Abstract
Antibiotic resistance is a problem that continues to challenge the healthcare sector, especially in clinically significant pathogens like methicillin-resistant Staphylococcus aureus (MRSA). Herein is described the isolation and structure elucidation of a bioactive compound from Allium stipitatum with antimicrobial activity. Crude Allium stipitatum dichloromethane extract (ASDE) was subjected to systematic purification by chromatographic procedures to afford various bioactive fractions. A fraction that exhibited anti-MRSA activity (4 µg·mL−1) was further characterized to determine the structure. The structure of the compound was elucidated as 2-(methyldithio)pyridine-3-carbonitrile (2-Medpy-3-CN). The 2-Medpy-3-CN compound, which was screened for antimicrobial activity, exhibited minimum inhibitory concentrations (MICs) in the range of 0.5 to >64 µg·mL−1 for tested bacterial species and 0.25 to 2 µg·mL−1 for Candida spp. Further studies are important to confirm the drug target and mechanism of action.
1. Introduction
The sustained increase in life-threatening infectious diseases and emerging infectious diseases (EID) due to the multidrug-resistant (MDR) pathogens is an immense and serious global challenge. Infections associated with MDR Staphylococcus aureus, including methicillin-resistant S. aureus (MRSA) and vancomycin-resistant S. aureus (VRSA) [1], and with vancomycin-resistant Enterococcus faecium [2], MDR Acinetobacter baumannii [3], trimethoprim/sulfamethoxazole (TMP-SMX)-resistant Stenotrophomonas maltophilia [4], and azole-resistant Candida albicans [5,6] are adequately reported worldwide.
Among the many clinically challenging microbes, S. aureus is a well-armed virulent pathogen that is currently the most common cause of hospital- and community-acquired infections worldwide. Its increasing resistance to antibiotics indicates that its prevalence will continue to rise. In addition, the high morbidity and mortality rates [7], and high treatment costs in combating MRSA infections [8,9,10] strongly underscore the pressing need for new classes of antimicrobials to overcome multidrug resistance.
There are a vast number of natural products that are a likely source of novel antibiotic compounds [11]. In particular, plants serve as main source of bioactive substances including antibacterial compounds, and there are noteworthy studies supporting their preliminary in vitro efficacy [12,13,14]. Allium has a long history of its use in traditional medicine with high therapeutic properties and its ethno-medicinal use is reported from most regions of the temperate world including Asia. An extensive review by Petrovska and Cekovska [15] clearly showcased the use of garlic (Allium sativum L.) for human health from the ancient times until today. Garlic was widely used as an antiseptic to prevent gangrene in the First and Second World Wars [15,16]. Several edible products including different species of Allium (garlic, onion, shallots) were shown to harbor remarkable antibacterial activities [17,18,19].
In our earlier study, we identified dichloromethane extract of Allium stipitatum or Persian shallot, popularly known as “Mooseer”, a wild edible plant mostly found in the cold mountains of central, south, and western Iran, some provinces of Turkey, and central Asia, to exhibit strong antimicrobial and wound-healing activity [20,21,22,23,24]. Pyridine-N-oxide alkaloids and analogs of disulfides from A. stipitatum were reported to possess moderate to strong antibacterial activity against MDR S. aureus, Mycobacterium tuberculosis, Escherichia coli, and Klebsiella and Proteus species [25,26]. The methyl disulfide analogs were also reported to inhibit the mycobacterial drug efflux systems and biofilm formation in M. tuberculosis H37Rv strains [25]. These data indicates that A. stipitatum retains intrinsic antimicrobial properties that likely contribute to the positive outcomes observed in in vivo and in vitro studies.
The promising in vitro and in vivo antimicrobial activities prompted us to explore the isolation of bioactive compounds from the bulbs of A. stipitatum. Therefore, the present study was aimed at purifying the bioactive compound from A. stipitatum that exhibits antimicrobial activity.
2. Results
2.1. Bioassay-Guided Fractionation
A total of 80 fractions were obtained through silica gel column chromatography (CC) and all the fractions were subjected for thin-layer chromatography (TLC) analysis. Fractions that displayed similar TLC patters were pooled together and divided into six major fractions (D1–D6). Screening for antibacterial activity of fractions D1–D6 showed fraction D3 to have strong anti-MRSA activity with a minimum inhibitory concentration (MIC) of 32 µg·mL−1. The MICs of fractions D2 and D4 were 128 µg·mL−1 and 256 µg·mL−1, respectively. However, fractions D1, D5, and D6 did not show any activity (Table 1). Fraction D3 which was subjected to further purification by preparative TLC (PTLC) showed eight major fractions (D3/1–D3/8). Screening for antibacterial activity of fractions D3/1–D3/8 showed fraction D3/6 to have strong anti-MRSA activity with an MIC of 16 µg·mL−1. The MICs of fractions D3/5 and D3/6 were 16 µg·mL−1, while the MICs of fractions D3/4 and D3/7 were 32 µg·mL−1. Fractions D3/1–D3/3 and D3/8 did not show any activity (Table 2). Fraction D3/6 was separated into 120 subfractions by CC, which were pooled into six subfractions (D3/6a–D3/6f) based on the TLC patterns. Screening for antibacterial activity of subfractions D3/6a–D3/6f showed D3/6e to have strong anti-MRSA activity with an MIC of 4 µg·mL−1. Subfraction D3/6d inhibited the growth of MRSA at 32 µg·mL−1, while subfractions D3/6a–c and D3/6f did not show any activity (Table 3).

