Abstract
The C3 direct arylation of 1H-indazole and 1H-7-azaindazole has been a significant challenge due to the lack of the reactivity at this position. In this paper, we describe a mild and an efficient synthesis of new series of C3-aryled 1H-indazoles and C3-aryled 1H-7-azaindazoles via a C3 direct arylation using water as solvent. On water, PPh3 was effective as a ligand along with a lower charge of the catalyst Pd(OAc)2 (5 mol%) at 100 °C, leading to C3-aryled 1H-indazoles or C3-aryled 1H-7-azaindazoles in moderate to good yields.
1. Introduction
In recent years, the C–H activation has risen as an increasingly powerful tool for molecular sciences with notable applications in organic synthesis [1,2,3,4,5]. This method has gained considerable recent momentum as a significantly environmentally and economically attractive alternative to classical cross-coupling such as Suzuki–Miyaura, Negishi, and Stille reactions [6,7,8,9,10]. With fewer steps and accessible reagents, complex organic molecules are nowadays easily accessible with C–H activation [11,12,13,14,15,16,17,18,19,20]. Nevertheless, this reaction usually requires high temperatures and organic solvents to achieve new C(sp2)–C(sp2) bonds [21]. For economic and environmental concerns, we are aiming at developing a new C–H direct arylation of 1H-indazole and 1H-7-azaindazole by using water as a green solvent. Water is nature’s primordial solvent to carry out synthesis [22]. It is known that water can increase the rate and affect the selectivity of a wide variety of organic reactions [23,24,25,26]. It was also found that on watery conditions, the heterogeneous mixture of substrates and catalyst can be very effective for direct arylation under mild conditions [27].
1H-indazoles and 2H-indazoles have widespread utility as highly bioactive molecules [28]. For such reasons, various researchers were inspired to develop and optimize new methods for their synthesis and functionalization [29,30,31,32]. Recently, different research groups have investigated the C3 direct arylation of indazole, including our own. In 2012, our group [33] in parallel with the Itami group [34] developed, for the first time, conditions to realize the direct arylation of 1H-indazole series. The key of this success was the use of bidentate ligand (1,10-phenanthroline) with a high catalyst loading (10 to 20 mol%). Later, the reaction conditions have been optimized by the Yu group by reducing catalyst and ligand loading [35]. In this report, only one example of C3 arylation of 1H-7-azaindazole was reported using Pd(OAc)2 as a catalyst, and Phen as a ligand in toluene at 160 °C (Scheme 1). Very recently, our group [36,37] and later Popowycz’s [38] group reported independently that the C3 direct arylation of azaindazoles was feasible but using again 1,10-phenanthroline (Phen) as a crucial ligand in the case of 1H series for both 4-azaindazoles and 7-azaindazoles. In 2017, Doucet and his group reported a phosphine free C3 arylation of 2H-indazoles in dimethylacetamide DMA using 5 mol% of Pd(OAc)2 as catalyst at 150 °C [39]. We noticed in their report that the C3 direct arylation of 1H-indazole was feasible for the first time without the use of bidentate ligands by employing PdCl(C3H5)(dppb) as catalyst and KOAc as base in DMA at 150 °C. Nevertheless, the expected products were obtained in moderate to low yields, and the reaction was achieved in organic solvent. It is noteworthy that all the reported methods on C3 arylation of 1H-indazole discussed above were carried out in organic solvents and at high temperatures. In 2010, Greaney et al. [32] reported that 2-phenyl-2H-indazole could be directly arylated at the C3 position in high yields using aryl bromides as coupling partners on water in the presence of Pd(dppf)Cl2 (5 mol%) and Ph3P (10 mol%) as catalyst and ligand, respectively. Unfortunately, under these reaction conditions, the authors failed to achieve direct arylation on 1-phenyl-1H-indazole, yet they concluded that, in this case, the C3 position is non-reactive toward substitution.
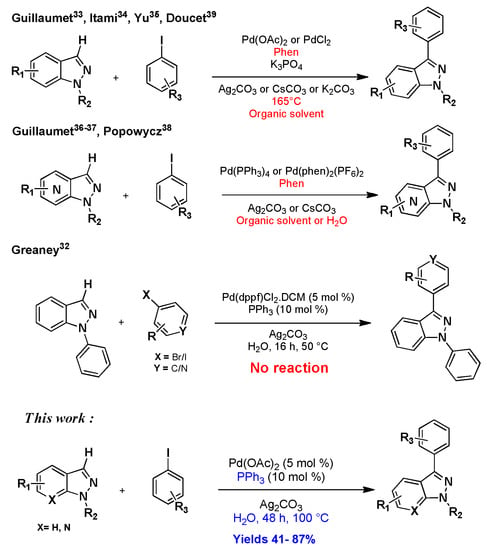
Scheme 1.
Reported methods on C3 arylation of 1H-indazoles and 1H-azaindazoles versus our new procedure.
Herein, we reported a new path for the direct and selective C3 arylation of 1H-indazoles on water. We showed, for the first time, that a phosphine ligand (PPh3), contrary to the bidentate (Phen) ligand used in the case of organic solvent, is crucial to achieve the arylation reaction (Scheme 1). We are striving also to report the first examples of “on water” C3 arylation of 1H-7-azaindazoles. We showed as well that a low charge of the catalyst (5 mol%) and ligand (10 mol%) leads to desired products in acceptable to good yields.
It is noticed that Knochel et al. used 3-zincated indazoles which undergo palladium-catalyzed Negishi cross-couplings to give the C3 substituted indazole derivatives [40], while, Burton et al. described a regioselective iridium-catalyzed C3 borylation of 1H-indazoles, followed by subsequent Suzuki–Miyaura coupling with aryl chlorides [41]. Compared to the methodology described in our manuscript, the two methodologies cited above are more difficult to implement and also less economical.
2. Results
Firstly, we tested Greaney’s reaction conditions [Ag2CO3 (1 equivalent), PPh3 (10 mol%), Pd(dppf)Cl2·DCM (5 mol%), 4-iodotoluene (1.1 equivalent), water at 50 °C for 16 h] [32] on 1-methyl-1H-indazole 1. The arylation reaction did not occur, and only starting material 1 was recovered (Table 1, entry 1).

Table 1.
Optimization of the ‘’on water’’ direct arylation of 1-methyl-7-nitro-1H-indazole.
