Simultaneous Study of Anti-Ferroptosis and Antioxidant Mechanisms of Butein and (S)-Butin
Abstract
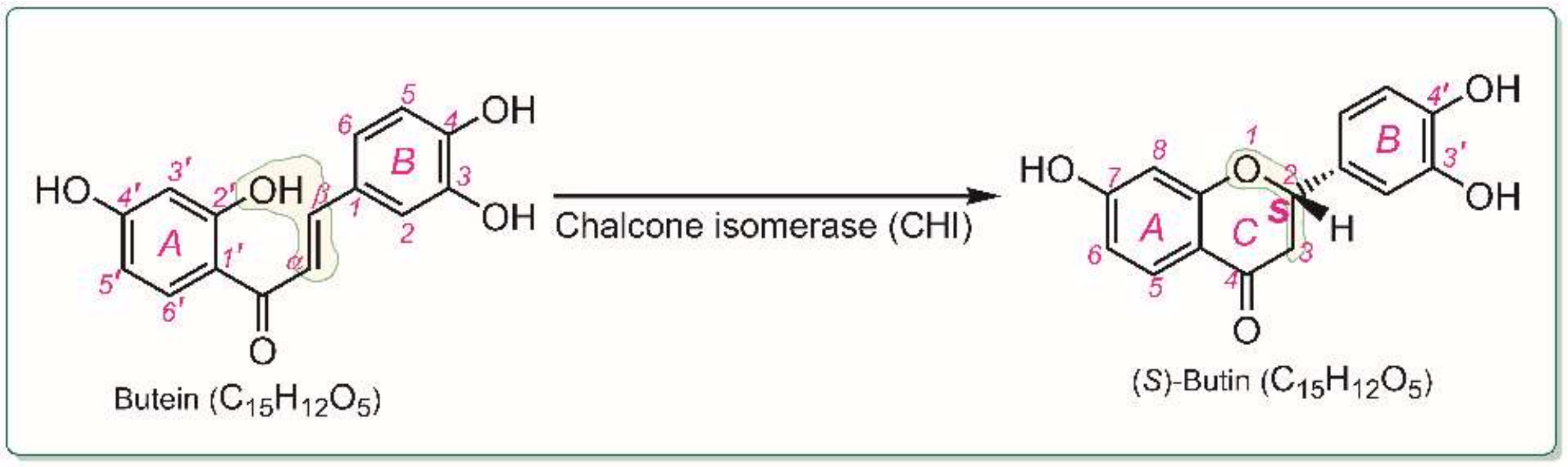
1. Introduction
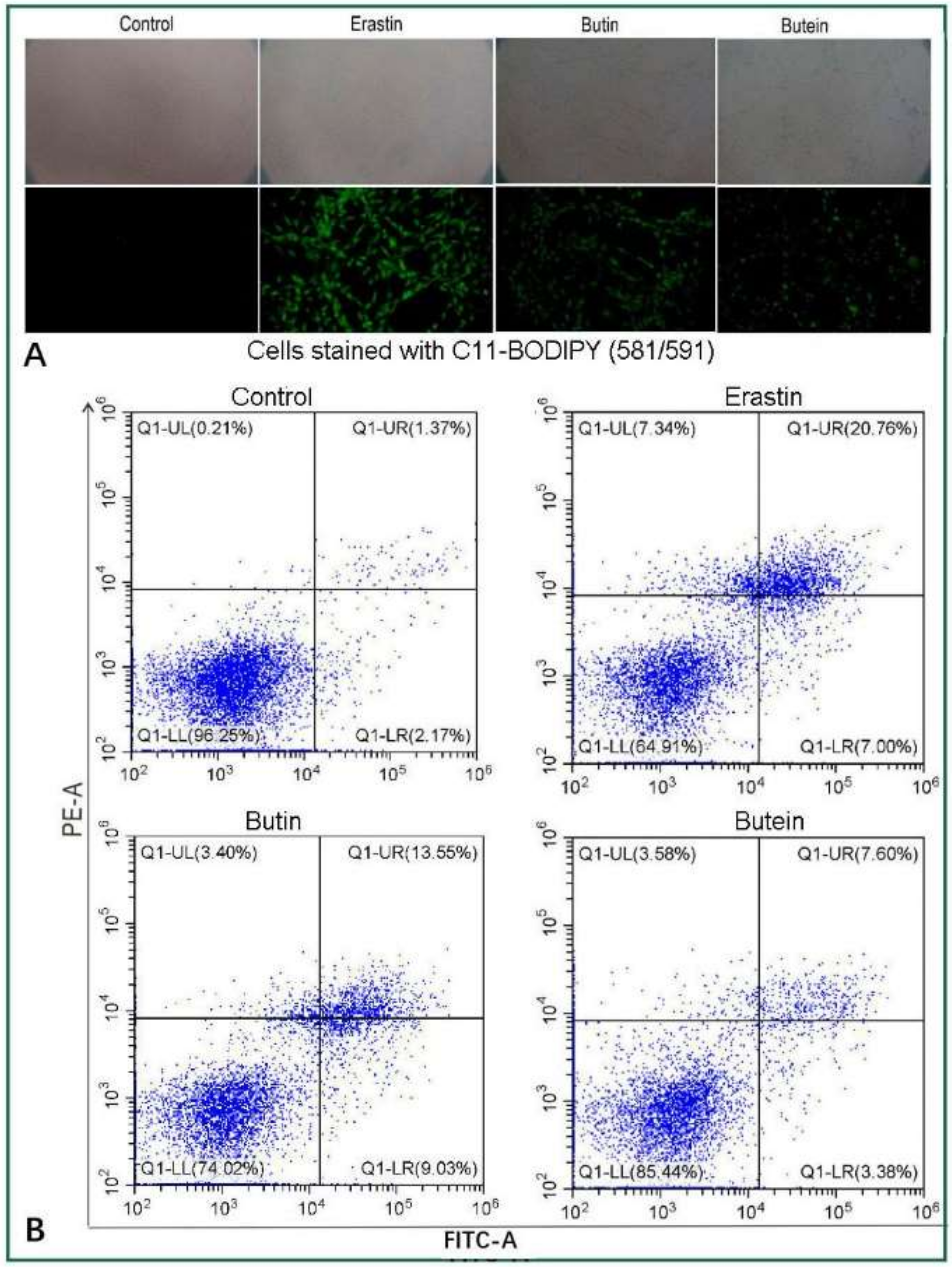
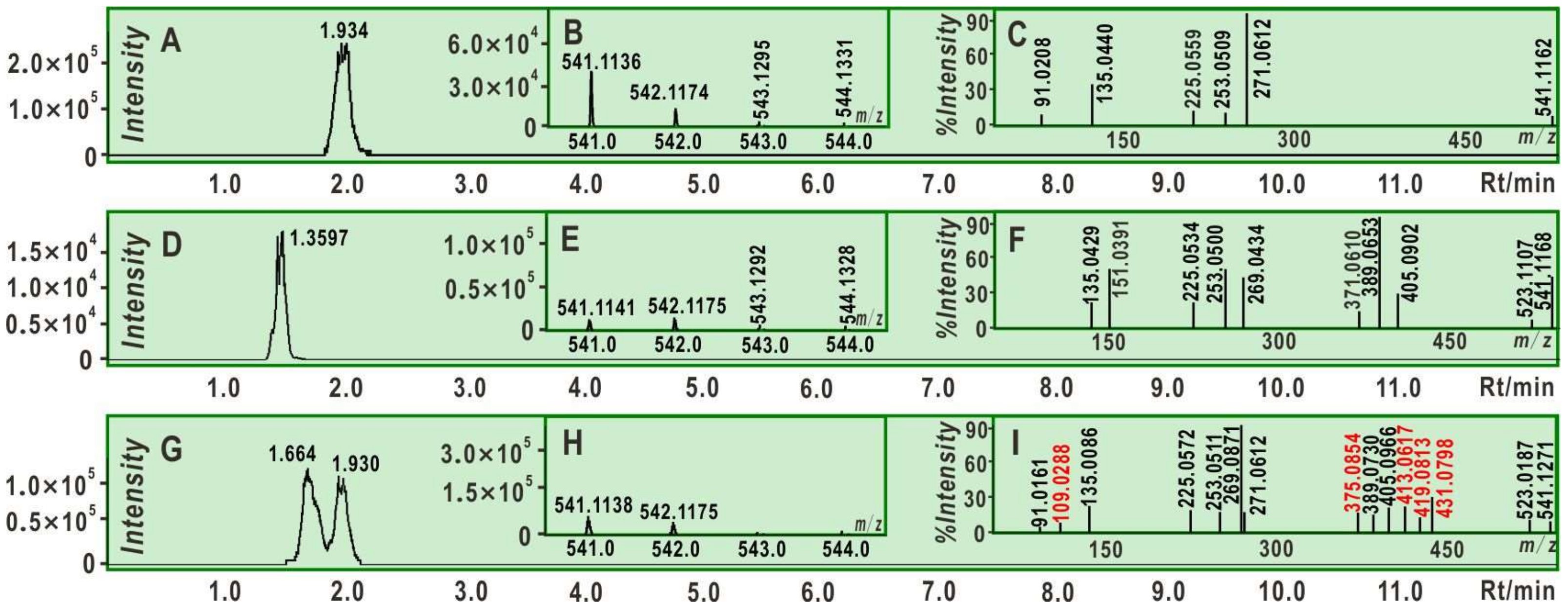
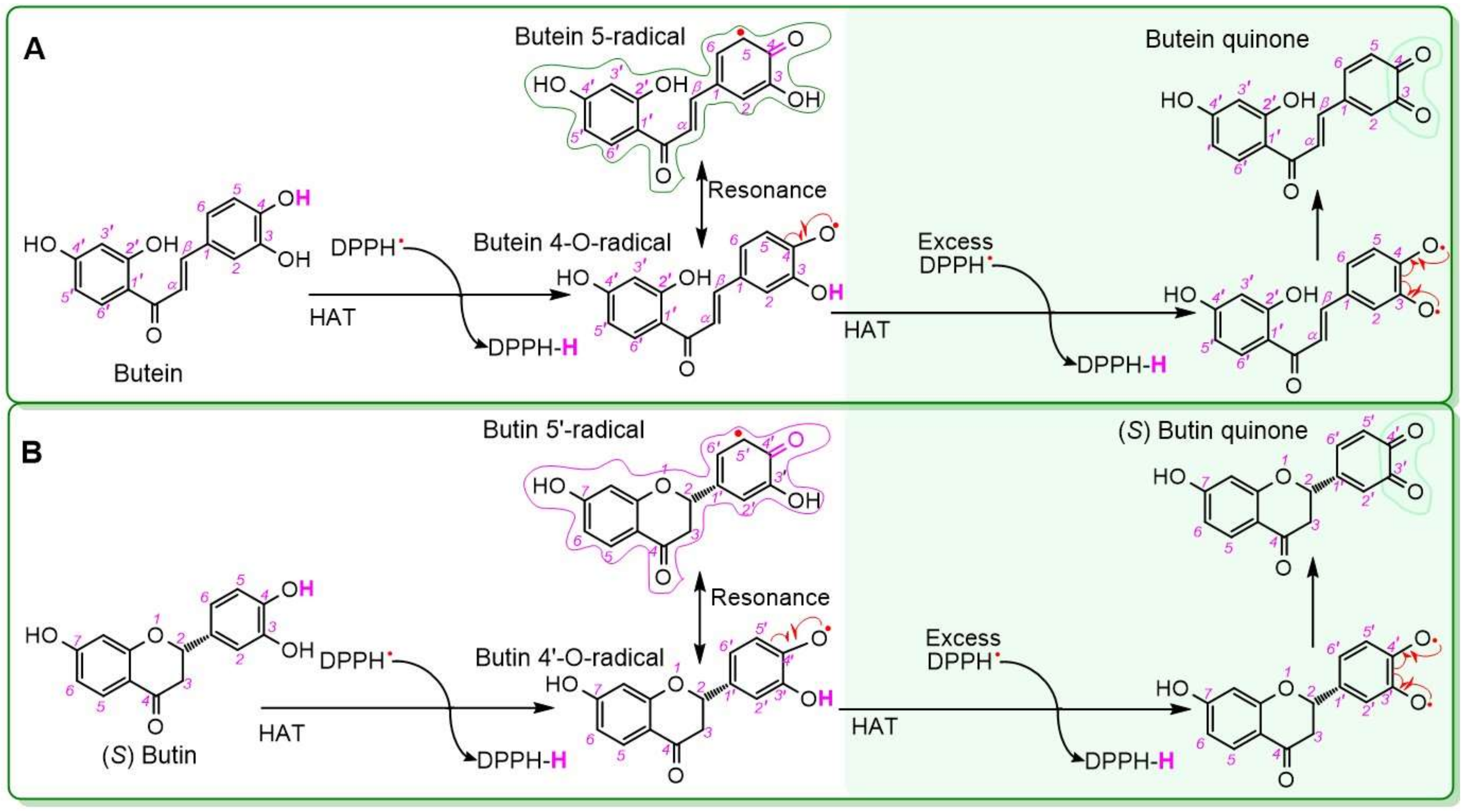
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals, Animals, and Biological Kits
3.2. Extraction and Culture of Bone Marrow-Derived Mesenchymal Stem Cells (BMSCs)
3.3. Prevention of Erastin-Induced Ferroptosis in BMSCs
3.4. Linoleic Acid Emulsion Assay
3.5. CUPRAC Assay
3.6. FRAP Assay
3.7. PTIO• Radical-Trapping Assay
3.8. DPPH• Radical-Trapping Assay
3.9. UPLC-ESI-Q-TOF-MS Analysis of RAF Products of Two Isomers Interacting with DPPH•
3.10. Preferential Conformation Analysis by Computational Chemistry and Molecular Weight Calculation
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| bMSCs | bone marrow-derived mesenchymal stem cells |
| CHI | Chalcone isomerase |
| CHS | Chalcone synthase |
| CUPRAC | Cu2+-reducing antioxidant power |
| C11-BODIPY | C11-BODIPY (581-591) |
| DPPH | α,α-diphenyl-β-picrylhydrazyl radical |
| ET | electron transfer |
| FBS | Fetal bovine serum |
| FRAP | Fe3+-reducing antioxidant power |
| GPx 4 | glutathione peroxidase 4 |
| HAT | hydrogen atom transfer |
| LOO | lipid peroxide radicals |
| LPO | lipid peroxides |
| PI | propidium iodide |
| PT | proton transfer |
| PTIO | 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide radical |
| RAF | radical adduct formation |
| ROS | reactive oxygen species |
| SD | standard deviation |
| SPSS | statistical product and service solutions |
| TPTZ | 2,4,6-Tripyridyl triazine |
| UPLC−ESI−Q−TOF−MS | ultra-performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry |
References
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Gaschler, M.M.; Andia, A.A.; Liu, H.; Csuka, J.M.; Hurlocker, B.; Vaiana, C.A.; Heindel, D.W.; Zuckerman, D.S.; Bos, P.H.; Reznik, E.; et al. FINO2 initiates ferroptosis through GPX4 inactivation and iron oxidation. Nat. Chem. Biol. 2018, 14, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Minikes, A.M.; Gao, M.; Bian, H.; Li, Y.; Stockwell, B.R.; Chen, Z.N.; Jiang, X. Intercellular interaction dictates cancer cell ferroptosis via NF2-YAP signaling. Nature 2019, 572, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Green, M.; Choi, J.E.; Gijon, M.; Kennedy, P.D.; Johnson, J.K.; Liao, P.; Lang, X.; Kryczek, I.; Sell, A.; et al. CD8(+) T cells regulate tumour ferroptosis during cancer immunotherapy. Nature 2019, 569, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascon, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef]
- Cong, L.; Dong, X.; Wang, Y.; Deng, Y.; Li, B.; Dai, R. On the role of synthesized hydroxylated chalcones as dual functional amyloid-beta aggregation and ferroptosis inhibitors for potential treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2019, 166, 11–21. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, Y.; Wang, G.; Hill, L.; Weng, J.; Chen, X.; Xue, H.; Martin, C. A specialized flavone biosynthetic pathway has evolved in the medicinal plant, Scutellaria baicalensis. Sci. Adv. 2016, 2, e1501780. [Google Scholar] [CrossRef]
- Li, X. Antioxidant Change in Biosynthesis from Naringenin Chalcone to Flavonoid Apingenin. ChemistrySelect 2019, 4, 5155–5159. [Google Scholar]