Table 1.
Allium stipitatum dichloromethane (ASDE) fractions and their respective minimum inhibitory concentrations (MICs) against methicillin-resistant Staphylococcus aureus (MRSA) American Type Culture Collection (ATCC) 43300.

Table 2.
ASDE subfractions of D3 and their respective MICs against MRSA ATCC 43300.

Table 3.
ASDE subfractions of D3/6 and their respective MICs against MRSA ATCC 43300.
2.2. Structure Elucidation and Identification
Fraction D3/6e was identified as 2-(methyldithio)pyridine-3-carbonitrile (2-Medpy-3-CN) (Figure 1) based on spectroscopic methods.
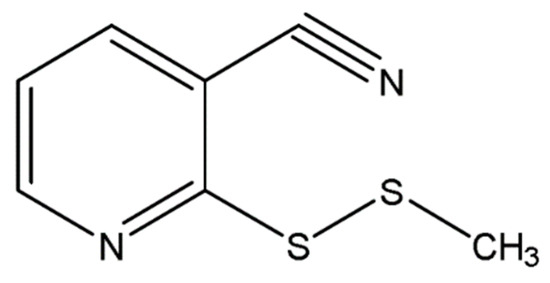
Figure 1.
Two-dimensional (2D) structure of 2-(methyldithio)pyridine-3-carbonitrile (2-Medpy-3-CN).
2-Medpy-3-CN: yellow crystalline; ultraviolet (UV) (MeOH) λmax nm (log ε): 221 (1.19), 272 (2.35); yield 85.86%; melting point (m.p.) 70.6–71.4 °C; infrared (IR) (KBr, disc) νmax cm−1: 3064, 2911, 2221, 1567, 1544, 1430, 1383, 1258, 1230, 1128, 1066, 957, 731; positive LC–MS/MS m/z 183.0047 [M + H]+, (calculated for C7H6N2S2, 182.27); m/e: 182 (100.0%), 183 (9.3%), 183.99 (9.5%); 1H-NMR (500 MHz, CDCl3) and 13C-NMR (125 MHz, CDCl3) spectral values were recorded in one-dimensional (1D) and two-dimensional (2D) models (Table 4).

Table 4.
One-dimensional (1D) and two-dimensional (2D) NMR spectral data of 2-(methyldithio)pyridine-3-carbonitrile (2-Medpy-3-CN).
2.3. Minimum Inhibitory Concentration (MIC), Minimum Bactericidal Concentration (MBC), and Minimum Fungicidal Concentration (MFC) of 2-Medpy-3-CN
Based on the susceptibility testing results obtained, 2-Medpy-3-CN exhibited strong antibacterial activity against six out of nine pathogens tested. The MICs of 2-Medpy-3-CN ranged from 0.5 to >64 µg·mL−1 (Table 5). Gram-positive MSSA (methicillin-sensitive S. aureus) and MRSA and Gram-negative Acinetobacter iwoffii and Acinetobacter baumannii were highly susceptible to 2-Medpy-3-CN. The lowest MIC was observed for A. iwoffii (0.5 µg·mL−1), while A. baumannii, MRSA, and MSSA showed MICs of 4 µg·mL−1. Escherichia coli and Stenotrophomonas maltophilia were susceptible at slightly high concentration (32 µg·mL−1). However, Pseudomonas aeruginosa, Salmonella typhi, and Shigella dysenteriae were not susceptible to 2-Medpy-3-CN even at 64 µg·mL−1 (highest concentration tested). In addition to its broad-spectrum antibacterial activity, the compound also exhibited excellent antifungal activity against all five Candida strains tested. The MICs for Candida spp. ranged from 0.25 to 2 µg·mL−1. Candida glabrata was susceptible to 2-Medpy-3-CN at 0.25 µg·mL−1 and Candida tropicalis at 2 µg·mL−1. The highest bactericidal and fungicidal concentrations that inhibited the growth of bacteria (MBC) and fungi (MFC) were 64 µg·mL−1 and 4 µg·mL−1, respectively.

Table 5.
Minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC), and minimum fungicidal concentration (MFC) determinations of 2-Medpy-3-CN against human pathogenic bacteria and Candida spp. DMSO—dimethyl sulfoxide.
2.4. Time-to-Kill Assay
Studies on the bacterial killing kinetics of 2-Medpy-3-CN on MRSA showed that the growth control in brain heart infusion(BHI) without any antibiotics (non-treated) maintained viability for 24 h, while MRSA treated with the test antibacterial compound at 1×, 2×, and 4× MIC showed significant reduction in growth, indicating that 2-Medpy-3-CN is strongly bactericidal in killing >90% of the cells at 2 h post-treatment. The compound did not exhibit a concentration-dependent activity, and no significant difference in log10 colony-forming units (CFU)·mL−1 reduction was observed. It was observed that, with an initial inoculum of ~106 CFU·mL−1, 2-Medpy-3-CN (1×, 2×, and 4× MIC) declined the growth of MRSA as the incubation time increased from 0 to 2 h. The average reductions in CFUs were found to be 1.8-log10, 2.1-log10, and 1.8-log10 decreases in CFU·mL−1 at 0.5, 1, and 2 h post-treatment, respectively (Figure 2).