Then, in the presence of palladium(II)acetate and 1,10-phenanthroline as a bidentate ligand, again no reaction was observed, and only starting material 1 was recovered (Table 1, entry 2). Changing the type of the ligand to triphenylphosphine, a monodentate ligand, which may form a stronger coordination with Pd(II) centers, led to the desired arylated product 1a in 40% yield (Table 1, entry 3). Encouraged by this result, we decided to improve the reaction’s conversion by screening different conditions. At first, we tried to increase the temperature and to change the heating system with our efforts leading to no avail (Table 1, entries 4–6). Water/ethanol solution was also used to increase the solubility, but only a decrease of the reaction yield (17%) was observed (Table 1, entry 7). Using (2 equiv.) of iodotoluene slightly improved the reaction yield (entry 8). Luckily, increasing the amount of iodotoluene to (3 equiv.) furnished 1a to 86% isolated yield and with a total conversion (Table 1, entry 9). Furthermore, we succeeded in reducing the loading of both palladium and PPh3 ligand to 5 and 10% respectively (Table 1, entry 11). When the reaction was run for 24 h instead of 48 h, the yield dramatically decreased (Table 1, entry 12). Notably, the reaction temperature can be reduced to 100 °C without affecting the reaction yield (Table 1, entry 13). Using Phen instead of Ph3P as a ligand, under the optimized reaction conditions, led to a total loss of the reactivity (Table 1, entry 14). The reaction under reflux of water in an open flask was not total and 10% of starting material 1 was recovered (Table 1, entry 15). In addition, when 4-iodotoluene was replaced by 4-bromotoluene, the arylation did not occur, and only starting material 1 was recovered (Table 1, entry 16).
The scope and limitation of the C3 arylation reaction of various substituted indazoles 1 and 2 were examined (their preparation is described in the Supporting Information). We used various iodoaryl derivatives carrying electron-donating or electron-withdrawing groups (such as methoxy, chlorine, ester, nitro, trifluoromethyl, or methyl groups) under the most optimal reaction conditions [5 mol% Pd(OAc)2 as the catalyst, 10% PPh3 as the ligand, and Ag2CO3 as the base in water at 100 °C (Scheme 2)]. The results showed that the expected C3-arylated products 1a–g were regioselectivity obtained. First, using indazole 1 with 4-iodotoluene gave 1a in 80% yield (Scheme 2). Afterwards, the coupling of 1 with various aryl iodides was studied. Thus, the reactions with iodobenzene, 4-iodoanisole, 4-iodobenzotrifluoride, 1-chloro-4-iodobenzene, ethyl 4-iodobenzoate, and 1-iodo-4-nitrobenzene afforded 1b–g in 54–87% yields (Scheme 2). A moderate yield was observed when 3-iodoanisole was used. In this case, the desired product 1h was isolated in 45% yield. No reaction was observed when indazole 1 was treated by 2-iodoanisole presumably as a result of its steric hindrance (methoxy group at the 2-position of the aromatic ring) and 4-iodopyridine (Scheme 2). It is noticeable that iodoaryl, containing electron-donating groups, was crucial for the achievement of C3 arylation reactions in good reaction yields (63–87%). In our second attempt, we used indazole 2 in order to see if the nitro group on position C7 affects the arylation regioselectivity. The treatment of indazole 2 with various substituted aryl iodides as coupling partners showed that the nature of the substituents (electron-donating or electron-withdrawing groups) did not affect the reaction yields or regioselectivity (compounds 2a–f). Thus, the desired products 2a–f were obtained in yields ranging between 41 and 68%. As described in the previous results, we also observed no arylation when indazole 2 was treated by 2-iodoanisole and hetero-aryl iodide as coupling partners (compounds 2g–h) (Scheme 2).
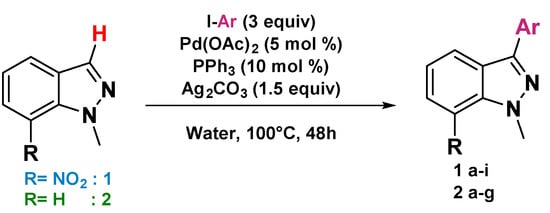
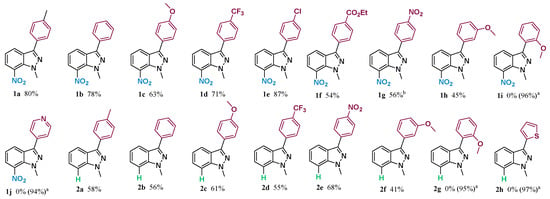
Scheme 2.
Scope and limitation of the ‘’on water’’ direct arylation of 1-methyl-7-nitro-1H-indazole and 1-methyl-1H-indazole. a Percent of recovered starting material, b 72 h.
Finally, we decided to run the reaction under our reaction conditions using the starting material 3 previously used without success by Greaney and his group [32]. As expected, we succeeded in achieving the desired arylated products 3a and 3b in 58 and 51% yield, respectively (Scheme 3).
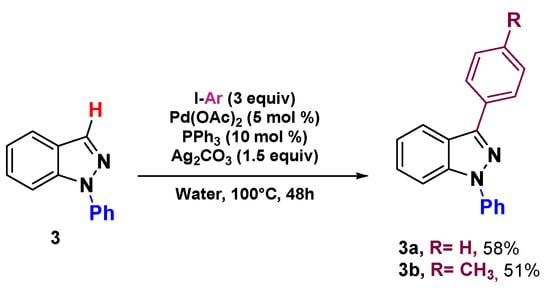
Scheme 3.
Direct arylation of 1-phenyl 1H-indazole 3.
After succeeding in developing a “greener” pathway to synthesize C3-arylated 1H-indazole, we extended the methodology to prepare C3-arylated pyrazolo[3,4-b]pyridine, also known as azaindazole, which is an 1H-indazole isostere and is often used as a key pharmacophore in drug design [42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57]. Despite the enormous biological interest of C3-arylated pyrazolo [3,4-b]pyridines, only one work has been reported by Lavard and Popowycz [38], addressing the functionalization of this motif via direct arylation. Although this reported method is efficient, it is relatively energy-wasting, using heating at 160 °C for a long period of 3 days and organic solvent. For this reason, we decided to test our conditions developed to prepare C3-arylated pyrazolo[3,4-b]pyridine using the starting material 4 containing a benzyl group (the preparation of starting material 4 is described in the Supporting Information) with various aryl iodides as arylating partners. As expected, this procedure also showed a very high tolerance to various substituents on the aryl rings and the arylated products 4b–d were isolated with yields ranging between 56 and 67% (Scheme 4).
