1,3,4-Thiadiazole-Containing Azo Dyes: Synthesis, Spectroscopic Properties and Molecular Structure
Abstract
1. Introduction
2. Results and Discussion
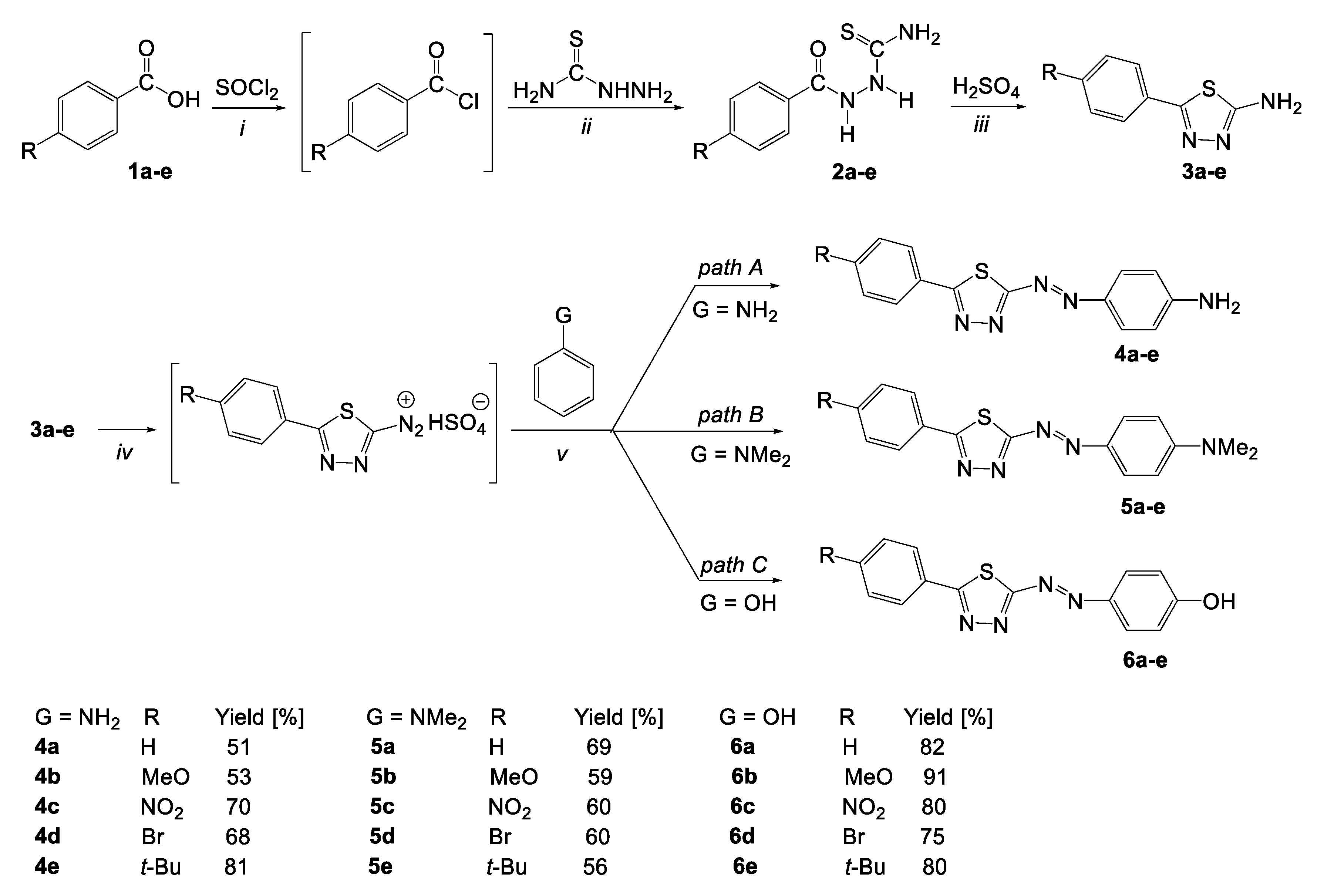
2.1. Synthesis
2.2. Spectral Characterization
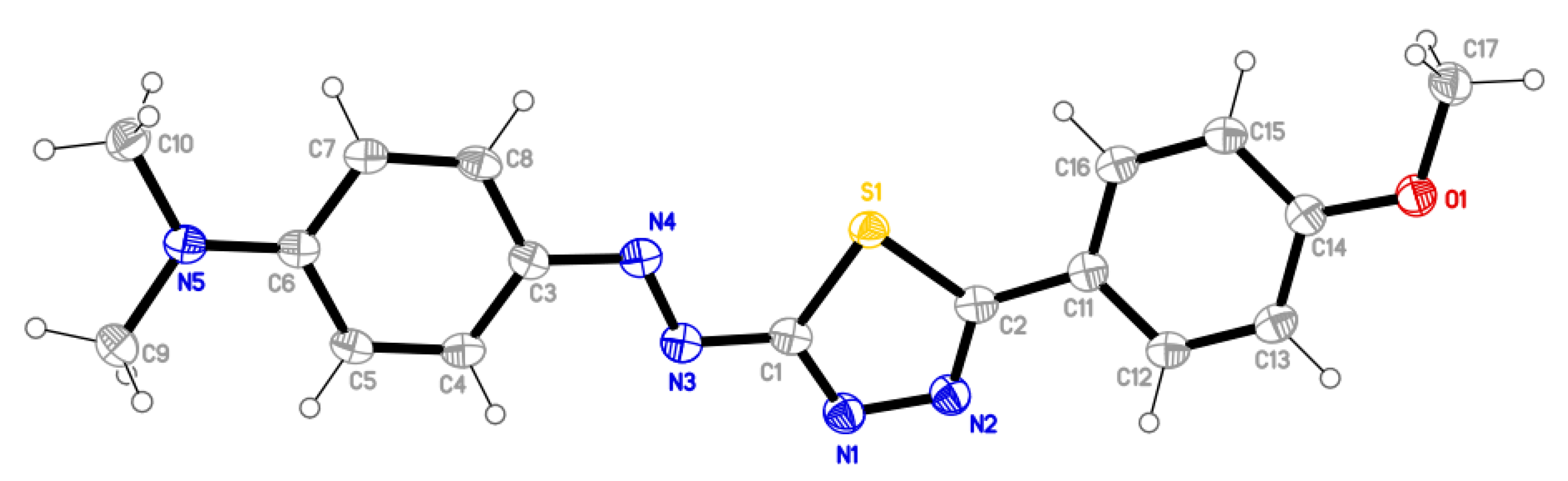
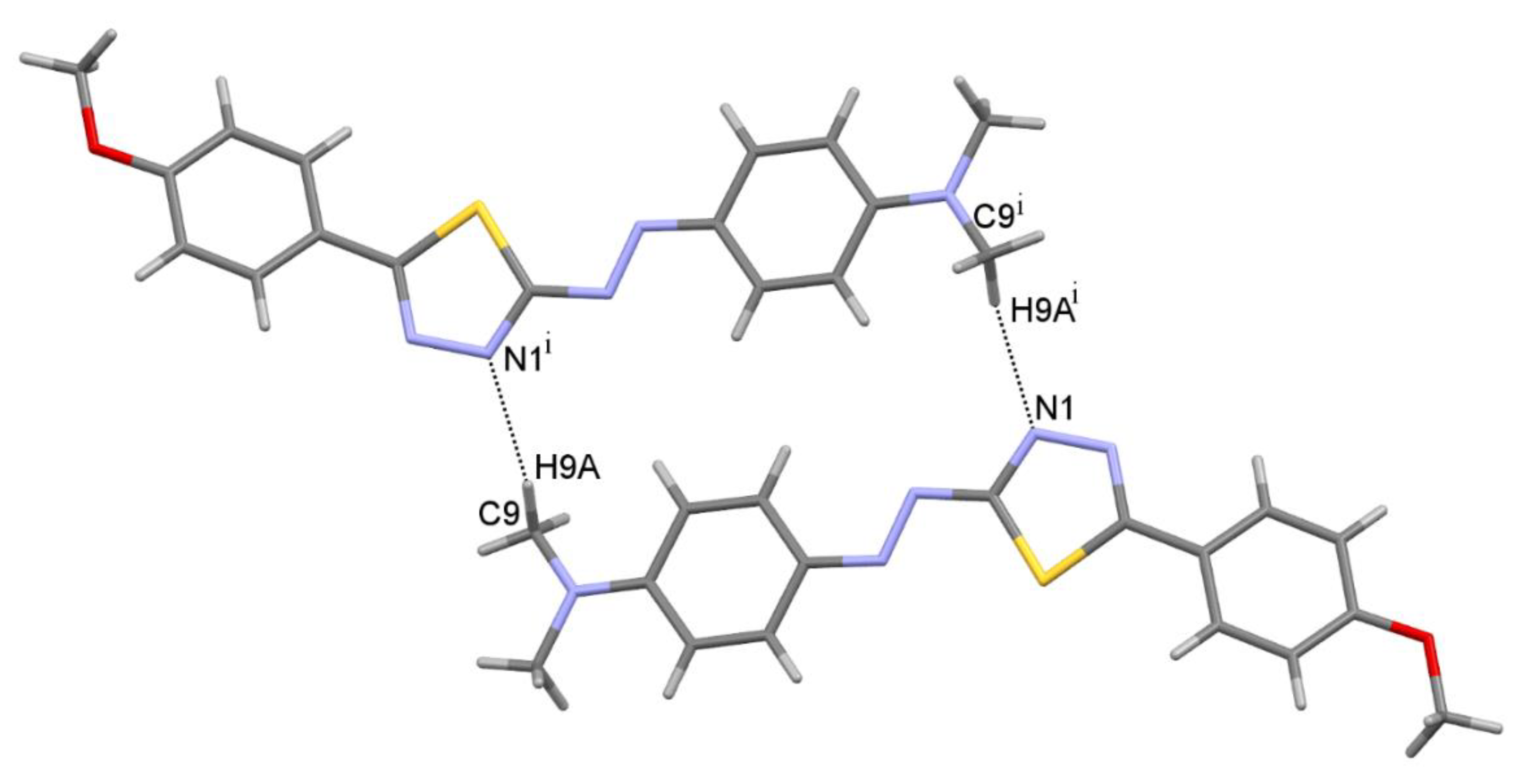
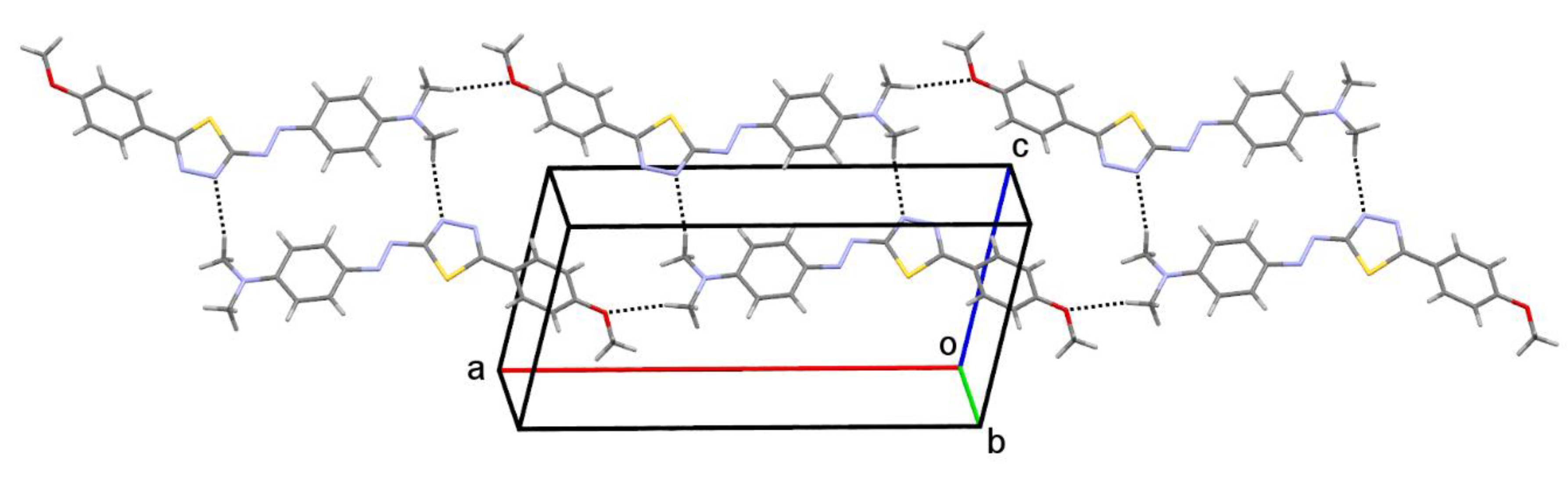
2.3. Molecular Structure
3. Experimental
3.1. General Information
3.2. Synthesis and Characterization
3.2.1. General Procedure for the Synthesis of 2-Benzoylhydrazinecarbothioamide Derivatives (2a–e)
3.2.2. General Procedure for the Synthesis of 2-Amino-1,3,4-thiadiazole Derivatives (3a–e)
3.2.3. General Procedure for the Synthesis of 2-(4-Aminophenylazo)-5-phenyl-1,3,4-thiadiazole Derivatives (4a–e)
3.2.4. General procedure for the synthesis of 2-[4-(N,N-dimethylamino)phenylazo]-5-phenyl-1,3,4-thiadiazole derivatives (5a–e)
3.2.5. General Procedure for the Synthesis of 2-(4-Hydroxyphenylazo)-5-phenyl-1,3,4-thiadiazole Derivatives (6a–e)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Koutentis, P.A.; Constantinides, C.P. 1,3,4-Thiadiazoles. In Comprehensive Heterocyclic Chemistry III; Katritzky, A., Ramsden, C.A., Scriven, E.F.V., Taylor, R.J.K., Eds.; Elsevier Science Ltd.: Oxford, UK, 2008; Volume 5, pp. 567–605. [Google Scholar]
- Hu, Y.; Li, C.; Wang, X.; Yang, Y.; Zhu, H. 1,3,4-Thiadiazole: Synthesis, reactions, and applications in medicinal, agricultural, and materials chemistry. Chem. Rev. 2014, 114, 5572–5610. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Geng, J.; Liu, Y.; Yu, S.; Zhao, G. Thiadiazole—A promising structure in medicinal chemistry. Chem. Med. Chem. 2013, 8, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Karaburun, A.; Acar Cevik, U.; Osmaniye, D.; Saglik, B.; Kaya Cavusoglu, B.; Levent, S.; Ozkay, Y.; Koparal, A.; Behcet, M.; Kaplancikli, Z.A. Synthesis and evaluation of new 1,3,4-thiadiazole derivatives as potent antifungal agents. Molecules 2018, 23, 3129. [Google Scholar] [CrossRef] [PubMed]
- Gur, M.; Sener, N.; Kastas, C.A.; Ozkan, O.E.; Muglu, H.; Elmaswaria, M.A.M. Synthesis and characterization of some new heteroaromatic compounds having chirality adjacent to a 1,3,4-thiadiazole moiety and their antimicrobial activities. J. Heterocycl. Chem. 2017, 54, 3578–3590. [Google Scholar] [CrossRef]