- Ayabe, S.; Uchiyama, H.; Aoki, T.; Akash, T. Plant Phenolics: Phenylpropanoids. In Comprehensive Natural Products; Elsevier Science: Amsterdam, The Netherlands, 2010; pp. 928–966. [Google Scholar]
- Qin, H.L.; Yu, D.Q. 1H-NMR Spectroscopic Databook of Natural Products; Chemical Industry Press: Beijing, China, 2011. [Google Scholar]
- Yang, J.S. 13C-NMR Spectroscopic Databook of Natural Products; Chemical Industry Press: Beijing, China, 2011. [Google Scholar]
- Chokchaisiri, R.; Suaisom, C.; Sriphota, S.; Chindaduang, A.; Chuprajob, T.; Suksamrarn, A. Bioactive flavonoids of the flowers of Butea monosperma. Chem. Pharm. Bull. (Tokyo) 2009, 57, 428–432. [Google Scholar] [CrossRef]
- Jeon, W.K.; Lee, J.H.; Kim, H.K.; Lee, A.Y.; Lee, S.O.; Kim, Y.S.; Ryu, S.Y.; Kim, S.Y.; Lee, Y.J.; Ko, B.S. Anti-platelet effects of bioactive compounds isolated from the bark of Rhus verniciflua Stokes. J. Ethnopharmacol. 2006, 106, 62–69. [Google Scholar] [CrossRef]
- Roux, D.G.; Paulus, E. Condensed tannins. Isolation of (-)-butin and butein from wattle heartwoods. Biochem. J. 1961, 80, 62–63. [Google Scholar] [CrossRef] [PubMed]
- Antal, D.S.; Schwaiger, S.; Ellmerer-Muller, E.P.; Stuppner, H. Cotinus coggygria wood: Novel flavanone dimer and development of an HPLC/UV/MS method for the simultaneous determination of fourteen phenolic constituents. Planta. Med. 2010, 76, 1765–1772. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Wang, B.; Yuk-Wai Lee, W.; Pong, U.K.; Leung, K.T.; Li, X.; Liu, Z.; Chen, R.; Lin, J.C.; Tsang, L.L.; et al. KDM3A and KDM4C Regulate Mesenchymal Stromal Cell Senescence and Bone Aging via Condensin-mediated Heterochromatin Reorganization. iScience 2019, 21, 375–390. [Google Scholar] [CrossRef] [PubMed]
- Leu, J.I.; Murphy, M.E.; George, D.L. Mechanistic basis for impaired ferroptosis in cells expressing the African-centric S47 variant of p53. Proc. Natl. Acad. Sci. USA 2019, 116, 8390–8396. [Google Scholar] [CrossRef] [PubMed]
- Doll, S.; Freitas, F.P.; Shah, R.; Aldrovandi, M.; da Silva, M.C.; Ingold, I.; Grocin, A.G.; Xavier da Silva, T.N.; Panzilius, E.; Scheel, C.; et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature 2019. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.H.; Cui, C.C.; Shan, C.; Li, Y.Z.; Sheng, D.H.; Sun, B.; Chen, D.Z. O-Phenylenediamine: A privileged pharmacophore of ferrostatins for radical-trapping reactivity in blocking ferroptosis. Org. Biomol. Chem. 2018, 16, 3952–3960. [Google Scholar] [CrossRef]
- Devisscher, L.; Van Coillie, S.; Hofmans, S.; Van Rompaey, D.; Goossens, K.; Meul, E.; Maes, L.; De Winter, H.; Van Der Veken, P.; Vandenabeele, P.; et al. Discovery of Novel, Drug-Like Ferroptosis Inhibitors with in Vivo Efficacy. J. Med. Chem. 2018, 61, 10126–10140. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, H.; Daniels, J.D.; Zandkarimi, F.; Liu, H.; Brown, L.M.; Uchida, K.; O’Connor, O.A.; Stockwell, B.R. Imidazole Ketone Erastin Induces Ferroptosis and Slows Tumor Growth in a Mouse Lymphoma Model. Cell Chem. Biol. 2019, 26, 623–633. [Google Scholar] [CrossRef]
- Chu, B.; Kon, N.; Chen, D.; Li, T.; Liu, T.; Jiang, L.; Song, S.; Tavana, O.; Gu, W. ALOX12 is required for p53-mediated tumour suppression through a distinct ferroptosis pathway. Nat. Cell Biol. 2019, 21, 579–591. [Google Scholar] [CrossRef]
- Hambright, W.S.; Fonseca, R.S.; Chen, L.; Na, R.; Ran, Q. Ablation of ferroptosis regulator glutathione peroxidase 4 in forebrain neurons promotes cognitive impairment and neurodegeneration. Redox Biol. 2017, 12, 8–17. [Google Scholar] [CrossRef]