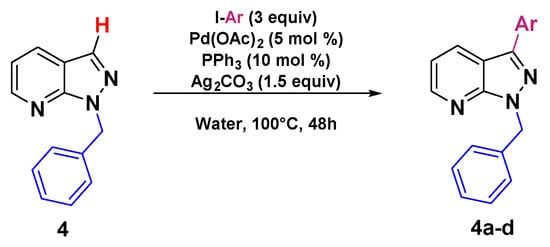
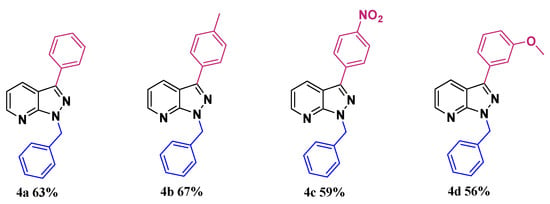
Scheme 4.
Direct arylation "on water" of pyrazolo [3,4-b] pyridine.
3. Materials and Methods
3.1. Instrumenttion
The reactions were monitored by thin-layer chromatography (TLC) analysis using silica gel (60 F254) plates. Compounds were visualized by UV irradiation. Flash column chromatography was performed on silica gel 60 (230–400.13 mesh, 0.040, 0.063 mm). Melting points (mp [°C]) were taken on samples in open capillary tubes and are uncorrected. The infrared spectra of compounds were recorded at room temperature on a Thermo Scientific Nicolet IS50 FT-IR. 1H and 13C NMR spectra were recorded on a Bruker Avance II 400 MHz (13C, 100 MHz) or on a Bruker Avance DPX 250 MHz (13C, 62.9 MHz). Chemical shifts are given in parts per million from tetramethylsilane (TMS) as internal standard. The multiplicities of the spectra are reported as follows: singlet (s), doublet (d), triplet (t), quartet (q), and multiplet (m). Coupling constants (J) are reported in hertz (Hz). High-resolution mass spectra (HRMS) were performed on a Maxis Bruker 4G.
3.2. Preparation of Starting Compounds 1–4
N-Methylation of indazole [33]: the indazole (1 g, 1 equivalent) was dissolved in acetone (10 mL) at 0 °C in a 50 mL flask. KOH (3 equivalent) was added, and then CH3I (1.5 equivalent) was added dropwise. The reaction mixture was filtered and separated by flash chromatography on silica gel (75% yield).
N-Arylation of indazole [42]: iodobenzene (204 mg, 1 mmol) and dimethyl sulfoxide 1 mL was added to the mixture of 1H-indazole (141.7 mg, 1.2 mmol), KOH (67.3 mg, 1.2 mmol), and copper iodide (I) (19.1 mg, 0.1 mmol), and the reaction was for 12 h at 120 °C. After the completion of the reaction, cooled to room temperature, 2 mL of water and ethyl acetate 2 mL was added, and liquid separation was done. 1-phenyl indazole was obtained as a main component of the organic layer (80% yield).
N-Benzylation of 1H-pyrazolo[3,4-b]pyridine [33]: 1H-pyrazolo[3,4-b]pyridine (1 g, 8.39 mmol, 1.00 equivalent) was dissolved in acetone (10 mL) at 0 °C in a 50 mL flask, along with KOH (1.41 g, 25.19 mmol, 3.00 equivalent). After few minutes of stirring, benzyl chloride (1.59 g, 12.59 mmol, 1.50 equivalent) was added dropwise. The reaction mixture was filtered, and the two isomers N1 and N2 were separated by flash chromatography on silica gel (N1: 50%/N2: 45%).
3.3. General Experimental Procedure for the Synthesis of Products 1a–h, 2a–f, 3a–b and 4a–d
A 5 mL sealed tube was charged with 1-methyl-7-nitro-1H-indazole 1, 1-methyl-1H-indazole 2, 1-phenyl-1H-indazole 3, or 1-benzyl-1H-pyrazolo[3,4-b]pyridine 4 (1.0 equivalent), iodoaryl (3.0 equivalent), Pd(OAc)2 (0.05 equivalent), PPh3 (0.1 equivalent) and Ag2CO3 (1.5 equivalent). The mixture of solids was stirred for a few seconds to ensure all solids were well mixed. Then, 3 mL of distilled water was added, the mixture was degassed for few minutes, and the vial was covered with a serum cap. Then, the vial and its contents were heated and stirred at 100 °C for 24 h. After it was cooled to room temperature, the mixture was filtered through celite, and the organic phase was extracted three times with ethyl acetate, dried over magnesium sulfate, and then concentrated under reduced pressure. The residue was purified by flash chromatography to provide the desired products.
1-Methyl-7-nitro-3-(p-tolyl)-1H-indazole1a: Yield: 80%; yellow solid; mp = 154–156 °C; 1H NMR (400 MHz, CDCl3) δ 8.15 (dd, J = 8.1, 1.0 Hz, 1H), 8.06 (dd, J= 8.1, 1.0 Hz, 1H), 7.66 (d, J = 7.6 Hz, 2H), 7.23 (d, J = 7.6 Hz, 2H), 7.20–7.12 (m, 1H), 4.18 (s, 3H), 2.35 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 145.5, 138.9, 135.3, 132.6, 129.8 (2 × C–H), 129.1, 128.3, 127.9 (2 × C–H), 126.9, 124.7, 119.9, 40.9, 21.5; HRMS (m/z) [M + H]+ calculated mass for C15H14N3O2, 268.1081, mass found 268.1079; IR (neat) ῠ = 1303, 1325, 1471, 1509, 2918, 3099 cm−1.
1-Methyl-7-nitro-3-phenyl-1H-indazole1b: Data for compound 1b were previously reported [11].