- Hafez, H.N.; Hegab, M.I.; Ahmed-Farag, I.S.; El-Gazzar, A.B.A. A facile regioselective synthesis of novel spiro-thioxanthene and spiro-xanthene-9′,2-[1,3,4]thiadiazole derivatives as potential analgesic and anti-inflammatory agents. Bioorg. Med. Chem. 2008, 18, 4538–4543. [Google Scholar] [CrossRef]
- Altintop, M.D.; Sever, B.; Ozdemir, A.; Iglin, S.; Alti, O.; Turan-Zitouni, G.; Kaplancikli, Z.A. Synthesis and evaluation of a series of 1,3,4-thiadiazole derivatives as potential anticancer agents. Anticancer Agents Med. Chem. 2019, 18, 1606–1616. [Google Scholar] [CrossRef]
- Richwine, J.R. 2,5-Dimercapto-1,3,4-thiadiazole as a Cross-Linker for Saturated Halogen-Containing Polymers. Inventor: Hercules Incorporated Wilmington, Assignee. U.S. Patent 4288576, 8 September 1981. [Google Scholar]
- Jin, L.; Wang, G.; Li, X. Poly(2,5-dimercapto-1,3,4-thiadiazole)/sulfonated graphene composite as cathode material for rechargeable lithium batteries. J. Appl. Electrochem. 2011, 41, 377–382. [Google Scholar] [CrossRef]
- Maradiya, H.R. Monoazo disperse dyes based on 2-amino-1,3,4-thiadiazole derivatives. J. Serb. Chem. Soc. 2002, 67, 709–718. [Google Scholar] [CrossRef]
- Yan, H.; Su, H.; Tian, D.; Miao, F.; Li, H. Synthesis of triazolo-thiadiazole fluorescent organic nanoparticles as primary sensor toward Ag+ and the complex of Ag+ as secondary sensor toward cysteine. Sens. Actuators B Chem. 2011, 160, 656–661. [Google Scholar] [CrossRef]
- Hipler, F.; Fischer, R.A.; Muller, J. Matrix-isolation pyrolysis investigation of mercapto-functionalized 1,3,4-thiadiazoles: Thermal stability of thiadiazole lubricant additives. Phys. Chem. Chem. Phys. 2005, 7, 731–737. [Google Scholar] [CrossRef]
- Dawson, J.F. Developments in disperse dyes. Color. Technol. 1978, 9, 25–35. [Google Scholar] [CrossRef]
- Arcoria, A.; De Giorgi, M.R.; Fatuzzo, F.; Longo, M.L. Dyeing properties of basic azo-dyes from 2-amino thiadiazole. Dyes Pigment. 1993, 21, 67–74. [Google Scholar] [CrossRef]
- De Giorgi, M.R.; Carpignano, R.; Cerniani, A. Structure optimization in a series of thiadiazole disperse dyes using a chemometric approach. Dyes Pigment. 1998, 37, 187–196. [Google Scholar] [CrossRef]
