Reactivity and Stability of Metalloporphyrin Complex Formation: DFT and Experimental Study
Abstract
1. Introduction
2. Results and Discussion
2.1. Calculated Structure of Metalloporphyrin Complex
2.2. Calculation of Binding Constants
3. Experimental Section
3.1. Computational Methods
3.2. Calculation of Binding Constant
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, Z.; Pan, J. Advances in synthesis and application of the derivatives of porphyrin as a reagent in analytical chemistry. Rev. Anal. Chem. 2020, 21, 167–230. [Google Scholar]
- Giovannetti, R. The use of spectrophotometry UV-VIS for the study of porphyrins. In Macro to Nano Spectroscopy; Uddin, J., Ed.; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Ishi, H.; Katsuhiko, S.; Yasuhiro, S.; Hidemasa, K. Spectrophotometric and analogue derivative spectrophotometric determination of ultramicro amounts of cadmium with cationic porphyrins. Talanta 1982, 29, 545–550. [Google Scholar] [CrossRef]
- Hu, Q.; Yang, G.; Li, H.; Tai, X. Study on determination of seven transition metal ions in water in food by microcolumn high performance liquid chromatography. Bull. Korean Chem. Soc. 2004, 2, 694–698. [Google Scholar]
- Zhang, L.; Zhao, Y.H.; Bai, R.B. Development of a multifunctional membrane for chromatic warning and enhanced adsorptive removal of heavy metal ions: Application to cadmium. J. Membr. Sci. 2011, 379, 69–79. [Google Scholar] [CrossRef]
- Daryono, H.T.; Takehiro, A.; Naoki, Y.; Hidenari, I. Cationic porphyrins bearing diazolium rings: Synthesis and their interaction with calf thymus DNA. Biochim. Biophys. Acta 1999, 1472, 333–343. [Google Scholar]
