Ethylene Adsorption Using Cobalt Oxide-Loaded Polymer-Derived Nanoporous Carbon and Its Application to Extend Shelf Life of Fruit
Abstract
1. Introduction
2. Results and Discussion
2.1. Adsorbent Characterization
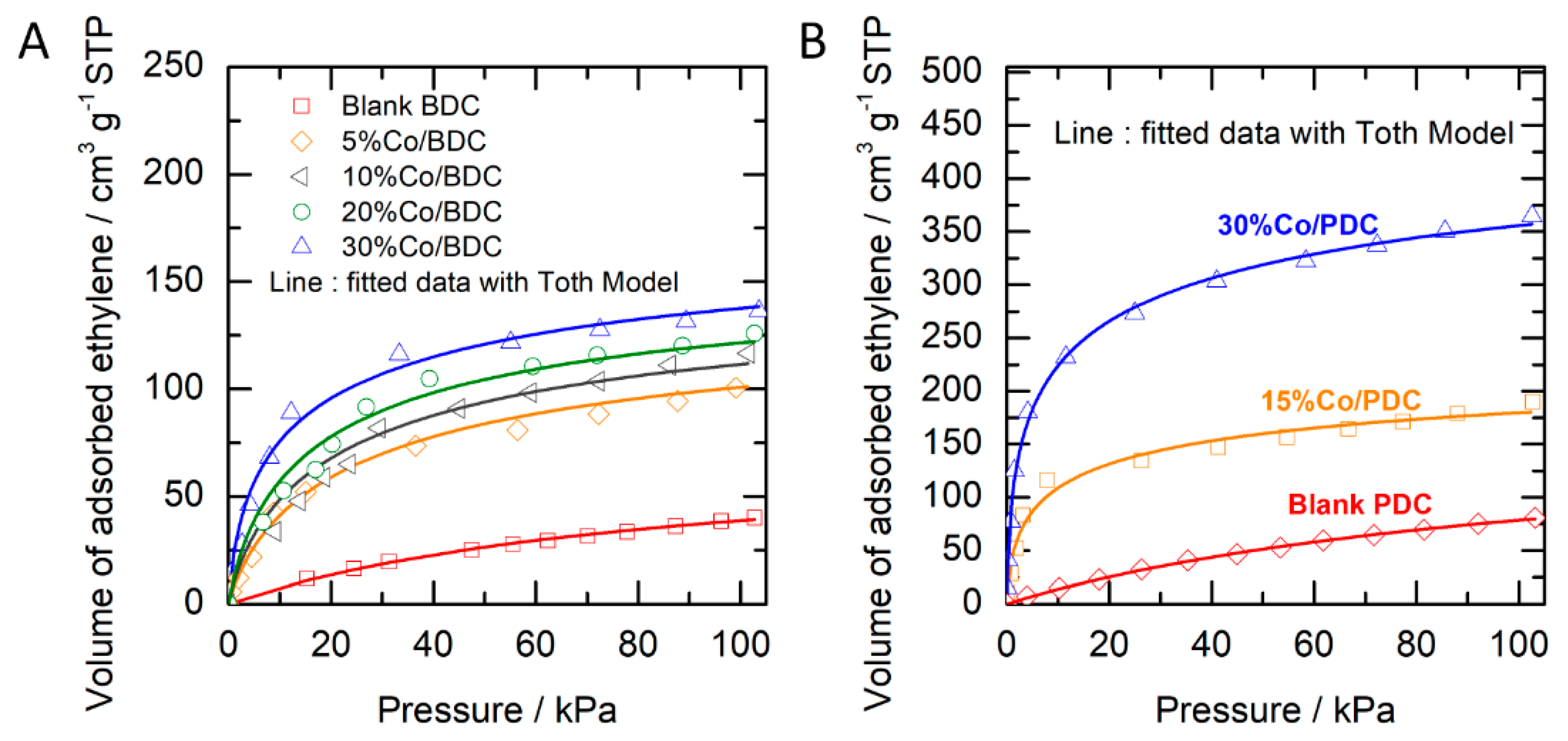
2.2. Ethylene Adsorption
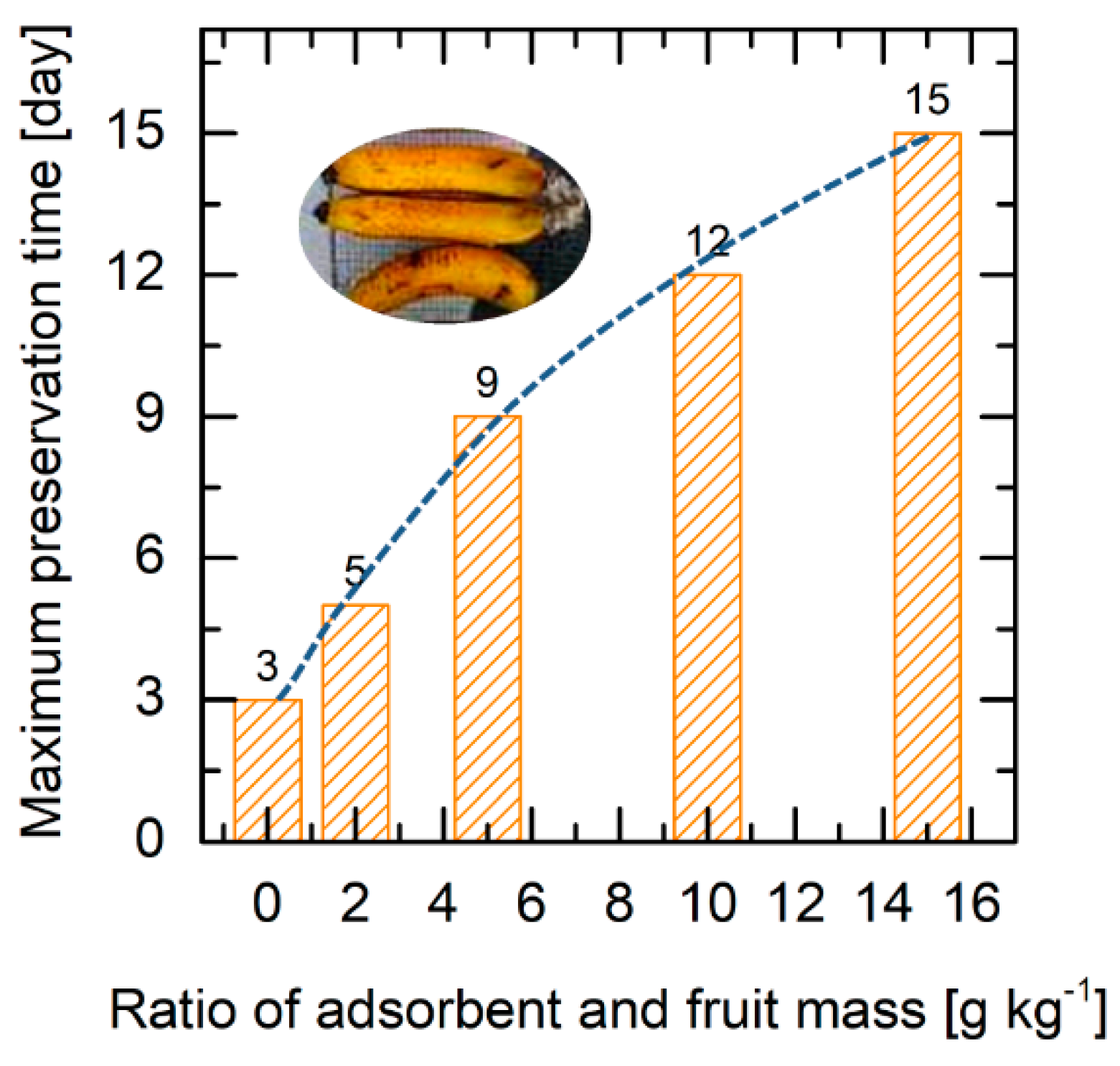
2.3. Fruit Preservation Test
3. Materials and Methods
3.1. Nanoporous Carbon Preparation
3.2. Cobalt Oxide-Impregnated Nanoporous Carbon Preparation
3.3. Characterization Methods
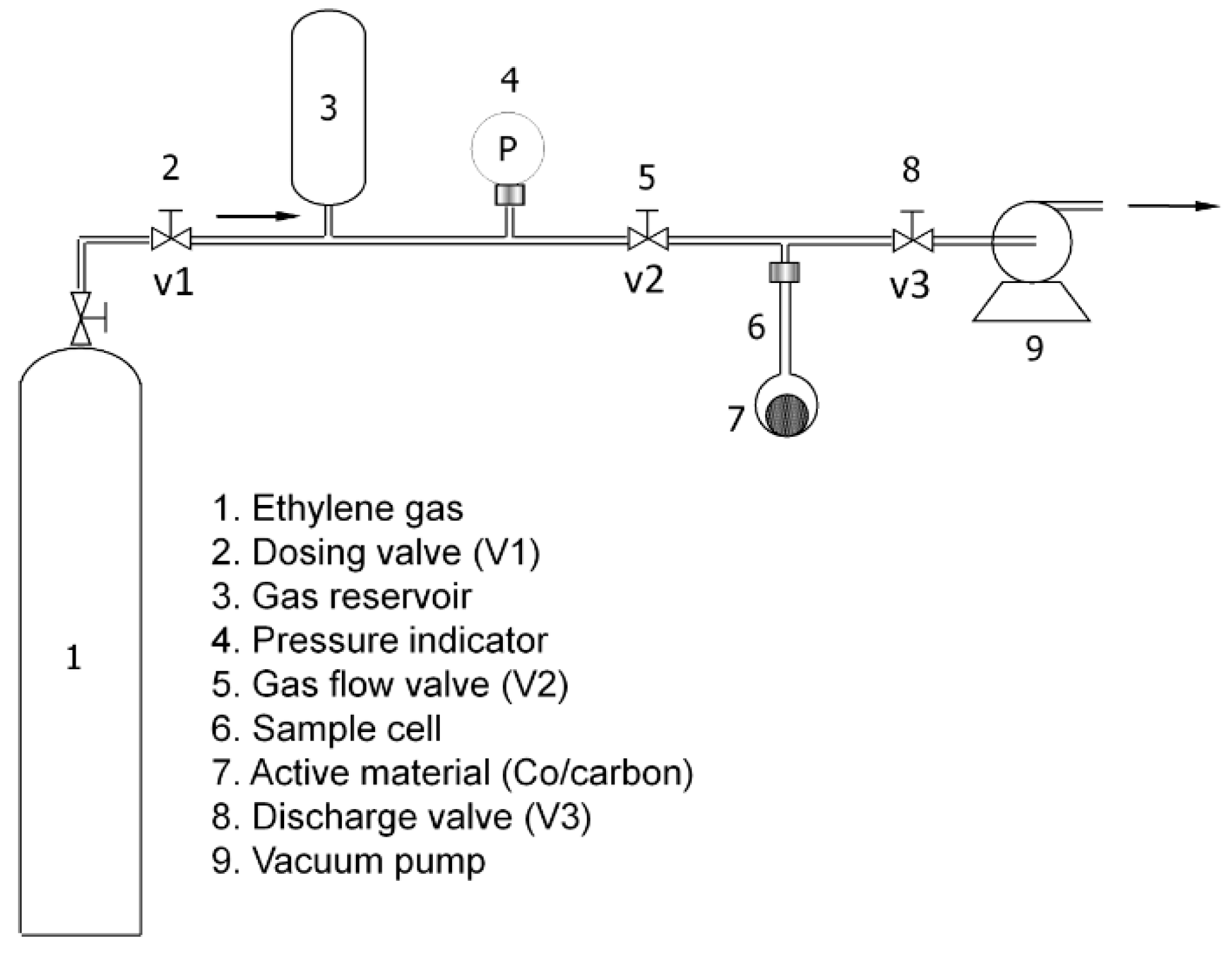
3.4. Ethylene Adsorption Measurement
3.5. Preservation Test
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dermesonluoglu, E.; Katsaros, G.; Tsevdou, M.; Giannakourou, M.; Taoukis, P. Kinetic study of quality indices and shelf life modelling of frozen spinach under dynamic conditions of the cold chain. J. Food Eng. 2015, 148, 13–23. [Google Scholar] [CrossRef]
- Paull, R.E. Effect of temperature and relative humidity on fresh commodity quality. Postharvest Biol. Technol. 1999, 15, 263–277. [Google Scholar] [CrossRef]
- Martínez-Romero, D.; Bailén, G.; Serrano, M.; Guillén, F.; Valverde, J.M.; Zapata, P.; Castillo, S.; Valero, D. Tools to maintain postharvest fruit and vegetable quality through the inhibition of ethylene action: A review. Crit. Rev. Food Sci. Nutr. 2007, 47, 543–560. [Google Scholar] [CrossRef] [PubMed]
