Stearic Acid/Layered Double Hydroxides Composite Thin Films Deposited by Combined Laser Techniques
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
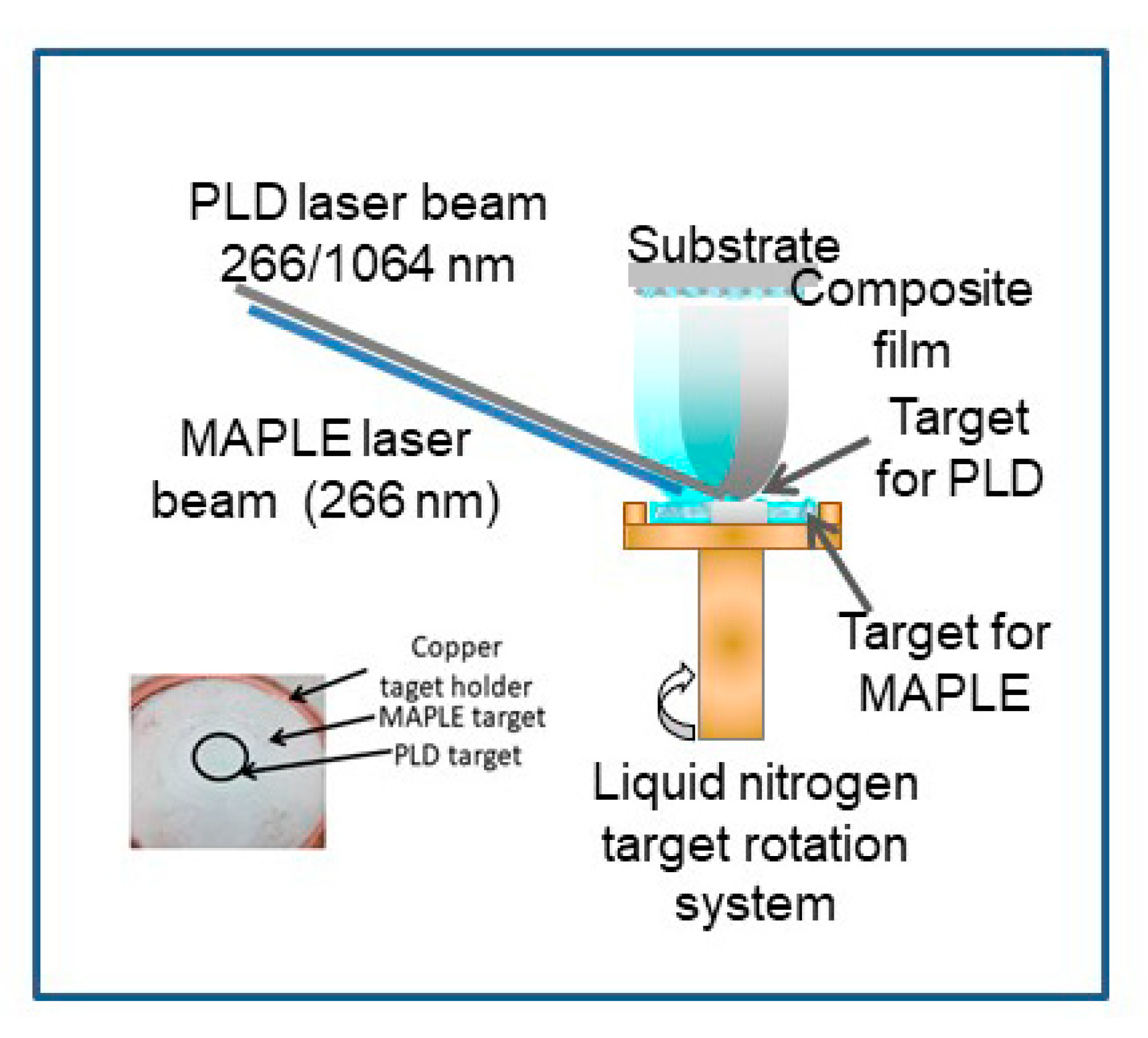
2.2. Thin Films Deposition
2.3. Characterization
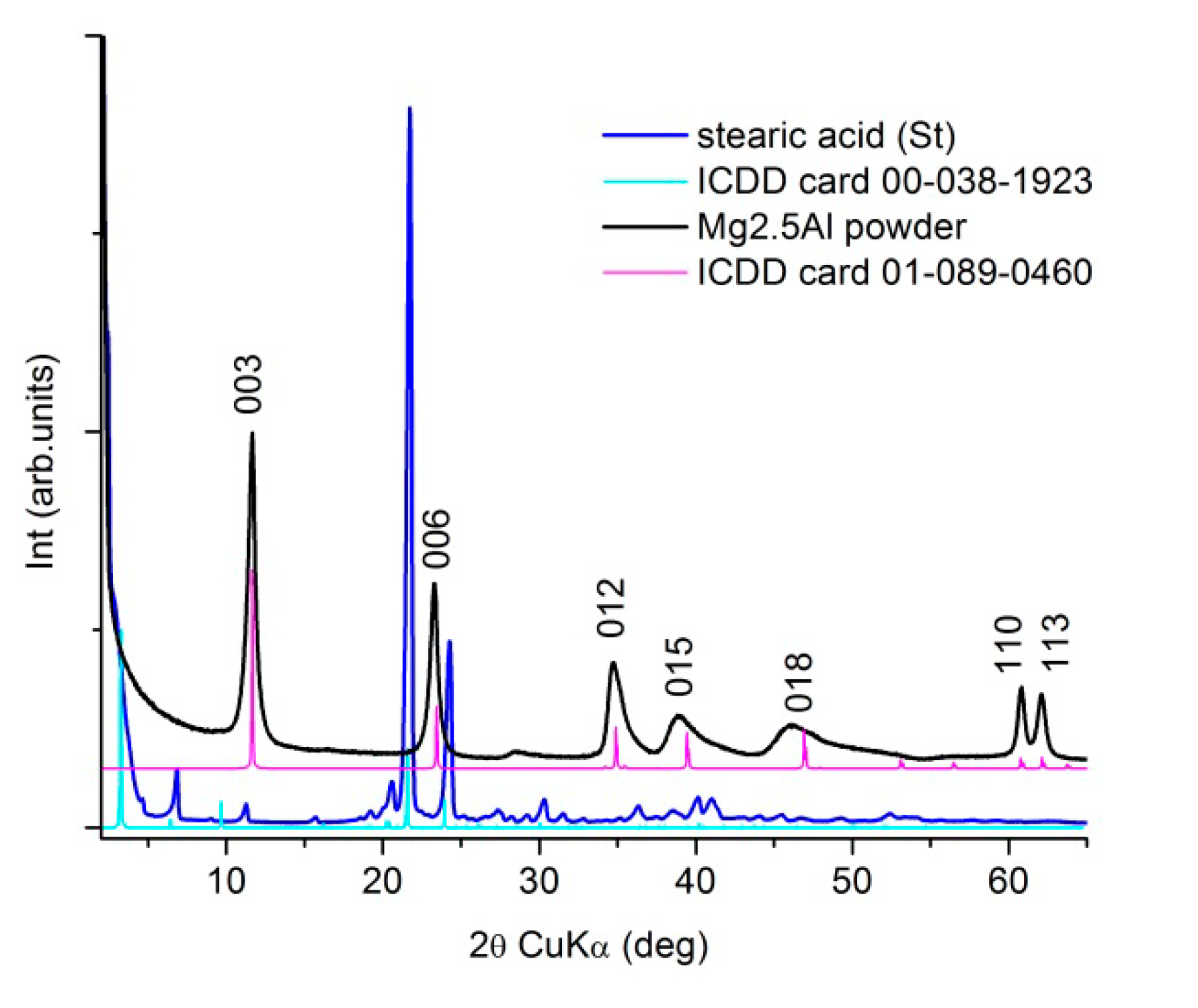
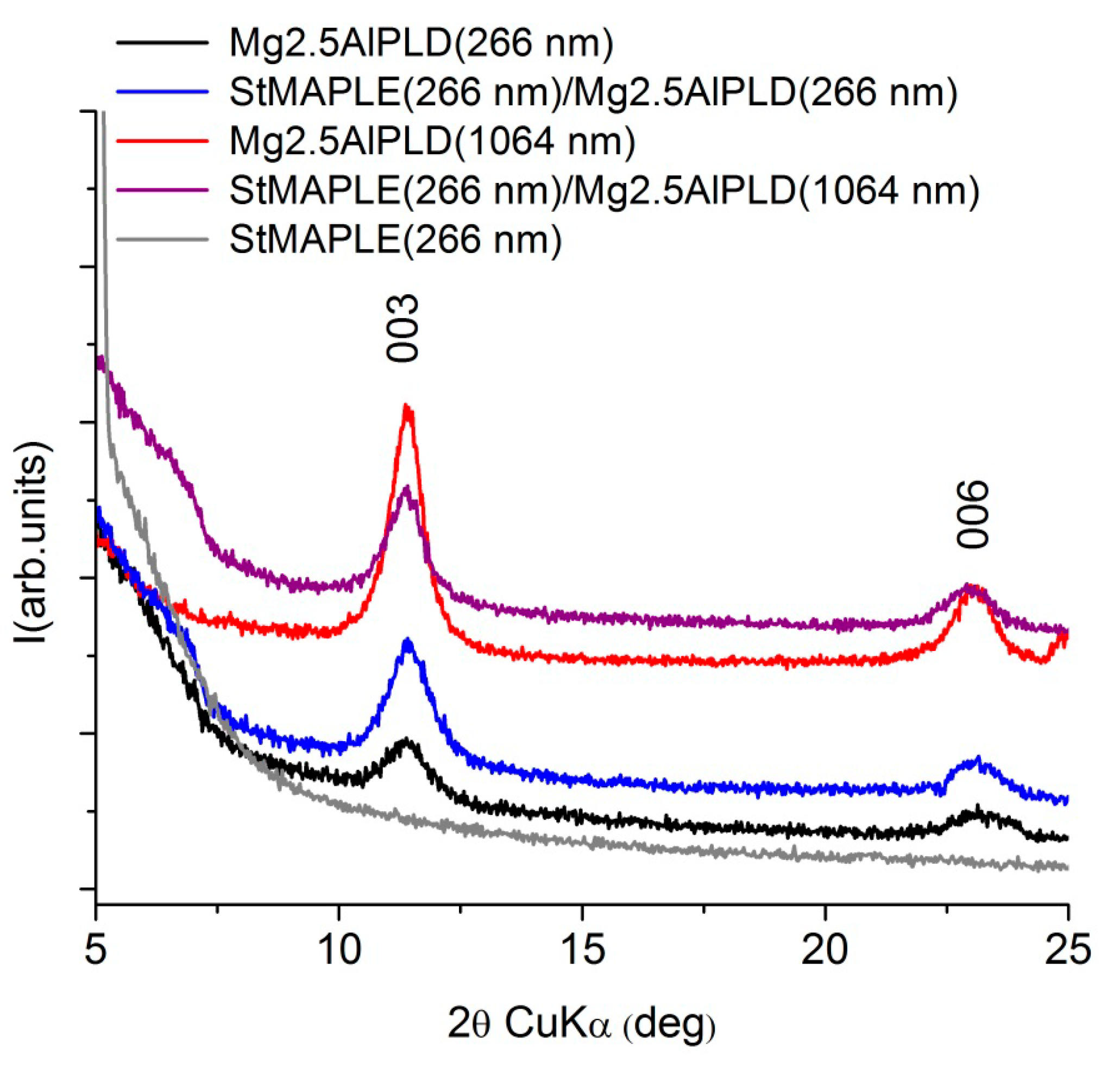
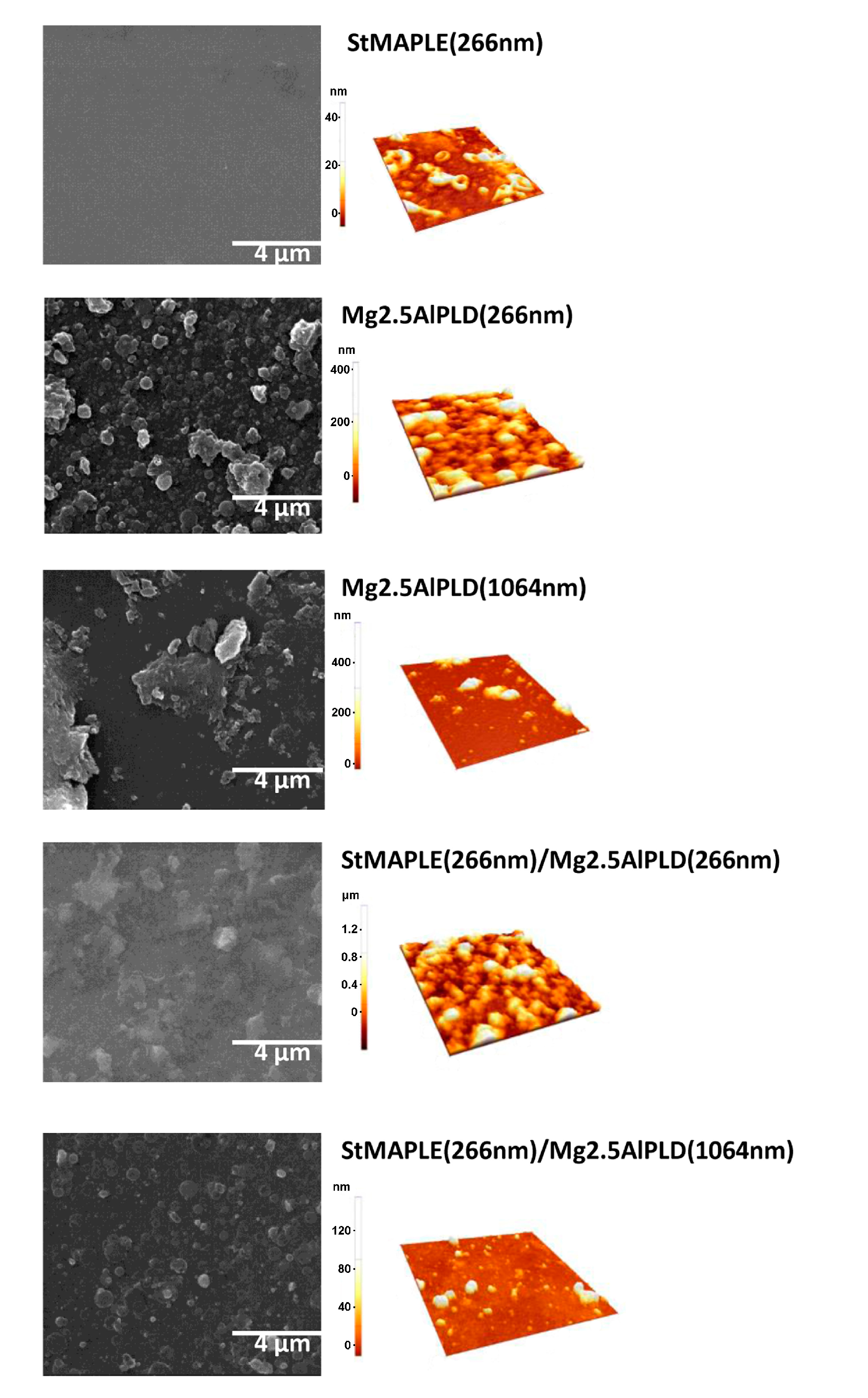
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Williams, G.R.; O’Hare, D. Towards understanding, control and application of layered double hydroxide chemistry. J. Mater. Chem. 2006, 16, 3065–3074. [Google Scholar] [CrossRef]
- Costantino, U.; Ambrogi, V.; Nocchetti, M.; Perioli, L. Hydrotalcite-like compounds: Versatile layered hosts of molecular anions with biological activity. Micropor. Mesopor. Mat. 2008, 107, 149–160. [Google Scholar] [CrossRef]
- Forano, C.; Hibino, T.; Leroux, F.; Taviot-Guého, C. Layered double hydroxides. In Handbook of Clay Science; Bergaya, F., Theng, B.K.G., Lagaly, G., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2006; pp. 1021–1095. [Google Scholar]
- Costantino, U.; Gallipoli, A.; Nocchetti, M.; Camino, G.; Bellucci, F.; Frache, A. New nanocomposites constituted of polyethylene and organically modified ZnAl-hydrotalcites. Polym. Degrad. Stab. 2005, 90, 586–590. [Google Scholar] [CrossRef]
- He, F.; Zhang, L.; Yang, F.; Chen, L.; Wu, Q. New nanocomposites based on syndiotactic polystyrene and organo-modified ZnAl layered double hydroxide. J. Polym. Res. 2006, 13, 483–493. [Google Scholar] [CrossRef]
- Aguzzi, A.; Ambrogi, V.; Costantino, U.; Marmottini, F. Intercalation of acrylate anions into the galleries of Zn–Al layered double hydroxide. J. Phys. Chem. Solids 2007, 68, 808–812. [Google Scholar] [CrossRef]