- Pratiwi, R.; Nguyen, M.P.; Ibrahim, S.; Yoshioka, N.; Henry, C.S.; Tjahjono, D.H. A selective distance-based paper analytical device for copper(II) determination using a porphyrin derivative. Talanta 2017, 174, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.C.; Mondal, S.; Ocayo, F.; Maturana, R.G.; Muñoz-Castro, A. Nature of C60 and C70 Fullerene Encapsulation in a Porphyrin-and Metalloporphyrin-Based Cage: Insights from Dispersion Corrected DFT Calculations. Int. J. Quant. Chem. 2019, 120, e26080. [Google Scholar]
- Ulloa, C.O.; Ponce-Vargas, M.; Muñoz-Castro, A. Formation of Coinage-Metal Fullerene Adducts. Evaluation of the Interaction Nature between Triangular Coinage Metal Complexes (M3 = Cu, Ag, and Au) and C60 through Relativistic Density Functional Theory Calculations. J. Phys. Chem. C 2018, 122, 25110. [Google Scholar] [CrossRef]
- Merian, E.; Anke, M.; Inhat, M.; Stoeppler, M. Metals and their compound. In Elements and Their Compounds in the Environment; Merian, E., Anke, M., Inhat, M., Stoeppler, M., Eds.; Wiley-VCH: Weinheim, Germany, 2004; Volume 2. [Google Scholar]
- Chandrakumar, K.; Sourav, P. The concept of density functional theory based descriptors and its relation with the reactivity of molecular systems: A semi-quantitative study. Int. J. Mol. Sci. 2002, 3, 324–337. [Google Scholar] [CrossRef]
- Smith, D.W. Inorganic Substances: A Prelude to the Study of Descriptive Inorganic Chemistry; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
- Chattaraj, P.K.; Vijayaraj, R.; Subramanian, V. Comparison of global reactivity descriptors calculated using various density functionals: A QSAR perspective. J. Chem. Theory Comput. 2009, 5, 2744–2753. [Google Scholar]
- Parr, R.G.; Pearson, R.G. Absolute hardness: Companion parameter to absolute electronegativity. J. Am. Chem. Soc. 1983, 105, 7512–7516. [Google Scholar] [CrossRef]
- Pearson, R.G. Chemical hardness and density functional theory. J. Chem. Sci. 2005, 117, 369–377. [Google Scholar] [CrossRef]