- Wills, R.B.H.; Warton, M.A. Efficacy of potassium permanganate impregnated into alumina beads to reduce atmospheric ethylene. J. Am. Soc. Hortic. Sci. 2004, 129, 433–438. [Google Scholar] [CrossRef]
- Jayaraman, K.S.; Raju, P.S. Development and evaluation of a permanganate-based ethylene scrubber for extending the shelf life of fresh fruits and vegetables. J. Food Sci. Technol. India 1992, 29, 77–83. [Google Scholar]
- Singh, R.; Giri, S. Shelf-life study of Guava under active packaging: An experiment with potassium permanganate salt as ethylene absorbent. J. Food Saf. Food Qual. 2014, 65, 32–39. [Google Scholar]
- Cao, J.; Li, X.; Wu, K.; Jiang, W.; Qu, G. Preparation of a novel PdCl2–CuSO4–based ethylene scavenger supported by acidified activated carbon powder and its effects on quality and ethylene metabolism of broccoli during shelf-life. Postharvest Biol. Technol. 2015, 99, 50–57. [Google Scholar] [CrossRef]
- Sue-aok, N.; Srithanratana, T.; Rangsriwatananon, K.; Hengrasmee, S. Study of ethylene adsorption on zeolite NaY modified with group I metal ions. Appl. Surf. Sci. 2010, 256, 3997–4002. [Google Scholar] [CrossRef]