- Zollinger, H. Azo dyes and Pigments. In Color Chemistry: Syntheses, Properties, and Applications of Organic Dyes and Pigments, 3rd ed.; Viley-VCH: Zurich, Switzerland, 2003; pp. 165–254. [Google Scholar]
- Ullrich, R.; Grewer, T. Decomposition of aromatic diazonium compounds. Thermochim. Acta 1993, 225, 201–211. [Google Scholar] [CrossRef]
- Zhao, R.; Tan, C.; Xie, Y.; Gao, C.; Liu, H.; Jiang, Y. One step synthesis of azo compounds from nitroaromatics and anilines. Tetrahedron Lett. 2011, 52, 3805–3809. [Google Scholar] [CrossRef]
- Chung, T.F.; Wu, Y.M.; Cheng, C.H. Reduction of aromatic nitro compounds by ethylenediamine. A new selective reagent for the synthesis of symmetric azo compounds. J. Org. Chem. 1984, 49, 1215–1217. [Google Scholar] [CrossRef]
- Srinivasa, G.R.; Abiraj, K.; Channe Gowda, D. Lead-catalyzed synthesis of azo compounds by ammonium acetate reduction of aromatic nitro compounds. Synth. Commun. 2003, 33, 4221–4227. [Google Scholar] [CrossRef]
- Noureldin, N.A.; Bellegarde, J.W. A novel method. The synthesis of ketones and azobenzenes using supported permanganate. Synthesis 1999, 6, 939–942. [Google Scholar] [CrossRef]
- Bhatnagar, I.; George, M.V. Oxidation with metal oxides. III. Oxidation of diamines and hydrazines with manganese dioxide. J. Org. Chem. 1968, 33, 2407–2411. [Google Scholar] [CrossRef]
- Faustino, H.; El-Shisthawy, R.M.; Reis, L.V.; Santos, P.F.; Almeida, P. 2-Nitrosobenzothiazoles: Useful synthons for new azobenzothiazole dyes. Tetrahedron Lett. 2008, 49, 6907–6909. [Google Scholar] [CrossRef]
- Tambe, S.M.; Tasaganva, R.G.; Inamdar, S.R.; Kariduraganavar, M.Y. Synthesis and characterization of nonlinear optical side-chain polyimides containing the thiadiazole chromophores. J. Appl. Pol. Sci. 2012, 125, 1049–1058. [Google Scholar] [CrossRef]
- Tomi, I.H.R.; Al-Daraji, A.H.R.; Al-Qaysi, R.R.T.; Hasson, M.M.; Al-Dulaimy, K.H.D. Synthesis, characterization and biological activities of some azo derivatives of aminothiadiazole derived from nicotinic and isonicotinic acids. Arab. J. Chem. 2014, 7, 687–694. [Google Scholar] [CrossRef]
- Kumar, C.T.K.; Keshavayya, J.; Rajesk, T.N.; Peethambar, S.K.; Ali, A.R.S. Synthesis, characterization, and biological activity of 5-phenyl-1,3,4-thiadiazole-2-amine incorporated azo dye derivatives. Org. Chem. Int. 2013, 370626. [Google Scholar] [CrossRef]
