Coordination Chemistry of Ru(II) Complexes of an Asymmetric Bipyridine Analogue: Synergistic Effects of Supporting Ligand and Coordination Geometry on Reactivities
Abstract
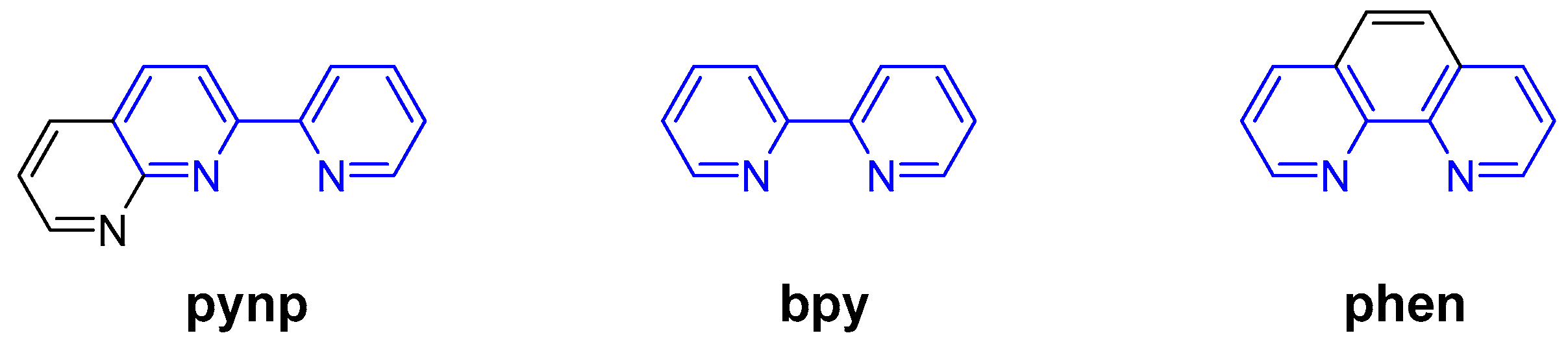
1. Introduction
2. Results and Discussion
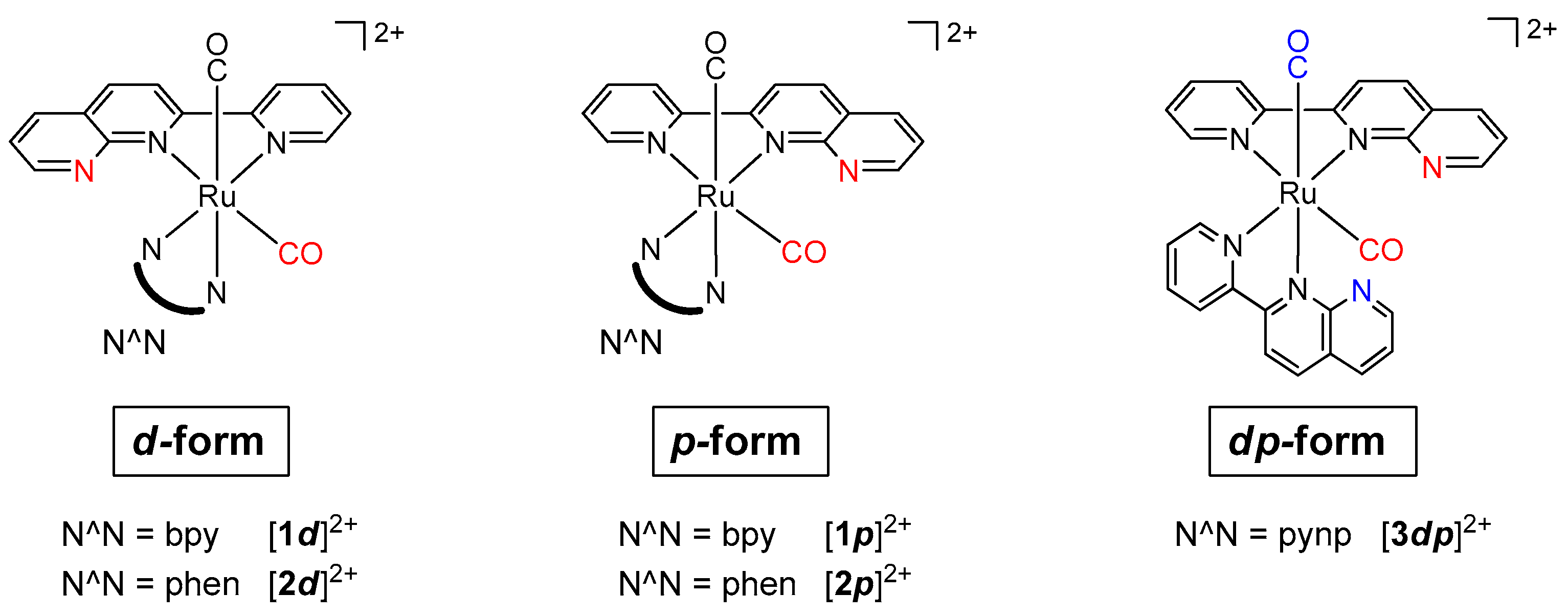
2.1. Diastereoselective Synthesis and Characterization of Ruthenium Complexes with the Asymmetric pynp Ligand
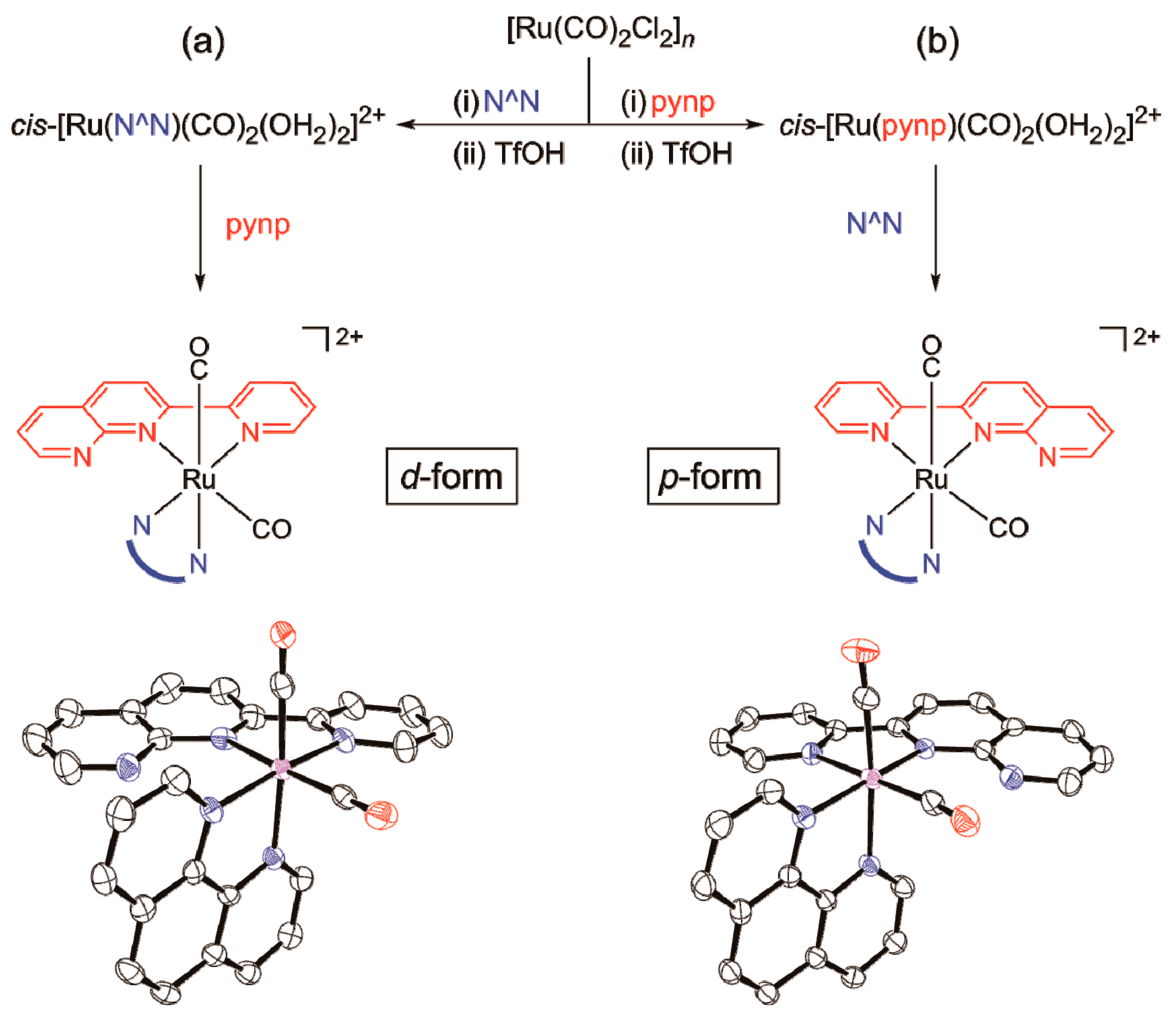
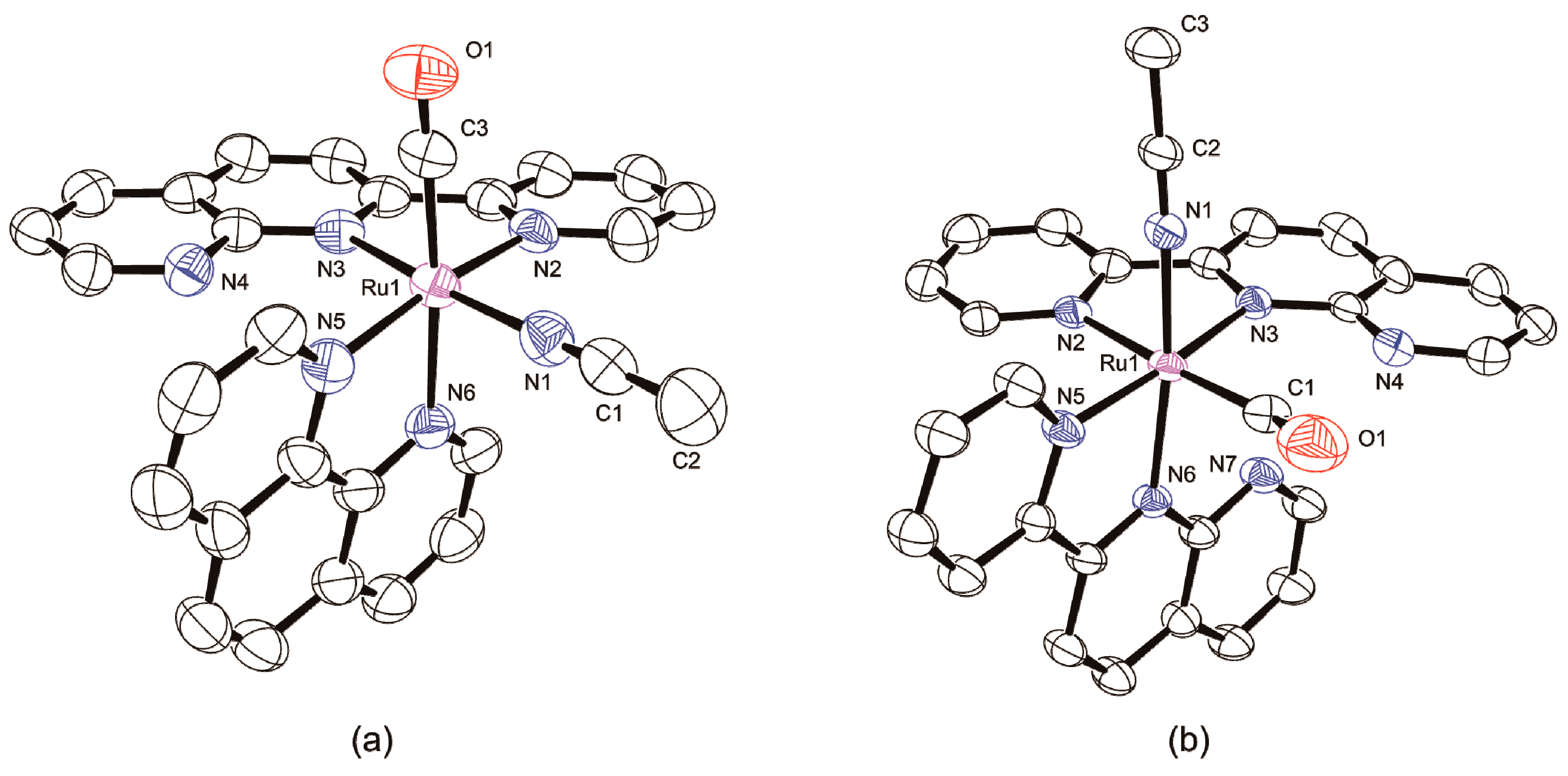
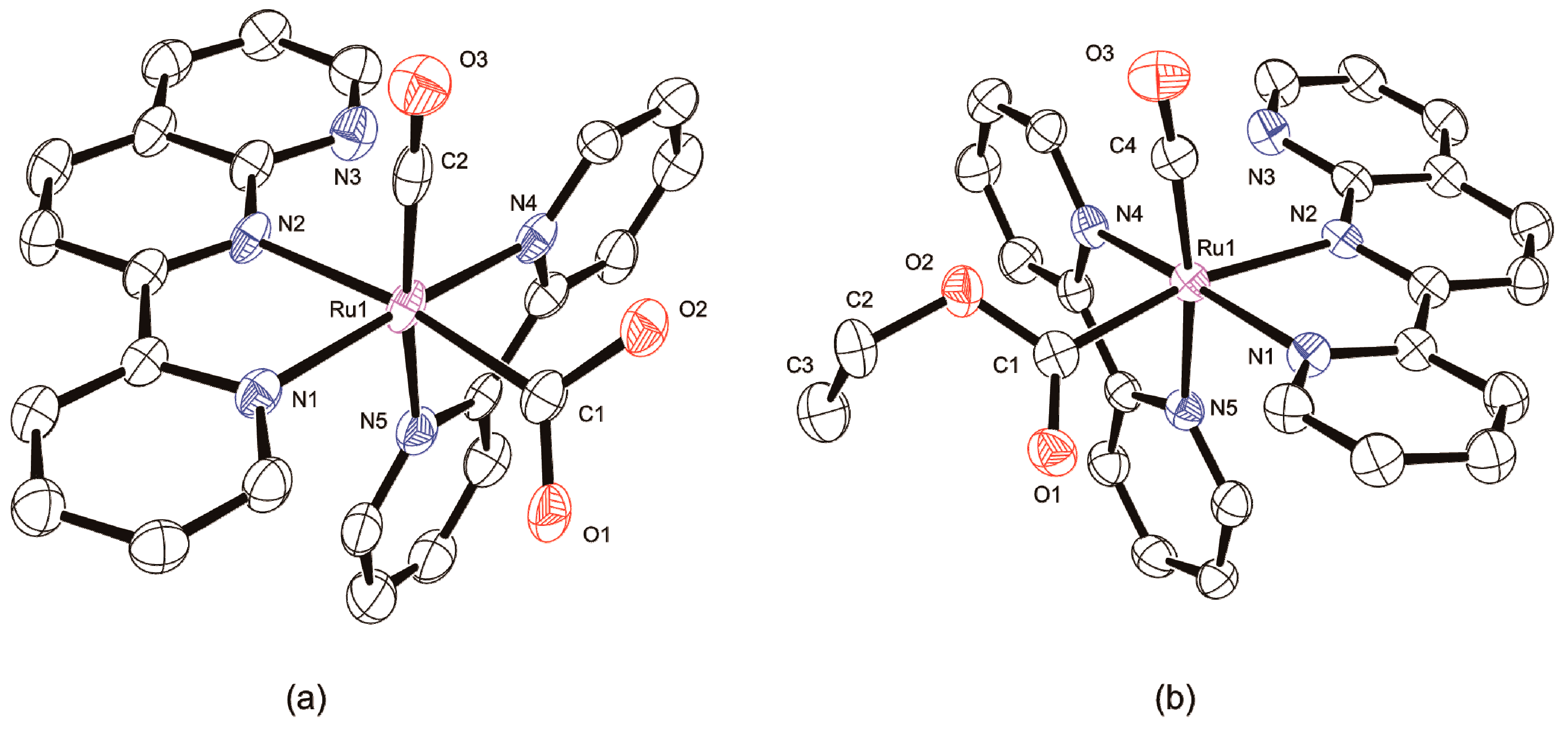
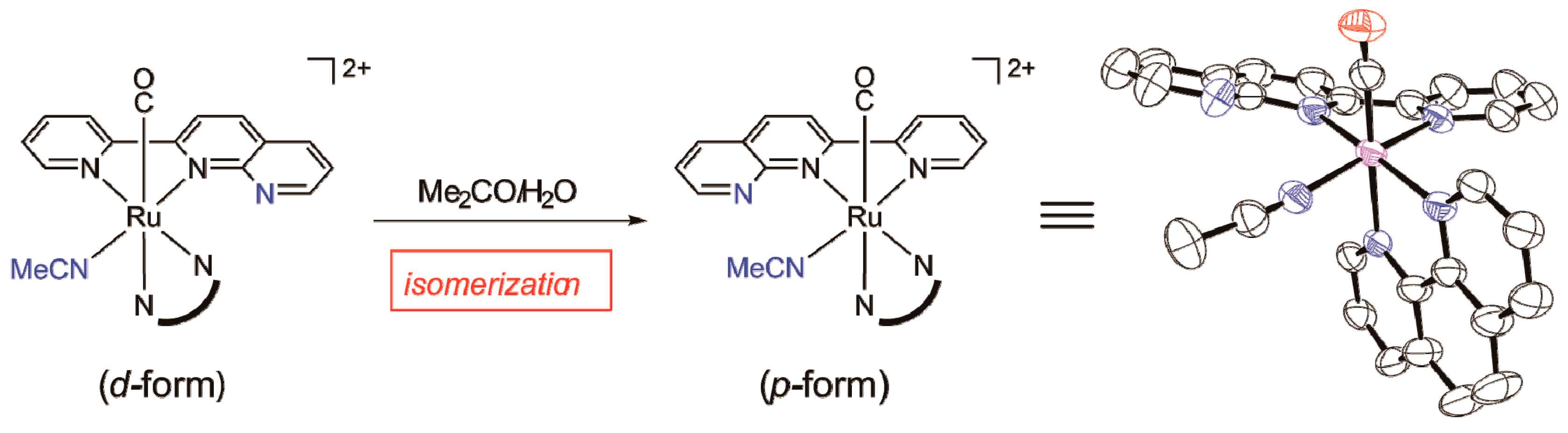
2.1.1. Synthesis and Structure of Complexes
2.1.2. Characterization of Complexes
2.2. Reactivities of Complexes
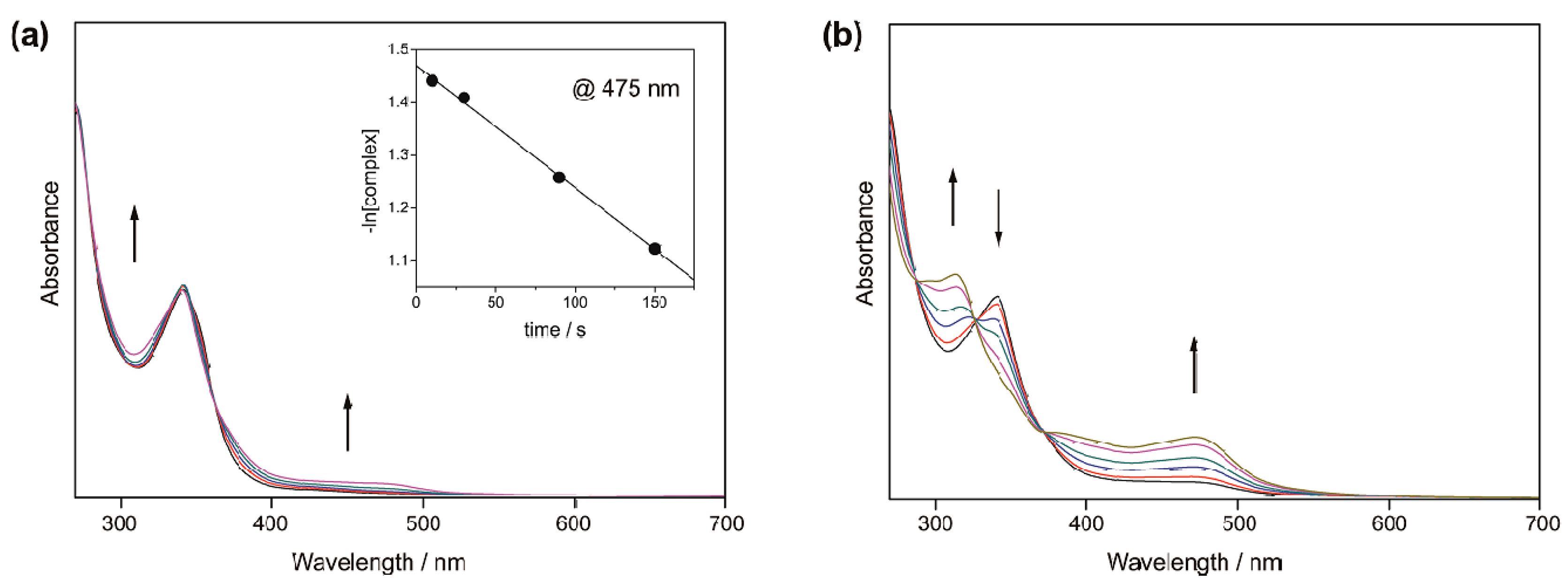
2.2.1. Photochemical Reactions
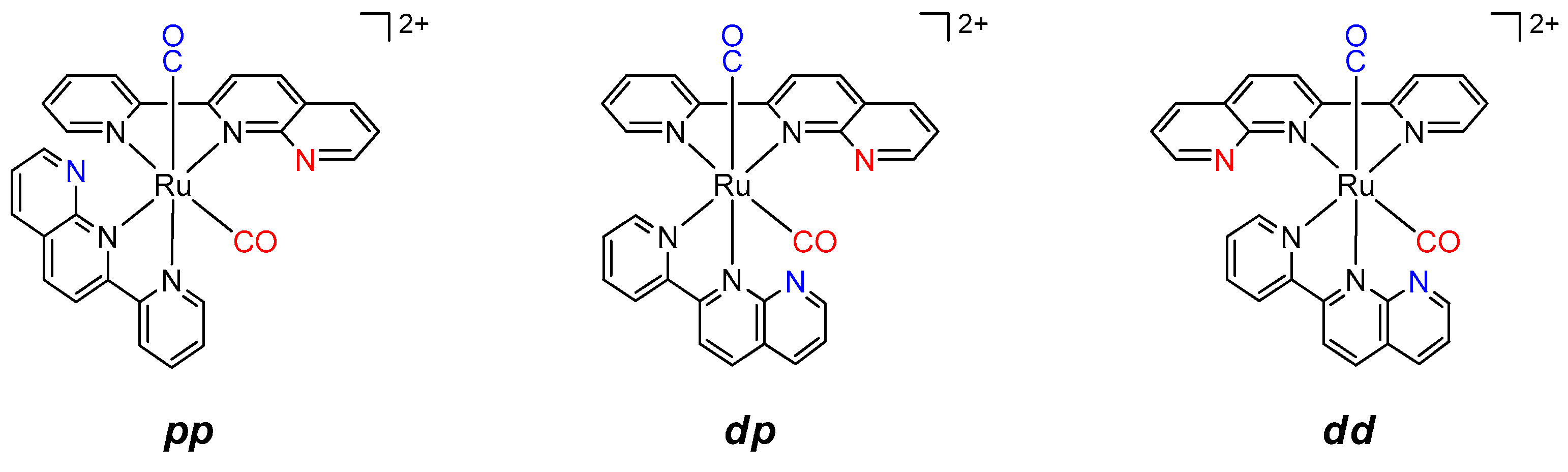
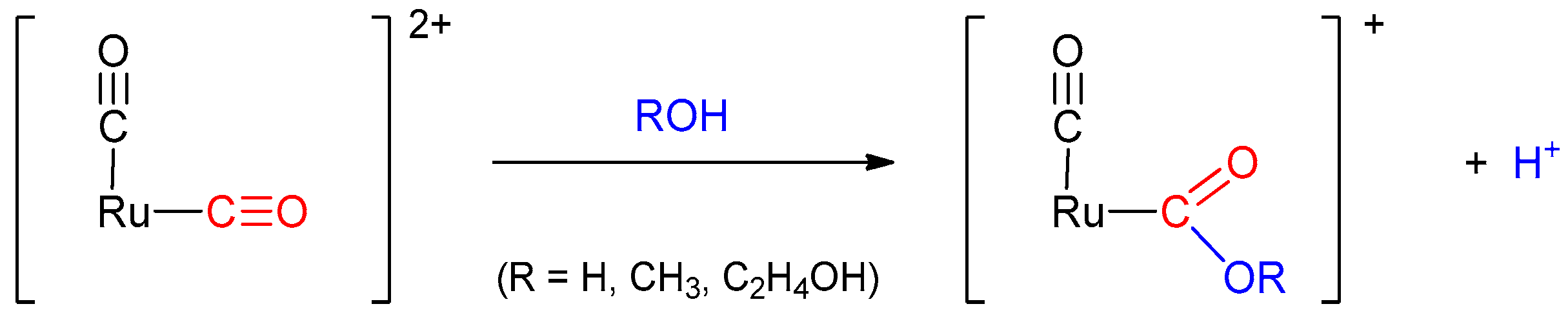
2.2.2. Thermochemical Reactions
3. Materials and Methods
3.1. General Remarks
3.2. Synthesis of Complexes
3.2.1. Synthesis of the Distal Isomers ([1d](PF6)2 and [2d](PF6)2)
3.2.2. Synthesis of the Proximal Isomers ([1p](PF6)2 and [2p](PF6)2)
3.2.3. Synthesis of [3dp](PF6)2
3.3. Photochemical Reactions
3.4. Thermochemical Reactions
3.4.1. Reaction of the Coordinated CO in [1d]2+ and [2d]2+ with Solvents
3.4.2. Thermal-induced Isomerization Using Monocarbonyl Complexes d-[Ru(pynp)(N^N)(CO)(CH3CN)]2+ (N^N = bpy or phen) and dp-[Ru(pynp)2(CO)(CH3CN)]2+
3.5. X-ray Crystallographic Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References and Notes