- Mukti, N.I.F.; Prasetyo, I.; Mindaryani, A. Preparasi karbon teremban oksida cobalt dari limbah kulit manggis sebagai adsorben penjerap etilen untuk pengawetan buah. Reaktor 2015, 15, 165–174. [Google Scholar] [CrossRef][Green Version]
- Mukti, N.I.F. Pemanfaatan Limbah Sisa Ekstraksi Kulit Manggis Sebagai Bahan Pembuat Adsorben Penjerap Etilen Untuk Pengawetan Buah. Master’s Thesis, Universitas Gadjah Mada, Yogyakarta, Indonesia, 2015. [Google Scholar]
- Zhao, X.S.; Bao, X.Y.; Guo, W.; Lee, F.Y. Immobilizing catalysts on porous materials. Mater. Today 2006, 9, 32–39. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, H.; Deng, J.; Xie, S.; Yang, H.; Tan, W.; Han, W.; Jiang, Y.; Guo, G. Mesoporous Co3O4-supported gold nanocatalysts: Highly active for the oxidation of carbon monoxide, benzene, toluene, and o-xylene. J. Catal. 2014, 309, 408–418. [Google Scholar] [CrossRef]
- Zuo, S.; Liu, F.; Tong, J.; Qi, C. Complete oxidation of benzene with cobalt oxide and ceria using the mesoporous support SBA-16. Appl. Catal. A Gen. 2013, 467, 1–6. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Z.; Wang, J.; Qiao, W.; Long, D.; Ling, L. Low temperature catalytic combustion of ethylene over cobalt oxide supported mesoporous carbon spheres. Chem. Eng. J. 2016, 293, 243–251. [Google Scholar] [CrossRef]