- Cozzi, A.; Orellana, D.I.; Santambrogio, P.; Rubio, A.; Cancellieri, C.; Giannelli, S.; Ripamonti, M.; Taverna, S.; Di Lullo, G.; Rovida, E.; et al. Stem Cell Modeling of Neuroferritinopathy Reveals Iron as a Determinant of Senescence and Ferroptosis during Neuronal Aging. Stem. Cell Reports 2019. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Wang, N.; Liu, N.; Dong, H. Lipid Peroxidation and GPX4 Inhibition Are Common Causes for Myofibroblast Differentiation and Ferroptosis. DNA Cell Biol. 2019, 38, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Doll, S.; Proneth, B.; Tyurina, Y.Y.; Panzilius, E.; Kobayashi, S.; Ingold, I.; Irmler, M.; Beckers, J.; Aichler, M.; Walch, A.; et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 2017, 13, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Galadari, S.; Rahman, A.; Pallichankandy, S.; Thayyullathil, F. Reactive oxygen species and cancer paradox: To promote or to suppress? Free Radic. Biol. Med. 2017, 104, 144–164. [Google Scholar] [CrossRef]
- Song, X.; Xie, Y.; Kang, R.; Hou, W.; Sun, X.; Epperly, M.W.; Greenberger, J.S.; Tang, D. FANCD2 protects against bone marrow injury from ferroptosis. Biochem. Biophys. Res. Commun. 2016, 480, 443–449. [Google Scholar] [CrossRef]
- Shah, R.; Margison, K.; Pratt, D.A. The Potency of Diarylamine Radical-Trapping Antioxidants as Inhibitors of Ferroptosis Underscores the Role of Autoxidation in the Mechanism of Cell Death. ACS Chem. Biol. 2017, 12, 2538–2545. [Google Scholar] [CrossRef]
- Shintoku, R.; Takigawa, Y.; Yamada, K.; Kubota, C.; Yoshimoto, Y.; Takeuchi, T.; Koshiishi, I.; Torii, S. Lipoxygenase-mediated generation of lipid peroxides enhances ferroptosis induced by erastin and RSL. Cancer Sci. 2017, 108, 2187–2194. [Google Scholar] [CrossRef]
- Alim, I.; Caulfield, J.T.; Chen, Y.; Swarup, V.; Geschwind, D.H.; Ivanova, E.; Seravalli, J.; Ai, Y.; Sansing, L.H.; Ste Marie, E.J.; et al. Selenium Drives a Transcriptional Adaptive Program to Block Ferroptosis and Treat Stroke. Cell 2019, 177, 1262–1279. [Google Scholar] [CrossRef]
- Wang, X.; Ma, S.; Yang, B.; Huang, T.; Meng, N.; Xu, L.; Xing, Q.; Zhang, Y.; Zhang, K.; Li, Q.; et al. Resveratrol promotes hUC-MSCs engraftment and neural repair in a mouse model of Alzheimer’s disease. Behav. Brain. Res. 2018, 339, 297–304. [Google Scholar] [CrossRef]
- Pisati, F.; Bossolasco, P.; Meregalli, M.; Cova, L.; Belicchi, M.; Gavina, M.; Marchesi, C.; Calzarossa, C.; Soligo, D.; Lambertenghi-Deliliers, G.; et al. Induction of neurotrophin expression via human adult mesenchymal stem cells: Implication for cell therapy in neurodegenerative diseases. Cell Transplant. 2007, 16, 41–55. [Google Scholar] [CrossRef]
- Greene, L.E.; Lincoln, R.; Cosa, G. Rate of Lipid Peroxyl Radical Production during Cellular Homeostasis Unraveled via Fluorescence Imaging. J. Am. Chem. Soc. 2017, 139, 15801–15811. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Farmer, L.A.; Zilka, O.; Van Kessel, A.T.M.; Pratt, D.A. Beyond DPPH: Use of Fluorescence-Enabled Inhibited Autoxidation to Predict Oxidative Cell Death Rescue. Cell Chem. Biol. 2019, 26, 1594–1607. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Peng, Y.; Xie, Y.; Zhou, B.; Sun, X.; Kang, R.; Tang, D. Antiferroptotic activity of non-oxidative dopamine. Biochem. Biophys. Res. Commun. 2016, 480, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Suemori, Y.; Nagata, M.; Kondo, M.; Ishigure, S.; Dewa, T.; Ohtsuka, T.; Nango, M. Phospholipid-linked quinones-mediated electron transfer on an electrode modified with lipid bilayers. Colloids. Surf. B Biointerfaces 2008, 61, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Smirnov, A.Y.; Mourokh, L.G.; Nori, F. Electrostatic models of electron-driven proton transfer across a lipid membrane. J. Phys. Condens. Matter. 2011, 23, 234101. [Google Scholar] [CrossRef]