3-(4-Methoxyphenyl)-1-methyl-7-nitro-1H-indazole1c: Yield: 63%; yellow solid; mp = 182–184 °C; 1H NMR (400 MHz, CDCl3) δ 8.27 (dd, J = 8.1, 1.0 Hz, 1H), 8.17 (dd, J = 8.1, 1.0 Hz, 1H), 7.84 (d, J = 8.7 Hz, 2H), 7.31 (t, J = 7.9 Hz, 1H), 7.11 (d, J = 8.7 Hz, 2H), 4.31 (s, 3H), 3.93 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 160.3, 145.3, 139.2, 132.7, 129.3 (2 × C–H), 128.3, 126.9, 124.7, 124.5, 119.9, 114.6 (2 × C–H), 55.5, 29.8; HRMS (m/z) [M + H]+ calculated mass for C15H14N3O3, 284.1030, mass found 284.1030; IR (neat) ῠ = 1321, 1361, 1514, 1530, 1612, 2849, 2919 cm−1.
1-Methyl-7-nitro-3-(4-(trifluoromethyl)phenyl)-1H-indazole1d: Yield: 55%; yellow solid; mp = 201–203 °C; 1H NMR (400 MHz, CDCl3) δ 8.25 (dd, J = 8.1, 1.0 Hz, 1H), 8.15 (dd, J = 8.1, 1.0 Hz, 1H), 8.07–7.95 (m, 2H), 7.85–7.73 (m, 2H), 7.32 (t, J = 7.9 Hz, 1H), 4.30 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 143.8, 139.4, 135.6, 132.5, 131.0 (q, JCq-F = 32.3 Hz, Cq-F), 128.1 (2 × C–H), 127.6, 126.5, 126.1 (q, 3JCHAr-F = 3.8 Hz, 2C, CHAr), 125.0 (q, 1JC-F = 272.1 Hz, CF3), 124.8, 120.1, 29.8;HRMS (m/z) [M + H]+ calculated mass for C15H11F3N3O2, 322.0798 mass found 322.0796; IR (neat) ῠ = 1275, 1329, 1346, 1498, 1540, 3020 cm−1.
3-(4-Chlorophenyl)-1-methyl-7-nitro-1H-indazole1e: Yield: 87%; yellow solid; mp = 172–173 °C; 1H NMR (400 MHz, CDCl3) δ 8.22 (dd, J = 8.1, 1.0 Hz, 1H), 8.15 (dd, J = 8.1, 1.0 Hz, 1H), 7.82 (d, J = 8.5 Hz, 2H), 7.51 (d, J = 8.5 Hz, 2H), 7.34–7.23 (m, 1H).4.29 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 144.2, 135.5, 134.9, 132.7, 130.5, 129.4 (2 × C–H), 129.2 (2 × C–H), 127.8, 126.6, 124.8, 120.4, 29.8; HRMS (m/z) [M + H]+ calculated mass for C14H11ClN3O2, 288.0534, mass found 288.0534; IR (neat) ῠ =763, 1336, 1358, 1488, 1510, 3018 cm−1.
Ethyl 4-(1-methyl-7-nitro-1H-indazol-3-yl)benzoate1f: Yield: 54%; yellow solid; mp = 206–208 °C; 1H NMR (400 MHz, CDCl3) δ 8.27 (dd, J = 8.1, 1.0 Hz, 1H), 8.20 (m, 2H), 8.17 (d, J = 8.1 Hz, 1H), 7.98 (m, 2H), 7.32 (t, J = 7.9 Hz, 1H), 4.43 (q, J = 7.1 Hz, 2H), 4.31 (s, 3H), 1.44 (t, J = 7.1 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 166.3, 144.2, 136.3, 135.6, 132.7, 130.6, 130.3 (2 × C–H), 127.9, 127.7 (2 × C–H), 126.7, 124.8, 120.6, 61.3, 41.2, 14.5; HRMS (m/z) [M + H]+ calculated mass for C17H16N3O4, 326.1135, mass found 326.1135; IR (neat) ῠ = 1100, 1259, 1320, 1365, 1514, 1708, 2850, 2919, 2986, 3104 cm−1.
1-Methyl-7-nitro-3-(4-nitrophenyl)-1H-indazole1g: Yield: 56%; yellow solid; mp = 242–244 °C; 1H NMR (400 MHz, CDCl3) δ 8.40 (d, J = 8.9 Hz, 2H), 8.28 (dd, J = 8.1, 1.0 Hz, 1H), 8.18 (dd, J = 8.1, 1.0 Hz, 1H), 8.10 (d, J = 8.9 Hz, 2H), 7.37 (t, J = 7.9 Hz, 1H), 4.32 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 148.5, 142.8, 138.5 (2 × C–H), 132.8, 132.2, 128.4 (2 × C–H), 127.4, 124.9, 124.4 (2 × C–H), 121.2, 29.8; HRMS (m/z) [M + H]+ calculated mass for C14H11N4O4, 299.0775 mass found 299.0774; IR (neat) ῠ = 1325, 1345, 1455, 1518, 3076, 1601 cm−1.
3-(3-Methoxyphenyl)-1-methyl-7-nitro-1H-indazole1h: Yield: 45%; yellow solid; mp = 164–166°C; 1H NMR (400 MHz, CDCl3) δ 8.27 (dd, J = 8.1, 1.0 Hz, 1H), 8.14 (dd, J = 8.1, 1.0 Hz, 1H), 7.51–7.37 (m, 3H), 7.28 (d, J = 7.9 Hz, 1H), 7.00 (m, 1H), 4.29 (s, 3H), 3.91 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 160.2, 145.3, 133.3, 132.7, 130.2, 128.7,128.2, 126.8, 124.7, 120.5, 120.1, 114.7, 113.4, 55.5, 41.0; HRMS (m/z) [M + H]+ calculated mass for C15H14N3O3, 284.1030, mass found 284.1029; IR (neat) ῠ = 1250, 1319, 1472, 1514, 1596, 2850, 2920 cm−1.
1-Methyl-3-(p-tolyl)-1H-indazole2a: Data for compound 2a were previously reported [35].
1-Methyl-3-phenyl-1H-indazole2b: Data for compound 2b were previously reported [35].
3-(4-Methoxyphenyl)-1-methyl-1H-indazole2c: Data for compound 2c were previously reported [35].
1-Methyl-3-(4-(trifluoromethyl)phenyl)-1H-indazole2d: Data for compound 2d were previously reported [35].