- Prasetyo, I.; Mukti, N.I.F.; Fahrurrozi, M.; Ariyanto, T. Removing Ethylene by Adsorption using Cobalt Oxide-Loaded Nanoporous Carbon. Asean J. Chem. Eng. 2018, 18, 9–16. [Google Scholar]
- Sharma, S.; Pollet, B.G. Support materials for PEMFC and DMFC electrocatalysts—A review. J. Power Sources 2012, 208, 96–119. [Google Scholar] [CrossRef]
- Sigot, L.; Fontseré Obis, M.; Benbelkacem, H.; Germain, P.; Ducom, G. Comparing the performance of a 13X zeolite and an impregnated activated carbon for H2S removal from biogas to fuel an SOFC: Influence of water. Int. J. Hydrogen Energy 2016, 41, 18533–18541. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, G.; Zhang, J.; Wang, Y.; Yao, M. Activated Carbon Preparation from Lignin by H3PO4 Activation and Its Application to Gas Separation. Chem. Eng. Technol. 2011, 35, 309–316. [Google Scholar] [CrossRef]
- Yi, H.; Li, F.; Ning, P.; Tang, X.; Peng, J.; Li, Y.; Deng, H. Adsorption separation of CO2, CH4, and N2 on microwave activated carbon. Chem. Eng. J. 2013, 215, 635–642. [Google Scholar] [CrossRef]