- Plank, J.; Dai, Z.; Andres, P.R. Preparation and characterization of new Ca-Al-polycarboxylate layered double hydroxides. Mater. Lett. 2006, 60, 3614–3617. [Google Scholar] [CrossRef]
- Rives, V.; Ulibarri, M.A. Layered double hydroxides (LDH) intercalated with metal coordination compounds and oxometalates. Coord. Chem. Rev. 1999, 181, 61–120. [Google Scholar] [CrossRef]
- Jin, S.; Fallgren, P.H.; Morris, J.M.; Chen, Q. Removal of bacteria and viruses from waters using layered double hydroxide nanocomposites. Sci. Technol. Adv. Mater. 2007, 8, 67–70. [Google Scholar] [CrossRef]
- Vlad, A.; Birjega, R.; Matei, A.; Luculescu, C.; Mitu, B.; Dinescu, M.; Zavoianu, R.; Pavel, O.D. Retention of heavy metals on layered double hydroxides thin films deposited by pulsed laser deposition. Appl. Surf. Sci. 2014, 302, 99–104. [Google Scholar] [CrossRef]
- Matei, A.; Birjega, R.; Nedelcea, A.; Vlad, A.; Colceag, D.; Ionita, M.D.; Luculescu, C.; Dinescu, M.; Zavoianu, R.; Pavel, O.D. Mg-Al layered double hydroxides (LDHs) and their derived mixed oxides grown by laser techniques. Appl. Surf. Sci. 2011, 257, 5308–5311. [Google Scholar]
- Matei, A.; Birjega, R.; Vlad, A.; Filipescu, M.; Nedelcea, A.; Luculescu, C.; Zavoianu, R.; Pavel, O.D.; Dinescu, M. Adsorption properties of Mg-Al layered double hydroxides thin films grown by laser based techniques. Appl. Surf. Sci. 2012, 258, 9466–9470. [Google Scholar] [CrossRef]
- Matei, A.; Birjega, R.; Vlad, A.; Luculescu, C.; Epurescu, G.; Stokker-Cheregi, F.; Dinescu, M.; Zavoianu, R.; Pavel, O.D. Pulsed laser deposition of Mg–Al layered double hydroxide with Ag nanoparticles. Appl. Phys. A Mater. 2013, 110, 841–846. [Google Scholar] [CrossRef]