- Kedzia, A.; Kudelko, A.; Swiatkowski, M.; Kruszynski, R. Microwave-promoted synthesis of highly luminescent s-tetrazine-1,3,4-oxadiazole and s-tetrazine-1,3,4-thiadiazole hybrids. Dyes Pigment. 2020, 172, 107865. [Google Scholar] [CrossRef]
- Wróblowska, M.; Kudelko, A.; Kuźnik, N.; Łaba, K.; Łapkowski, M. Synthesis of Extended 1,3,4-Oxadiazole and 1,3,4-Thiadiazole Derivatives in the Suzuki Cross-Coupling Reactions. J. Heterocycl. Chem. 2017, 54, 1550–1557. [Google Scholar] [CrossRef]
- Wróblowska, M.; Kudelko, A.; Łapkowski, M. Efficient Synthesis of Conjugated 1,3,4-Thiadiazole Hybrids through Palladium-Catalyzed Cross Coupling of 2,5-Bis(4-bromophenyl)-1,3,4-thiadiazole with Boronic Acids. Synlett 2015, 26, 2127–2130. [Google Scholar] [CrossRef]
- Mavrova, A.T.; Wesselinova, D.; Tsenov, Y.A.; Denkova, P. Synthesis, cytotoxity and effects of some 1,2,4-triazole and 1,3,4-thiadiazole derivatives on immunocompetent cells. Eur. J. Med. Chem. 2009, 44, 63–69. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. B 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Cowley, J.M. Scattering factors for the diffraction of electrons by crystalline solids. In International Tables for Crystallography, Volume C: Mathematical, Physical and Chemical Tables, 3rd ed.; Prince, E., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; pp. 259–262. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
- Desiraju, G.R.; Steiner, T. The Weak Hydrogen Bond in Structural Chemistry and Biology; Oxford University Press: Oxford, UK, 1999; pp. 293–342. [Google Scholar]
- Kruszynski, R.; Sieranski, T. Can stacking interactions exist beyond the commonly accepted limits? Cryst. Growth Des. 2016, 16, 587–595. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision, D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comp. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum mechanical continuum solvation models. Chem. Rev. 2005, 105, 2999–3093. [Google Scholar] [CrossRef]
- Campaigne, E.; Selby, T.P. Thiazoles and thiadiazines. The condensation of ethyl 4-chloroacetoacetate with thiosemicarbazide. J. Heterocycl. Chem. 1978, 15, 401–411. [Google Scholar] [CrossRef]