- Lenghaus, K.; GuangHua Qiao, G.; Solomon, D.H.; Gomez, C.; Rodriguez-Reinoso, F.; Sepulveda-Escribano, A. Controlling carbon microporosity: The structure of carbons obtained from different phenolic resin precursors. Carbon 2002, 40, 743–749. [Google Scholar] [CrossRef]
- Glasel, J.; Diao, J.Y.; Feng, Z.B.; Hilgart, M.; Wolker, T.; Su, D.S.; Etzold, B.J.M. Mesoporous and graphitic carbide-derived carbons as selective and stable catalysts for the dehydrogenation reaction. Chem. Mater. 2015, 27, 5719–5725. [Google Scholar] [CrossRef]
- Moreira, R.F.P.M.; José, H.J.; Rodrigues, A.E. Modification of pore size in activated carbon by polymer deposition and its effects on molecular sieve selectivity. Carbon 2001, 39, 2269–2276. [Google Scholar] [CrossRef]
- Prasetyo, I.; Rochmadi, R.; Wahyono, E.; Ariyanto, T. Controlling synthesis of polymer-derived carbon molecular sieve and its performance for CO2/CH4 separation. Eng. J. 2017, 21, 83–94. [Google Scholar] [CrossRef]
- Choi, B.-U.; Choi, D.-K.; Lee, Y.-W.; Lee, B.-K.; Kim, S.-H. Adsorption equilibria of methane, ethane, ethylene, nitrogen, and hydrogen onto activated carbon. J. Chem. Eng. Data 2003, 48, 603–607. [Google Scholar] [CrossRef]