- Smith, A.P.; Fraser, C.L. Bipyridine ligands. In Comprehensive Coordination Chemistry II; McClevety, J.A., Meyer, T.J., Eds.; Elsevier: Oxford, UK, 2004; Volume 1, pp. 1–23. [Google Scholar]
- Winter, A.; Hoeppener, S.; Newkome, G.M.; Schubert, U.S. Terpyridine-functionalized surfaces: Redox-active, switchable, and electroactive nanoarchitectures. Adv. Mater. 2011, 23, 3484–3498. [Google Scholar] [CrossRef]
- Constable, E.C.; Housecroft, C.E. More hydra than Janus – Non-classical coordination modes in complexes of oligopyridine ligands. Coord. Chem. Rev. 2017, 350, 84–104. [Google Scholar] [CrossRef]
- Fukushima, T.; Ghosh, D.; Kobayashi, K.; Ohtsu, H.; Kitagawa, S.; Tanaka, K. Four-electron reduction of a new ruthenium dicarbonyl complex having two NAD model ligands through decarboxylation in water. Inorg. Chem. 2016, 55, 11613–11616. [Google Scholar] [CrossRef]
- Kobayashi, K.; Tanaka, K. Reactivity of CO2 activated on transition metals and sulfur ligands. Inorg. Chem. 2015, 54, 5085–5095. [Google Scholar] [CrossRef]
- Machan, C.W.; Sampson, M.D.; Kubiak, C.P. A molecular ruthenium electrocatalyst for the reduction of carbon dioxide to CO and formate. J. Am. Chem. Soc. 2015, 137, 8564–8571. [Google Scholar] [CrossRef]
- Kuramochi, Y.; Itabashi, J.; Fukaya, K.; Enomoto, A.; Yoshida, M.; Ishida, H. Unexpected effect of catalyst concentration on photochemical CO2 reduction by trans(Cl)-Ru(bpy)(CO)2Cl2: new mechanistic insight into the CO/HCOO– selectivity. Chem. Sci. 2015, 6, 3063–3074. [Google Scholar] [CrossRef] [PubMed]