- Geerlings, P.; Proft, F.D.; Langenaeker, W. Conceptual density functional theory. Chem. Rev. 2003, 103, 1793–1873. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.G. Chemical Hardness: Density Functional Theory; Wiley-VCH: Weinheim, Germany, 1997. [Google Scholar]
- Chattaraj, P.K.; Nath, S.; Maiti, B. Reactivity Descriptors. In Computational Medicinal Chemistry for Drug Discovery; Patrick, B., Hans, D.W., Wilfried, L., Jan, P.T., Eds.; Marcel Dekker: New York, NY, USA, 2004. [Google Scholar]
- Mebi, C.A. DFT study on structure, electronic properties, and reactivity of cis-isomers of [(NC5H4-S)2Fe(CO)2]. J. Chem. Sci. 2011, 123, 727–731. [Google Scholar] [CrossRef]
- Feng, X.T.; Yu, J.G.; Lei, M.; Fang, W.H.; Liu, S.B. Toward understanding metal-binding specificity of porphyrin: A conceptual density functional theory study. J. Phys. Chem. B 2009, 113, 13381–13389. [Google Scholar] [CrossRef] [PubMed]
- Chattaraj, P.K.; Santanab, G. Electrophilicity index within a conceptual DFT framework. Annu. Rep. Prog. Chem. Sect. C Phys. Chem. 2009, 105, 13–39. [Google Scholar] [CrossRef]
- Romera, C.; Sabater, L.; Garofalo, A.; Dixon, I.M.; Pratviel, G. Interaction of cationic nickel and manganese porphyrins with the minor groove of DNA. Inorg. Chem. 2010, 49, 18. [Google Scholar] [CrossRef] [PubMed]
- Pasternack, R.F.; Huber, P.R.; Boyd, P.; Engasser, G.; Francesconi, L.; Gibbs, E.; Fasella, P.; Venturo, G.C.; Hinds, L.d. On the aggregation of meso-substituted water-soluble porphyrins. J. Am. Chem. Soc. 1971, 94, 13. [Google Scholar] [CrossRef] [PubMed]
- Valeur, B.; Bernard-Santos, M.N. Molecular Fluorescence: Principles and Application; Wiley-VCH: Weinheim, Germany, 2001. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |
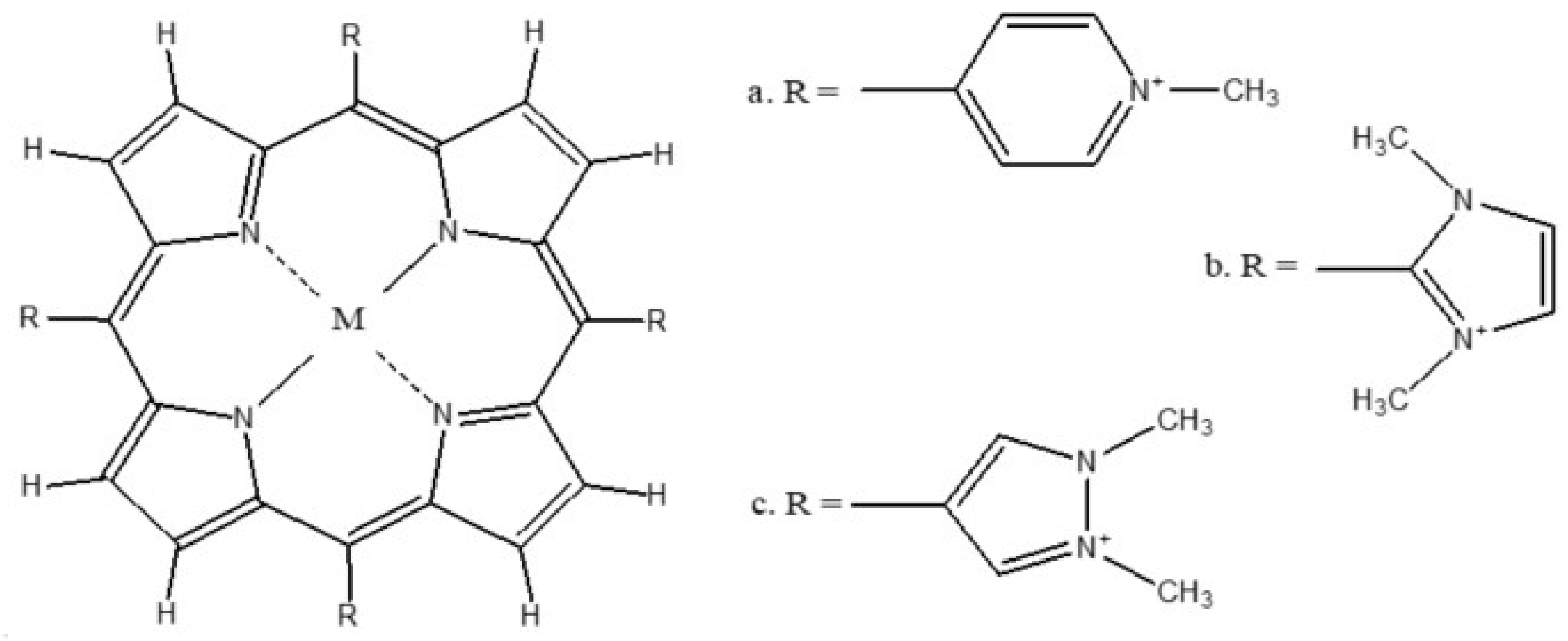
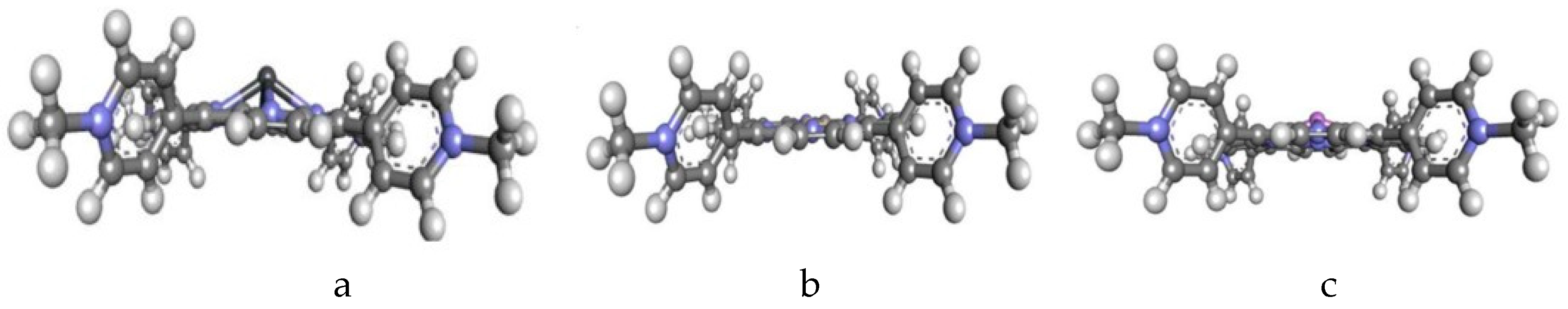
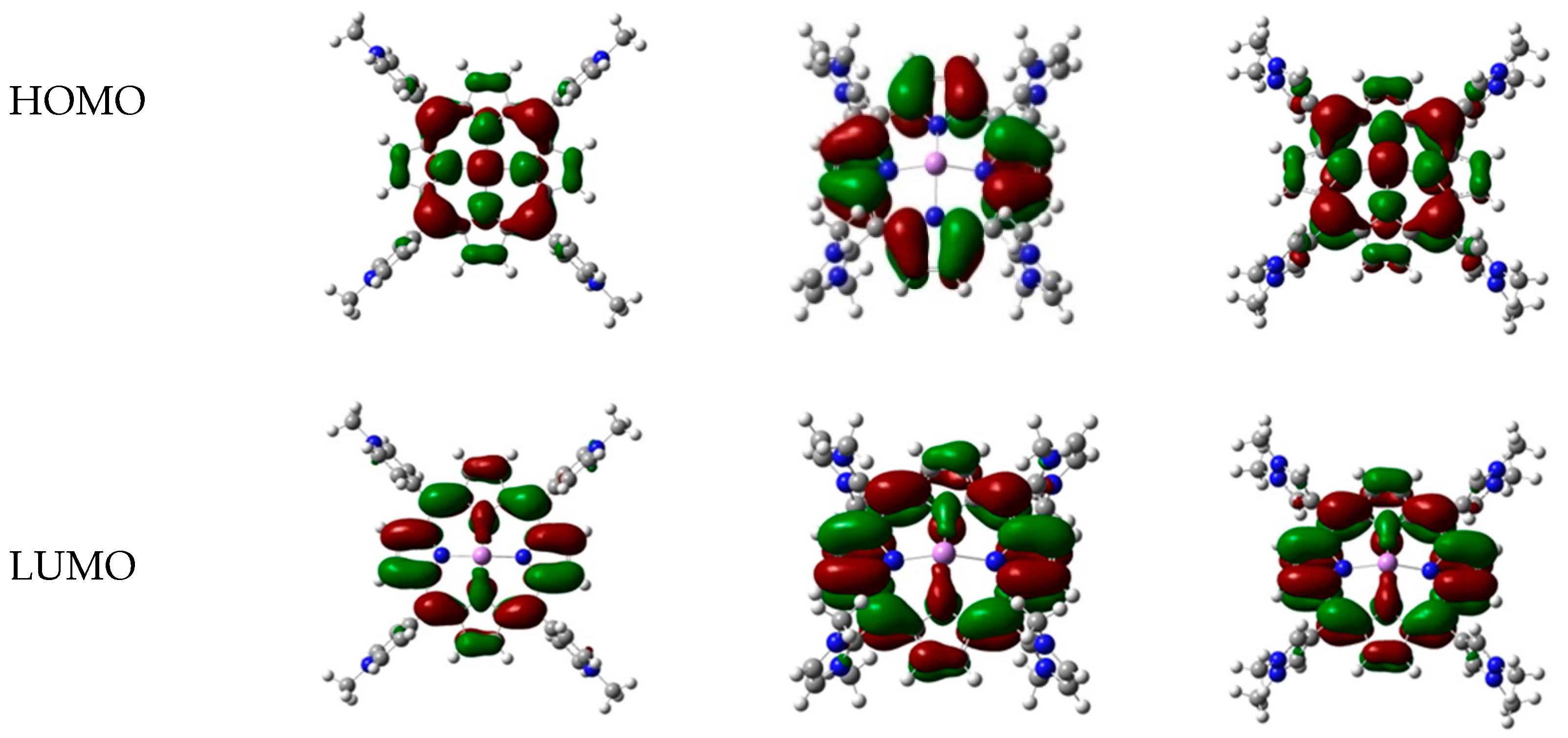
| No. | TMPyP (Energy, Hartree) | TDMImP (Energy, Hartree) | TDMPzP (Energy, Hartree) | |||
