative 17.
Finally, condensation of chalcone 11 with thiosemicarbazide gave pyrazoline derivative 20 as shown in Scheme 7. The selectivity that leads to the formation of intermediate 18 results from 1,2-addition of thiosemicarbazide to the carbonyl group of chalcone 11 and subsequent N-H intermolecular cycloaddition to the double bond of 18, which leads to the formation of 20. As a result and in accordance with the currently accepted mechanism [32], the formation of 20 is formed via hydrazone formation under the reaction condition; the product structure is determined via steps 19–20 where a regioselective enamine-imine tautomerism [33] may take place to form the more stable 2-pyrazoline 20. The structures of 20 were established on the basis of their elemental analysis and spectral data (MS, IR, 1H-NMR, and 13C-NMR). Structure 20 was supported by its mass spectrum (m/z 454) [M+], which agrees with its molecular formula C23H18N8OS. Its IR spectrum (KBr/cm−1) showed an absorption band at v 3475, 3436 cm−1due to NH2, a band at v 2924, 2890 cm−1 due to CH-alphatic, a band at v 1562 cm−1 due to C=C and a due to v1616 cm−1 due to C=N Its 1H-NMR spectrum displayed three singlet signals at δ = 2.54, 3.11 and 4.61 ppm due to CH3, OCH3-NH2 group protons respectively, one doublet at δ = 3.40-3. 47 related to CH2-pyrazole, and two multiples at δ = 3.74–3.82 and 7.01–7.72 ppm. related to CH2 protons and 15 protons of aromatic.
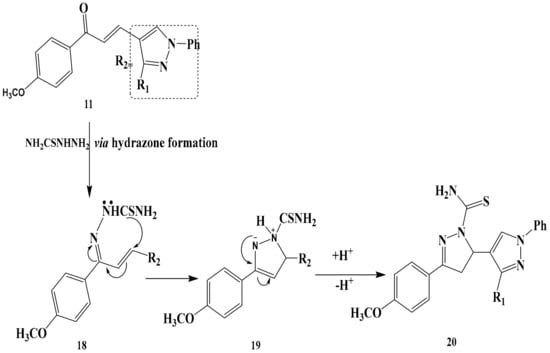
Scheme 7.
The reaction mechanism of chalcone 11 with thiosemicarbazid to form pyrazoline derivative 20.
2.2. Pharmacological Screening
2.2.1. α-Reductase Inhibitors
Circulating testosterone and dihydrotestosterone hormone levels or tissue concentrations were measured after the administration of 5α-reductase inhibitor radioimmuno assays. All new synthesized compounds 1–11, 13, 14, 17, and 20 were screened for their 5α-reductase inhibitor activity in vivo. All the tested compounds showed 5α-reductase inhibitor activities with good IC50 (µM); compounds (4–11, 13, 14, 17 and 20) indicated a significant activity compared to the control drug anastrozole. IC50 values were determined. From the results, it is evident that 12 of the investigated compounds have displayed a potent activity as a 5α-Reductase inhibitor (Table 1); the potency of the investigated compounds decreases in the order 13 > 8 > 11 > 20 > 17 > 14 > 7 > 4 > 10 > 6 = 9 > 5. Compound 13 was found to be the most potent 5α-Reductase inhibitor compared to the other tested compounds, and the standard drug anastrozole may be due to the presence of the ester group and pyridine ring. Compound 8 showed high activity may be due to the presence of the cyano group; also compound 11 giving high activity may be attributed to the presence of α–β unsaturated ketone; compound 20 giving high reactivity may be due to the thioamide group; compound 17 giving high activity may be due to the pyrazoline ring system; and compound 14 may be giving high activity due to the cyano-pyridine ring system. Compound 7 giving high activity may be due to the presence of pyrazole and pyrazolone ring systems; compound 4 showing high reactivity may be due to the presence of the pyrazole ring system and ester group; compound 10 giving high reactivity may be due to the presence of the thioprymidone ring system; the activity of compound 6 may be due to the presence of pyrazole ring system and methyl group. Also, the reactivity of compound 9 may be due to the presence of the pyrazole ring system. Finally compound 5 give inhibition activity may be due to the presence of two amino groups, also all the tested compounds have the 1,2,3-triazole moiety, which made them close in structure to that of the standard drug anastrozole. This is all in addition to their physical properties (low melting point, high solubility and small molecular weight), which may increase their reactivity either.

Table 1.
5α-Reductase inhibitors activities and acute toxicity of the studied compounds.