- Kuramochi, Y.; Fukaya, K.; Yoshida, M.; Ishida, H. trans-(Cl)-[Ru(5,5’-diamide-2,2’-bipyridine)(CO)2Cl2]: Synthesis, structure, and photocatalytic CO2 reduction activity. Chem. Eur. J. 2015, 21, 10049–10060. [Google Scholar] [CrossRef] [PubMed]
- Voyame, P.; Toghill, K.E.; Méndez, M.A.; Girault, H.H. Photoreduction of CO2 using [Ru(bpy)2(CO)L]n+ catalysts in biphasic solution/supercritical CO2 systems. Inorg. Chem. 2013, 52, 10949–10957. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Majumdar, M.; Sinha, A.; Ghatak, T.; Patra, S.K.; Sadhukhan, N.; Rahaman, S.M.W.; Bera, J.K. Mapping the transformation [{RuII(CO)3Cl2}2] to [RuI2(CO)4]2+: Implications in binuclear water-gas shift chemistry. Chem. Eur. J. 2010, 16, 2574–2585. [Google Scholar] [CrossRef]
- Ishida, H.; Tanaka, K.; Morimoto, M.; Tanaka, T. Isolation of intermediates in the water gas shift reactions catalyzed by [Ru(bpy)2(CO)Cl]+ and [Ru(bpy)2(CO)2]2+. Organometallics 1986, 5, 724–730. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, P.; Aathira, M.S.; Singh, D.P.; Behera, B.; Jain, S.L. A bridged ruthenium dimer (Ru-Ru) for photoreduction of CO2 under visible light irradiation. Ind. End. Chem. 2018, 61, 381–387. [Google Scholar] [CrossRef]
- Son, A.; Kawasaki, A.; Hara, D.; Ito, T.; Tanabe, K. Phosphorescent ruthenium complexes with a nitroimidazole unit that image oxygen fluctuation in tumor tissue. Chem. Eur. J. 2014, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Carrington, S.J.; Chakraborty, I.; Alvarado, J.R.; Mascharak, P.K. Differences