- Limburg, B.; Laisne, G.; Bouwman, E.; Bonnet, S. Enhanced photoinduced electron transfer at the surface of charged lipid bilayers. Chemistry 2014, 20, 8965–8972. [Google Scholar] [CrossRef]
- Enomoto, T.; Brea, R.J.; Bhattacharya, A.; Devaraj, N.K. In Situ Lipid Membrane Formation Triggered by Intramolecular Photoinduced Electron Transfer. Langmuir 2018, 34, 750–755. [Google Scholar] [CrossRef]
- Wang, X.S.; Stocker, R. Detection of specifically oxidized apolipoproteins in oxidized HDL. Methods Mol. Biol. 2008, 477, 49–63. [Google Scholar]
- Lin, J.; Li, X.; Chen, B.; Wei, G.; Chen, D. E-Configuration Improves Antioxidant and Cytoprotective Capacities of Resveratrols. Molecules 2018, 23. [Google Scholar] [CrossRef]
- Cekic, S.D.; Baskan, K.S.; Tutem, E.; Apak, R. Modified cupric reducing antioxidant capacity (CUPRAC) assay for measuring the antioxidant capacities of thiol-containing proteins in admixture with polyphenols. Talanta 2009, 79, 344–351. [Google Scholar] [CrossRef]
- Gulcin, I. Fe3+-Fe2+ transformation method: An important antioxidant assay. Methods Mol. Biol. 2015, 1208, 233–246. [Google Scholar] [PubMed]
- Wright, J.S.; Johnson, E.R.; DiLabio, G.A. Predicting the activity of phenolic antioxidants: Theoretical method, analysis of substituent effects, and application to major families of antioxidants. J. Am. Chem. Soc. 2001, 123, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Shi, Y.; Jia, Z.; Zhao, C.; Zhang, Q.; Tan, X. Fast repair of DNA radicals. Chem. Soc. Rev. 2010, 39, 2827–2834. [Google Scholar] [CrossRef]
- Jin, W.B.; Wu, S.; Jian, X.H.; Yuan, H.; Tang, G.L. A radical S-adenosyl-L-methionine enzyme and a methyltransferase catalyze cyclopropane formation in natural product biosynthesis. Nat. Commun. 2018, 9, 2771. [Google Scholar] [CrossRef]
- Zhuravlev, A.V.; Zakharov, G.A.; Shchegolev, B.F.; Savvateeva-Popova, E.V. Antioxidant Properties of Kynurenines: Density Functional Theory Calculations. PLoS Comput. Biol. 2016, 12, e1005213. [Google Scholar] [CrossRef]
- Li, X.C. 2-Phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-Oxide (PTIO•) Radical Scavenging: A New and Simple Antioxidant Assay In Vitro. J. Agric. Food Chem. 2017, 65, 6288–6297. [Google Scholar] [CrossRef]
- Rabago Smith, M.; Kindl, E.D.; Williams, I.R.; Moorman, V.R. 5,7,3′,4′-Hydroxy substituted flavonoids reduce the heme of cytochrome c with a range of rate constants. Biochimie 2019, 162, 167–175. [Google Scholar] [CrossRef]
- Darshani, P.; Gumpu, M.B.; Thumpati, P.; Rayappan, J.B.B.; Ravichandiran, V.; Pazhani, G.P.; Veerapandian, M. Chemically synthesized butein and butin: Optical, structure and electrochemical redox functionality at electrode interface. J. Photochem. Photobiol. B 2018, 182, 122–129. [Google Scholar] [CrossRef]
- Galano, A.; Mazzone, G.; Alvarez-Diduk, R.; Marino, T.; Alvarez-Idaboy, J.R.; Russo, N. Food Antioxidants: Chemical Insights at the Molecular Level. Annu. Rev. Food Sci. Technol. 2016, 7, 335–352. [Google Scholar] [CrossRef]