- Plumitallo, A.; Cardia, M.C.; Distinto, S.; DeLogu, A.; Maccioni, E. Synthesis and anti-microbial activity evaluation of some new 1-benzoyl-isothiosemicarbazides. Farmaco 2004, 59, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Malbec, F.; Milcent, R.; Barbier, G. Dérivés de la dihydro-2,4 triazole-1,2,4 thione-3 et de l’amino-2 thiadiazole-1,3,4 à partir de nouvelles thiosemicarbazones d’esters. J. Heterocycl. Chem. 1984, 21, 1689–1698. [Google Scholar] [CrossRef]
- Giri, S.; Nizamuddin Srivastava, U.C. Synthesis of some N-(5-aryl/aryloxymethyl-1,3,4-thiadiazol-2-yl)glyoxylamide thiosemicarbazones as potential antiviral and antifungal agents. Agric. Biol. Chem. 1983, 47, 103–105. [Google Scholar] [CrossRef]
- Jatav, V.; Mishra, P.; Kashaw, S.; Stables, J.P. Synthesis and CNS depressant activity of some novel 3-[5-substituted 1,3,4-thiadiazole-2-yl]-2-styryl quinazoline-4(3H)-ones. Eur. J. Med. Chem. 2008, 43, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.S.; Gibaldi, D.; Pinto, A.C.; Bozza, M.; Boechat, N. Synthesis and trypanocidal evaluation of news 5-[N-(3-(5-substituted)-1,3,4-thiadiazolyl)]amino-1-methyl-4-nitroimidazoles. Lett. Drug Des. Discov. 2006, 3, 98–101. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 4a–e, 5a–e, 6a–e are available from the authors. |

| λmax (nm) | The Most Important Orbitals Involved in Electronic Transitions | Character of Transition | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Experimental | Calculated | ||||||||||
| 4 (G=NH2) | |||||||||||
| a | b | c | d | e | a | b | c | d | e | ||
| 237 (3.95) | 256 (4.05) | 267 (4.07) | 249 (3.90) | 248 (4.02) | 261.44 (0.0909) | 276.70 (0.1045) | 237.50 (0.0536) | 256.39 (0.0432) | 270.17 (0.2800) | (a, e)H-2→L+1 (b)H→L+2 (c)H-2→L+2 (d)H-8→L | (a, e)n(NH2)/π→π* (b)n(NH2)/n(MeO)/π→π*(C6-rings) (c)n(NH2)/n(NO2)/π→π* (d)n(Br)/π(S-ring)→π* |
| 262.74 (0.0776) | 280.67 (0.2599) | 253.28 (0.0806) | 274.45 (0.3976) | 273.29 (0.1360) | (a)H-6→L (b)H-1→L+1 (c)H-6→L+1 (d)H-2→L+1 (e) H→L+2 | (a)n(NH2)/π→π* (b)n(NH2)/n(MeO)/π→π* (c)π→π* (d)n(Br)/n(NH2)/π→π* (e)n(NH2)/π→π*(C6-rings) | |||||
| 266.87 (0.1051) | 268.23 (0.0893) | (a)H→L+3 (c)H→L+4 | (a, c)n(NH2)/π→π*(C6-rings) | ||||||||
| 272.57 (0.1083) | (a)H→L+2 | (a)n(NH2)/π→π*(C6-rings) | |||||||||
| 285 (3.72) | 286 (3.88) | 286 (3.63) | 284 (3.79) | ||||||||