in the CO photolability of cis- and trans-[RuCl2(azpy)(CO)2] complexes: Effect of metal-to-ligand back-bonding. Inorg. Chim. Acta. 2013, 407, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, G.; Kondo, M.; Crisalli, M.; Lee, S.K.; Shibata, A.; Ford, P.C.; Masaoka, S. Syntheses and properties of phosphine-substituted ruthenium(II) polypyridine complexes with nitrogen oxides. Dalton Trans. 2015, 44, 17189–17200. [Google Scholar] [CrossRef]
- Boyer, J.L.; Polyansky, D.E.; Szalda, D.J.; Zong, R.; Thummel, R.P.; Fujita, E. Effects of a proximal base on water oxidation and proton reduction catalyzed by geometric isomers of [Ru(tpy)(pynap)(OH2)]2+. Angew. Chem. Int. Ed. 2011, 50, 12600–12604. [Google Scholar] [CrossRef]
- Ashford, D.L.; Glasson, C.R.K.; Norris, M.R.; Concepcion, J.J.; Keinan, S.; Brennaman, M.K.; Templeton, J.L.; Meyer, T.J. Controlling ground and excited state properties through ligand changes in ruthenium polypyridyl complexes. Inorg. Chem. 2014, 53, 5637–5646. [Google Scholar] [CrossRef]
- Oyama, D.; Abe, R.; Takase, T. CO-ligand photodissociation in two Ru(II) complexes affected by different polypyridyl supporting ligands. Chem. Lett. 2017, 46, 1412–1414. [Google Scholar] [CrossRef]
- The distal and proximal isomers are defined by the relative positions of the monodentate ligand (L) and non-coordinating nitrogen atom of pynp: the d-isomer and p-isomer denote cis(L,py)- and trans(L,py)-, respectively (py: pyridyl group in pynp).