- Birjega, R.; Matei, A.; Filipescu, M.; Stokker-Cheregi, F.; Luculescu, C.; Colceag, R.; Zavoianu, R.; Pavel, O.D.; Dinescu, M. The investigation of Ni-Al and Co-Al based layered double hydroxides and their derived mixed oxides thin films deposited by pulsed laser deposition. Appl. Surf. Sci. 2013, 278, 122–126. [Google Scholar] [CrossRef]
- Birjega, R.; Matei, A.; Mitu, B.; Ionita, M.D.; Filipescu, M.; Stokker-Cheregi, F.; Luculescu, C.; Dinescu, M.; Zavoianu, R.; Pavel, O.D.; et al. Layered double hydroxides/polymer thin films grown by matrix assisted pulsed laser evaporation. Thin Solid Films 2013, 543, 63–68. [Google Scholar] [CrossRef]
- Purice, A.; Schou, J.; Kingshott, P.; Dinescu, M. Production of active lysozyme films by matrix assisted pulsed laser evaporation at 355 nm. Chem. Phys. Lett. 2007, 435, 350–353. [Google Scholar] [CrossRef]
- Dumitrescu, L.N.; Neacsu, P.; Necula, M.G.; Bonciu, A.; Marascu, V.; Cimpean, A.; Moldovan, A.; Rotaru, A.; Dinca, V.; Dinescu, M. Induced Hydrophilicity and In Vitro Preliminary Osteoblast Response of Polyvinylidene Fluoride (PVDF) Coatings Obtained via MAPLE Deposition and Subsequent Thermal Treatment. Molecules 2020, 25, 582. [Google Scholar] [CrossRef]
- Canulescu, S.; Schou, J.; Fæster, S.; Hansen, K.V.; Conseil, H. Deposition of matrix-free fullerene films with improved morphology by matrix-assisted pulsed laser evaporation (MAPLE). Chem. Phys. Lett. 2013, 588, 119–123. [Google Scholar] [CrossRef]
- Darwish, A.M.; Moore, S.; Mohammad, A.; Alexander, D.; Bastian, T.; Dorlus, W.; Sarkisov, S.; Patel, D.; Mele, P.; Koplitz, B.; et al. Polymer nano-composite films with inorganic upconversion phosphor and electro-optic additives made by concurrent triple-beam matrix assisted and direct pulsed laser deposition. Compos. Part B Eng. 2017, 109, 82–90. [Google Scholar] [CrossRef]