- Zheng, Y.Z.; Deng, G.; Guo, R.; Chen, D.F.; Fu, Z.M. Substituent Effects on the Radical Scavenging Activity of Isoflavonoid. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef]
- Li, X.; Lin, J.; Chen, B.; Xie, H.; Chen, D. Antioxidant and Cytoprotective Effects of Kukoamines A and B: Comparison and Positional Isomeric Effect. Molecules 2018, 23. [Google Scholar] [CrossRef]
- Li, X.; Chen, B.; Xie, H.; He, Y.; Zhong, D.; Chen, D. Antioxidant Structure-Activity Relationship Analysis of Five Dihydrochalcones. Molecules 2018, 23. [Google Scholar] [CrossRef]
- Zheng, Y.Z.; Deng, G.; Liang, Q.; Chen, D.F.; Guo, R.; Lai, R.C. Antioxidant Activity of Quercetin and Its Glucosides from Propolis: A Theoretical Study. Sci. Rep. 2017, 7, 7543. [Google Scholar] [CrossRef]
- Liu, J.J.; Li, X.C.; Lin, J.; Li, Y.R.; Wang, T.T.; Jiang, Q.; Chen, D.F. Sarcandra glabra (Caoshanhu) protects mesenchymal stem cells from oxidative stress: A bioevaluation and mechanistic chemistry. Bmc Complement. Altern. Med. 2016, 16, 423. [Google Scholar] [CrossRef]
- Maciel, E.N.; Soares, I.N.; da Silva, S.C.; de Souza, G.L.C. A computational study on the reaction between fisetin and 2,2-diphenyl-1-picrylhydrazyl (DPPH). J. Mol. Model. 2019, 25, 103. [Google Scholar] [CrossRef]
- Maciel, E.N.; Almeida, S.K.C.; da Silva, S.C.; de Souza, G.L.C. Examining the reaction between antioxidant compounds and 2,2-diphenyl-1-picrylhydrazyl (DPPH) through a computational investigation. J. Mol. Model. 2018, 24, 218. [Google Scholar] [CrossRef]
- Li, X.; Xie, H.; Jiang, Q.; Wei, G.; Lin, L.; Li, C.; Ou, X.; Yang, L.; Xie, Y.; Fu, Z.; et al. The mechanism of (+)-taxifolin’s protective antioxidant effect for •OH-treated bone marrow-derived mesenchymal stem cells. Cell Mol. Biol. Lett. 2017, 22, 231. [Google Scholar] [CrossRef]
- Li, X.C. Improved pyrogallol autoxidation method: A reliable and cheap superoxide-scavenging assay suitable for all antioxidants. J. Agric. Food Chem. 2012, 60, 6418–6424. [Google Scholar] [CrossRef]
- Liang, M.; Li, X.; Ouyang, X.; Xie, H.; Chen, D. Antioxidant Mechanisms of Echinatin and Licochalcone A. Molecules 2019, 24. [Google Scholar] [CrossRef]
- Li, X.; Ouyang, X.; Cai, R.; Chen, D. 3′,8′’-Dimerization Enhances the Antioxidant Capacity of Flavonoids: Evidence from Acacetin and Isoginkgetin. Molecules 2019, 24. [Google Scholar] [CrossRef]
- Caruso, F.; Tanski, J.; Villegas-Estrada, A.; Rossi, M. Structural basis for antioxidant activity of trans-resveratrol: Ab initio calculations and crystal and molecular structure. J. Agric. Food Chem. 2004, 52, 7279–7285. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Dai, F.; Sun, L.D.; Zhou, B. Toward an understanding of the role of a catechol moiety in cancer chemoprevention: The case of copper- and o-quinone-dependent Nrf2 activation by a catechol-type resveratrol analog. Mol. Nutr. Food Res. 2015, 59, 2395–2406. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jiang, Q.; Chen, B.; Luo, X.; Chen, D. Structure-Activity Relationship and Prediction of the Electron-Transfer Potential of the Xanthones Series. ChemistryOpen 2018, 7, 730–736. [Google Scholar] [CrossRef]
- Han, L.; Sun, C.; Lin, J.; Wu, Z.; Bai, Y.; Li, F.; Li, X.; Mai, W.; Chen, D. Herba Ecliptae Protects against Hydroxyl Radical-induced Damages to DNA and Mesenchymal Stem Cells via Antioxidant Mechanism. J. Chin. Chem. Soc. 2014, 1161–1167. [Google Scholar] [CrossRef]