- Oyama, D.; Abe, R.; Takase, T. Coordination chemistry of mononuclear ruthenium complexes bearing versatile 1,8-naphthyridine units: Utilization of specific reaction sites constructed by the secondary coordination sphere. Coord. Chem. Rev. 2018, 375, 424–433. [Google Scholar] [CrossRef]
- Ooyama, D.; Kobayashi, T.; Shiren, K. Tanaka, K. Regulation of electron donating ability to metal center: isolation and characterization of ruthenium carbonyl complexes with N,N- and/or N,O-donor polypyridyl ligands. J. Organomet. Chem. 2003, 665, 107–113. [Google Scholar] [CrossRef]
- Tanaka, H.; Tzeng, B.-C.; Nagao, H.; Peng, S.-M.; Tanaka, K. Comparative study on crystal structures of ruthenium bipyridine carbonyl complexes [Ru(bpy)2(CO)2](PF6)2, [Ru(bpy)2(CO)(C(O)OCH3)]B(C6H5)4·CH3CN, and [Ru(bpy)2(CO)(η1-CO2)]·3H2O (bpy = 2,2’-bipyridyl). Inorg. Chem. 1993, 32, 1508–1512. [Google Scholar] [CrossRef]
- Bondi, A. van der Waals volumes and radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Zhao, H.C.; Fu, B.-L.; Schweinfurth, D.; Harney, J.P.; Sarkar, B.; Tsai, M.-K.; Rochford, J. Tuning oxyquinolate non-innocence at the ruthenium polypyridyl core. Eur. J. Inorg. Chem. 2013, 4410–4420. [Google Scholar] [CrossRef]
- Campos-Fernández, C.S.; Ouyang, X.; Dunbar, K.R. A homologous series of redox-active, dinuclear cations with the bridging ligand 2-(2-pyridyl)-1,8-naphthyridine. Inorg. Chem. 2000, 39, 2432–2433. [Google Scholar] [CrossRef] [PubMed]
- Oyama, D.; Ukawa, N.; Hamada, T.; Takase, T. Reversible intramolecular cyclization in ruthenium complexes induced by ligand-centered one-electron transfer on bidentate naphthyridine: An important intermediate for both metal- and organo-hydride species. Chem. Lett. 2015, 44, 533–535. [Google Scholar] [CrossRef]
- Knoll, J.D.; Albani, B.A.; Turro, C. New Ru(II) complexes for dual photoreactivity: Ligand exchange and 1O2 generation. Acc. Chem. Res. 2015, 48, 2280–2287. [Google Scholar] [CrossRef]
- Liu, Y.; Turner, D.B.; Singh, T.N.; Angeles-Boza, A.M.; Chouai, A.; Dunbar, K.R.; Turro, C. Ultrafast ligand exchange: detection of a pentacoordinate Ru(II) intermediate and product formation. J. Am. Chem. Soc. 2009, 131, 26–27. [Google Scholar] [CrossRef]
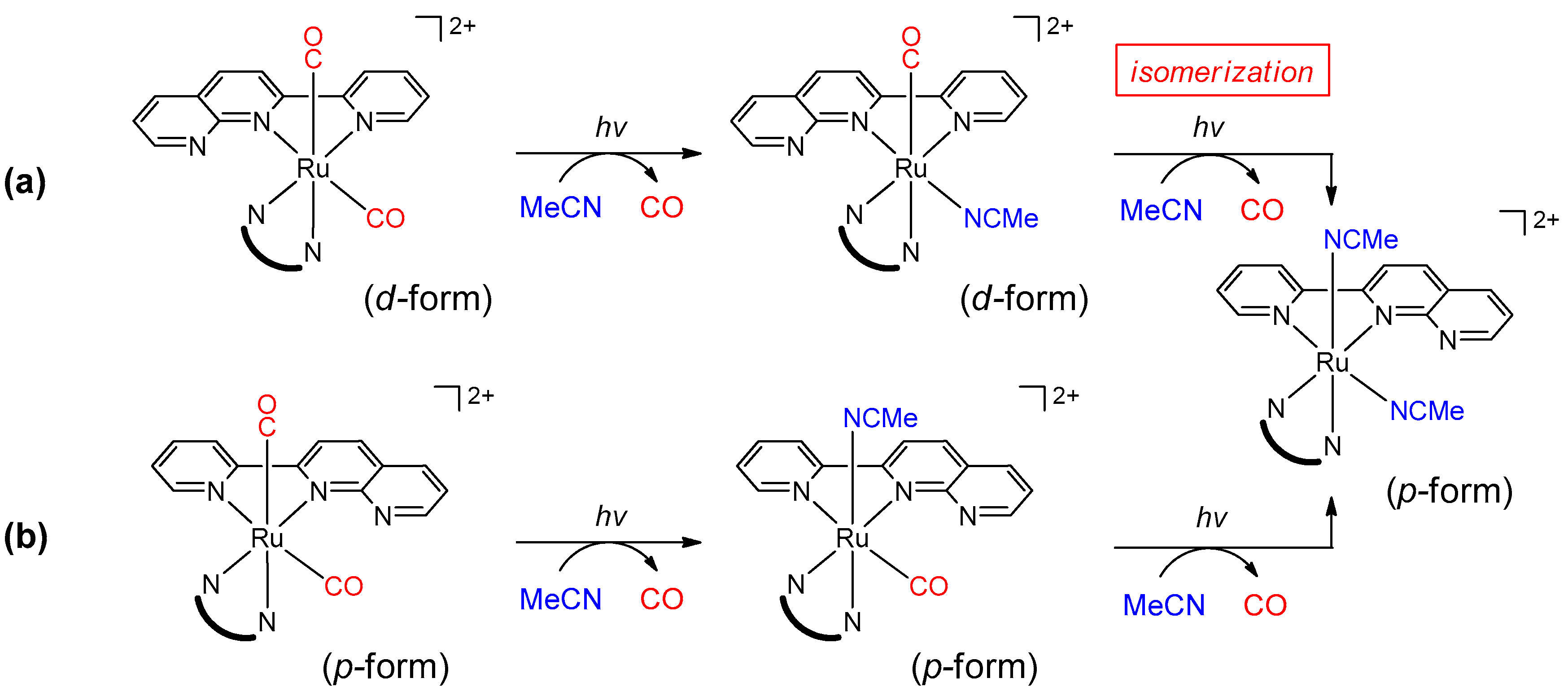
- Based on isolation and identification of the intermediates, we confirmed that the photoreaction proceeds through the two-step process as shown in Scheme 2.