2.2.2. Aromatase Activity Assay
All synthesized compounds 1–11, 13, 14, 17, and 20 were tested for their aromatase inhibitor activity in vivo. From the results given in Table 2, it was found that nine of the investigated compounds (7–11, 13, 14, 17, and 20) have given a potent activity as aromatase inhibitors compared to the standard drug letrozole. Compound 13 was found to be the most potent aromatase inhibitor compared to the tested compounds, and the standard drug Letrazole may be due to the presence of the ester group and pyridine ring. Compound 8 showed high activity, which may be due to the presence of the cyano group; also, compound 11 showing high activity may be due to the presence of α-β unsaturated ketone; compound 20 showing high reactivity may be due to the thioamide group; and compound 17 showing high activity may be due to the pyrazoline ring system. In addition, compound 14 may have showed high activity due to the cyano-pyridine ring system. Compound 7 showing high activity may be due to the presence of pyrazole and pyrazolone ring systems, and compound 10 showing high reactivity may be due to the presence of the thioprymidone ring system. Also, the reactivity of compound 9 may be due to the presence of the pyrazole ring system. This is all in addition to their physical properties (low melting point, high solubility and small molecular weight), which may increase their reactivity.

Table 2.
Aromatase inhibitors’ activities of compounds 1–14, 17 and 20.
3. Experimental
3.1. General Procedures
All melting points were determined on an electrothermal apparatus and are uncorrected. IR spectra were recorded (KBr discs) on a Shimadzu FT-IR 8201 PC spectrophotometer. H1-NMR spectra were recorded in CDCl3 and (CD3)2SO solutions on a Varian Gemini 400 MHz spectrometer, and chemical shifts are expressed in δ ppm units using TMS as an internal reference. Mass spectra were recorded on a GC-MS QP1000 EX Shimadzu. Elemental analyses were carried out at the Microanalytical Center of Cairo University.
3.2. Synthesis of Compounds 2–4
General Producer
A mixture of 1 [27] (3.3 gm, 10 mmol) and the appropriate amount of malononitrile, acetylacetone and diethylmalonate (10 mmol) was heated under reflux in ethanol (20 mL) containing a catalytic amount of triethyamine (5–10 drops) for 3 h. The reaction mixture was cooled, and the solid formed was separated and recrystallized from the proper solvent to give the corresponding derivatives 2–4, respectively, in a good yield.
2-((3-(5-Methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)methylene)malononitrile (2): Yellow crystals from acetic acid in yield 93%, m.p. 200–202 °C. H1-NMR (400 MHz, DMSO-d6): δ = 2.52 (s, 3H, CH3), 6.37 (s, 1H, CH-pyrazole), 8.34 (s, 1H, CH-aliphatic), 7.33–7.94 (m, 10H, Ar-H). IR (KBr, cm−1): v 1620 (C=C), 1640 (C=N), 2222 v (CN), 2854, 2927 (CH-aliphatic). MS m/z (M+ 377,54%), (M+1, 378, 26%), (M+2, 379, 5%). Anal. Calcd. for C22H15N7 (377): C, 70.01; H, 4.01; N, 25.98; Found: C, 70.09; H, 4.08; N, 25.90%.
3-((3-(5-Methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)methylene)pentane-2,4-dione (3): Pale yellow crystals from acetic acid in yield 81%, m.p. 222–224 °C. H1-NMR (400 MHz, DMSO-d6): δ = 2.39 (s, 6H, 2CH3), 2.52 (s, 3H, CH3), 6.33 (s, 1H, CH-pyrazole), 8.34 (s, 1H, CH-aliphatic), 7.37–7.94 (m, 10H, Ar-H). IR (KBr, cm−1): v 1620 (C=C), 1639 (C=N), 1693 (C=O), 2927, 2974 (CH-aliphatic); MS m/z (M+ 411, 18%), (M+1, 412, 21%), (M+2, 413, 3%). Anal. Calcd. for C24H21N5O2 (411): C, 70.06; H, 5.14; N, 17.02; Found: C, 70.01; H, 5.10; N, 17.09%.
Diethyl2-((3-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)methylene) malonate (4): White crystals from ethanol in yield 87%, m.p. 210–212 °C. H1-NMR (400 MHz, DMSO-d6): δ = 1.24–129 (t, 6H, 2CH2CH3), 2.53(s, 3H, CH3), 4.16–4.23 (q, 4H, 2CH2CH3), 6.38 (s, 1H, CH-pyrazole), 8.33 (s, 1H, CH-aliphatic), 7.35–7.84 (m, 10H, Ar-H). IR (KBr, cm−1): v 1620 (C=C), 1632 (C=N), 1697 (C=O), 2862, 2927 (CH-aliphatic). MS: m/z (M+, 471, 23%), (M+1, 472, 6%), (M+2 473, 1%). Anal. Calcd. for C26H25N5O4 (471): C, 66.23; H, 5.34; N, 14.85; Found: C, 66.14; H, 5.25; N, 14.89%.
3.3. Synthesis of Compounds 5–7
General Producer
A mixture of the appropriate of compounds 2, 3 and 4 (5 mmol) and hydrazine hydrate (1 gm, 1 mL, 10 mmol) was heated under reflux in absolute ethanol (20 mL) for (3 h); the reaction mixture was cooled, and the solid formed was collected and recrystallized to afford the corresponding derivatives 5, 6 and 7, respectively.