1-Methyl-3-(4-nitrophenyl)-1H-indazole2e: Yield: 68%; white solid; mp = 209–211 °C; 1H NMR (400 MHz, CDCl3) δ 8.37 (d, J = 8.8 Hz, 2H), 8.18 (d, J = 8.8 Hz, 2H), 8.03 (dd, J = 8.1, 1.1 Hz, 1H), 7.48 (d, J = 3.4 Hz, 2H), 7.30 (dt, J = 8.1, 3.4 Hz, 1H), 4.17 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 147.1, 141.7, 141.2, 140.4, 127.5 (2 × C–H), 126.8, 124.3 (2 × C–H), 122.1, 121.7, 120.9, 109.8, 29.8; HRMS (m/z) [M + H]+ calculated mass forC14H12N3O2, 254.0924, mass found 254.0922; IR (neat) ῠ = 2931, 1543, 1486, 1352, 1347, 1260, 780 cm−1.
3-(3-Methoxyphenyl)-1-methyl-1H-indazole2f: Yield: 41%; white solid; mp = 112–113 °C; 1H NMR (400 MHz, CDCl3) δ 8.04 (d, J = 8.1 Hz, 1H), 7.57 (dd, J = 7.4, 1.4 Hz, 1H), 7.54 (d, J = 1.4 Hz, 1H), 7.49–7.37 (m, 3H), 7.22 (ddd, J = 8.1, 5.1, 2.7 Hz, 1H), 6.96 (d, J = 5.8 Hz, 1H), 4.13 (s, 3H), 3.91 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 160.1, 143.6, 141.5, 135.1, 129.9, 126.3, 121.7, 121.4, 121.0, 120.0, 113.9, 112.6, 109.3, 55.4, 29.8; HRMS (m/z) [M + H]+ calculated mass for C15H15N2O, 239.1179 mass found 239.1176; IR (neat) ῠ =2931, 1486, 1347, 1289, 1260, 780 cm−1.
1,3-Diphenyl-1H-indazole3a: Data for compound 3a were previously reported [34].
1-Phenyl-3-(p-tolyl)-1H-indazole3b: Data for compound 3b were previously reported [34].
1-Benzyl-3-phenyl-1H-pyrazolo[3,4-b]pyridine4a: Yield: 63%; white solid; mp = 133–135 °C; 1H NMR (400 MHz, CDCl3) δ 8.60 (dd, J = 4.5, 1.5 Hz, 1H), 8.35 (dd, J = 8.1, 1.5 Hz, 1H), 8.04–7.95 (m, 2H), 7.52 (dd, J = 8.3, 6.8 Hz, 2H), 7.46–7.40 (m, 3H), 7.35–7.25 (m, 3H), 7.18 (dd, J = 8.1, 4.5 Hz, 1H), 5.82 (s, 2H); 13C NMR (101 MHz, CDCl3) δ 151.45, 148.90, 143.11, 137.24, 133.33, 130.50, 128.99 (2 × C–H), 128.65 (2 × C–H), 128.38, 127.98 (2 × C–H), 127.72 (2 × C–H), 127.22, 117.20, 113.77, 50.87, 14.25; HRMS (m/z) [M + H]+ calculated mass for C19H17N3, 286.1339, mass found 286.1338; IR (neat) ῠ = 3050, 3031, 1610, 1330 cm−1.
1-Benzyl-3-(p-tolyl)-1H-pyrazolo[3,4-b]pyridine4b: Yield: 67%; Yellow solid; mp = 85–90 °C; 1H NMR (400 MHz, CDCl3) δ 8.59 (dd, J = 4.5, 1.5 Hz, 1H), 8.34 (dd, J = 8.1, 1.5 Hz, 1H), 7.98–7.79 (m, 2H), 7.45–7.38 (m, 2H), 7.35–7.24 (m, 5H), 7.18 (dd, J = 8.1, 4.5 Hz, 1H), 5.80 (s, 2H), 2.44 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 151.49, 148.87, 143.25, 138.32, 137.34, 130.59, 130.54, 129.72 (2 × C–H), 128.66 (2 × C–H), 127.98 (2 × C–H), 127.70, 127.14 (2 × C–H), 117.09, 113.81, 50.84, 21.48; HRMS (m/z) [M + H]+ calculated mass for C20H18N3, 300.1495, mass found 300.1499; IR (neat) ῠ = 2922, 2852, 1735, 1465, 772, 511 cm−1.
1-Benzyl-3-(4-nitrophenyl)-1H-pyrazolo[3,4-b]pyridine4c: Yield: 59%; Yellow solid; mp = 157–162 °C; 1H NMR (400 MHz, CDCl3) δ 8.66 (dd, J = 4.5, 1.5 Hz, 1H), 8.42–8.32 (m, 3H), 8.17 (d, J = 8.8 Hz, 2H), 7.45 (dd, J = 6.7, 1.7 Hz, 2H), 7.36–7.27 (m, 4H), 5.83 (s, 2H); 13C NMR (101 MHz, CDCl3) δ 151.50, 149.39, 147.38, 140.52, 139.71, 136.70, 130.04, 128.79 (2 × C–H), 128.17 (2 × C–H), 128.03, 127.39 (2 × C–H), 124.35 (2 × C–H), 118.14, 113.76, 51.23; HRMS (m/z) [M + H]+ calculated mass for C19H15N4O2, 331.1190, mass found 331.1191; IR (neat) ῠ = 2923, 2852, 1516, 1342, 771 cm−1.
1-Benzyl-3-(3-methoxyphenyl)-1H-pyrazolo[3,4-b]pyridine4d: Yield: 56%; Yellow oil; 1H NMR (400 MHz, CDCl3) δ 8.58 (dd, J = 4.5, 1.5 Hz, 1H), 8.35 (dd, J = 8.1, 1.5 Hz, 1H), 7.58–7.48 (m, 2H), 7.46–7.36 (m, 3H), 7.30 (t, J = 7.2 Hz, 2H), 7.19 (dd, J = 8.1, 4.5 Hz, 1H), 6.96 (ddd, J = 8.2, 2.6, 1.0 Hz, 1H), 5.79 (s, 2H), 3.90 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 160.19, 151.51, 148.99, 143.02, 137.26, 134.68, 130.58, 130.06, 128.70 (2 × C–H), 128.01 (2 × C–H), 127.76, 119.78, 117.30, 114.24, 113.87, 112.63, 55.55, 50.92; HRMS (m/z) [M + H]+ calculated mass for C20H18N3O, 316.1444, mass found 316.1443; IR (neat) ῠ = 2936, 2805, 1601, 1280 cm−1.