- Wang, T.; Li, L.; Li, Y.; Wu, J.; Huang, Y.; Wei, G.; Chen, D. Mechanistic Chemistry of Extraordinary Capacity of Salvianolic Acid B on Oxidatively damaged Mesenchymal Stem Cells. J. Chin. Chem. Soc. 2016, 63, 924–929. [Google Scholar] [CrossRef]
- Li, X. Comparative study of 1,1-Diphenyl-2-picryl-hydrazl Radical (DPPH•) Scavenging Capacity of Antioxidant Xanthones Family. ChemistrySelect 2018, 2, 13081–13086. [Google Scholar] [CrossRef]
- Gross, J.H. Mass spectrometry; Science press: Beijing, China, 2013. [Google Scholar]
- Li, X.; Xie, Y.; Li, K.; Wu, A.; Xie, H.; Guo, Q.; Xue, P.; Maleshibek, Y.; Zhao, W.; Guo, J.; et al. Antioxidation and Cytoprotection of Acteoside and Its Derivatives: Comparison and Mechanistic Chemistry. Molecules 2018, 23, 498. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; He, Y.; Lu, F. The structure-antioxidant activity relationship of dehydrodiferulates. Food Chem. 2018, 269, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Li, X.O.X.; Liang, M.; Chen, D. Comparative Analysis of Radical Adduct Formation (RAF) Products and Antioxidant Pathways between Myricetin-3-O-Galactoside and Myricetin Aglycone. Molecules 2019, 24, 2769. [Google Scholar] [CrossRef]
- Lomakin, I.B.; Dmitriev, S.E.; Steitz, T.A. Crystal structure of the DENR-MCT-1 complex revealed zinc-binding site essential for heterodimer formation. Proc. Natl. Acad. Sci. USA 2019, 116, 528–533. [Google Scholar] [CrossRef]
- Wang, L.; Yang, X.; Zhang, Y.; Chen, R.; Cui, Y.; Wang, Q. Anti-inflammatory Chalcone–Isoflavone Dimers and Chalcone Dimers from Caragana jubata. J. Nat. Prod. 2019. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.-H.; Yan, D.-X.; Huang, X.-Y.; Hou, B.; Ma, Y.-B.; Peng, H.; Zhang, X.-M.; Chen, J.-J.; Geng, C.-A. Termipaniculatones AF, chalcone-flavonone heterodimers from Terminthia paniculata, and their protective effects on hyperuricemia and acute gouty arthritis. Phytochemistry 2019, 164, 228–235. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; Zhao, Z.-X.; Wang, T.; Lin, J.; Chen, D. Effects of Natural Chalcone–Tannin Hybrids Protecting Mesenchymal Stem Cells against ROS-mediated Oxidative Damage and Indexes for Antioxidant Mechanisms. Chem. Lett. 2016, 45, 743–745. [Google Scholar] [CrossRef]
- Pechamat, L.; Zeng, L.; Jourdes, M.; Ghidossi, R.M.; Teissedre, P.-L. Occurrence and formation kinetics of pyranomalvidin-procyanidin dimer pigment in merlot red wine: Impact of acidity and oxygen concentrations. J. Agr. Food Chem. 2014, 62, 1701–1705. [Google Scholar] [CrossRef] [PubMed]
- Fossen, T.; Rayyan, S.; Holmberg, M.H.; Nimtz, M.; Andersen, Ø.M. Covalent anthocyanin–flavone dimer from leaves of Oxalis triangularis. Phytochemistry 2007, 68, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Hou, Y.; Zhu, L.; Chen, G.; Xu, D.; Zhang, S.; He, Y.; Xie, W. A bio-inspired synthesis of hybrid flavonoids from 2-hydroxychalcone driven by visible light. Rsc Adv. 2019, 9, 29005. [Google Scholar] [CrossRef]
- Distefano, A.M.; Martin, M.V.; Cordoba, J.P.; Bellido, A.M.; D’Ippolito, S.; Colman, S.L.; Soto, D.; Roldan, J.A.; Bartoli, C.G.; Zabaleta, E.J.; et al. Heat stress induces ferroptosis-like cell death in plants. J. Cell Biol. 2017, 216, 463–476. [Google Scholar] [CrossRef]
- Ghosh, S.; Mahapatra, A.; Chattopadhyay, K. Modulation of alpha-Synuclein Aggregation by Cytochrome c Binding and Hetero-dityrosine Adduct Formation. ACS Chem. Neurosci. 2019, 10, 1300–1310. [Google Scholar] [CrossRef]