4-((3-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)methylene)-4H-pyrazole-3,5-diamine (5): White crystals from ethanol in yield 78%, m.p. 240–242 °C. H1-NMR (400 MHz, DMSO-d6): δ = 2.53 (s, 3H, CH3), 5.70 (s, 4H, 2NH2), 6.39 (s, 1H, CH-pyrazole), 8.33 (s, 1H, CH-aliphatic), 7.35–7.84 (m, 10H, Ar-H). IR (KBr, cm−1): v 1608 (C=C), 1626 (C=N), 2862, 2927 (CH-aliphatic), 3310, 3244 (NH2); MS: m/z (M+ 409, 3%), (M+1, 410, 61%), (M+2, 411, 12%). Anal. Calcd. for C22H19N9 (409): C, 64.53; H, 4.68; N, 30.79; Found: C, 64.38; H, 4.55; N, 30.65%.
5-methyl-4-(4-((3,5-dimethyl-4H-pyrazol-4-ylidene)methyl)-1-phenyl-1H-pyrazol-3-yl)-1-phenyl-1H-1,2,3-triazole (6): White crystals from ethanol in yield 87%, m.p. 261–263 °C. H1-NMR (400 MHz, DMSO-d6): δ = 2.53 (s, 3H, CH3), 2.75 (s, 6H, 2CH3), 6.37 (s, 1H, CH-pyrazole), 8.35 (s, 1H, CH-aliphatic), 7.35–7.84 (m, 10H, Ar-H). IR (KBr, cm−1): v 1600 (C=C), 1620 (C=N), 2862, 2924 (CH-aliphatic) MS: m/z (M+ 407, 65%), (M+1 408, 12%), (M+2 409, 11%). Anal. Calcd. for C24H21N7 (407): C, 70.74; H, 5.19; N, 24.06; Found: C, 70.55; H, 5.11; N, 23.95%.
4-((3-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)methylene) pyrazolidine-3,5-dione (7): Grey crystals from ethanol in yield 82%, m.p. 282–284 °C. H1-NMR (400MHz, DMSO-d6): δ = 2.53 (s, 3H, CH3), 6.67 (s, 1H, CH-pyrazole), 8.35 (s, 1H, CH-aliphatic), 7.35–7.84 (m, 10H, Ar-H), 11.00 (s, 2H, 2NH). IR (KBr, cm−1): v 1600 (C=C), 1620 (C=N), 1640 (C=O), 2862, 2924 (CH-aliphatic), 3236 (NH). MS: m/z (M+, 411, 51%), (M+1, 412, 1.2%), (M+2, 413, 17%). Anal. Calcd. for C22H17N7O2 (411): C, 64.23; H, 4.16; N, 23.83; Found: C, 64.32; H, 4.09; N, 23.92%.
3.4. Synthesis of Compounds 8 and 9
General Producer
A mixture of compound number 1 (3.29 gm, 10 mmol) and the appropriate amount of 3-amino-4-cyano-1H-pyrazole and 3-amino-5-phenyl-1H-pyrazole was heated under reflux in acetic acid for 2 h. The solid formed through heating was separated and recrystallized from the appropriate solvent to give the corresponding compounds 8 and 9, respectively.
5-((3-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)methyleneamino)-1H-pyrazole-4-carbonitrile (8): Orange crystals from Dioxane in yield 91%, m.p. 161–163 °C. H1-NMR (400 MHz, DMSO-d6): δ = 2.53 (s, 3H, CH3), 6.32 (s, 1H, CH-pyrazole), 6.67 (s, 1H, CH-pyrazole), 7.35–7.84 (m, 10H, Ar-H), 8.50 (s, 1H, CH-aliphatic), 9.11 (s, 1H, NH). IR (KBr, cm−1): v 1600v (C=C), 1616 (C=N), 2222 (CN), 2858, 2974 (CH-aliphatic), 3105 (NH). MS: m/z (M+ 419, 21%), (M+1, 420, 15%), (M+2, 421, 4%). Anal. Calcd. for C23H17N9 (419): C, 65.86; H, 4.09; N, 30.05; Found: C, 65.92; H, 4.03; N, 30.01%.
N-((3-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)methylene)-3-phenyl-1H-pyrazol-5-amine (9): Beige crystals from Dioxane in yield 85%, m.p. 140–142 °C. H1-NMR (400 MHz, DMSO-d6): δ = 2.53 (s, 3H, CH3), 6.31 (s, 1H, CH-pyrazole), 6.68 (s, 1H, CH-pyrazole), 7.15–7.54 (m, 15H, Ar-H), 8.51 (s, 1H, CH-aliphatic), 9.11 (s, 1H, NH). IR (KBr, cm−1): v 1600 (C=C), 1616 (C=N), 2858, 2984 (CH-aliphatic), 3109 (NH). MS: m/z (M+, 470, 31%), (M+1, 471, 19%), (M+2, 472, 9%). Anal. Calcd. for C28H22N8 (470): C, 71.47; H, 4.71; N, 23.81; Found: C, 71.46; H, 4.72; N, 23.82%.