- Lim, M.S.; Feng, K.; Chen, X.; Wu, N.; Raman, A.; Nightingale, J.; Gawalt, E.S.; Korakakis, D.; Hornak, L.A.; Timperman, A.T. Adsorption and Desorption of Stearic Acid Self-Assembled Monolayers on Aluminum Oxide. Langmuir 2007, 23, 2444–2452. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Zhong, L.; Wang, J.; Jiang, Q.; Guo, X. Super-hydrophobic surface on pure magnesium substrate by wet chemical method. Appl. Surf. Sci. 2010, 256, 3837–3840. [Google Scholar] [CrossRef]
- Wu, R.; Liang, S.; Pan, A.; Yuan, Z.; Tang, Y.; Tan, X.; Guan, D.; Yu, Y. Fabrication of nano-structured super-hydrophobic film on aluminum by controllable immersing method. Appl. Surf. Sci. 2012, 258, 5933–5937. [Google Scholar] [CrossRef]
- Chu, Q.; Liang, J.; Hao, J. Facile fabrication of a robust super-hydrophobic surface on magnesium alloy. Colloids Surf. Physicochem. Eng. Aspects 2014, 443, 118–122. [Google Scholar] [CrossRef]
- Cui, X.; Lin, X.; Liu, C.; Yang, R.; Zheng, X.; Gong, M. Fabrication and corrosion resistance of a hydrophobic micro-arc oxidation coating on AZ31 Mg alloy. Corros. Sci. 2015, 90, 402–415. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, S.; Hu, J.; Li, T. Formation mechanism and corrosion resistance of the hydrophobiccoating on anodized magnesium. Corros. Sci. 2016, 111, 334–343. [Google Scholar] [CrossRef]
- Mojiri Forooshani, H.; Aliofkazraei, M.; Sabour Rouhaghdam, A. Superhydrophobic copper surfaces by shot penning and chemical treatment. Surf. Rev. Lett. 2017, 24, 1750093. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, S.; Cheng, Y.F. Stearic acid modified zinc nano-coatings with superhydrophobicity and enhanced antifouling performance. Surf. Coat. Technol. 2018, 340, 55–65. [Google Scholar] [CrossRef]