4. Conclusions
In summary, we have developed a new palladium catalyzed direct C3 arylation of 1H-indazole using a low charge of 5 mol% of Pd(OAc)2 along with 10 mol % of PPh3 on water as solvent at 100 °C. This new procedure afforded a board of arylated indazoles differently substituted at the C3 position in moderate to good yields. The application of this protocol on 1H 7-azaindazole was fruitful, and four examples of C3-arylated pyrazolo[3,4-b]pyridine were successfully reported.
Supplementary Materials
The following are available online, Spectral Data of All the Synthesized Products, NMR Spectra of All the Products.
Author Contributions
Conceptualization, S.E.K.; G.G.; M.A.; and F.S.; methodology, S.E.K., G.G., M.A. and F.S.; validation, S.E.K.; G.G.; M.A.; M.B.; and F.S.; formal analysis, S.E.K.; G.G.; M.A.; M.B.; and F.S.; investigation, S.E.K.; G.G.; M.A.; M.B.; and F.S.; resources, K.G.; A.E.A.; S.N.; data curation, K.G.; A.E.A.; S.N.; writing—Original draft preparation, K.G.; A.E.; S.N.; S.E.K., G.G.; writing—Review and editing, K.G.; A.E.A.; S.N.; S.E.K., G.G.; visualization, K.G.; A.E.A.; S.N.; S.E.K., G.G.; supervision, S.E.K., G.G., M.A.; and F.S. project administration, S.E.K., G.G; funding acquisition, S.E.K., G.G., M.A., M.B. and F.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Euromed University of Fes, CNRST of Morocco and University of Orleans.
Acknowledgments
This work was supported by CNRST of Morocco, University of Orleans, University Hassan II of Casablanca, and the Euromed University of Fes.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gutekunst, W.R.; Baran, P.S. C–H functionalization logic in total synthesis. Chem. Soc. Rev. 2011, 40, 1976–1991. [Google Scholar] [CrossRef] [PubMed]
- McMurray, L.; O’Hara, F.; Gaunt, M.J. Recent developments in natural product synthesis using metal-catalysed C–H bond functionalization. Chem. Soc. Rev. 2011, 40, 1885–1898. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.Y.K.; Youn, S.W. C–H activation: A complementary tool in the total synthesis of complex natural products. Chem. Eur. J. 2012, 18, 9452–9474. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, J.; Yamaguchi, A.D.; Itami, K. C–H Bond Functionalization: Emerging Synthetic Tools for Natural Products and Pharmaceuticals. Angew. Chem. Int. Ed. 2012, 51, 8960–9009. [Google Scholar] [CrossRef]
- Noisier, A.F.M.; Brimble, M.A. C–H Functionalization in the Synthesis of Amino Acids and Peptides. Chem. Rev. 2014, 114, 8775–8806. [Google Scholar] [CrossRef]
- Miyaura, N.; Yamada, K.; Suzuki, A. A new stereospecific cross-coupling by the palladium-catalyzed reaction of 1-alkenylboranes with 1-alkenyl or 1-alkynyl halides. Tetrahedron Lett. 1979, 20, 3437–3440. [Google Scholar] [CrossRef]
- Miyaura, N.; Suzuki, A. Stereoselective synthesis of arylated (E)-alkenes by the reaction of alk-1-enylboranes with aryl halides in the presence of palladium catalyst. J. Chem. Soc., Chem. Commun. 1979, 866–867. [Google Scholar] [CrossRef]
- Milstein, D.; Stille, J.K. Palladium-catalyzed coupling of tetraorganotin compounds with aryl and benzyl halides. Synthetic utility and mechanism. J. Am. Chem. Soc. 1979, 101, 4992–4998. [Google Scholar] [CrossRef]
- King, A.O.; Okukado, N.; Negishi, E. Highly general stereo-, regio-, and chemo-selective synthesis of terminal and internal conjugated enynes by the Pd-catalysed reaction of alkynylzinc reagents with alkenyl halides. J. Chem. Soc. Chem. Commun. 1977, 683–684. [Google Scholar] [CrossRef]
- Negishi, E.; King, A.O.; Okukado, N. Selective carbon-carbon bond formation via transition metal catalysis. 3. A highly selective synthesis of unsymmetrical biaryls and diarylmethanes by the nickel- or palladium-catalyzed reaction of aryl- and benzylzinc derivatives with aryl halides. J. Org. Chem. 1977, 42, 1821–1823. [Google Scholar] [CrossRef]
- Sharma, A.; Vacchani, D.; Van Der Eycken, E. Developments in Direct C–H Arylation of (Hetero)Arenes under Microwave Irradiation. Chem. Eur. J. 2013, 19, 1158–1168. [Google Scholar] [CrossRef] [PubMed]
- Naas, M.; El Kazzouli, S.; Essassi, E.M.; Bousmina, M.; Guillaumet, G. Palladium-Catalyzed Direct C7-Arylation of Substituted Indazoles. J. Org. Chem. 2014, 79, 7286–7293. [Google Scholar] [CrossRef] [PubMed]
- El Kazzouli, S.; Koubachi, J.; El Brahmi, N.; Guillaumet, G. Advances in direct C–H arylation of 5,5- 6,5- and 6,6-fused-heterocycles containing heteroatoms (N, O, S). RSC Adv. 2015, 5, 15292–15327. [Google Scholar] [CrossRef]
- Rossi, R.; Lessi, M.; Manzini, C.; Marianetti, G.; Bellina, F. Transition Metal-Free Direct C-H (Hetero)arylation of Heteroarenes: A Sustainable Methodology to Access (Hetero)aryl-Substituted Heteroarenes. Adv. Synth. Catal. 2015, 357, 3777–3814. [Google Scholar] [CrossRef]