4-amino-1,6-dihydro-3-((3-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)methylene)-6-thioxopyridin-2(3H)-one (10): A mixture of compound number 1 (3.29, 10 mmol), ethylcyanoacetate (10 mmol) and thiourea (10 mmol) was heated under reflux in absolute ethanol (20 mL) containing a catalytic amount of hydrochloric acid (1 mL) for (15 h); the solid formed while heating was separated and recrystallized from dioxane as yellow crystals in 87% yield. m.p. 230–232 °C. H1-NMR (400 MHz, DMSO-d6): δ = 2.53 (s, 3H, CH3), 6.31 (s, 1H, CH-pyrazole), 6.82 (s, 2H, NH2), 7.5–7.8 (m, 10H, Ar-H), 8.21 (s, 1H, CH-aliphatic), 9.50 (s, 1H, NH). IR (KBr, cm−1): v 1400 (C=S), 1600 (C=C), 1620 (C=N), 1639 (C=O), 2852, 2984 (CH-aliphatic), 3228 (NH), 3317, 3348 (NH2). MS: m/z (M+, 454, 51%), (M+1, 455, 14%), (M+2, 456, 12%). Anal. Calcd. for C23H18N8OS (454): C, 60.78; H, 3.99; N, 24.65; Found: C, 60.76; H, 3.98; N, 24.64%.
1-(4-methoxyphenyl)-3-(3-(5-methyl-2-phenyl-2H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)prop-2-en-1-one (11): Sodium hydroxide (10 mL, 10%) was added dropwise to a mixture of compound number 3 (3.29, 10 mmol) and p-methoxy acetophenone (10 mmol) in ethanol (20 mL) while stirring at 0–5 °C. The stirring was continued for 2 h. The resulting solid was collected and recrystallized from ethanol to give compound number 11 as white crystals in 94% yield. m.p. 168–170 °C. H1-NMR (400 MHz, DMSO-d6): δ = 2.53 (s, 3H, CH3), 3.11 (s, 3H, OCH3), 6.51 (s, 1H, CH-pyrazole), 7.3–7.69 (m, 15H, Ar-H and CH=), 796–7.99 (d, 1H, J=12.0Hz, CH=). IR (KBr, cm−1): v 1662 (C=O), 2842, 2921 (CH-aliphatic). MS: m/z (M+, 461, 31%), (M+1, 462, 10%), (M+2, 463, 2%). Anal. Calcd. for C28H23N5O2 (461): C, 72.87; H, 5.02; N, 15.17; Found: C, 72.81; H, 5.06; N, 15.13%.
3.5. Synthesis of Compounds 13 and 14
General Producer
Method A: a mixture of the compound 11 (10 mmol) and the appropriate amount of ethyl cyanoacetate or malononitrile (10 mmol) and ammonium acetate (0.77 gm, 10 mmol) was heated under reflux in acetic acid (20 mL) for 5 h. On cooling, the separated solid was filtrated, washed with water and recrystallized from the proper solvent to give the corresponding compounds 13 and 14 respectively in a good yield.
Method B: A mixture of the compound 1 (10 mmol), p-methoxy acetophenone (10 mmol), the appropriate amount of ethyl cyanoacetate or malononitrile (10 mmol), and an excess amount of ammonium acetate (4 gm) was heated under reflux in n-butanol (20 mL) for 3 h. On cooling, the separated solid was filtrated, washed with water and recrystallized from the proper solvent to give compounds identical in all aspects (m.p., IR, Mass, NMR) with that obtained from method A.
Ethyl 2-amino-6-(4-methoxyphenyl)-4-(3-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)pyridine-3-carboxylate (13): Yellow crystals from ethanol in yield 73%, m.p. 250–252 °C. H1-NMR (400 MHz, DMSO-d6): δ = 1.02–1.21 (t, 3H, CH2CH3), 2.53 (s, 3H, CH3), 3.11 (s, 3H, OCH3), 3.90–4.12 (q, 2H, CH2CH3), 6.34 (s, 1H, CH-pyrazole), 7.07–7.67 (m, 14H, Ar-H), 6.15 (s, 2H, NH2), 8.50 (s, 1H, CH-pyridine). IR (KBr, cm−1): v 1590 (C=C), 1601 (C=N), 1740 (C=O), 3066, 3133 (NH2). MS: m/z (M+, 571, 12%), (M+1, 572, 3%). Anal. Calcd. for C33H29N7O3 (571): C, 69.34; H, 5.11; N,17.15; Found: C, 69.28; H, 5.03; N, 17.10%.