- Xiao, H.; Hu, A.; Hang, T.; Li, M. Electrodeposition nanostructured cobalt film and its dual modulation on both superhydrophobic property and adhesiveness. Appl. Surf. Sci. 2014, 324, 319–323. [Google Scholar] [CrossRef]
- Geng, W.; Hu, A.; Li, M. Super-hydrophilicity to super-hydrophobicity transition of a surface with Ni micro–nano cones array. Appl. Surf. Sci. 2012, 263, 821–824. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, T.-Y. Oxygen adsorption induced superhydrophilic-to-superhydrophobic transition on hierarchical nanostructured CuO surface. J. Colloid. Interface Sci. 2012, 377, 438–441. [Google Scholar] [CrossRef]
- Khorsand, S.; Raeissi, K.; Ashrafizadeh, F. Corrosion resistance and long-term durability of super-hydrophobic nickel film prepared by electrodeposition process. Appl. Surf. Sci. 2014, 305, 498–505. [Google Scholar] [CrossRef]
- Khorsand, S.; Raeissi, K.; Ashrafizadeh, F.; Arenas, M.A. Super-hydrophobic nickel-cobalt alloy coating with micro-nano flower-like structure. Chem. Eng. 2015, 273, 638–646. [Google Scholar] [CrossRef]
- Khorsand, S.; Raeissi, K.; Ashrafizadeh, F.; Arenas, M.A. Relationship between the structure and water repellency of nickel–cobalt alloy coatings prepared by electrodeposition process. Surf. Coat. Technol. 2015, 276, 296–304. [Google Scholar] [CrossRef]
- Liu, P.; Cao, P.; Zhao, W.; Xia, Y.; Huang, W.; Li, Z. Insights into the superhydrophobicity of metallic surfaces prepared by electrodeposition involving spontaneous adsorption of airborne hydrocarbons. Appl. Surf. Sci. 2015, 324, 576–583. [Google Scholar] [CrossRef]