| 316 (3.56) | 319 (3.78) | 321 (4.03) | 318 (3.56) | 319 (3.68) | 325.66 (0.0203) | 322.13 (0.0500) | 312.40 (0.0522) | 333.77 (0.0204) | 324.73 (0.0294) | (a, b, d, e)H→L+1 (c)H-2→L+1 | (a, c, e)n(NH2)/π→π* (b)n(NH2)/n(MeO)/π→π* (d)n(Br)/n(NH2)/π→π* |
| 344.08 (0.1372) | (c)H-2→L | (c)n(NH2)/π→π* | |||||||||
| 491 (4.52) | 491 (4.46) | 507 (4.35) | 496 (4.41) | 492 (4.47) | 383.91 (0.1281) | 428.57 (0.7203) | (b)H-1→L (c)H→L+1 | (b)n(NH2)/n(MeO)/π→π* (c)n(NH2)/π→π* | |||
| 459.54 (1.4831) | 478.70 (1.3233) | 529.34 (0.8884) | 464.44 (1.4918) | 464.82 (1.4522) | H→L | (a, c, e)n(NH2)/π→π* (b) n(NH2)/n(MeO)/π→π* (d)n(Br)/n(NH2)/π→π* | |||||
| 5 (G=NMe2) | |||||||||||
| a | b | c | d | e | a | b | c | d | e | ||
| 243 (3.96) | 256 (3.83) | 249 (3.73) | 251 (3.80) | 245 (4.06) | 264.95 (0.1359) | 278.83 (0.0592) | 241.40 (0.0442) | 256.30 (0.0430) | 270.21 (0.0655) | (a)H-2→L+1 (b, e) H-1→L+1 (c)H-2→L+2 (d)H-8→L | (a)π→π* (b) n(MeO)/n(NMe2)/π→π* (c)n(NO2)/π→π* (d)n(Br)/π→π* (e)σ(t-Bu)/n(Me2)/π→π* |
| 275.87 (0.1374) | 283.75 (0.1408) | 261.09 (0.0967) | 274.64 (0.1508) | 270.38 (0.0853) | (a)H-6→L (b)H→L+2 (c)H-6→L+1 (d)H-2→L+1 (e)H-1→L+1 | (a, c)π→π* (b)n(MeO)/n(NMe2)/π→π*(C6-ring near MeO) (d)n(Br)/n(NMe2)/π→π* (e)σ(t-Bu)/n(Me2)/π→π* | |||||
| 281.40 (0.0925) | 287.77 (0.2354) | 277.34 (0.1087) | 282.30 (0.2530) | 279.49 (0.2116) | (a)H→L+3 (b, d)H-5→L (c)H→L+4 (e)H-6→L | (a)n(NMe2)/π→π* (b)n(MeO)/π→π* (c)n(NMe2)/π→π*(C6-ring near NMe2) (d)n(Br)/π→π* (e)σ(t-Bu)/π→π* | |||||
| 281.87 (0.0869) | (e)H→L+2 | (e)n(NMe2)/π→π*(C6-rings) | |||||||||
| 293 (3.79) | 293 (3.73) | 299 (3.85) | 290 (3.61) | 295 (3.92) | |||||||
| 320 (3.68) | 334 (3.46) | 325 (3.49) | 323 (3.80) | 337.91 (0.0197) | 333.19 (0.0329) | 353.80 (0.1689) | 346.81 (0.0206) | 336.73 (0.0263) | (a, b, d, e)H→L+1 (c)H-2→L | (a, d, e)n(NMe2)/π→π* (b)n(MeO)/n(NMe2)/π→π* (c)n(NO2)/π→π* | |
| 515 (4.50) | 515 (4.25) | 530 (3.95) | 520 (4.30) | 515 (4.41) | 393.91 (0.0395) | 453.15 (0.8353) | (b)H-1→L (c)H→L+1 | (b)n(MeO)/n(NMe2)/π→π* (c)n(NMe2)/π→π* | |||
| 483.86 (1.4831) | 497.00 (1.1993) | 568.89 (0.8657) | 488.69 (1.5864) | 487.35 (1.5439) | H→L | (a, c, d, e)n(NMe2)/π→π* (b)n(MeO)/n(NMe2)/π→π* | |||||
| 6 (G=OH) | |||||||||||
| a | b | c | d | e | a | b | c | d | e | ||