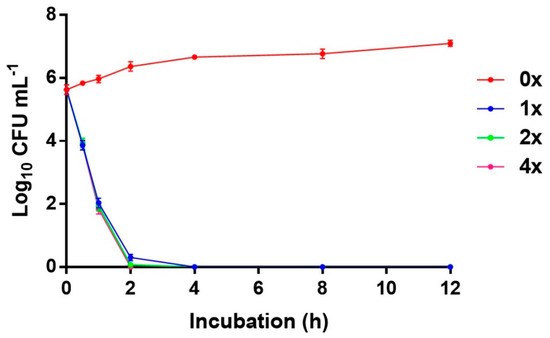
Figure 2.
Effect of 2-Medpy-3-CN on the viability of methicillin-resistant Staphylococcus aureus (MRSA) grown in BHI at concentrations of 1×, 2×, and 4× MIC with control (0× MIC). MIC—minimum inhibitory concentration; CFU—colony-forming units.
3. Discussion
The 2-Medpy-3-CN compound was isolated as a yellow-colored oil (later formed as yellow crystals). The LC–MS data displayed a molecular mass of 183.00471 m/z, signifying a molecular formula of C7H6N2S2, and the spectrum also gave the isotype motif for two sulfur atoms. The 1H-NMR spectrum (Table 5; Figure S1, Supplementary Materials) showed a deshielded methyl singlet at δ 2.60 and the presence of four hydrogens represented a characteristic ABCD aromatic structure/scheme. The 13C-NMR and DEPT-135 spectra (Table 5; Figures S2 and S8, Supplementary Materials) showed the presence of three methine aromatic carbons at δ 152.18–120.58, a deshielded quaternary carbon at δ 120.58S, and a methyl carbon at δ 23.23. This deshielded nature of the hydrogen atom (δ 8.77, H-6) of 2-Medpy-3-CN points out the phenomenon of a typical coupling of an alpha hydrogen to the nitrogen of a pyridine structure [27]. Hence, this pattern of coupling and correlation strongly underscores the possibility that 2-Medpy-3-CN could be a pyridine ring-containing natural compound.
Correlation spectroscopy (COSY) revealed the correlations of the aromatic hydrogens of 2-Medpy-3-CN (Figure S6, Supplementary Materials). In particular, the double-doublet of H-3 (δ 8.77) was coupled to a H-4 triplet (δ 7.89), which finally coupled to αH-6 doublet (δ 7.26). At δ 2.60, the presence of a methyl group appeared as a singlet featuring without couplings, indicating that it belongs to the aromatic nucleus. Its selected appearance in the 1H spectrum at 2.60 ppm determined that it was deshielded slightly and was attached to one of the sulfur atoms (apparently as a heteroatom). Heteronuclear multiple bond correlation spectroscopy (HMBC) showed the weak correlation (4J) between the methyl hydrogens and the C-2 quaternary carbon (HSQC), which suggested its terminal position on a disulfide side-chain α to the nitrogen (Figures S3 and S4, Supplementary Materials). Stereochemistry on the nuclear Overhauser spectroscopy (NOESY) effect of methyl hydrogens to H-3 was also determined (Figure S5, Supplementary Materials).
The IR data indicated the presence of nitrile (2221 cm−1) and pyridine (1544, 1430, 1230, 731 cm−1) moieties (Figure S10, Supplementary Materials). The molecular formula of 2-Medpy-3-CN was C7H6N2S2 (Figure S11, Supplementary Materials), which left the placement of the nitrile group in the skeleton of 2-Medpy-3-CN. Thus, the structure of the pure fraction D3/6e was determined as 2-(methyldithio)pyridine-3-carbonitrile (Figure 1). Previously, pyridine-N-oxide compounds were reported from A. stipitatum [25,26,28]; however, this is the first report on a naturally existing pyridine compound with a nitrile as a functional group from A. stipitatum.