- Tiwari, M.K.; Leinisch, F.; Sahin, C.; Moller, I.M.; Otzen, D.E.; Davies, M.J.; Bjerrum, M.J. Early events in copper-ion catalyzed oxidation of α-synuclein. Free Radic. Biol. Med. 2018, 121, 38–50. [Google Scholar] [CrossRef]
- Belavgeni, A.; Bornstein, S.R.; von Massenhausen, A.; Tonnus, W.; Stumpf, J.; Meyer, C.; Othmar, E.; Latk, M.; Kanczkowski, W.; Kroiss, M.; et al. Exquisite sensitivity of adrenocortical carcinomas to induction of ferroptosis. Proc. Natl. Acad. Sci. USA 2019, 116, 22269–22274. [Google Scholar] [CrossRef]
- Mao, X.Y. Lipoxygenases as Targets for Drug Development. Methods Mol. Biol. 2020, 2089, 251–256. [Google Scholar] [PubMed]
- Li, X.; Chen, B.; Zhao, X.; Chen, D. 2-Phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide Radical (PTIO•) Trapping Activity and Mechanisms of 16 Phenolic Xanthones. Molecules 2018, 23. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xie, Y.; Xie, H.; Yang, J.; Chen, D. π -π Conjugation Enhances Oligostilbene’s Antioxidant Capacity: Evidence from α-Viniferin and Caraphenol A. Molecules 2018, 23, 694. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xie, H.; Zhan, R.; Chen, D. Effect of Double Bond Position on 2-Phenyl-benzofuran Antioxidants: A Comparative Study of Moracin C and Iso-Moracin C. Molecules 2018, 23, 754. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Li, X.C.; Ren, Z.X.; Qiu, W.M.; Chen, J.L.; Jiang, Q.; Chen, B.; Chen, D.F. Antioxidant and Cytoprotective Effects of Tibetan Tea and Its Phenolic Components. Molecules 2018, 23. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, P.; Garcia-Beltran, O.; Tapia, V.; Munoz, Y.; Cassels, B.K.; Nunez, M.T. Neuroprotective Effect of a New 7,8-Dihydroxycoumarin-Based Fe2+/Cu2+ Chelator in Cell and Animal Models of Parkinson’s Disease. ACS Chem. Neurosci. 2017, 8, 178–185. [Google Scholar] [CrossRef]
- Forkink, M.; Basit, F.; Teixeira, J.; Swarts, H.G.; Koopman, W.J.H.; Willems, P. Complex I and complex III inhibition specifically increase cytosolic hydrogen peroxide levels without inducing oxidative stress in HEK293 cells. Redox Biol. 2015, 6, 607–616. [Google Scholar] [CrossRef]
- Yu, H.; Yang, C.; Jian, L.; Guo, S.D.; Chen, R.; Li, K.; Qu, F.; Tao, K.; Fu, Y.; Luo, F.; et al. Sulfasalazine-induced ferroptosis in breast cancer cells is reduced by the inhibitory effect of estrogen receptor on the transferrin receptor. Oncol. Rep. 2019, 42, 826–838. [Google Scholar]
- Li, X.; Ren, Z.; Wu, Z.; Fu, Z.; Xie, H.; Deng, L.; Jiang, X.; Chen, D. Steric Effect of Antioxidant Diels-Alder-Type Adducts: A Comparison of Sanggenon C with Sanggenon D. Molecules 2018, 23, 2610. [Google Scholar] [CrossRef]
- Li, X.C.; Wang, T.T.; Liu, J.J.; Liu, Y.L.; Zhang, J.; Lin, J.; Zhao, Z.X.; Chen, D.F. Effect and mechanism of wedelolactone as antioxidant-coumestan on •OH-treated mesenchymal stem cells. Arab. J. Chem. 2020, 13, 184–192. [Google Scholar] [CrossRef]
- Chen, D.; Eyupoglu, I.Y.; Savaskan, N. Ferroptosis and Cell Death Analysis by Flow Cytometry. Methods Mol. Biol. 2017, 1601, 71–77. [Google Scholar] [PubMed]
- Li, X.; Gao, Y.; Han, W.; Lin, J.; Hu, Q.; Chen, D. Antioxidant Activity and Mechanism in Flower of Hylocereus Undatus (Haw.) Britt. Et Rose. Acta Biol. Crac. Ser. Bot. 2013, 55, 80–85. [Google Scholar]
- Apak, R.; Guclu, K.; Ozyurek, M.; Bektas Oglu, B.; Bener, M. Cupric ion reducing antioxidant capacity assay for food antioxidants: Vitamins, polyphenolics, and flavonoids in food extracts. Methods Mol. Biol. 2008, 477, 163–193. [Google Scholar] [PubMed]