- Basu, K.; Poirier, T.; Ruck, R.T. Solution to the C3–Arylation of Indazoles: Development of a Scalable Method. Org. Lett. 2016, 18, 3218–3221. [Google Scholar] [CrossRef] [PubMed]
- Hameury, S.; Kunz, S.; Sommer, M. Expanding the Scope of Electron-Deficient C−H Building Blocks: Direct Arylation of Pyromellitic Acid Diimide. ACS Omega. 2017, 2, 2483–2488. [Google Scholar] [CrossRef]
- Kim, J.; Hong, S.H. Ligand-Promoted Direct C–H Arylation of Simple Arenes: Evidence for a Cooperative Bimetallic Mechanism. ACS Catal. 2017, 7, 3336–3343. [Google Scholar] [CrossRef]
- Shoji, T.; Araki, T.; Sugiyama, S.; Ohta, A.; Sekiguchi, R.; Ito, S.; Okujima, T.; Toyota, K. Synthesis of 2-Azulenyltetrathiafulvalenes by Palladium-Catalyzed Direct Arylation of 2-Chloroazulenes with Tetrathiafulvalene and Their Optical and Electrochemical Properties. J. Org. Chem. 2017, 82, 1657–1665. [Google Scholar] [CrossRef]
- Roudesly, F.; Oble, J.; Poli, G. Metal-catalyzed C-H activation/functionalization: The fundamentals. J. Mol. Catal. A Chem. 2017, 426, 275–296. [Google Scholar] [CrossRef]
- Narayan, S.; Muldoon, J.; Finn, M.G.; Fokin, V.V.; Kolb, H.C.; Sharpless, K.B. On water: Unique reactivity of organic compounds in aqueous suspension. Angew. Chem., Int. Ed. 2005, 44, 3275–3279. [Google Scholar] [CrossRef]
- Li, C.J.; Chan, T.H. Organic reactions in aqueous media. J. Chem. Educ. 2000, 77, 707–708. [Google Scholar]
- Grieco, P.A. Organic Synthesis in Water; Blackie Academic & Professional: London, UK, 1998. [Google Scholar]
- Ferrer Flegeau, E.F.; Popkin, M.E.; Greaney, M.F. Direct arylation of oxazoles at C2. A concise approach to consecutively linked oxazoles. Org. Lett. 2008, 10, 2717–2720. [Google Scholar] [CrossRef] [PubMed]
- Ohnmacht, S.A.; Mamone, P.; Culshaw, A.J.; Greaney, M.F. Direct arylations on water: Synthesis of 2,5-disubstituted oxazolesbalsoxin and texaline. Chem. Commun. 2008, 1241–1243. [Google Scholar] [CrossRef] [PubMed]
- Turner, G.L.; Morris, J.A.; Greaney, M.F. Direct arylation of thiazoles on water. Angew. Chem., Int. Ed. 2007, 46, 7996–8000. [Google Scholar] [CrossRef]
- Schmidt, A.; Beutler, A.; Snovydovych, B. Recent advances in the chemistry of indazoles. Eur. J. Org. Chem. 2008, 24, 4073–4095. [Google Scholar] [CrossRef]
- Stadlbauer, W. Houben-Weyl, Methoden der Organischen Chemie: Indazole (Benzopyrazole); Schaumann, E., Ed.; Georg-Thieme-Verlag Stuttgart: New York, NY, USA, 1994; Volume 3, pp. 764–864. [Google Scholar]
- Elguero, J. Comprehensive Heterocyclic Chemistry; Katritzky, A.R., Rees, C.W., Scriven, E.F.V., Eds.; Pergamon: Oxford, UK, 1996; Volume 3, pp. 1–75. [Google Scholar]
- Elguero, J. Comprehensive Heterocyclic Chemistry; Katritzky, A.R., Rees, C.W., Eds.; Pergamon: Oxford, UK, 1984; Volume 5, pp. 167–303. [Google Scholar]
- Behr, L.C. The Chemistry of Heterocyclic Compounds; Weissberger, A., Taylor, E.C., Eds.; Wiley-Interscience: New York, NY, USA, 1967; Volume 22, pp. 289–382. [Google Scholar]
- Cerecetto, H.; Gerpe, A.; González, M.; Arán, V.J.; Ochoa de Ocáriz, C. Pharmacological properties of indazole derivatives: Recent developments. Mini-Rev. Med. Chem. 2005, 5, 869–878. [Google Scholar] [CrossRef]
- Ohnmacht, A.S.; Culshaw, A.J.; Greaney, M.F. Direct Arylations of 2H-Indazoles On Water. Org. Lett. 2010, 12, 224–226. [Google Scholar] [CrossRef]
- Ben-Yahia, A.; Naas, M.; El Kazzouli, S.; Essassi, E.M.; Guillaumet, G. Direct C-3-arylations of 1H-indazoles. Eur. J. Org. Chem 2012, 2012, 7075–7081. [Google Scholar] [CrossRef]
- Hattori, K.; Yamaguchi, K.; Yamaguchi, J.; Itami, K. Pd- and Cu- catalyzed C-H arylation of indazoles. Tetrahedron 2012, 68, 7605–7612. [Google Scholar] [CrossRef]
- Ye, M.; Edmunds, A.J.; Morris, J.A.F.; Sale, D.; Zhang, Y.; Yu, J.Q. A robust protocol for Pd(II)-catalyzed C-3 arylation of (1H) indazoles and pyrazoles: Total synthesis of nigellidinehydrobromide. Chem. Sci. 2013, 4, 2374–2379. [Google Scholar] [CrossRef]
- Faarasse, S.; El Kazzouli, S.; Naas, M.; Jouha, J.; Suzenet, F.; Guillaumet, G. “On water” direct C-3 arylation of 2H-pyrazolo[3,4-b]pyridines. J. Org. Chem. 2017, 82, 12300–12306. [Google Scholar] [CrossRef]
- Faarasse, S.; El Kazzouli, S.; Suzenet, F.; Guillaumet, G. Palladium-catalyzed C3 arylations of 1H and 2H pyrazolo[4,3-b]pyridines on water. J. Org. Chem. 2018, 83, 12847–12854. [Google Scholar] [CrossRef]
- Lavrard, H.; Popowycz, F. Regioselective late-stage C-3 functionalization of pyrazolo[3,4-b]pyridines. Synthesis 2018, 50, 998–1006. [Google Scholar]
- Belkessam, F.; Aidene, M.; Soule, J.F.; Doucet, H. Direct C3-Arylation of 2H-Indazole Derivatives with Aryl Bromides by using Low Loading of a Phosphine-free Palladium Catalyst. ChemCatChem 2017, 9, 2239–2249. [Google Scholar] [CrossRef]
- Unsinn, A.; Knochel, P. Regioselective zincation of indazoles using TMP2Zn and Negishi cross-coupling with aryl and heteroaryl iodides. Chem. Commun. 2012, 48, 2680–2682. [Google Scholar] [CrossRef] [PubMed]