- Tanaka, S.; Takahashi, K.; Hirahara, M.; Yagi, M.; Onda, K. Characterization of the excited states of distal- and proximal-[Ru(tpy)(pynp)OH2]2+ in aqueous solution using time-resolved infrared spectroscopy. J. Photochem. Photobiol. A 2015, 313, 87–98. [Google Scholar] [CrossRef]
- Hirahara, M.; Ertem, M.Z.; Komi, M.; Yamazaki, H.; Cramer, C.J.; Yagi, M. Mechanisms of photoisomerization and water-oxidation catalysis of mononuclear ruthenium(II) monoaquo complexes. Inorg. Chem. 2013, 52, 6354–6364. [Google Scholar] [CrossRef]
- Oyama, D.; Yuzuriya, K.; Naoi, R.; Hamada, T.; Takase, T. Syntheses of geometrical isomers for comparison of properties caused by steric and electronic effects in carbonylruthenium(II) complexes. Bull. Chem. Soc. Jpn. 2014, 87, 1107–1115. [Google Scholar] [CrossRef]
- Constable, E.C.; Housecroft, C.E. The early years of 2,2’-bipyridine – A ligand in its own lifetime. Molecules 2019, 24, 3951. [Google Scholar] [CrossRef]
- Oyama, D.; Hamada, T.; Ukawa, N.; Mochizuki, R.; Takase, T. Isolation and characterization of a metallacyclic compound by selective protection of a single CO ligand in a ruthenium complex. Bull. Chem. Soc. Jpn. 2015, 88, 1572–1574. [Google Scholar] [CrossRef]
- Haukka, M.; Kiviaho, J.; Ahlgren, M.; Pakkanen, A.T. Studies on catalytically active ruthenium carbonyl bipyridine systems. Synthesis and structural characterization of [Ru(bpy)(CO)2Cl2], [Ru(bpy)(CO)2Cl(C(O)OCH3)], [Ru(bpy)(CO)2Cl]2 and [Ru(bpy)(CO)2ClH] (bpy = 2,2’-bipyridine). Organometallics 1995, 14, 825–833. [Google Scholar] [CrossRef]
- Kobayashi, K.; Kikuchi, T.; Kitagawa, S.; Tanaka, K. Selective generation of formamides through photocatalytic CO2 reduction catalyzed by ruthenium carbonyl compounds. Angew. Chem. Int. Ed. 2014, 53, 11813–11817. [Google Scholar] [CrossRef] [PubMed]
- Oyama, D.; Hamada, T.; Takase, T. Stereospecific synthesis and redox properties of ruthenium(II) carbonyl complexes bearing a redox-active 1,8-naphthyridine unit. J. Organomet. Chem. 2011, 696, 2263–2268. [Google Scholar] [CrossRef]
- Oyama, D.; Kainuma, S.; Akatsuka, K.; Abe, R.; Takase, T. Solvent mediated complete trans-to-cis isomerization of [Ru(polypyridine)(CO)2Cl2] complexes. J. Organomet. Chem. 2019, 900, 120883. [Google Scholar] [CrossRef]
- Campos-Fernández, C.S.; Thomson, L.M.; Galán-Mascarós, J.R.; Ouyang, X.; Dunbar, K.R. Homologous series of redox-active, dinuclear cations [M2(O2CCH3)2(pynp)2]2+ (M = Mo, Ru, Rh) with the bridging ligand 2-(2-pyridyl)-1,8-naphthyridine (pynp). Inorg. Chem. 2002, 41, 1523–1533. [Google Scholar] [CrossRef]
- Anderson, P.A.; Deacon, G.B.; Haarmann, K.H.; Keene, F.R.; Meyer, T.J.; Reitsma, D.A.; Skelton, B.W.; Strouse, G.F.; Thomas, N.C.; Treadway, J.A.; et al. Designed synthesis of mononuclear tris(heteroleptic) ruthenium complexes containing bidentate polypyridyl ligands. Inorg. Chem. 1995, 34, 6145–6157. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09W, revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Francl, M.M.; Pietro, W.J.; Hehre, W.J.; Binkley, J.S.; Gordon, M.S.; DeFrees, D.J.; Pople, J.A. Self-consistent molecular orbital methods. XXIII. A polarization-type basis set for second-row elements. J. Chem. Phys. 1982, 77, 3654–3665. [Google Scholar] [CrossRef]