- Liu, Y.; Yin, X.; Zhang, J.; Yu, S.; Han, Z.; Ren, L. A electro-deposition process for fabrication of biomimetic super-hydrophobic surface and its corrosion resistance on magnesium alloy. Electrochim. Acta 2014, 125, 395–403. [Google Scholar] [CrossRef]
- Liang, M.; Wei, Y.; Hou, L.; Wang, H.; Li, Y.; Guo, C. Fabrication of a super-hydrophobic surface on a magnesium alloy by a simple method. J. Alloys Compounds 2016, 656, 311–317. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, Z.; Liu, Y.; Qi, C.; Zhang, J. Reducing Friction and Wear of a Zinc Substrate by Combining a Stearic Acid Overcoat with a Nanostructured Zinc Oxide Underlying Film: Perspectives to Super-Hydrophobicity. Tribol. Lett. 2011, 44, 327–333. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, B.; Li, L.; Zeng, Z.; Zhao, W.; Wang, G.; Guan, X. Simple and green fabrication of a superhydrophobic surface by one-step immersion for continuous oil/water separation. J. Phys. Chem. A 2016, 120, 5617–5623. [Google Scholar] [CrossRef]
- Maege, I.; Jaehne, E.; Henke, A.; Adler, H.-J.P.; Bram, C.; Jung, C.; Stratmann, M. Self-assembling adhesion promoters for corrosion resistant metal polymer interface. Prog.Org. Coat. 1998, 34, 1–12. [Google Scholar] [CrossRef]
- Pertays, K.M.; Thompson, G.E.; Alexander, M.R. Self-assembly of stearic acid on aluminium: the importance of oxide surface chemistry. Surf. Interface Anal. 2004, 36, 1361–1366. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, C.; Zeng, R.; Song, L.; Guo, L.; Huang, X. Corrosion resistance of the superhydrophobic Mg(OH)2/Mg-Al layered double hydroxide coatings on magnesium alloys. Metals 2016, 6, 85. [Google Scholar] [CrossRef]