- Egan, B.A.; Burton, P.M. Synthesis of 3-aryl-1H-indazoles via iridium-catalysed C–H borylation and Suzuki–Miyaura coupling. RSC Adv. 2014, 4, 27726–27729. [Google Scholar] [CrossRef]
- Nishida, M.; Uehata, Y. Method for Producing Arylpyrazole. Japan Patent JP2006342127A, 21 December 2006. [Google Scholar]
- Zhai, M.; Liu, S.; Gao, M.; Wang, L.; Sun, J.; Du, J.; Guan, Q.; Bao, K.; Zuo, D.; Wu, Y.; et al. 3,5-Diaryl-1H-pyrazolo[3,4-b]pyridines as potent tubulin polymerization inhibitors: Rational design, synthesis and biological evaluation. Euro. J. Med. Chem. 2019, 168, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.L.; Liu, Y.P.; Xu, B.H.; Wang, X.H.; Jiang, B.; Tu, S.J. Microwave-assisted chemoselective reaction: A divergent synthesis of pyrazolopyridine derivatives with different substituted patterns. Tetrahedron. 2011, 67, 9417–9425. [Google Scholar] [CrossRef]
- Bharate, S.B.; Mahajan, T.R.; Gole, Y.R.; Nambiar, M.; Matan, T.T.; Kulkarni-Almeida, A.; Balachandran, S.; Junjappa, H.; Balakrishnan, A.; Vishwakarma, R.A. Synthesis and evaluation of pyrazolo[3,4-b]pyridines and its structural analogues as TNF-α and IL-6 inhibitors. Bioorg. Med. Chem. 2008, 16, 7167–7176. [Google Scholar] [CrossRef]
- Sharma, P.K.; Singh, K.; Kumar, S.; Dhawan, S.N.; Lal, S.; Ulbrich, H.; Dannhardt, G. Synthesis and anti-inflammatory evaluation of some pyrazolo[3,4-b]pyridines. Med. Chem. Res. 2011, 20, 239–244. [Google Scholar] [CrossRef]
- Oe, T.; Kawasaki, K.; Terasawa, M.; Imayoshi, T.; Yasunaga, Y. Pyrazolopyridine compounds, their preparation and use as platelet aggregation inhibitors. U.S. Patent US4808620A, 28 February 1989. [Google Scholar]
- Straub, A.S.; Alonso-Alija, J.P.C. NO-independent Stimulators of Soluble Guanylate Cyclase. Bioorg. Med. Chem. Lett. 2001, 11, 781–784. [Google Scholar] [CrossRef]
- Cappelli, A.; Nannicini, C.; Gallelli, A.; Giuliani, G.; Valenti, S.; Mohr, G.P.; Anzini, M.; Mennuni, L.; Ferrari, F.; Caselli, G.; et al. Design, Synthesis, and Biological Evaluation of AT1 Angiotensin II Receptor Antagonists Based on the Pyrazolo[3,4-b]pyridine and Related Heteroaromatic Bicyclic Systems. J. Med. Chem. 2008, 51, 2137–2146. [Google Scholar] [CrossRef] [PubMed]
- Höhn, H.; Polacek, I.; Schulze, E. Potential antidiabetic agents. Pyrazolo[3,4-b]pyridines. J. Med. Chem. 1973, 16, 1340–1346. [Google Scholar] [CrossRef]
- Lin, R.; Connolly, P.J.; Lu, Y.; Chiu, G.; Li, S.; Yu, Y.; Huang, S.; Li, X.; Emanuel, S.L.; Middleton, S.A.; et al. Synthesis and evaluation of pyrazolo[3,4-b]pyridine CDK1 inhibitors as anti-tumor agents. Bioorg. Med. Chem. Lett. 2007, 17, 4297. [Google Scholar] [CrossRef] [PubMed]
- Bare, T.M.; McLaren, C.D.; Campbell, D.J.B.; Firor, J.W.; Resch, J.F.; Walters, C.P.; Salama, A.I.; Meiners, B.A.; Patel, J.B. Synthesis and structure-activity relationships of a series of anxioselective pyrazolopyridine ester and amide anxiolytic agents. J. Med. Chem. 1989, 32, 2561–2573. [Google Scholar] [CrossRef] [PubMed]
- Leal, B.; Afonso, I.F.; Rodrigues, C.R.; Abreu, P.A.; Garrett, R.; Pinheiro, L.C.; Azevedo, A.R.; Borges, J.C.; Vegi, P.F.; Santos, C.C.C.; et al. Antibacterial profile against drug-resistant Staphylococcus epidermidis clinical strain and structure–activity relationship studies of 1H-pyrazolo[3,4-b]pyridine and thieno[2,3-b]pyridine derivatives. Bioorg. Med. Chem. 2008, 16, 8196–8204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Balan, G.; Barreiro, G.; Boscoe, B.P.; Chenard, L.K.; Cianfrogna, J.; Claffey, M.M.; Chen, L.; Coffman, K.J.; Drozda, S.E.; et al. Discovery and Preclinical Characterization of 1-Methyl-3-(4-methylpyridin-3-yl)-6-(pyridin-2-ylmethoxy)-1H-pyrazolo-[3,4-b]pyrazine (PF470): A Highly Potent, Selective, and Efficacious Metabotropic Glutamate Receptor 5 (mGluR5) Negative Allosteric Modulator. J. Med. Chem. 2014, 57, 861–877. [Google Scholar] [PubMed]
- Halank, M.; Tausche, K.; Grünig, E.; Ewert, R.; Preston, I.R. Practical management of riociguat in patients with pulmonary arterial hypertension. Ther. Adv. Respir. Dis. 2019, 13, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Bandarage, U.K.; Clark, M.P.; Perola, E.; Gao, H.; Jacobs, M.D.; Tsai, A.; Gillespie, J.; Kennedy, J.M.; Maltais, F.; Ledeboer, M.W.; et al. Novel 2-Substituted 7-Azaindole and 7-Azaindazole Analogues as Potential Antiviral Agents for the Treatment of Influenza. ACS Med. Chem. Lett. 2017, 8, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Anand, D.; Yadav, P.K.; Patel, O.P.S.; Parmar, N.; Maurya, R.K.; Vishwakarma, P.; Raju, K.S.R.; Taneja, I.; Wahajuddin, M.; Kar, S.; et al. Antileishmanial Activity of Pyrazolopyridine Derivatives and Their Potential as an Adjunct Therapy with Miltefosine. J. Med. Chem. 2017, 60, 1041–1059. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).