The promising antibacterial activities of pyridine and thiopyridines derivatives ere adequately reported [29,30,31]. The 2-Medpy-3-CN compound demonstrated outstanding potency with low MIC values ranging from 0.5 to >64 µg·mL−1. The MICs of 2-Medpy-3-CN are consistent with the earlier data on pyridine based compounds from other research groups [25,26,28]. O’Donnell and his colleagues were the first to isolate and report pyridine-N-oxide alkaloids with disulfide functional groups from natural sources [26]. Three compounds named 2-(methyldithio)pyridine-N-oxide, 2[(methylthiomethyl)dithio]pyridine-N-oxide, and 2,2’-dithio-bis-pyridine-N-oxide (dipyrithione) were reported. Of the three compounds, 2-(methyldithio)pyridine-N-oxide and 2[(methylthiomethyl)dithio]pyridine-N-oxide were shown to possess strong antibacterial activity against fast-growing Mycobacterium sp., MRSA, and MDR variants of S. aureus with MICs of 0.5–8 µg·mL−1. These results were in accordance with the present study results where an MIC of 4 µg·mL−1 was observed for MSSA and MRSA strains. Following O’Donnell’s discovery of pyridine N-oxide compounds, Krejčová and his colleagues reported the anti-inflammatory and neuroprotective effects of five naturally occurring sulfur-containing pyridine-N-oxides from A. stipitatum [28]. The compound of the present study, 2-Medpy-3-CN, harbors nitrile as a functional group, which is another likely perception for its strong antimicrobial properties. Carbonitrile-containing semisynthetic derivatives of pyridine molecules exhibit strong antibacterial activity [32,33,34]. In addition to antibacterial activity, 2-Medpy-3-CN also exhibited very strong anticandidal activity, attributed to a broad-spectrum antimicrobial activity at a very low MIC level of 2 µg·mL−1 for C. tropicalis. Semisynthetic pyrimidine derivatives with carbonitrile as the functional group at the fifth position were reported to have strong antibacterial and antifungal activities at 12.5 µg·mL−1 [33,35]. The extreme potency of 2-Medpy-3-CN foreshadows its possibility in clinical use.
Time kill study was used to explore the bactericidal activity of 2-Medpy-3-CN using MRSA American Type Culture Collection (ATCC) 43300. The killing kinetics of 2-Medpy-3-CN demonstrated time-dependent killing instead of a dose-dependent pattern. At 1× MIC (4 µg·mL−1), 2-Medpy-3-CN effected a time-dependent reduction in the viability of the tested bacterial strain. Increasing the concentration of 2-Medpy-3-CN did not show any change in the bactericidal action. The killing rates were very much similar at 2× and 4× MIC, with 1.8-log10, 2.1-log10, and 1.8-log10 reductions in the number of CFU·mL−1 after 0.5, 1, and 2 h of incubation, respectively. For the first time, our study reports the killing kinetics or the rate of killing of pyridine compounds on MRSA. A recent study on novel 2-thiopyridines against actively growing and dormant cells of M. tuberculosis for seven days at 10 µg·mL−1 showed more than 2-log-unit killing effect [31]. Remarkably, the tested concentration and the CFU reductions were similar to the results of the present study. Testing 2-Medpy-3-CN on a diverse panel of antibiotic-resistant S. aureus isolates at various MICs disclosed the consistency of anti-MRSA activity.
Absorption, distribution, metabolism, and excretion (ADME) properties were used to determine the “drug-like” characteristics of the ligand molecule. ADME predictions can be used to focus lead optimization efforts in enhancing the desired properties of a given compound. The expected ADME property of the tested compound was evaluated with the QikProp module of Schrodinger. Almost all the predicted properties of the tested compound were in the range as predicted by QikProp for 100% (>80% is high) of known oral drugs, and the compound also satisfies Lipinski’s rule of five to be considered as having drug-like potential.
4. Materials and Methods
4.1. Chemistry
4.1.1. Plant Material and Preparation of Extracts
The source and the preparation of the extract were described earlier [22]. Briefly, dried bulbs (5 kg) of A. stipitatum were ground into fine powder and extracted with 10 L of dichloromethane for 72 h (for each solvent) by maceration at room temperature. The extract was filtered through Whatman No.1 paper to remove solid plant materials, and the filtrate was dried under vacuum (BÜCHI Rotovapor R-200, Flawil, Switzerland) at 40 °C. Upon filtration and solvent volatilization, the dichloromethane extract yielded 164 g (3.28%) of residue.
4.1.2. General Experimental Procedures
Analytical-grade solvents, including hexane and dichloromethane used for extraction and isolation, were purchased from (Merck KGaA, Darmstadt, Germany). Column chromatography was carried out using silica gel Merck 7734 (70–230 mesh ASTM) and Merck 9385 (230–400 mesh ASTM). Thin-layer chromatography (TLC) analyses were carried out on Merck silica gel DC-plastikfolien 60 F254 plastic sheets and TLC spots were visualized using a UV lamp at 254 and 366 nm, respectively. Melting point was recorded using an electrothermal digital melting point apparatus. Ultraviolet (UV) and IR spectra were recorded on a Varian UV–visible light (UV–Vis) 50 and Perkin-Elmer 100 Fourier-transform infrared (FTIR) spectrophotometers, respectively. The isolated compound was dissolved in CDCl3 (deuterated chloroform), and the NMR spectra (1D and 2D NMR) were recorded on a Bruker AVANCE 500 Ultrashield NMR spectrometer. The molecular weight of the isolated compound was determined on an Agilent 1290 Infinity II LC system (Agilent Technologies, Santa Carla, CA, USA) coupled to a 6520 Q-TOF tandem mass spectrometer for separation. The chemical shifts (δ) were determined from the residual solvent peaks.