- Chen, C.; Ruengkajorn, K.; Buffet, J.-C.; O’Hare, D. Water adsorbancy of high surface area layered double hydroxides (AMO-LDHs). RCS Adv. 2018, 8, 34650. [Google Scholar] [CrossRef]
Sample Availability: Samples of thin films are available from the authors. |
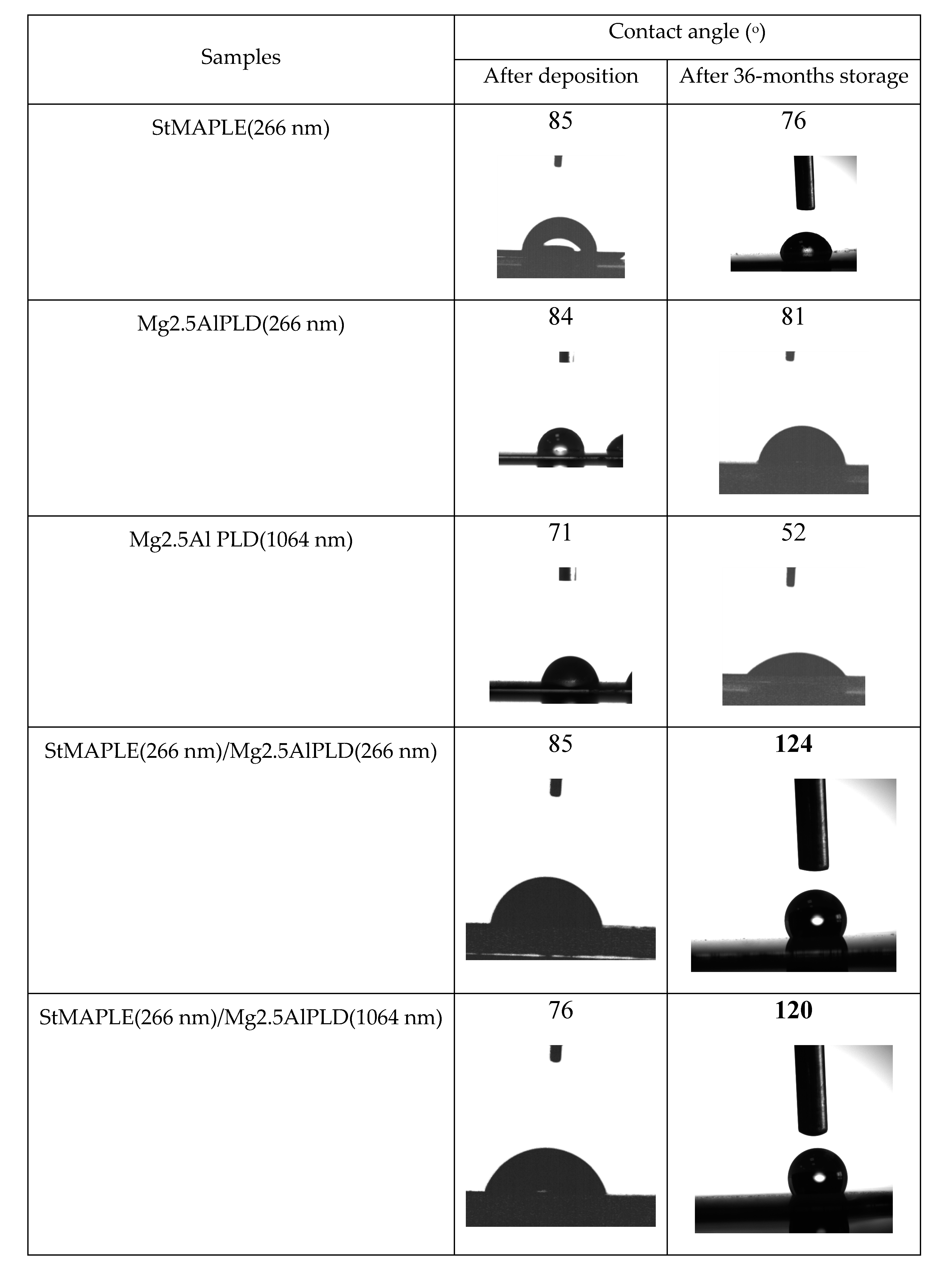
| Labels | Deposition Conditions |
|---|---|
| StMAPLE(266 nm) | Film of Pure Stearic Acid Deposited by MAPLE, at 266 nm Wavelength |
| Mg2.5AlPLD(266 nm) | Films of pristine Mg2.5Al deposited by standard PLD, at 266 nm wavelength |
| Mg2.5AlPLD(1064 nm) | Films of pristine Mg2.5Al deposited by standard PLD, at 1064 nm wavelength |
| StMAPLE (266 nm)/Mg2.5AlPLD(266 nm) | Stearic acid/layered double hydroxide composite thin films deposited by combined MAPLE-PLD: MAPLE at 266 nm and PLD at 266 nm wavelength |
| StMAPLE (266 nm)/Mg2.5AlPLD(1064 nm) | Stearic acid/layered double hydroxide composite thin films deposited by combined MAPLE-PLD: MAPLE at 266 nm and PLD at 1064 nm wavelength |
| Samples | Structural Data | RMS (nm) | |
|---|---|---|---|
| StMAPLE(266 nm) | amorphous | 8 | |
| c-oriented LDH | |||
| c (Å) | D003 (nm) | ||
| Mg2.5AlPLD(266 nm) | 23.355 | 8.9 | 80 |
| Mg2.5AlPLD(1064 nm) | 23.303 | 10.0 | 23 |
| StMAPLE (266 nm)/Mg2.5AlPLD(266 nm) | 23.188 | 7.7 | 102 |
| StMAPLE (266 nm)/Mg2.5AlPLD(1064 nm) | 23.262 | 10.1 | 18 |
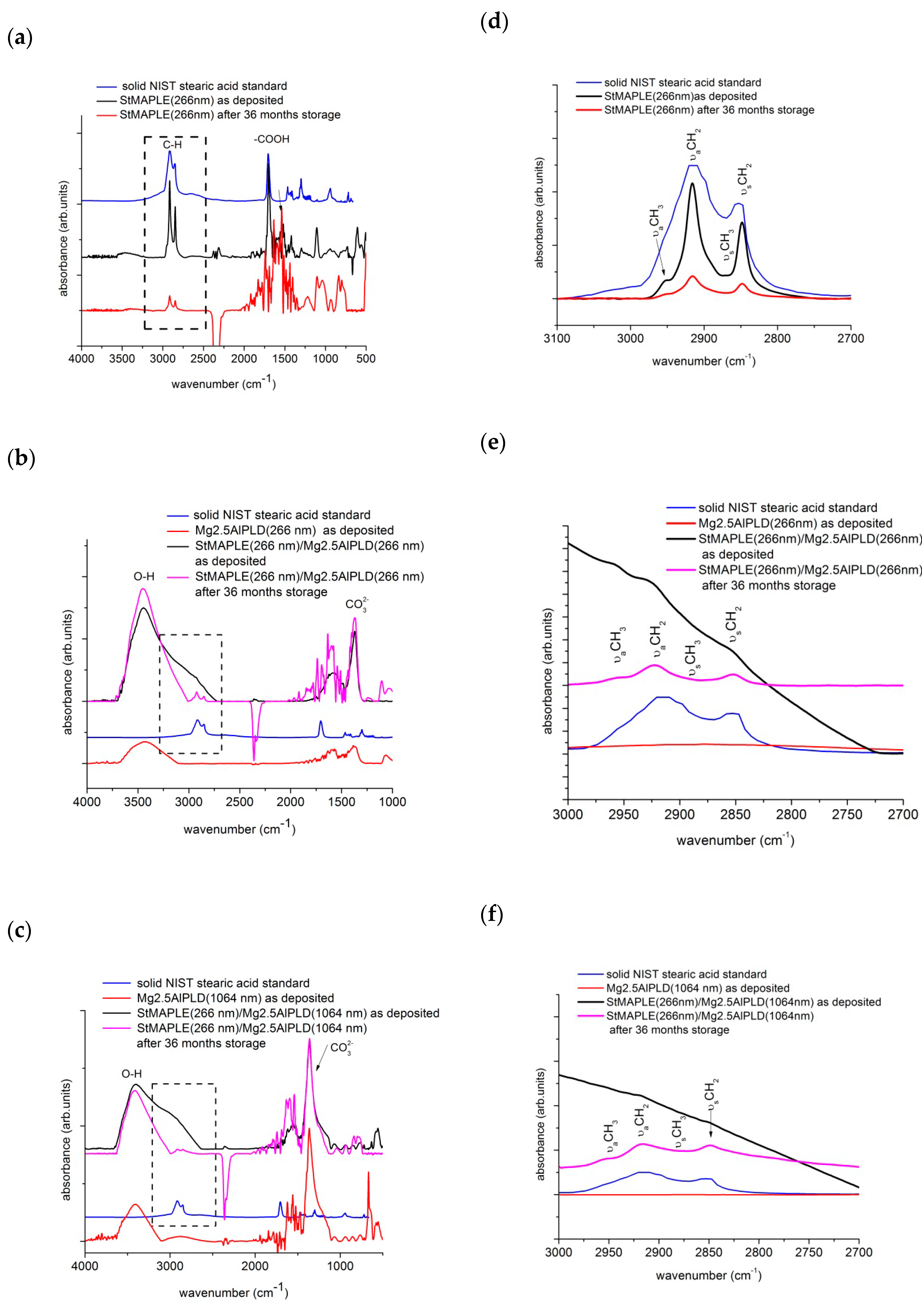
| Samples | Time | O-H Vibrations of LDH Component | C-H of Stearic Acid Vibrations | |||
|---|---|---|---|---|---|---|
| OH-M | H2O-H2O bridges | CO32--H | νaCH2 | νaCH2/νaCH3 | ||
| Stearic acid NIST standard | 2915 | 0.9 | ||||
| StMAPLE (266 nm) | as-deposited | 2916 | 17.02 | |||
| 36 months storage | 2916 | 2.62 | ||||
| Mg2.5AlPLD(266 nm) | as-deposited | 3561 cm−1 (0.04%) | 3411 cm−1 (0.83%) | 3230 cm −1 (0.13%) | ||