| 220.52 (0.0983) | (b)H-4→L+1 | (b)π(C6-ring near OH)→π* | |||||||||
| 236 (4.00) | 231 (3.68) | 234.73 (0.0439) | 232.01 (0.0382) | (a)H→L+3 (c)H-2→L+2 | (a)n(OH)/π→π*(C6-ring near S-ring) (c)n(NO2)/(OH)/π→π* | ||||||
| 256 (3.90) | 254 (4.04) | 260 (3.72) | 245 (4.04) | 243 (4.10) | 251.23 (0.0758) | 234.51 (0.0525) | 249.20 (0.0821) | 238.98 (0.0306) | 234.61 (0.0464) | (a, e)H→L+2 (b)H-3→L+1 (c)H-8→L (d)H-4→L+1 | (a)n(OH)/π→π*(C6-ring near OH) (b)π(C6-ring near MeO)→π* (c)n(NO2)/(OH)/π→π* (d)π(C6-rings)→π* (e)σ(t-Bu)/n(OH)/π→π*(C6-ring near t-Bu) |
| 254.98 (0.1078) | 271.99 (0.1002) | 264.75 (0.0369) | 251.23 (0.0556) | 265.07 (0.2413) | (a)H-2→L+1 (b, c, e)H-6→L (d)H→L+3 | (a, c)n(OH)/π→π* (b)n(MeO)/n(OH)/π→π* (d)n(Br)/n(OH)/π→π*(C6-ring near OH) (e)σ(t-Bu)/n(OH)/π→π* | |||||
| 262.06 (0.1766) | 266.81 (0.1192) | (a)H-6→L+1 (d)H-2→L+1 | (a)n(OH)/π→π* (d)n(Br)/n(OH)/π→π* | ||||||||
| 270.78 (0.1692) | (d)H-6→L | (d)n(Br)/n(OH)/π→π* | |||||||||
| 298 (3.54) | 291 (3.74) | 290 (3.76) | 290 (3.64) | 297.41 (0.0427) | 302.67 (0.1542) | 304.17 (0.0596) | 298.30 (0.0985) | (a, b, c, e)H→L+1 | (a)n(OH)/π→π* (b)n(MeO)/n(OH)/π→π* (d)n(Br)/n(OH)/π→π* (e)σ(t-Bu)/n(OH)/π→π* | ||
| 325.44 (0.0438) | (a)H-4→L | (a)π(C6-ring near OH)→π* | |||||||||
| 310 (3.74) | 305.19 (0.1361) | (c)H-2→L+1 | (c)n(NO2)/(OH)/π→π* | ||||||||
| 405 (4.46) | 415 (4.35) | 410 (4.13) | 409 (4.28) | 409 (3.96) | 341.08 (0.0630) | (c)H-3→L | (c)π(C6-ring near OH)→π* | ||||
| 345.44 (0.0461) | 370.43 (0.3618) | 385.35 (0.2630) | 355.56 (0.0770) | 355.42 (0.1288) | (a, d, e)H-2→L (b)H-1→L (c)H-1→L+1 | (a, c)n(OH)/π→π* (b)n(MeO)/n(OH)/π→π* (d)n(Br)/n(OH)/π→π* (e)σ(t-Bu)/n(OH)/π→π* | |||||
| 432.77 (1.1904) | 471.46 (0.9677) | 458.02 (1.1983) | 439.27 (1.2728) | 444.00 (1.1983) | H→L | (a, c)n(OH)/π→π* (b)n(MeO)/n(OH)/π→π* (d)n(Br)/n(OH)/π→π* (e)σ(t-Bu)/n(OH)/π→π* | |||||
| i—j | dij [Å] | i—j—k | αijk [°] | i—j—k | αijk [°] |
|---|---|---|---|---|---|
| S1—C1 | 1.7462(14) | C1—S1—C2 | 86.22(7) | C2—C11—C16 | 122.25(13) |
| S1—C2 | 1.7366(14) | S1—C2—N2 | 114.29(11) | C5—C6—N5 | 121.16(12) |
| C1—N1 | 1.3092(18) | C2—N2—N1 | 112.80(12) | C7—C6—N5 | 121.27(12) |
| C2—N2 | 1.3124(18) | N2—N1—C1 | 112.25(12) | C6—N5—C9 | 120.76(12) |