4.1.3. Isolation of Potential Antimicrobial Compounds
TLC analysis of the Allium stipitatum dichloromethane (ASDE) extract showed the presence of large, clear spots when visualized under a UV chamber and iodine staining. The crude dichloromethane extract of A. stipitatum bulbs (164 g) was subjected to silica gel column chromatography (CC) using CH2Cl2 as the mobile phase. A total of 80 fractions (50 mL in volume) were collected in 100-mL conical flasks. All fractions were subjected to TLC analysis. Fractions that showed similar TLC patterns were pooled together which yielded six major fractions (D1–D6). The six fractions (D1–D6) were tested for their antibacterial activity against MRSA. Fraction D3 (1.354 g) which exerted remarkable antibacterial activity, was subjected to preparative thin-layer chromatography (PTLC) using CH2Cl2 as the mobile phase to yield eight subfractions (D3/1–D3/8). Subfraction D3/6 (302 mg) was further subjected to column chromatography (CC) using CH2Cl2 as the mobile phase to yield 120 fractions (10 mL in volume). Fractions were pooled together based on TLC patterns. Subfraction D3/6e (31 mg) was identified as a pure fraction and subjected to NMR and mass analysis. The purification scheme of fraction D3/6e is illustrated in Scheme 1. At each purification step, the fractions and subfractions were tested for their antibacterial activity using the micro-broth dilution method.
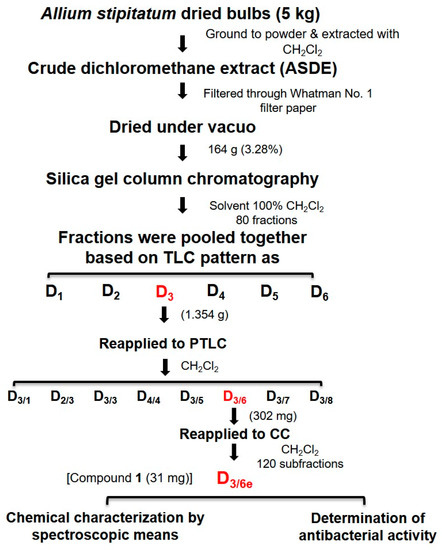
Scheme 1.
Purification scheme of fraction D3/6e from the bulbs of Allium stipitatum.
4.2. Biological Evaluation with Methicillin-Resistant S. aureus (MRSA)
4.2.1. Test Microorganisms
MRSA ATCC 43300 was used as model organism to screen for the antimicrobial activity. For the anti-MRSA activity, the confirmed compound was further screened for broad-spectrum activity against other pathogenic bacteria including Acinetobacter baumannii, A. iwoffii, Enterobacter sp., Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, Salmonella typhi, Shigella dysenteriae, and Stenotrophomonas maltophilia through MIC (minimum inhibitory concentration) and MBC (minimum bactericidal concentration) determinations. The compound was also tested for antifungal activity against five different pathogenic forms of Candida species, namely C. albicans, C. glabrata, C. krusei, C. parapsilosis, and C. tropicalis, for MIC and MFC (minimum fungicidal concentration).
4.2.2. MIC, MBC, and MFC Measurements
For the determination of MIC, MBC, and MFC, the compound was dissolved in 10% dimethyl sulfoxide (DMSO) with a graded concentration in the range of 64–0.125 µg·mL−1 with MHB for bacteria and Roswell Memorial Park Institute (RPMI-1640) medium for Candida strains. The MIC of the compound was determined using microbroth dilution method as described for bacteria and yeast [36,37]. Fluconazole was used as an antifungal standard and its MIC was determined using the Etest method. Appropriate antibiotic controls were included as positive controls based on the recommendations by the Clinical Laboratory Standards Insititute CLSI (2012). DMSO (10%) was used as a negative control and broth cultures without antibiotics served as growth controls.
4.2.3. Time-To-Kill Assay for Detecting the Bactericidal Effect of the Compound for MRSA
The killing kinetics of the compound on MRSA ATCC 43300 at 0×, 1× (4 µg·mL−1), 2× (8 µg·mL−1), and 4× (16 µg·mL−1) MIC was determined according to the method described previously [38] with some modifications. Bacterial and yeast suspensions were diluted to 1 × 106 CFU·mL−1. The compound concentrations were adjusted to 1× MIC, 2× MIC, and 4× MIC. Cultures treated with the varying concentrations of the compound were incubated at 37 °C for 0, 0.5, 1, 2, 4, 8, and 12 h. Aliquots of 100 µL were pipetted out from each tube at each time point, serially diluted in phosphate-buffered saline (PBS) and spread onto Mueller Hinton Agar MHA plates. Tubes without the compound served as growth controls (0×). The plates were incubated at 37 °C for 24 h followed by the enumeration of the colonies. Killing curves were constructed by plotting the log10 CFU·mL−1 versus time over a 24 h time period. A decline in bacterial/fungal growth to ≥3log10 CFU·mL−1 from the initial inoculum was considered to be bactericidal/fungicidal.