2-Amino-6-(4-methoxyphenyl)-4-(3-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)pyridine-3-carbonitrile (14): Yellow crystals from ethanol in yield 71%, m.p. 271–273 °C. H1-NMR (400 MHz, DMSO-d6): δ = 2.53 (s, 3H, CH3), 3.11 (s, 3H, OCH3), 6.11 (s, 2H, NH2), 6.34 (s, 1H, CH-pyrazole), 7.37–8.10 (m, 14H, Ar-H), 8.50 (s, 1H, CH-pyridine). IR (KBr, cm−1): v 1592 (C=C), 1610 (C=N), 2212 (CN), 3350, 3417 (NH2). MS: m/z (M+, 524, 36%). Anal. Calcd. for C31H24N8O (524): C, 70.98; H, 4.16; N, 21.36; Found: C,70.91; H, 4.12; N, 21.25%.
4-(4-(4,5-dihydro-3-(4-methoxyphenyl)-1H-pyrazol-5-yl)-1-phenyl-1H-pyrazol-3-yl)-5-methyl-1-phenyl-1H-1,2,3-triazole (17): A mixture of compound number 11 (5 mmol) and hydrazine hydrate (1 gm, 1 mL, 10 mmol) was heated under reflux in ethanol (10 mL) for 3 h. The reaction mixture was cooled and the resulting solid collected and recrystallized from ethanol as white crystals in yield 76%. Yellow crystals from ethanol in yield 71%, m.p. 291–293 °C. H1-NMR (400 MHz, DMSO-d6): δ = 2.53 (s, 3H, CH3), 3.11 (s, 3H, OCH3), 3.44 (dd, 1H, J = 18.1, 5.8Hz, CH2-pyrazole), 3.89 (dd, 1H, J = 18.1, 12Hz, CH2-pyrazole), 4.99 (dd, 1H, J = 12.2,5.8, CH-pyrazole), 6.34 (s, 1H, CH-pyrazole), 7.07–7.66 (m, 15H, Ar-H and NH). IR (KBr, cm−1): v 1597 (C=C), 1620 (C=N), 3311 (NH). MS: m/z (M+475, 22%), (M+2477, 14%). Anal. Calcd. for C28H25N7O (475): C,70.72; H, 5.30; N, 20.62; Found: C,70.72; H,5.29; N,20.61%.
4,5-Dihydro-3-(4-methoxyphenyl)-5-(3-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)pyrazole-1-carbothioamide (20): A mixture of 1-(4-methoxyphenyl)-3-(3-(5-methyl-2-phenyl-2H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)prop-2-en-1-one 11. (2.5 g, 5 mmol), thiosemicarbazide (0.46 g, 5 mmol) in ethanol (20 mL) containing a catalytic amount of hydrochloric acid (0.5 mL) was heated under reflux for 3 h. The resulting solid was collected and recrystallized from dioxane to give 20 as white crystals in Yield: 82%, m.p. 207–209 °C. H1-NMR (400 MHz, DMSO-d6): δ = 2.54 (s, 3H, CH3), 3.11 (s, 3H, OCH3) 3.40–3.47 (dd, 1H, J = 18.1, 5.8Hz, CH2-pyrazole), 3.74–3.82 (m, 2H, CH2-pyrazole), 4.61 (s, broad, 2H, NH2), 7.01–7.72 (m, 15H, Ar-H, CH-pyrazole). IR (KBr, cm−1): v 3475, 3436 (NH2), 2924, 2890 (CH-alphatic), 1562 (C=C), 1616 (C=N). MS: m/z (M+534, 96%), (M+1 535, 3%), (M+2 536, 11%). Anal. Calcd. for C29H26N8OS (534): C, 65.15; H, 4.90; N, 20.69; Found: C, 65.14; H, 4.91; N, 20.69%.
4. Biological Experiments
4.1. α-Reductase Inhibitors
4.1.1. Treatment of Animals
Animals were obtained from the animal house colony of the NRC Cairo Egypt. All animals were allowed free access to water and were kept on a constant standard diet. Nineteen Groups, each of 12 male Sprague–Dawley rats in the postnatal 3rd day were treated subcutaneously with the 5α-reductase inhibitor (tested compound or reference standard). The tested compounds were suspended in 5% Tween 80 in water. The vehicle was used for both the standard and negative control group, beginning on the postnatal 3rd day until the age of 7 weeks. Eighteen Groups were used to test the activities, of which one was used as the positive control for anastrozole and another served as the negative control group. After scarifying, blood was withdrawn for testosterone and dihydrotestosterone (DHT) determination [34]. Moreover, intraprostatic concentrations of testosterone and DHT were determined [35]. The biological experiments were performed according to the official standards.