- Tian, Y.; Li, X.; Xie, H.; Wang, X.; Xie, Y.; Chen, C.; Chen, D. Protective Mechanism of the Antioxidant Baicalein toward Hydroxyl Radical-Treated Bone Marrow-Derived Mesenchymal Stem Cells. Molecules 2018, 23, 223. [Google Scholar] [CrossRef] [PubMed]
- Li, X.C.; Mai, W.Q.; Chen, D.F. Chemical study on protective effect against hydroxyl-induced DNA damage and antioxidant mechanism of myricitrin. J. Chin. Chem. Soc. 2014, 61, 383–391. [Google Scholar] [CrossRef]
- Chen, B.; Li, X.; Liu, J.; Qin, W.; Liang, M.; Liu, Q.; Chen, D. Antioxidant and Cytoprotective effects of Pyrola decorata H. Andres and its five phenolic components. BMC Complement Altern. Med. 2019, 19, 275. [Google Scholar] [CrossRef]
- Ouyang, X.; Li, X.; Lu, W.; Zhao, X.; Chen, D. A Null B-Ring Improves the Antioxidant Levels of Flavonol: A Comparative Study between Galangin and 3,5,7-Trihydroxychromone. Molecules 2018, 23, 3083. [Google Scholar] [CrossRef]
- Liu, Z.; Barigye, S.J.; Shahamat, M.; Labute, P.; Moitessier, N. Atom Types Independent Molecular Mechanics Method for Predicting the Conformational Energy of Small Molecules. J. Chem. Inf. Model. 2018, 58, 194–205. [Google Scholar] [CrossRef]
- Kaminsky, J.; Jensen, F. Conformational Interconversions of Amino Acid Derivatives. J. Chem. Theory Comput. 2016, 12, 694–705. [Google Scholar] [CrossRef]
- Xie, Y.; Li, X.; Chen, J.; Deng, Y.; Lu, W.; Chen, D. pH Effect and Chemical Mechanisms of Antioxidant Higenamine. Molecules 2018, 23, 2176. [Google Scholar] [CrossRef]
Sample Availability: Sample of the compound butein is available from the authors. |
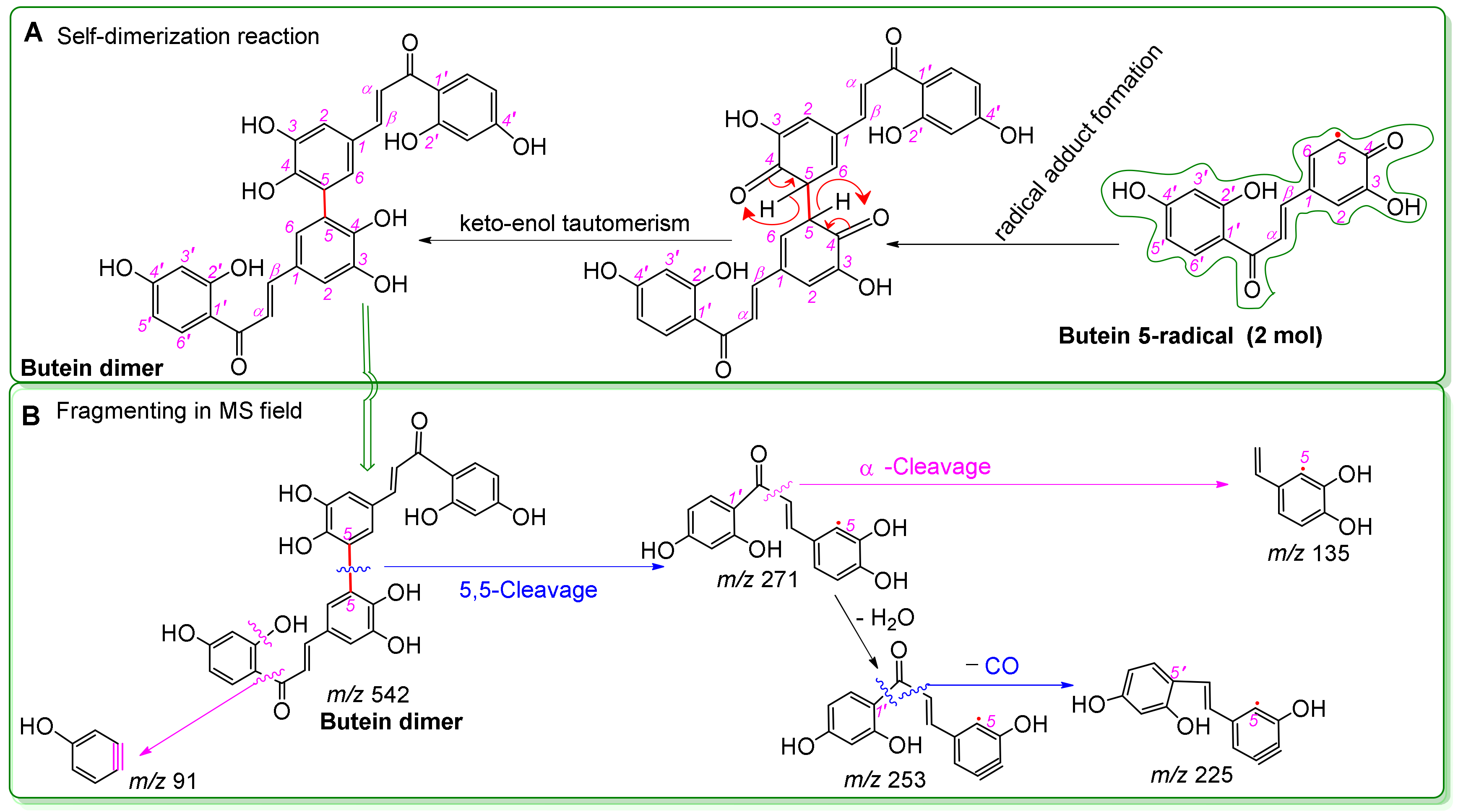

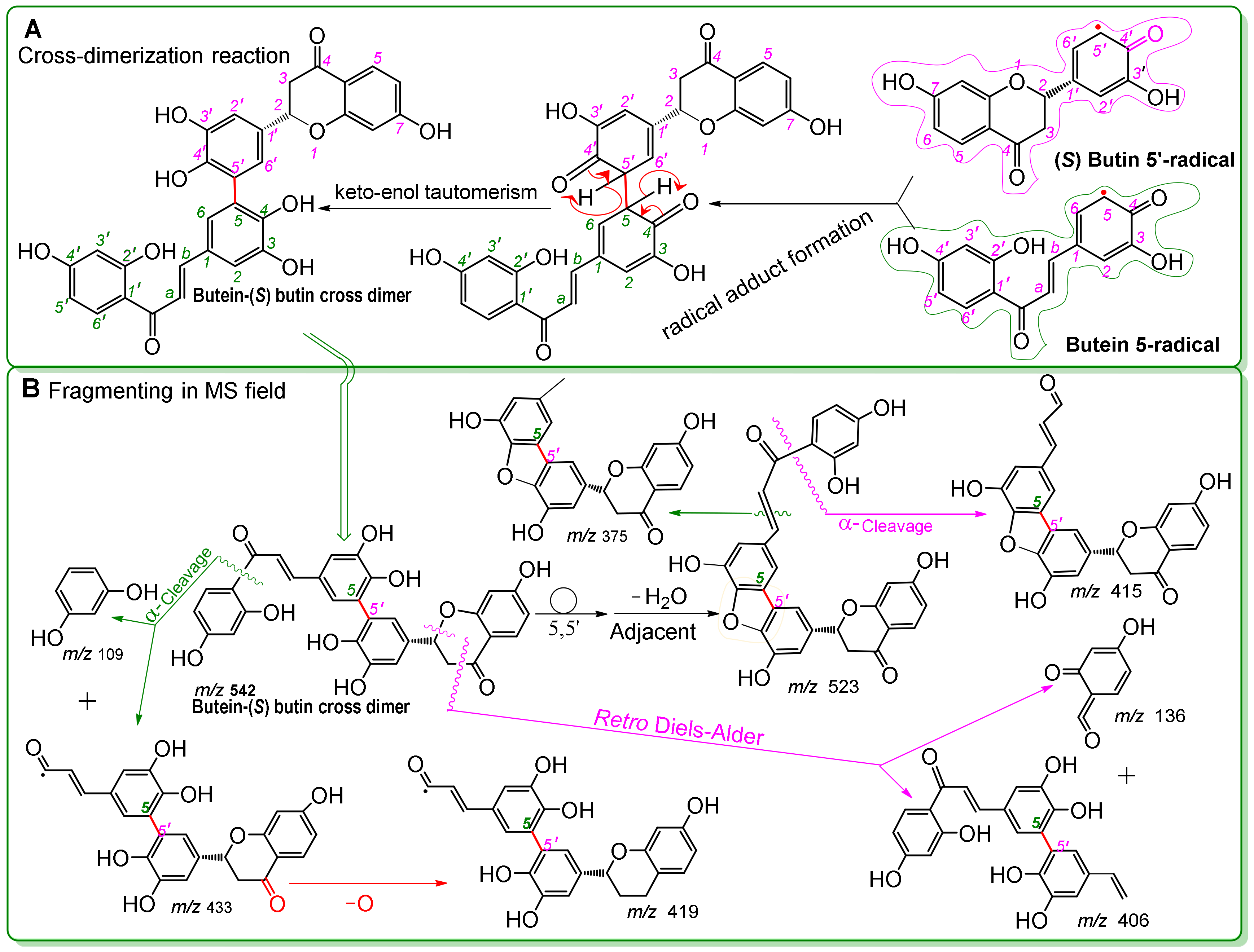
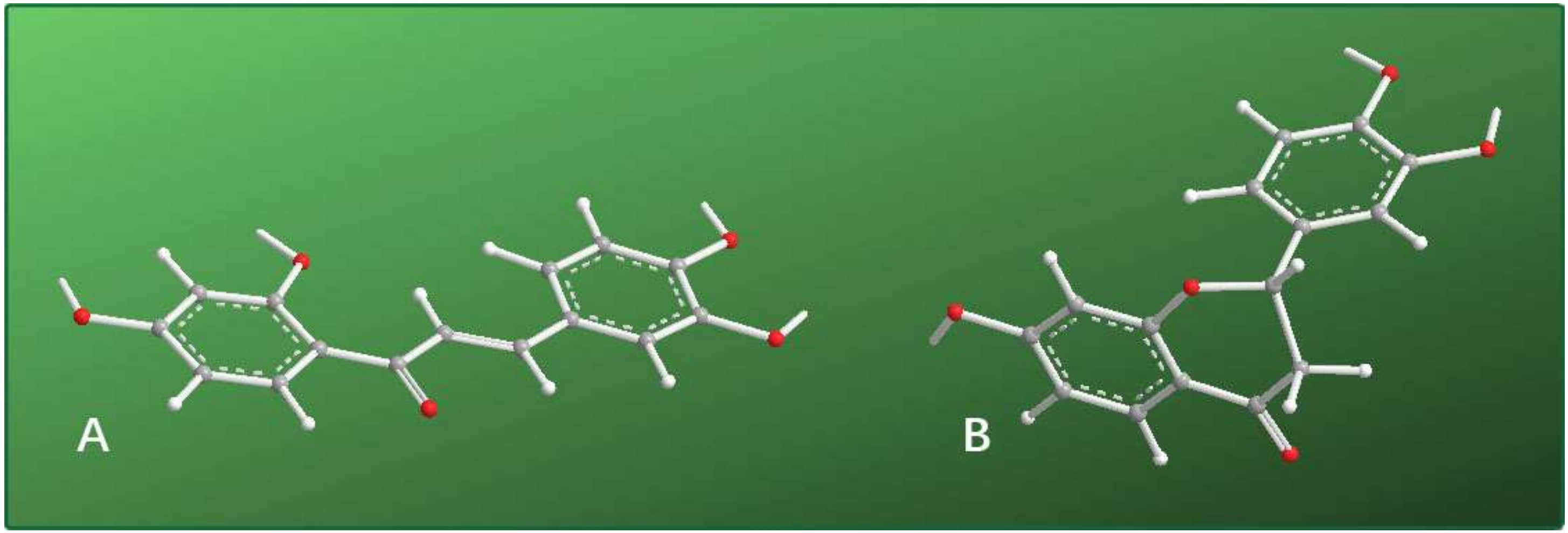
| Antioxidant Assays | Butein | (S)-Butin | Trolox | l-Ascorbic Acid |
|---|---|---|---|---|
| Linoleic acid emulsion | 3.2 ± 1.4 a | 46.7 ± 7.5 b | 1.0± 1.5 | N.D. |
| Cu2+-reducing | 36.2 ± 0.1 a | 44.4 ± 1.9 b | 91.6 ± 3.8 | 83.2 ± 1.2 |
| Fe3+-reducing | 5.3 ± 0.1 a | 5.6 ± 0.1 b | 8.7 ± 0.2 | 4.5 ± 0.2 |
| PTIO•-trapping pH 4.5 | 16.4 ± 0.9 a | 18.4 ± 0.4 b | 9.1 ± 0.2 | 8.2 ± 0.2 |
| PTIO•-trapping pH 6.0 | 9.3 ± 0.9 a | 47.6 ± 7.1 b | 11.1 ± 0.5 | 8.3 ± 0.5 |
| PTIO•-trapping pH 7.4 | 12.9 ± 0.4 a | 17.4 ± 0.4 b | 5.2 ± 2.8 | 4.7 ± 1.1 |
| DPPH•-trapping | 15.8 ± 0.5 a | 29.9 ± 0.5 b | 23.3 ± 0.7 | 27.0 ± 0.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Li, X.; Cai, R.; Ren, Z.; Zhang, A.; Deng, F.; Chen, D. Simultaneous Study of Anti-Ferroptosis and Antioxidant Mechanisms of Butein and (S)-Butin. Molecules 2020, 25, 674. https://doi.org/10.3390/molecules25030674
Liu J, Li X, Cai R, Ren Z, Zhang A, Deng F, Chen D. Simultaneous Study of Anti-Ferroptosis and Antioxidant Mechanisms of Butein and (S)-Butin. Molecules. 2020; 25(3):674. https://doi.org/10.3390/molecules25030674
Chicago/Turabian StyleLiu, Jie, Xican Li, Rongxin Cai, Ziwei Ren, Aizhen Zhang, Fangdan Deng, and Dongfeng Chen. 2020. "Simultaneous Study of Anti-Ferroptosis and Antioxidant Mechanisms of Butein and (S)-Butin" Molecules 25, no. 3: 674. https://doi.org/10.3390/molecules25030674
APA StyleLiu, J., Li, X., Cai, R., Ren, Z., Zhang, A., Deng, F., & Chen, D. (2020). Simultaneous Study of Anti-Ferroptosis and Antioxidant Mechanisms of Butein and (S)-Butin. Molecules, 25(3), 674. https://doi.org/10.3390/molecules25030674