4.3. Prediction of ADME Properties
Schrodinger’s QikProp module is an accurate, quick, and easy-to-use absorption, distribution, metabolism, and excretion (ADME) prediction program that was designed to produce certain ADME-related descriptors. QikProp consists of two modes: normal mode and fast mode. In the present study, the QikProp program, version 3.4 was run in normal processing mode with default options [39]. To set up the calculation, a pose viewer file (generated after docking with Glide) was used to consider the receptor and source of ligands. The program was run (with default options) and reasonable descriptors were produced.
4.4. Statistical Analysis
All experiments were performed in triplicate except for the time-to-kill assay. Differences between the treated and untreated (control) groups were analyzed using GraphPad Prism 5.0. One-way ANOVA was performed and a Dunnett’s post hoc test was used for the comparison of multiple means. Significance was set at a p-value of <0.05.
5. Conclusions
The present study isolated a novel broad-spectrum antimicrobial compound 2-(methyldithio)pyridine-3-carbonitrile. The 2-Medpy-3-CN compound showed excellent antimicrobial activity against a panel of bacterial and fungal pathogens. The compound inhibited the growth of Gram-positive and Gram-negative pathogens along with Candida species at MICs ranging from 0.5 to >64 µg·mL−1. The 2-Medpy-3-CN compound was strongly bactericidal against MRSA in less than 2 h post-treatment. Further studies are vital to identify the drug target and mechanism of action for this highly potent antimicrobial compound.
Supplementary Materials
The following are available online at https://www.mdpi.com/1420-3049/24/6/1003/s1, 1H and 13C-NMR data and other related spectra for 2-Medpy-3-CN within this article, along with ADME toxicity data, can be found in the Supplementary Materials.
Author Contributions
Conceptualization, A.K., E.G.-R., and V.N.; methodology, A.K., E.G.-R., and J.J.N.; software, A.K., S.C., and M.R.P.; validation, A.K., N.J., J.J.N., S.C., M.R.P., R.A.H., L.T.T.L., A.v.B., and V.N.; formal analysis, A.K., J.J.N., S.C., M.R.P., L.T.T.L., and V.N.; investigation, A.K., E.G.-R., J.J.N., S.C., and V.N.; resources, A.K., E.G.-R., J.J.N., M.R.P., R.A.H., L.T.T.L., A.v.B., and V.N.; data curation, A.K., J.J.N., and V.N.; writing—original draft preparation, A.K. and V.N.; writing—review and editing, A.K., S.C., F.M.F., R.A.H., L.T.T.L., A.v.B., and V.N.; visualization, A.K. and V.N.; supervision, M.R.P., R.A.H., L.T.T.L., A.v.B., and V.N.; project administration, A.K., R.A.H., and V.N.; funding acquisition, A.K. and V.N.
Funding
This research was funded by Universiti Putra Malaysia through the Research University Grant Scheme (RUGS, Grant No. 04-02-1756 RU and GP/2018/9612800).
Acknowledgments
We thank the Pharmacotherapeutics Unit, Department of Medicine and Department of Medical Microbiology and Parasitology, Faculty of Medicine and Health Sciences, UPM for providing research facilities. The authors are also thankful to the staff at Atta-ur-Rahman Institute for Natural Products Discovery, Universiti Teknologi MARA for providing the NMR spectra.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pfeltz, R.F.; Wilkinson, B.J. The escalating challenge of vancomycin resistance in Staphylococcus Aureus. Curr. Drug Targets 2004, 4, 273–294. [Google Scholar] [CrossRef]
- Bonten, M.J.; Willems, R.; Weinstein, R.A. Vancomycin-resistant enterococci: Why are they here, and where do they come from? Lancet Infect. Dis. 2001, 15, 314–325. [Google Scholar] [CrossRef]
- Dijkshoorn, L.; Nemec, A.; Seifert, H. An increasing threat in hospitals: Multidrug-resistant Acinetobacter Baumannii. Nat. Rev. Microbiol. 2007, 5, 939–951. [Google Scholar] [CrossRef] [PubMed]
- Neela, V.; Rankouhi, S.Z.R.; van Belkum, A.; Goering, R.V.; Hamat, R.A. Stenotrophomonas maltophilia in Malaysia: Molecular epidemiology and trimethoprim-sulfamethoxazole resistance. Int. J. Infect. Dis. 2012, 16, e603–e607. [Google Scholar] [CrossRef]