4.1.2. Aromatase Activity Assay
Inhibitory potencies of compounds were determined according to an established procedure using a commercially available aromatase test kit from BD Gentest [36]. This fluorescence-based assay measures the rate at which recombinant human aromatase (baculovirus/insect cell-expressed) converts the substrate 7-methoxy-trifluoromethylcoumarin (MFC) into a fluorescent product 7-ethynyl-trifluoromethylcoumarin (HFC) (λex = 409 nm, λem = 530 nm) in a NADPH-regenerating system. Briefly, concentrated stock solutions of test compounds were prepared in acetonitrile.
One-hundred microliter samples containing serial dilutions of test compounds (dilution factor of 3 between samples) and cofactor mixture (0.4 U/mL glucose-6-phosphate dehydrogenase; 16.2 µM NADP+; 825 µM MgCl2; 825 µM glucose -6-phosphate; 50 µM citrate buffer, pH 7.5) were prepared in a 96-well plate. After incubating the plate for 10 min at 37 °C, 100 µL of an aromatase/P450 reductase/substrate solution (105 µg protein/mL enzyme; 50 µM MFC; 20 mM phosphate buffer, pH 7.4) were added to each well. The plate was covered and incubated for 30 min at 37 °C. Seventy-five microliters of 0.5 M Tris base were then added to stop the reaction, and the fluorescence of the formed de-methylated MFC was measured with a plate reader (SpectraMax Gemini, Molecular Devices).
Fluorescence intensities, which were proportional to the amount of reaction product generated by aromatase, were graphed as a function of inhibitor concentration and then fit to a three-parameter logistic function. Inhibitory potencies were expressed in terms of the IC50 value, the inhibitor concentration necessary to reduce the enzyme activity by half. Each experiment was performed at least in triplicate.
5. Conclusions
Results of the present study demonstrate that compound 1 reacts with different active methylene and amino pyrazoles to yield a new series of pyrazole derivatives derived from 1,2,3-triazole derivative. Newly synthesized compounds 1–11, 13, 14, 17, and 20 were evaluated in vivo as 5α-reductase and aromatase inhibitors; most of the tested compounds exhibited more potent activity than the standard references drugs. Compound 13 has the highest potency amongst the tested compounds and the reference drugs, and this may be due to the presence of the pyridine moiety linked with pyrazoline and 1,2,3-trizole moieties. The combination between 1,2,3-triazole and pyrazole was found to be essential for 5α-reductase and aromatase inhibitor activities. Future work will involve the design of steroidal molecules of such features.
Author Contributions
H.R.M.R., M.E.-N., A.S.A.E.-A. and S.I.A.E.-N. and. designed and synthesized the new organic compounds and they confirmed the chemical composition of these compounds using physical and chemical analysis. M.M.A. evaluated the enzyme inhibition potency of the newly synthesized compounds. All authors analyzed the data and wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interests.
References
- Zhang, Z.; Wang, X.; Zhao, X.; Liu, B.; Yi, L.; Zuo, L.; Wen, Q.; Liu, F.; Xu, J.; Hu, S. A 2010 update of National Land Use/Cover Database of China at 1: 100000 scale using medium spatial resolution satellite images. Remote Sens. Environ. 2014, 149, 142–154. [Google Scholar] [CrossRef]
- Shoombuatong, W.; Prachayasittikul, V.; Prachayasittikul, V.; Nantasenamat, C. Prediction of aromatase inhibitory activity using the efficient linear method (ELM). Excli J. 2015, 14, 452. [Google Scholar] [PubMed]
- Njar, V.C.; Kato, K.; Nnane, I.P.; Grigoryev, D.N.; Long, B.J.; Brodie, A.M. Novel 17-azolyl steroids, potent inhibitors of human cytochrome 17α-hydroxylase-C17, 20-lyase (P45017α): Potential agents for the treatment of prostate cancer. J. Med. Chem. 1998, 41, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Pet, C.; Batta, G.; Györgydeák, Z.; Sztaricskai, F. Glycoside Synthesis with Anomeric 1-N-Glycobiosyl-1, 2, 3-triazoles1. J. Carbohydr. Chem. 1996, 15, 465–483. [Google Scholar]