|---|---|---|---|---|---|---|
| 1. | Base | −2135.8 | Base | −2204.7 | Base | −2204.6 |
| 2. | Pb-TMPyP | −2138.3 | Pb-TDMImP | −2207.3 | Pb-TDMPzP | −2207.2 |
| 3. | Cd-TMPyP | −2182.9 | Cd-TDMImP | −2251.9 | Cd-TDMPzP | −2251.8 |
| 4. | Hg-TMPyP | −2177.5 | Hg-TDMImP | −246.5 | Hg-TDMPzP | −2246.4 |
| 5. | Sn-TMPyP | −2137.1 | Sn-TDMImP | −2205.9 | Sn-TDMPzP | −2206.0 |
| 6. | As3+-TMPyP | −2140.4 | As3+-TDMImP | −2209.3 | As3+-TDMPzP | −2209.3 |
| 7. | As5+-TMPyP | −2138.9 | As5+-TDMImP | −2207.8 | As5+-TDMPzP | −2207.9 |
| Average | M-TMPyP | −2152.5 | M-TDMImP | −2221.5 | M-TDMPzP | −2221.4 |
| Parameter | Pb2+ | Cd2+ | Hg2+ | Sn4+ | As3+ | As5+ | |
|---|---|---|---|---|---|---|---|
| TMPyP | M-Nph (Å) | 2.370 | 2.163 | 2.244 | 2.046 | 2.128 | 2.107 |
| M-Plane (Å) | 1.215 | 0 | 0.540 | 0 | 0.648 | 0.686 | |
| Bond Angle (M-Plane) | 30.84° | 0 | 13.92° | 0 | 17.73° | 19.00° | |
| TDMImP | M-Nph (Å) | 2.370 | 2.159 | 2.247 | 2.048 | 2.128 | 2.136 |
| M-Plane (Å) | 1.108 | 0 | 0.576 | 0 | 0.652 | 0.660 | |
| Bond Angle (M-Plane) | 27.87° | 0 | 14.85° | 0 | 17.84° | 18.00° | |
| TDMPzP | M-Nph (Å) | 2.366 | 2.158 | 2.243 | 2.045 | 2.127 | − |
| M-Plane (Å) | 1.101 | 0 | 0.544 | 0 | 0.651 | − | |
| Bond Angle (M-Plane) | 27.73° | 0 | 14.04° | 0 | 17.82 | − |
| Parameter | HOMO | LUMO | µ | η | ω | |
|---|---|---|---|---|---|---|
| TMPyP | P | −0.509 | −0.414 | −0.462 | 0.048 | 2.223 |
| P-Pb | −0.504 | −0.417 | −0.461 | 0.044 | 2.415 | |
| P-Cd | −0.513 | −0.418 | −0.466 | 0.048 | 2.262 | |
| P-Hg | −0.512 | −0.418 | −0.465 | 0.047 | 2.300 | |
| P-Sn | −0.747 | −0.638 | −0.693 | 0.055 | 4.366 | |
| P-As3+ | −0.626 | −0.523 | −0.575 | 0.052 | 3.179 | |
| P-As5+ | −0.862 | −0.813 | −0.838 | 0.025 | 14.045 | |
| Average | −0.627 | −0.538 | −0.583 | 0.045 | 4.761 | |
| TDMImP | I | −0.555 | −0.450 | −0.503 | 0.053 | 2.387 |