- Horn, D.L.; Neofytos, D.; Anaissie, E.J.; Fishman, J.A.; Steinbach, W.J.; Olyaei, A.J.; Marr, K.A.; Pfaller, M.A.; Chang, C.H.; Webster, K.M. Epidemiology and outcomes of candidemia in 2019 patients: Data from the prospective antifungal therapy alliance registry. Clin. Infect. Dis. 2009, 48, 1695–1703. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Messer, S.A.; Moet, G.J.; Jones, R.N.; Castanheira, M. Candida bloodstream infections: Comparison of species distribution and resistance to echinocandin and azole antifungal agents in Intensive Care Unit (ICU) and non-ICU settings in the SENTRY Antimicrobial Surveillance Program (2008–2009). Int. J. Antimicrob. Agents 2011, 38, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Van Hal, S.J.; Jensen, S.O.; Vaska, V.L.; Espedido, B.A.; Paterson, D.L.; Gosbell, I.B. Predictors of mortality in Staphylococcus aureus Bacteremia. Clin. Microbiol. Rev. 2012, 25, 362–386. [Google Scholar] [CrossRef]
- Reed, S.D.; Friedman, J.Y.; Engemann, J.J.; Griffiths, R.I.; Anstrom, K.J.; Kaye, K.S.; Stryjewski, M.E.; Szczech, L.A.; Reller, L.B.; Corey, G.R.; et al. Costs and outcomes among hemodialysis-dependent patients with methicillin-resistant or methicillin-susceptible Staphylococcus aureus bacteremia. Infect. Control Hosp. Epidemiol. 2005, 26, 175–183. [Google Scholar] [CrossRef]
- McHugh, C.G.; Riley, L.W. Risk factors and costs associated with methicillin-resistant Staphylococcus aureus bloodstream infections. Infect. Control Hosp. Epidemiol. 2004, 25, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, S.E.; Qi, Y.; Kaye, K.S.; Harbarth, S.; Karchmer, A.W.; Carmeli, Y. The impact of methicillin resistance in Staphylococcus aureus bacteremia on patient outcomes: Mortality, length of stay, and hospital charges. Infect. Control Hosp. Epidemiol. 2005, 26, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Moloney, M.G. Natural Products as a Source for Novel Antibiotics. Trends Pharmacol. Sci. 2016, 37, 689–701. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Cheesman, M.J.; Ilanko, A.; Blonk, B.; Cock, I.E. Developing New Antimicrobial Therapies: Are Synergistic Combinations of Plant Extracts/Compounds with Conventional Antibiotics the Solution? Pharmacogn. Rev. 2017, 11, 57–72. [Google Scholar] [CrossRef]
- Gupta, P.D.; Birdi, T.J. Development of botanicals to combat antibiotic resistance. J. Ayurveda Integr. Med. 2017, 8, 266–275. [Google Scholar] [CrossRef]
- Petrovska, B.B.; Cekovska, S. Extracts from the history and medical properties of garlic. Pharmacogn. Rev. 2010, 4, 106–110. [Google Scholar] [CrossRef]
- Sivam, G.P. Protection against Helicobacter pylori and other bacterial infections by garlic. J. Nutr. 2001, 131, 1106S–1108S. [Google Scholar] [CrossRef]
- Li, G.; Ma, X.; Deng, L.; Zhao, X.; Wei, Y.; Gao, Z.; Jia, J.; Xu, J.; Sun, C. Fresh Garlic Extract Enhances the Antimicrobial Activities of Antibiotics on Resistant Strains in Vitro. Jundishapur J. Microbiol. 2015, 8, e14814. [Google Scholar] [CrossRef]
- Palaksha, M.N.; Ahmed, M.; Das, S. Antibacterial activity of garlic extract on streptomycin-resistant Staphylococcus aureus and Escherichia coli solely and in synergism with streptomycin. J. Nat. Sci. Biol. Med. 2010, 1, 12–15. [Google Scholar] [CrossRef]
- Reiter, J.; Levina, N.; van der Linden, M.; Gruhlke, M.; Martin, C.; Slusarenko, A.J. Diallylthiosulfinate (Allicin), a Volatile Antimicrobial from Garlic (Allium sativum), Kills Human Lung Pathogenic Bacteria, Including MDR Strains, as a Vapor. Molecules 2017, 22, 1711. [Google Scholar] [CrossRef]
- Asili, A.; Behravan, J.; Reza Naghavi, M.; Asili, J. Genetic diversity of persian shallot (Allium hirtifolium) ecotypes based on morphological traits, allicin content and RAPD markers. Open Access J. Med. Aromat. Plants 2010, 1, 1–6. [Google Scholar]
- Ebrahimi, R.; Zamani, Z.; Kashi, A. Genetic diversity evaluation of wild Persian shallot (Allium hirtifolium Boiss.) using morphological and RAPD markers. Sci. Hortic. 2009, 119, 345–351. [Google Scholar] [CrossRef]
- Karunanidhi, A.; Ghaznavi-Rad, E.; Jeevajothi Nathan, J.; Abba, Y.; van Belkum, A.; Neela, V. Allium stipitatum extract exhibits in vivo antibacterial activity against methicillin-resistant Staphylococcus aureus and accelerates burn wound healing in a full-thickness murine burn model. Evid. Based Complement. Alternat. Med. 2017, 2017, 1914732. [Google Scholar] [CrossRef] [PubMed]