- Rashdan, H.R.; Gomha, S.M.; El-Gendey, M.S.; El-Hashash, M.A.; Soliman, A.M.M. Eco-friendly one-pot synthesis of some new pyrazolo [1, 2-b] phthalazinediones with antiproliferative efficacy on human hepatic cancer cell lines. Green Chem. Lett. Rev. 2018, 11, 264–274. [Google Scholar] [CrossRef]
- Rashdan, H.R.; Farag, M.M.; El-Gendey, M.S.; Mounier, M.M. Toward Rational Design of Novel Anti-Cancer Drugs Based on Targeting, Solubility, and Bioavailability Exemplified by 1, 3, 4-Thiadiazole Derivatives Synthesized Under Solvent-Free Conditions. Molecules 2019, 24, 2371. [Google Scholar] [CrossRef]
- Khatri, P.; Yeatts, S.D.; Mazighi, M.; Broderick, J.P.; Liebeskind, D.S.; Demchuk, A.M.; Amarenco, P.; Carrozzella, J.; Spilker, J.; Foster, L.D. Time to angiographic reperfusion and clinical outcome after acute ischaemic stroke: An analysis of data from the Interventional Management of Stroke (IMS III) phase 3 trial. Lancet Neurol. 2014, 13, 567–574. [Google Scholar] [CrossRef]
- Oishi, T.; Koyama, K.; Alam, S.; Tanaka, M.; Lee, J.-C. Recovery of high purity copper cathode from printed circuit boards using ammoniacal sulfate or chloride solutions. Hydrometallurgy 2007, 89, 82–88. [Google Scholar] [CrossRef]
- Polkam, N.; Rayam, P.; Anireddy, J.S.; Yennam, S.; Anantaraju, H.S.; Dharmarajan, S.; Perumal, Y.; Kotapalli, S.S.; Ummanni, R.; Balasubramanian, S. Synthesis, in vitro anticancer and antimycobacterial evaluation of new 5-(2, 5-dimethoxyphenyl)-1, 3, 4-thiadiazole-2-amino derivatives. Bioorganic Med. Chem. Lett. 2015, 25, 1398–1402. [Google Scholar] [CrossRef]
- Malladi, S.; Isloor, A.M.; Isloor, S.; Akhila, D.; Fun, H.-K. Synthesis, characterization and antibacterial activity of some new pyrazole based Schiff bases. Arab. J. Chem. 2013, 6, 335–340. [Google Scholar] [CrossRef]
- Gallardo, A.; Marui, A. Modeling the dynamics of the freshwater-saltwater interface in response to construction activities at a coastal site. Int. J. Environ. Sci. Technol. 2007, 4, 285–294. [Google Scholar] [CrossRef]
- Helser, T.E.; Stewart, I.; Fleischer, G.; Martell, S. Stock assessment of Pacific hake (whiting) in US and Canadian waters in 2006. Pac. Fish. Manag. Counc. 2006, 7700, 97220-1384. [Google Scholar]
- Merolla, P.; Arthur, J.; Akopyan, F.; Imam, N.; Manohar, R.; Modha, D.S. A digital neurosynaptic core using embedded crossbar memory with 45pJ per spike in 45nm. In Proceedings of the 2011 IEEE custom integrated circuits conference (CICC), San Jose, CA, USA, 19–21 September 2011; pp. 1–4. [Google Scholar]
- Madamba, P.S.; Driscoll, R.H.; Buckle, K.A. The thin-layer drying characteristics of garlic slices. J. Food Eng. 1996, 29, 75–97. [Google Scholar] [CrossRef]
- Ribeiro, K.L.; Shaban, Y.R. Theoretical Studies no Laser Pumping for the 1.15 {mu} m Wavelength from the He {sup 3}(n, p) H {sup 3} Reaction; Estudos Teoricos do Bombeamento laser Para o Comprimento de onda 1.15 {mu} ma Partir da Reacao He {sup 3}(n, p) H {sup 3}; Associacao Brasileira de Energia Nuclear: Rio de Janeiro, RJ, Brazil, 1997. [Google Scholar]
- Kume, S.; Muto, A.; Aruga, J.; Nakagawa, T.; Michikawa, T.; Furuichi, T.; Nakade, S.; Okano, H.; Mikoshiba, K. The Xenopus IP3 receptor: Structure, function, and localization in oocytes and eggs. Cell 1993, 73, 555–570. [Google Scholar] [CrossRef]
- Moltzen, E.K.; Pedersen, H.; Boegesoe, K.P.; Meier, E.; Frederiksen, K.; Sanchez, C.; Lemboel, H.L. Bioisosteres of arecoline: 1, 2, 3, 6-tetrahydro-5-pyridyl-substituted and 3-piperidyl-substituted derivatives of tetrazoles and 1, 2, 3-triazoles. Synthesis and muscarinic activity. J. Med. Chem. 1994, 37, 4085–4099. [Google Scholar] [CrossRef] [PubMed]
- Shaban, M.A.; Nasr, A.Z. The chemistry of C-nucleosides and their analogs I: C-nucleosides of hetero monocyclic bases. Adv. Heterocycl. Chem. 1997, 68, 225. [Google Scholar]
- Hugelius, G.; Strauss, J.; Zubrzycki, S.; Harden, J.W.; Schuur, E.; Ping, C.-L.; Schirrmeister, L.; Grosse, G.; Michaelson, G.J.; Koven, C.D. Estimated stocks of circumpolar permafrost carbon with quantified uncertainty ranges and identified data gaps. Biogeosciences (Online) 2014, 11. [Google Scholar] [CrossRef]