- Hehre, W.J.; Ditchfield, R.; Pople, J.A. Self-consistent molecular orbital methods. XII. Further extensions of Gaussian-type basis sets for use in molecular orbital studies of organic molecules. J. Chem. Phys. 1972, 56, 2257–2261. [Google Scholar] [CrossRef]
- Wadt, W.R.; Hay, P.J. Ab initio effective core potentials for molecular calculations. Potentials for main group elements Na to Bi. J. Chem. Phys. 1985, 82, 284–298. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds[1d]2+, [1p]2+, [2d]2+, [2p]2+, and [3dp]2+ are available from the authors. |
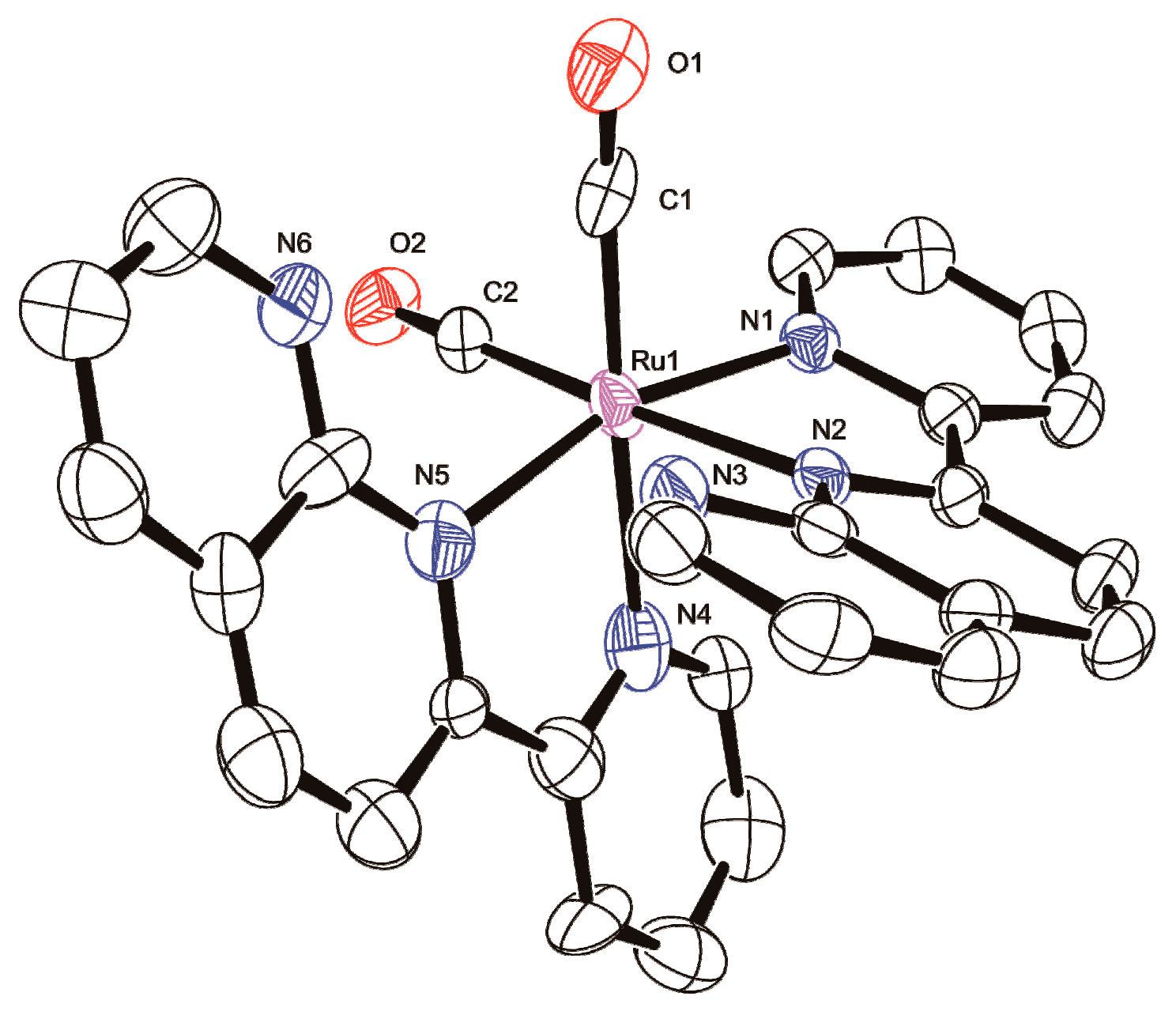
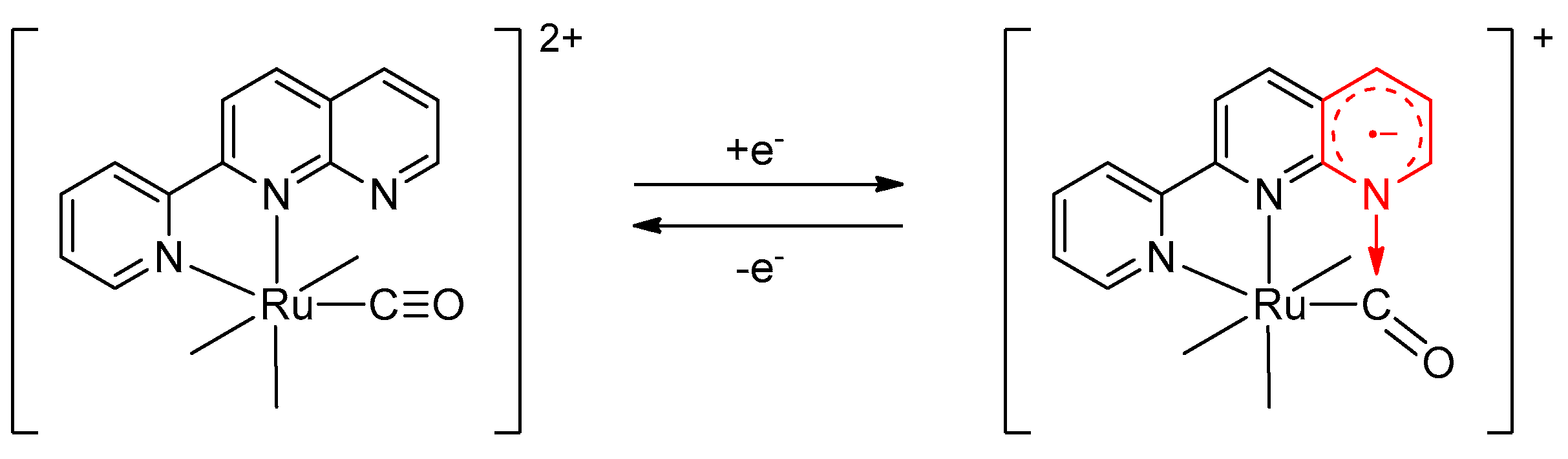
| Parameter | [1d]2+ 2 | [1p]2+ 3 | [2d]2+ | [2p]2+ | [3dp]2+ |
|---|---|---|---|---|---|
| Ru–C | 1.905 (3) | 1.925 (11) | 1.903 (9) | 1.917 (4) | 1.919 (10) |
| 1.900 (3) | 1.910 (10) | 1.888 (9) | 1.911 (4) | 1.886 (8) | |
| 1.909 (9) | |||||
| 1.895 (10) | |||||
| C–O | 1.134 (4) | 1.123 (13) | 1.145 (11) | 1.133 (5) | 1.132 (10) |
| 1.124 (4) | 1.119 (12) | 1.123 (11) | 1.122 (5) | 1.120 (11) | |
| 1.133 (11) | |||||
| 1.121 (11) | |||||
| Ru–C–O | 176.9 (3) | 177.6 (8) | 177.0 (8) | 176.0 (4) | 175.7 (7) |
| 176.6 (3) | 174.3 (9) | 175.5 (8) | 171.6 (4) | 175.6 (8) | |
| 177.4 (8) | |||||
| 177.1 (8) | |||||
| C…N 1 | - | 2.671 (14) | - | 2.607 (5) | 2.754 (14) |
| Technique | [1d]2+ | [1p]2+ | [2d]2+ | [2p]2+ | [3dp]2+ |
|---|---|---|---|---|---|
| IR (νCO/cm−1) | 2097 | 2097 | 2099 | 2093 | 2094 |
| 2044 | 2045 | 2045 | 2044 | 2040 | |
| UV–vis (λmax/nm) | 339 (1.78) | 343 (2.90) | 342 (1.64) | 349 (2.05) | 336 (2.93) |
| (ε/×104 M−1 cm−1) | 314 (2.22) | 315 (2.63) | 273 (3.19) | 272 (3.79) | |
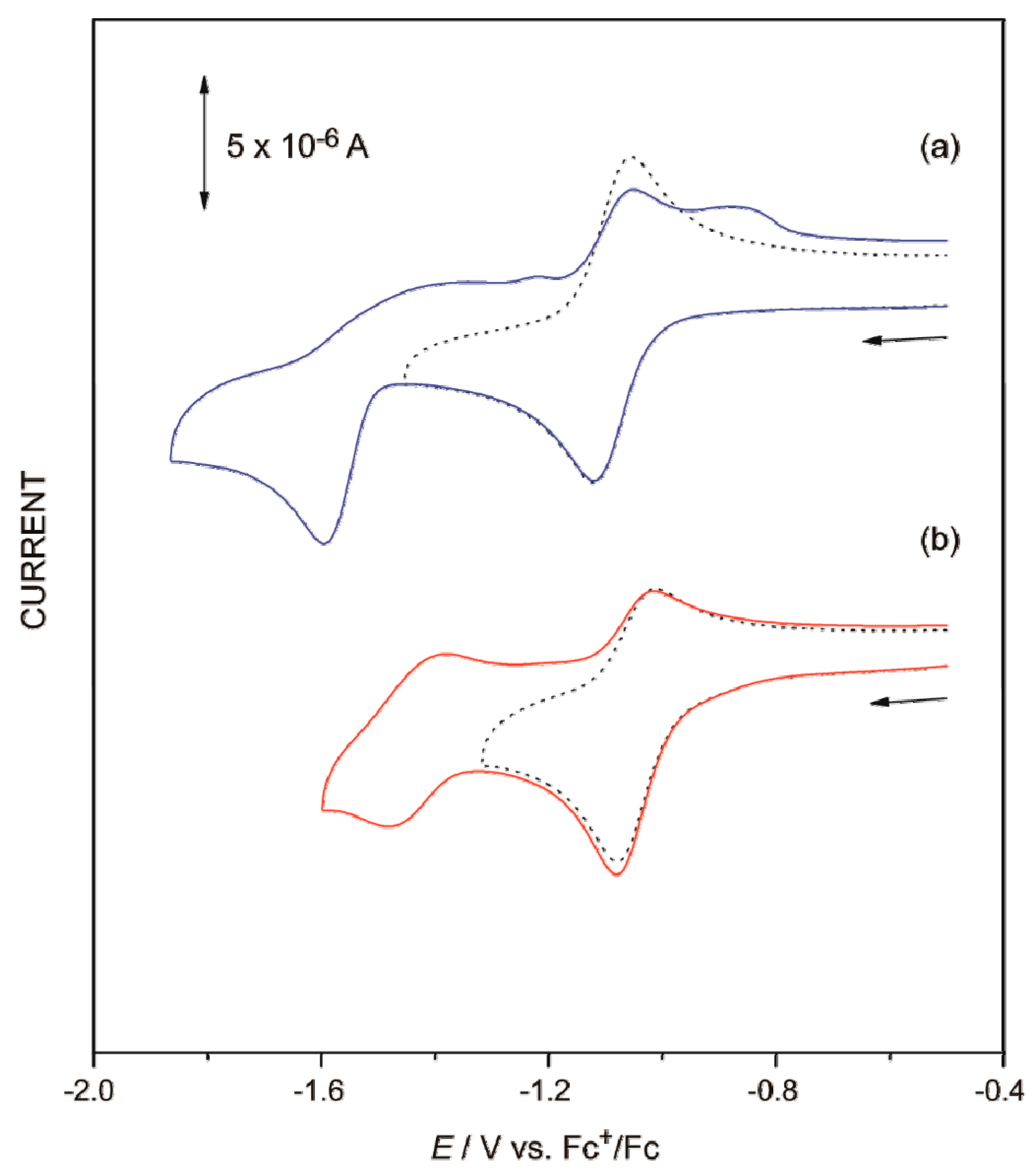
| CV (E/V vs. Fc+/Fc) | −1.09 1 | −1.07 1 | −1.12 1 | −1.09 1 | −1.11 1 |
| −1.45 1 | −1.48 1 | −1.59 1 | −1.49 1 | −1.29 1 | |
| −0.98 2 | −1.05 2 | −1.03 2 | −1.03 2 | ||
| (−0.78) 3 | (−0.87) 3 | (−0.79) 3 |
| Rate | [1d]2+ | [1p]2+ | [2d]2+ | [2p]2+ | [3dp]2+ |
|---|---|---|---|---|---|
| kobs/sec−1 | 12 × 10−3 | 6.5 × 10−3 | 2.4 × 10−3 | 1.3 × 10−3 | 7.6 × 10−3 |
| Parameter | [2d] | [2p] | [3dp] |
|---|---|---|---|
| Chemical formula | C55H34F21N10O8P3Ru2S | C29H20F12N6O2P2Ru | C30H18F6N6O8RuS2 |
| Formula weight | 1689.02 | 875.51 | 869.69 |
| Temperature (K) | 93 | 93 | 93 |
| Crystal system | monoclinic | triclinic | triclinic |
| Space group | P21/c | P-1 | P-1 |
| a (Å) | 13.2730(3) | 8.57310(10) | 9.68729(18) |
| b (Å) | 22.5773(6) | 12.7558(3) | 13.0592(3) |
| c (Å) | 20.2047(5) | 15.8850(4) | 13.6489(3) |
| α (°) | 90 | 82.856(6) | 83.1931(7) |
| β (°) | 93.7571(8) | 75.919(6) | 81.5465(7) |
| γ (°) | 90 | 74.843(6) | 72.0749(7) |
| V (Å3) | 6041.7(3) | 1622.90(8) | 1620.10(6) |
| Z | 4 | 2 | 2 |
| Calcd density (g/cm3) | 1.857 | 1.792 | 1.783 |
| μ (Mo Kα) (mm–1) | 0.744 | 0.691 | 0.709 |
| No. unique reflns | 62,024 | 16,768 | 16,909 |
| No. obsd reflns | 13,818 | 7331 | 7304 |
| Refinement method | Full-matrix least-squares on F2 | ||
| Parameters | 901 | 470 | 478 |
| R (I > 2σ(I)) 1 | 0.0941 | 0.0552 | 0.1119 |