- Karunanidhi, A.; Ghaznavi-Rad, E.; Hamat, R.A.; Pichika, M.R.; Lung, L.T.T.; Mohd Fauzi, F.; Chigurupati, S.; van Belkum, A.; Neela, V. Antibacterial and Antibiofilm Activities of Nonpolar Extracts of Allium stipitatum Regel. against Multidrug Resistant Bacteria. Biomed Res. Int. 2018, 2018, 9845075. [Google Scholar] [CrossRef] [PubMed]
- Karunanidhi, A.; Ghaznavi-Rad, E.; Jeevajothi Nathan, J.; Mohd Fauzi, F.; Lung, L.T.T.; Hamat, R.A.; Neela, V. Antifungal and antibiofilm activity of Persian shallot (Allium stipitatum Regel.) against clinically significant Candida spp. Trop. Biomed. 2018, 35, 1–11. [Google Scholar]
- Danquah, C.A.; Kakagianni, E.; Khondkar, P.; Maitra, A.; Rahman, M.; Evangelopoulos, D.; McHugh, T.D.; Stapleton, P.; Malkinson, J.; Bhakta, S.; et al. Analogues of Disulfides from Allium stipitatum Demonstrate Potent Anti-tubercular Activities through Drug Efflux Pump and Biofilm Inhibition. Sci. Rep. 2018, 8, 1150. [Google Scholar] [CrossRef]
- O’Donnell, G.; Poeschl, R.; Zimhony, O.; Gunaratnam, M.; Moreira, J.B.C.; Neidle, S.; Evangelopoulos, D.; Bhakta, S.; Malkinson, J.P.; Boshoff, H.I.; et al. Bioactive pyridine-N-oxide disulfides from Allium stipitatum. J. Nat. Prod. 2009, 72, 360–365. [Google Scholar] [CrossRef]
- Jinbo, Z.; Mingan, W.; Wenjun, W.; Zhiqing, J.; Zhaonong, H. Insecticidal sesquiterpene pyridine alkaloids from Euonymus species. Phytochemistry 2002, 61, 699–704. [Google Scholar] [CrossRef]
- Krejčová, P.; Kučerová, P.; Stafford, G.I.; Jäger, A.K.; Kubec, R. Antiinflammatory and neurological activity of pyrithione and related sulfur-containing pyridine N-oxides from Persian shallot (Allium stipitatum). J. Ethnopharmacol. 2014, 154, 176–182. [Google Scholar] [CrossRef]
- Miron, T.; Shin, I.; Feigenblat, G.; Weiner, L.; Mirelman, D.; Wilchek, M.; Rabinkov, A. A spectrophotometric assay for allicin, alliin, and alliinase (Alliin lyase) with a chromogenic thiol: Reaction of 4-mercaptopyridine with thiosulfinates. Anal. Biochem. 2002, 307, 76–83. [Google Scholar] [CrossRef]
- Salina, E.G.; Ryabova, O.; Vocat, A.; Nikonenko, B.; Cole, S.T.; Makarov, V. New 1-hydroxy-2-thiopyridine derivatives active against both replicating and dormant Mycobacterium tuberculosis. J. Infect. Chemother. 2017, 23, 794–797. [Google Scholar] [CrossRef]
- Salina, E.; Ryabova, O.; Kaprelyants, A.; Makarov, V. New 2-thiopyridines as potential candidates for killing both actively growing and dormant Mycobacterium tuberculosis cells. Antimicrob. Agents Chemother. 2014, 58, 55–60. [Google Scholar] [CrossRef]
- Kotb, E.R.; Anwar, M.M.; Abbas, H.-A.S.; Abd El-Moez, S.I. A concise synthesis and antimicrobial activity of a novel series of naphthylpyridine-3-carbonitrile compounds. Acta Pol. Pharm. 2013, 70, 667–679. [Google Scholar]
- Alam, M.M.; Akhter, M.; Husain, A.; Marella, A.; Tanwar, O.P.; Ali, R.; Hasan, S.M.; Kumar, H.; Haider, R.; Shaquiquzzaman, M. Anti-inflammatory and antimicrobial activity of 4,5-dihydropyrimidine-5-carbonitrile derivatives: Their synthesis and spectral elucidation. Acta Pol. Pharm. 2012, 69, 1077–1085. [Google Scholar]
- Sayed, H.H.; Abbas, H.-A.S.; Morsi, E.M.H.; Amr, A.E.-G.E.; Abdelwahad, N.A.M. Antimicrobial activity of some synthesized glucopyranosyl-pyrimidine carbonitrile and fused pyrimidine systems. Acta Pharm. 2010, 60, 479–491. [Google Scholar] [CrossRef]
- Al-Abdullah, E.S.; Al-Turkistani, A.A.; Al-Deeb, O.A.; El-Brollosy, N.R.; Habib, E.E.; El-Emam, A.A. Pyrimidine-5-carbonitriles II: Synthesis and antimicrobial activity of novel 6-alkyl-2,4-disubstituted pyrimidine-5-carbonitriles. Drug Res. 2014, 64, 31–39. [Google Scholar] [CrossRef]
- Schwalbe, R.; Steele-Moore, L.; Goodwin, A.C. Macro- and microdilution methods of antimicrobial susceptibility testing. In Antimicrobial Susceptibility Testing Protocols; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2007. [Google Scholar]
- Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Second Informational Supplement; Clinical Laboratory Standards Institutes: Wayne, PA, USA, 2012; Volume 32, ISBN 1562387855.
- Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fifth Informational Supplement; Clinical Laboratory Standards Institutes: Wayne, PA, USA, 2015; ISBN 1562388657.
- Schrödinger. QikProp, version 3.4; Schrödinger LLC: New York, NY, USA, 2013. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).