- Ariyanto, T.; Kern, A.; Etzold, B.J.M.; Zhang, G.-R. Carbide-derived carbon with hollow core structure and its performance as catalyst support for methanol electro-oxidation. Electrochem. Commun. 2017, 82, 12–15. [Google Scholar] [CrossRef]
- Fraga, M.A.; Jordao, E.; Mendes, M.J.; Freitas, M.M.A.; Faria, J.L.; Figueiredo, J.L. Properties of carbon-supported platinum catalysts: Role of carbon surface sites. J. Catal. 2002, 209, 355–364. [Google Scholar] [CrossRef]
- Do, D.D. Adsorption Analysis: Equilibria and Kinetics, 2nd ed.; Imperial College Press: London, UK, 1998. [Google Scholar]
- Fogler, H.S. Essentials of Chemical Reaction Engineering, 5th ed.; Pearson Education: London, UK, 2010. [Google Scholar]
- Prasetyo, I.; Rochmadi; Ariyanto, T.; Yunanto, R. Simple method to produce nanoporous carbon for various applications by pyrolysis of specially synthesized phenolic resin. Indones. J. Chem. 2013, 13, 95–100. [Google Scholar] [CrossRef]
- Becker, H.; Turek, T.; Güttel, R. Study of temperature-programmed calcination of cobalt-based catalysts under NO-containing atmosphere. Catal. Today 2013, 215, 8–12. [Google Scholar] [CrossRef]
- Wang, C.-B.; Lin, H.-K.; Tang, C.-W. Thermal Characterization and Microstructure Change of Cobalt Oxides. Catal. Lett. 2004, 94, 69–74. [Google Scholar] [CrossRef]