| StMAPLE(266 nm)/Mg2.5AlPLD(266 nm) | as-deposited | 3488 cm−1 (0.31%) | 3342 cm−1 (0.26%) | 3055cm−1 (0.43%) | ||
| 36 months storage | 3474 cm−1 (0.54%) | 3286 cm−1 (0.46 %) | - | 2923 | 10.76 | |
| Mg2.5AlPLD(1064 nm) | as-deposited | 3571 cm−1 (0.13%) | 3426 cm−1 (0.70%) | 3247 cm −1 (0.17%) | ||
| StMAPLE9266 nm)/Mg2.5AlPLD(1064 nm) | as-deposited | 3461 cm−1 (0.20%) | 3318 cm−1 (0.25%) | 3023 cm −1 (0.55%) | ||
| 36 months storage | 3445 cm−1 (0.45%) | 3271 cm−1 (0.55%) | - | 2917 | 10.25 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Birjega, R.; Matei, A.; Marascu, V.; Vlad, A.; Ionita, M.D.; Dinescu, M.; Zăvoianu, R.; Corobea, M.C. Stearic Acid/Layered Double Hydroxides Composite Thin Films Deposited by Combined Laser Techniques. Molecules 2020, 25, 4097. https://doi.org/10.3390/molecules25184097
Birjega R, Matei A, Marascu V, Vlad A, Ionita MD, Dinescu M, Zăvoianu R, Corobea MC. Stearic Acid/Layered Double Hydroxides Composite Thin Films Deposited by Combined Laser Techniques. Molecules. 2020; 25(18):4097. https://doi.org/10.3390/molecules25184097
Chicago/Turabian StyleBirjega, Ruxandra, Andreea Matei, Valentina Marascu, Angela Vlad, Maria Daniela Ionita, Maria Dinescu, Rodica Zăvoianu, and Mihai Cosmin Corobea. 2020. "Stearic Acid/Layered Double Hydroxides Composite Thin Films Deposited by Combined Laser Techniques" Molecules 25, no. 18: 4097. https://doi.org/10.3390/molecules25184097
APA StyleBirjega, R., Matei, A., Marascu, V., Vlad, A., Ionita, M. D., Dinescu, M., Zăvoianu, R., & Corobea, M. C. (2020). Stearic Acid/Layered Double Hydroxides Composite Thin Films Deposited by Combined Laser Techniques. Molecules, 25(18), 4097. https://doi.org/10.3390/molecules25184097