| wR (all data) 2 | 0.2536 | 0.1483 | 0.2633 |
| S | 1.155 | 1.077 | 1.189 |
| Parameter | d-(phen) | p-(phen) | dp-(pynp) |
|---|---|---|---|
| Chemical formula | C28H20F12N6OP2Ru | C29H20F12N6O2P2Ru | C32H27F12N7O2P2Ru |
| Formula weight | 847.50 | 875.51 | 932.61 |
| Temperature (K) | 93 | 93 | 93 |
| Crystal system | orthorhombic | triclinic | monoclinic |
| Space group | Pna21 | P-1 | P21/n |
| a (Å) | 13.8734(9) | 11.9822(4) | 10.067(4) |
| b (Å) | 24.7954(15) | 12.5252(3) | 32.588(12) |
| c (Å) | 9.0869(5) | 13.2975(4) | 11.329(5) |
| α (°) | 90 | 84.1114(11) | 90 |
| β (°) | 90 | 87.5838(8) | 109.861(5) |
| γ (°) | 90 | 63.326(2) | 90 |
| V (Å3) | 3125.9(3) | 1773.87(9) | 3496(2) |
| Z | 4 | 2 | 4 |
| Calcd density (g/cm3) | 1.801 | 1.639 | 1.772 |
| μ (Mo Kα) (mm−1) | 0.712 | 0.632 | 0.648 |
| No. unique reflns | 30,815 | 18,631 | 35,054 |
| No. obsd reflns | 7100 | 8064 | 7963 |
| Refinement method | Full-matrix least-squares on F2 | ||
| Parameters | 452 | 436 | 508 |
| R (I > 2σ(I)) 1 | 0.0506 | 0.0832 | 0.0745 |
| wR (all data) 2 | 0.1237 | 0.2503 | 0.1623 |
| S | 1.028 | 1.049 | 1.113 |
| Parameter | R = OH | R = OC2H5 |
|---|---|---|
| Chemical formula | C28H24F6N5O4PRu | C30H28F6N5O4PRu |
| Formula weight | 740.56 | 768.62 |
| Temperature (K) | 93 | 93 |
| Crystal system | triclinic | monoclinic |
| Space group | P-1 | P21/n |
| a (Å) | 8.35369(15) | 17.2474(5) |
| b (Å) | 12.9208(3) | 8.1493(2) |
| c (Å) | 13.3343(3) | 22.1009(6) |
| α (°) | 88.9616(7) | 90 |
| β (°) | 79.0700(7) | 102.3249(8) |
| γ (°) | 88.2204(7) | 90 |
| V (Å3) | 1412.36(5) | 3034.77(14) |
| Z | 2 | 4 |
| Calcd density (g/cm3) | 1.741 | 1.682 |
| μ (Mo Kα) (mm−1) | 0.697 | 0.652 |
| No. unique reflns | 12,245 | 30,159 |
| No. obsd reflns | 4934 | 6960 |
| Refinement method | Full-matrix least-squares on F2 | |
| Parameters | 406 | 463 |
| R (I > 2σ(I)) 1 | 0.0583 | 0.0475 |
| wR (all data) 2 | 0.1331 | 0.1132 |
| S | 1.219 | 1.067 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akatsuka, K.; Abe, R.; Takase, T.; Oyama, D. Coordination Chemistry of Ru(II) Complexes of an Asymmetric Bipyridine Analogue: Synergistic Effects of Supporting Ligand and Coordination Geometry on Reactivities. Molecules 2020, 25, 27. https://doi.org/10.3390/molecules25010027
Akatsuka K, Abe R, Takase T, Oyama D. Coordination Chemistry of Ru(II) Complexes of an Asymmetric Bipyridine Analogue: Synergistic Effects of Supporting Ligand and Coordination Geometry on Reactivities. Molecules. 2020; 25(1):27. https://doi.org/10.3390/molecules25010027
Chicago/Turabian StyleAkatsuka, Komi, Ryosuke Abe, Tsugiko Takase, and Dai Oyama. 2020. "Coordination Chemistry of Ru(II) Complexes of an Asymmetric Bipyridine Analogue: Synergistic Effects of Supporting Ligand and Coordination Geometry on Reactivities" Molecules 25, no. 1: 27. https://doi.org/10.3390/molecules25010027
APA StyleAkatsuka, K., Abe, R., Takase, T., & Oyama, D. (2020). Coordination Chemistry of Ru(II) Complexes of an Asymmetric Bipyridine Analogue: Synergistic Effects of Supporting Ligand and Coordination Geometry on Reactivities. Molecules, 25(1), 27. https://doi.org/10.3390/molecules25010027