- Gor, G.Y.; Thommes, M.; Cychosz, K.A.; Neimark, A. V Quenched solid density functional theory method for characterization of mesoporous carbons by nitrogen adsorption. Carbon 2012, 50, 1583–1590. [Google Scholar] [CrossRef]
- Scherdel, C.; Reichenauer, G.; Wiener, M. Relationship between pore volumes and surface areas derived from the evaluation of N2-sorption data by DR-, BET- and t-plot. Microporous Mesoporous Mater. 2010, 132, 572–575. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
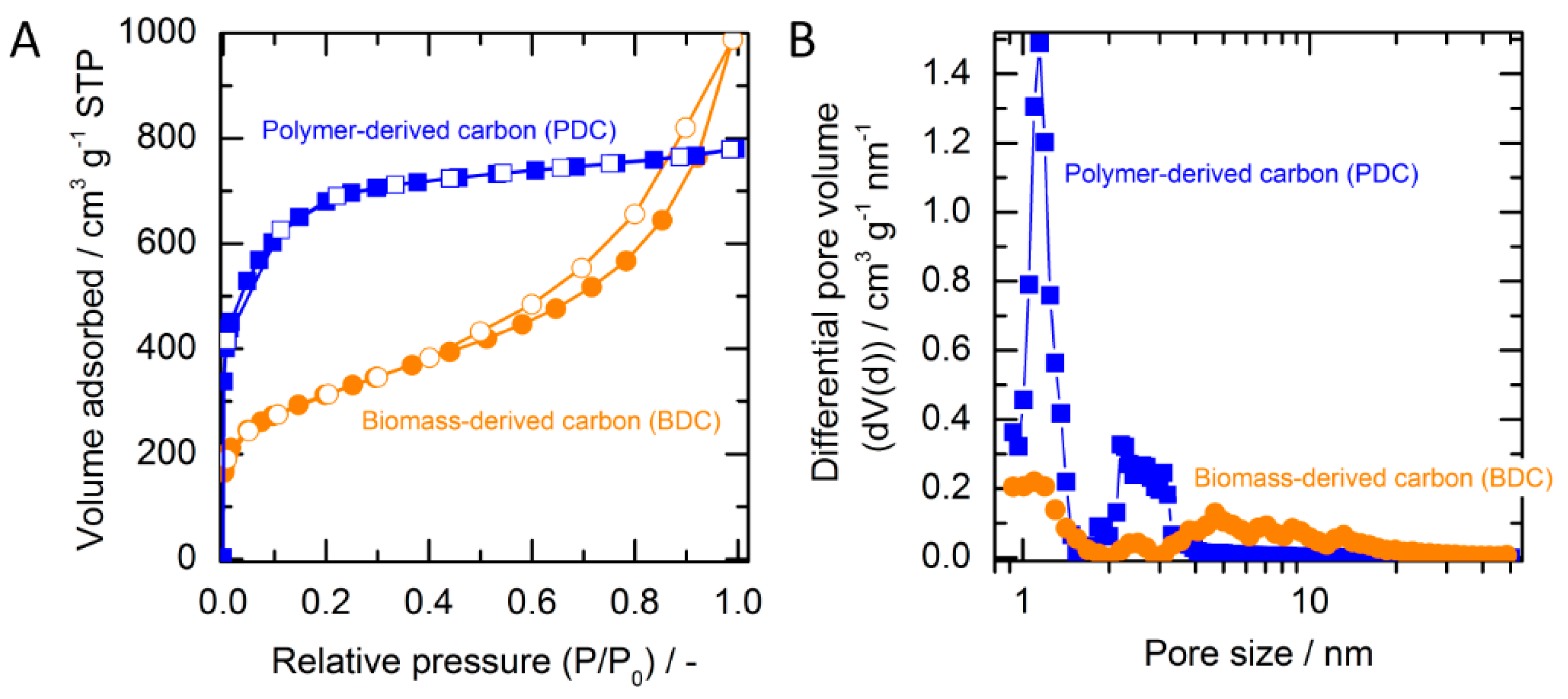
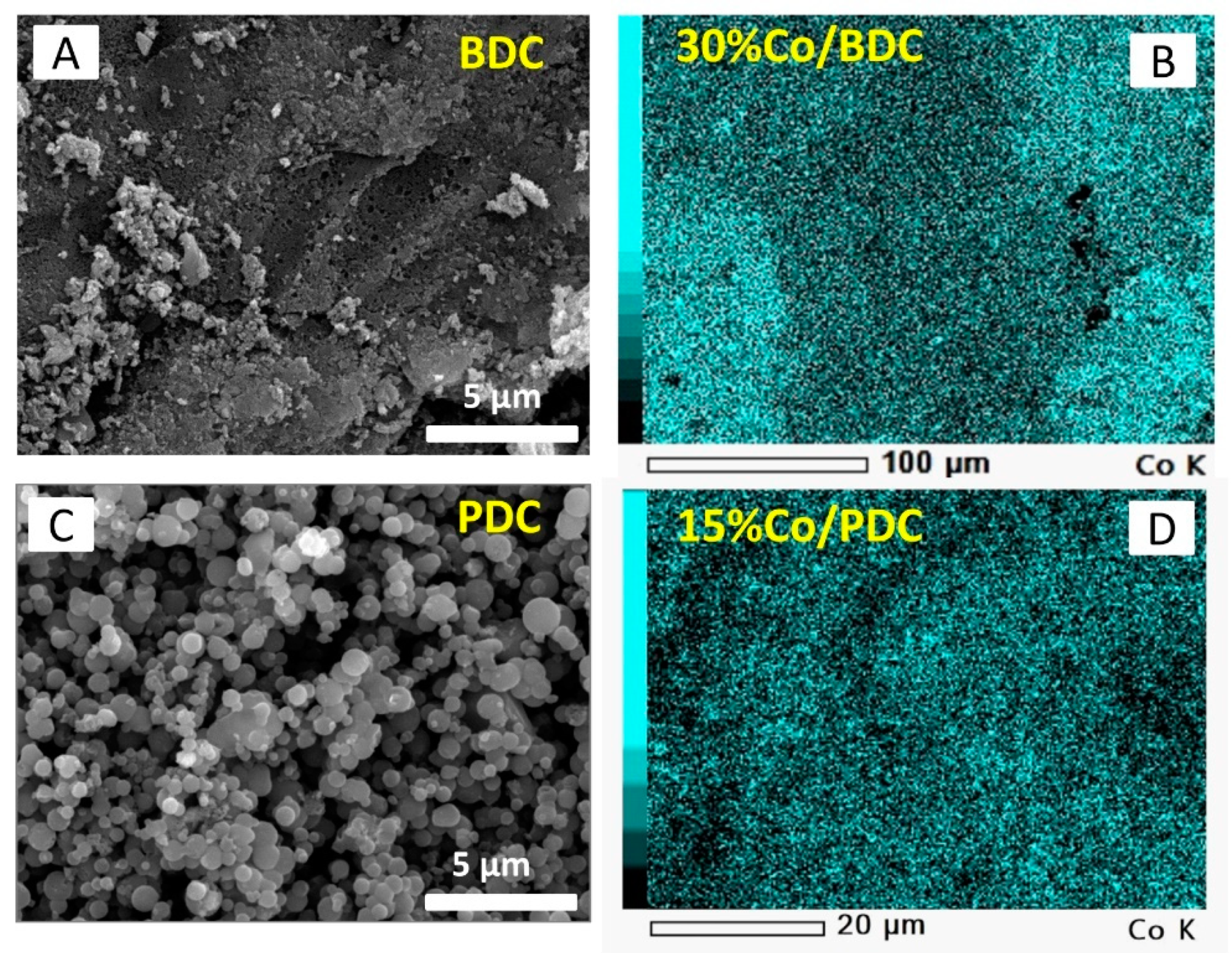

| Porous Textural Parameter | SSAa, m2 g−1 | %SSAMic b, % | VTc, cm3 g−1 | %Vmic d, % | dve, nm |
|---|---|---|---|---|---|
| Commercial activated carbon | 1050 | 55 | 0.46 | 82 | 1.75 |
| Biomass-derived carbon (BDC) | 1080 | 32 | 1.53 | 10 | 5.66 |
| Polymer-derived carbon (PDC) | 2390 | 84 | 1.21 | 60 | 2.03 |
| 15% Co/PDC | 1469 | 92 | 0.70 | 70 | 1.92 |
| 30% Co/PDC | 928 | 87 | 0.49 | 63 | 2.10 |
| Parameter | Cobalt Oxide/Biomass-Derived Carbon | Cobalt oxide/Polymer-Derived Carbon | ||||||
|---|---|---|---|---|---|---|---|---|
| Blank BDC | 5%Co/BDC | 10%Co/BDC | 20%Co/BDC | 30%Co/BDC | Blank PDC | 15%Co/PDC | 30%Co/PDC | |
| Cµs, cm3 g−1 | 76.57 | 147.08 | 159.35 | 164.38 | 195.51 | 174.24 | 273.10 | 553.70 |