- Khloya, P.; Kumar, P.; Mittal, A.; Aggarwal, N.K.; Sharma, P.K. Synthesis of some novel 4-arylidene pyrazoles as potential antimicrobial agents. Org. Med. Chem. Lett. 2013, 3, 9. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, X.; Nepovimova, E.; Miron, A.; Liu, Q.; Wang, Y.; Su, D.; Yang, H.; Li, L.; Kuca, K. Trichothecenes: Immunomodulatory effects, mechanisms, and anti-cancer potential. Arch. Toxicol. 2017, 91, 3737–3785. [Google Scholar] [CrossRef]
- Bouazzi, H. Contribution à l’identification de nouveaux gènes impliqués dans la Déficience intellectuelle liée au Sexe (X-LID) par séquençage à haut débit de l’exome du chromosome X avec la technologie SOLiD; Inserm: Paris, France, 2016. [Google Scholar]
- El-Hashash, M.A.; Sherif, S.M.; Badawy, A.A.; Rashdan, H.R. Facial synthesis of some new pyrazolopyridine, barbituric and thiobarbituric acid derivatives with antimicrobial activities. Inter. J. Adv. Res 2014, 2, 900–913. [Google Scholar]
- El-Hashash, M.A.; Sherif, S.M.; Badawy, A.A.; Rashdan, H.R. Synthesis of some new antimicrobial 5, 6, 7, 8-tetrahydro-pyrimido [4, 5-b] quinolone derivatives. Der Pharm. Chem 2014, 6, 23–29. [Google Scholar]
- Rashdan, H.R.; Roaiah, H.M.; Muhammad, Z.A.; Wietrzyk, J.; Milczarek, M.; Soliman, A.M. Design, efficient synthesis, mechanism of reaction and antiproliferative activity against cancer and normal cell lines of a novel class of fused pyrimidine derivatives. Acta Pol. Pharm. 2018, 75, 679–688. [Google Scholar]
- El-Hashash, M.A.; Sherif, S.M.; Badawy, A.A.; Rashdan, H.R. Synthesis of Potent Antimicrobial Pyrrole Derivatives. Int. J. 2014, 2, 1022–1035. [Google Scholar]
- Abdelhamid, A.O.; Abdel-Riheem, N.A.; El-Idreesy, T.T.; Rashdan, H.R. Synthesis of some new azolotriazine, 4-arylazopyrazole and pyridine derivatives containing 1, 2, 3-triazole moiety. Int. J. 2013, 1, 729–745. [Google Scholar]
- Rashdan, H.R.; Abdel-Aziem, A.; El-Naggar, D.H.; Nabil, S. synthesis and biological evaluation of some new pyridines, isoxazoles and isoxazolopyridazines bearing 1, 2, 3-triazole moiety. Acta Pol. Pharm. 2019, 76, 469–482. [Google Scholar]
- El-Naggar, M.; Mohamed, M.E.; Mosallam, A.M.; Salem, W.; Rashdan, H.R.; Abdelmonsef, A.H. Synthesis, Characterization, Antibacterial Activity, and Computer-Aided Design of Novel Quinazolin-2, 4-dione Derivatives as Potential Inhibitors Against Vibrio cholerae. Evol. Bioinform. 2020, 16, 1176934319897596. [Google Scholar] [CrossRef]
- Elnaggar, D.H.; Abdel Hafez, N.A.; Rashdan, H.R.; Abdelwahed, N.A.; Awad, H.M.; Ali, K.A. Synthesis, Antimicrobial and Antitumor Evaluations of a New Class of Thiazoles Substituted on the Chromene Scaffold. Mini Rev. Med. Chem. 2019, 19, 1717–1725. [Google Scholar] [CrossRef]
- Rashdan, H.; Nasr, S.; El-Refai, H.; Abdel-Aziz, M. A novel approach of potent antioxidant and antimicrobial agents containing coumarin moiety accompanied with cytotoxicity studies on the newly synthesized derivatives. J. Appl. Pharm. Sci. 2017, 7, 186–196. [Google Scholar]
- Anniyappan, M.; Muralidharan, D.; Perumal, P.T. Synthesis of Hantzsch 1, 4-dihydropyridines under microwave irradiation. Synth. Commun. 2002, 32, 659–663. [Google Scholar] [CrossRef]
- Shepard, R.N. Attention and the metric structure of the stimulus space. J. Math. Psychol. 1964, 1, 54–87. [Google Scholar] [CrossRef]
- George, A. Western State Terrorism; Polity Press Cambridge: Cambridge, UK, 1991. [Google Scholar]
- Lo, D.C.; McAllister, A.K.; Katz, L.C. Neuronal transfection in brain slices using particle-mediated gene transfer. Neuron 1994, 13, 1263–1268. [Google Scholar] [CrossRef]
- Das, S.; Gautam, N.; Dey, S.K.; Maiti, T.; Roy, S. Oxidative stress in the brain of nicotine-induced toxicity: Protective role of Andrographis paniculata Nees and vitamin E. Appl. Physiol. Nutr. Metab. 2009, 34, 124–135. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).