| I-Pb | −0.551 | −0.452 | −0.502 | 0.050 | 2.520 | |
| I-Cd | −0.557 | −0.452 | −0.505 | 0.053 | 2.406 | |
| I-Hg | −0.556 | −0.452 | −0.504 | 0.052 | 2.442 | |
| I-Sn | −0.768 | −0.688 | −0.728 | 0.040 | 6.625 | |
| I-As3 | −0.676 | −0.572 | −0.624 | 0.052 | 3.744 | |
| I-As5 | −0.866 | −0.830 | −0.848 | 0.018 | 19.975 | |
| Average | −0.662 | −0.574 | −0.618 | 0.044 | 6.285 | |
| TDMPzP | Pz | −0.496 | −0.391 | −0.444 | 0.053 | 1.860 |
| Pz-Pb | −0.490 | −0.396 | −0.443 | 0.047 | 2.088 | |
| Pz-Cd | −0.499 | −0.395 | −0.447 | 0.052 | 1.921 | |
| Pz-Hg | −0.496 | −0.396 | −0.446 | 0.050 | 1.989 | |
| Pz-Sn | −0.740 | −0.632 | −0.686 | 0.054 | 4.357 | |
| Pz-As3 | −0.619 | −0.515 | −0.567 | 0.052 | 3.091 | |
| Pz-As5 | −0.830 | −0.780 | −0.805 | 0.025 | 12.961 | |
| Average | −0.612 | −0.519 | −0.566 | 0.046 | 4.401 |
| Complexes | Binding Constant (M−1) |
|---|---|
| Pb-TDMPzP | 3.0 × 107 |
| Hg-TDMPzP | 3.3 × 107 |
| Cd-TDMPzP | 5.1 × 107 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pratiwi, R.; Ibrahim, S.; Tjahjono, D.H. Reactivity and Stability of Metalloporphyrin Complex Formation: DFT and Experimental Study. Molecules 2020, 25, 4221. https://doi.org/10.3390/molecules25184221
Pratiwi R, Ibrahim S, Tjahjono DH. Reactivity and Stability of Metalloporphyrin Complex Formation: DFT and Experimental Study. Molecules. 2020; 25(18):4221. https://doi.org/10.3390/molecules25184221
Chicago/Turabian StylePratiwi, Rimadani, Slamet Ibrahim, and Daryono H. Tjahjono. 2020. "Reactivity and Stability of Metalloporphyrin Complex Formation: DFT and Experimental Study" Molecules 25, no. 18: 4221. https://doi.org/10.3390/molecules25184221
APA StylePratiwi, R., Ibrahim, S., & Tjahjono, D. H. (2020). Reactivity and Stability of Metalloporphyrin Complex Formation: DFT and Experimental Study. Molecules, 25(18), 4221. https://doi.org/10.3390/molecules25184221