| b, kPa−1 | 0.01 | 0.06 | 0.08 | 0.10 | 0.27 | 0.01 | 0.92 | 1.76 |
| t, - | 0.94 | 0.67 | 0.65 | 0.66 | 0.51 | 0.95 | 0.39 | 0.35 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prasetyo, I.; Mukti, N.I.F.; Ariyanto, T. Ethylene Adsorption Using Cobalt Oxide-Loaded Polymer-Derived Nanoporous Carbon and Its Application to Extend Shelf Life of Fruit. Molecules 2019, 24, 1507. https://doi.org/10.3390/molecules24081507
Prasetyo I, Mukti NIF, Ariyanto T. Ethylene Adsorption Using Cobalt Oxide-Loaded Polymer-Derived Nanoporous Carbon and Its Application to Extend Shelf Life of Fruit. Molecules. 2019; 24(8):1507. https://doi.org/10.3390/molecules24081507
Chicago/Turabian StylePrasetyo, Imam, Nur Indah Fajar Mukti, and Teguh Ariyanto. 2019. "Ethylene Adsorption Using Cobalt Oxide-Loaded Polymer-Derived Nanoporous Carbon and Its Application to Extend Shelf Life of Fruit" Molecules 24, no. 8: 1507. https://doi.org/10.3390/molecules24081507
APA StylePrasetyo, I., Mukti, N. I. F., & Ariyanto, T. (2019). Ethylene Adsorption Using Cobalt Oxide-Loaded Polymer-Derived Nanoporous Carbon and Its Application to Extend Shelf Life of Fruit. Molecules, 24(8), 1507. https://doi.org/10.3390/molecules24081507