| N1—N2 | 1.3757(17) | N1—C1—S1 | 114.42(11) | C6—N5—C10 | 120.34(12) |
| C1—N3 | 1.3821(18) | N1—C1—N3 | 120.62(13) | C9—N5—C10 | 118.16(12) |
| N3—N4 | 1.2907(16) | S1—C1—N3 | 124.91(10) | C13—C14—O1 | 115.86(12) |
| N4—C3 | 1.3851(18) | C1—N3—N4 | 111.67(11) | C15—C14—O1 | 124.38(13) |
| C2—C11 | 1.4651(19) | N3—N4—C3 | 115.29(11) | C14—O1—C17 | 117.14(11) |
| N5—C6 | 1.3520(18) | N4—C3—C4 | 125.47(12) | ||
| N5—C9 | 1.4635(18) | N4—C3—C8 | 116.30(12) | ||
| N5—C10 | 1.4606(18) | S1—C2—C11 | 122.98(10) | ||
| O1—C14 | 1.3610(17) | N2—C2—C11 | 122.55(13) | ||
| O1—C17 | 1.4346(17) | C2—C11—C12 | 118.81(13) |
| D-H•••A | d(D-H) [Å] | d(H•••A) [Å] | d(D•••A) [Å] | <(DHA) [°] | Gda(n) |
|---|---|---|---|---|---|
| C9—H9A•••N1 i | 0.98 | 2.58 | 3.5351(1) | 165.4 | R22(22) |
| C10—H10B•••O1 ii | 0.98 | 2.54 | 3.5031(1) | 166.3 | C(17) |
| C16—H16•••S1 | 0.95 | 2.86 | 3.2088(1) | 102.7 | S(5) |
| C7—H7•••Cg(C3) iii | 0.95 | 2.97 | 3.8190(1) | 148.8 | C(2) |
| R(I)•••R(J) | d(Cg•••Cg) [Å] | α [°] | β [°] | dp [Å] |
|---|---|---|---|---|
| C3•••C3 iv | 3.6217(1) | 0 | 15.5 | 3.4908 |
| C11•••C11 v | 3.5355(1) | 0 | 22.3 | 3.2703 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudelko, A.; Olesiejuk, M.; Luczynski, M.; Swiatkowski, M.; Sieranski, T.; Kruszynski, R. 1,3,4-Thiadiazole-Containing Azo Dyes: Synthesis, Spectroscopic Properties and Molecular Structure. Molecules 2020, 25, 2822. https://doi.org/10.3390/molecules25122822
Kudelko A, Olesiejuk M, Luczynski M, Swiatkowski M, Sieranski T, Kruszynski R. 1,3,4-Thiadiazole-Containing Azo Dyes: Synthesis, Spectroscopic Properties and Molecular Structure. Molecules. 2020; 25(12):2822. https://doi.org/10.3390/molecules25122822
Chicago/Turabian StyleKudelko, Agnieszka, Monika Olesiejuk, Marcin Luczynski, Marcin Swiatkowski, Tomasz Sieranski, and Rafal Kruszynski. 2020. "1,3,4-Thiadiazole-Containing Azo Dyes: Synthesis, Spectroscopic Properties and Molecular Structure" Molecules 25, no. 12: 2822. https://doi.org/10.3390/molecules25122822
APA StyleKudelko, A., Olesiejuk, M., Luczynski, M., Swiatkowski, M., Sieranski, T., & Kruszynski, R. (2020). 1,3,4-Thiadiazole-Containing Azo Dyes: Synthesis, Spectroscopic Properties and Molecular Structure. Molecules, 25(12), 2822. https://doi.org/10.3390/molecules25122822









