Abstract
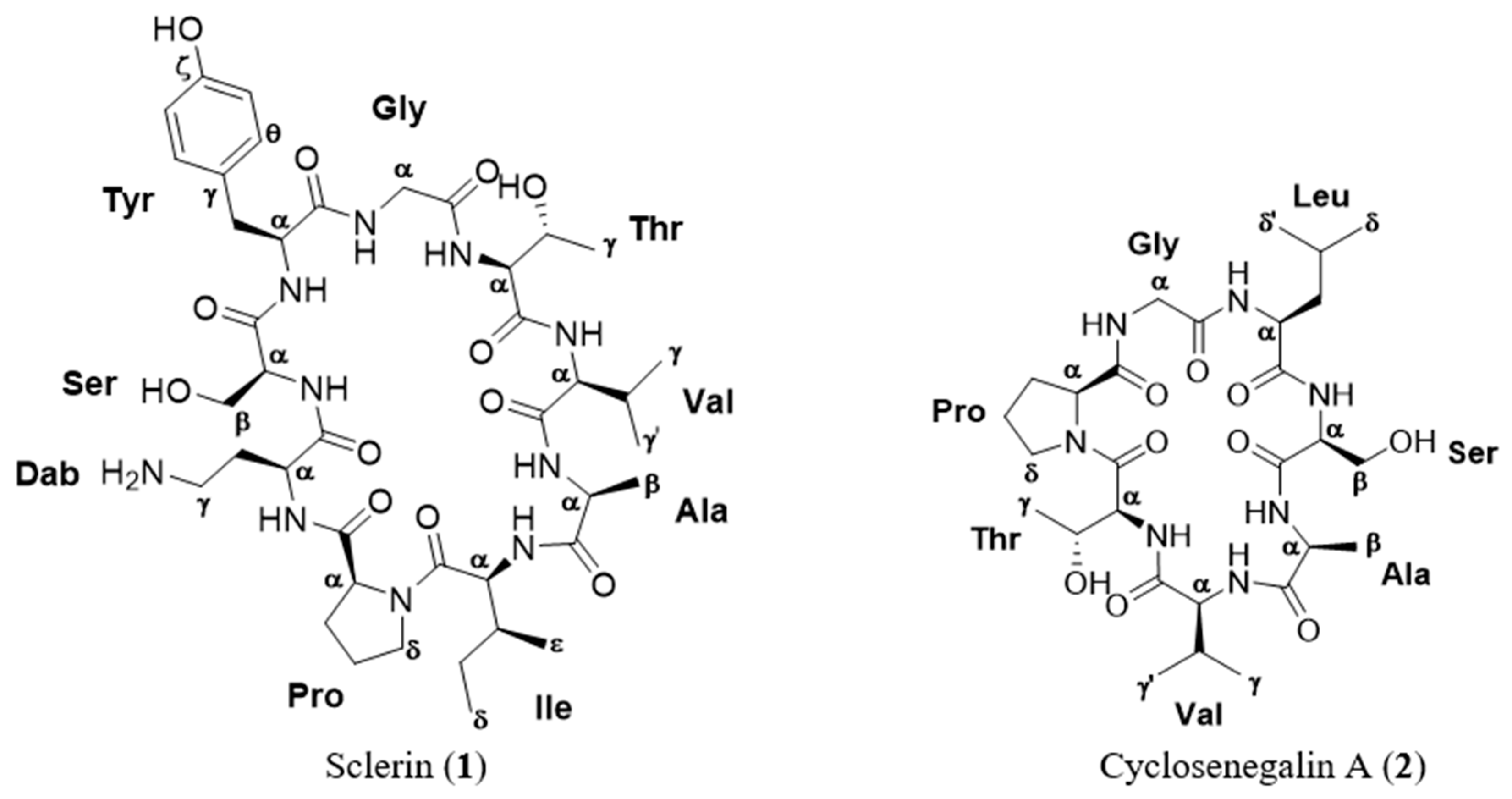
A new cytotoxic cyclononapeptide, sclerin, cyclo(–Dab1–Ser2–Tyr3–Gly4–Thr5–Val6–Ala7– Ile8–Pro9–) (1), was isolated from the methanol extract of the seeds of Annona scleroderma, together with the known metabolite, cyclosenegalin A, cyclo(–Pro1–Gly2–Leu3–Ser4–Ala5–Val6–Thr7–) (2). The planar structures for the two compounds were established by comprehensive analysis of NMR and ESI-HRMS data, and the absolute stereochemistry was stablished by Marfey’s method. Compound 1 showed moderate cytotoxic activity against the human prostate carcinoma cell line DU-145 at µM concentration.
1. Introduction
Terrestrial natural products have played a fundamental role in drug development during the last decades, either directly as drugs or lead structures that were further optimized by medicinal chemistry [1,2]. Within the natural products, cyclic peptides constitute an important class of natural molecules with a great diversity of ring sizes; some of them have been submitted to clinical trials or come near to that phase, because of their attractive pharmacological properties [3,4]. On the other hand, many cyclopeptides represent research tools in molecular biology for investigating several processes involved in cellular regulation [5]. These metabolites have been isolated from higher plants as well as microorganisms and marine sources [3,6]. Phytochemical studies on species of the genus Annona have demonstrated that the plants belonging to this genus produce an amazing variety of cyclopeptide derivatives. Within this genus, Annona scleroderma is distributed in tropical and subtropical latitudes worldwide. In México, A. scleroderma, commonly named “cawesh”, “cahuex”, or “poshté”, grows in warm climates areas, such as Tabasco, Chiapas, Quintana Roo, Nayarit, Michoacán, Yucatán, and Veracruz [7]. This study describes the investigation on seeds of A. scleroderma, leading to the isolation and structural elucidation of a new cyclopeptide, sclerin (1), together with the known metabolite, cyclosenegalin A (2) (Figure 1). Their planar structures were determined based on detailed spectroscopic NMR studies and ESI-HRMS data. The absolute stereochemistry of each amino acid residue in compounds 1 and 2 were determined by Marfey’s method [8]. The cytotoxicity bioassays indicated that these compounds possess activity against human prostate cancer cell line DU-145.
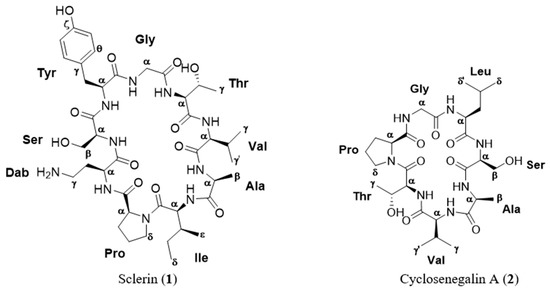
Figure 1.
Structure of the cyclopeptides isolated from the methanol extract of Annona scleroderma.
2. Results and Discussion
Seeds of Annona scleroderma (700.3 g) were extracted with MeOH (4 × (3 L × 3 h)) at room temperature, and the resulting extract (17.5 g) was first fractionated by liquid–liquid extraction using the Kupchan method [9,10]. The ethyl acetate fraction was then subjected to sequential Lobar LiChroprep-RP18 and μ-Bondapack C-18 column chromatography to afford one new cyclopeptide sclerin (1) (1.3 mg) and the known compound cyclosenegalin A (2) (3.1 mg).
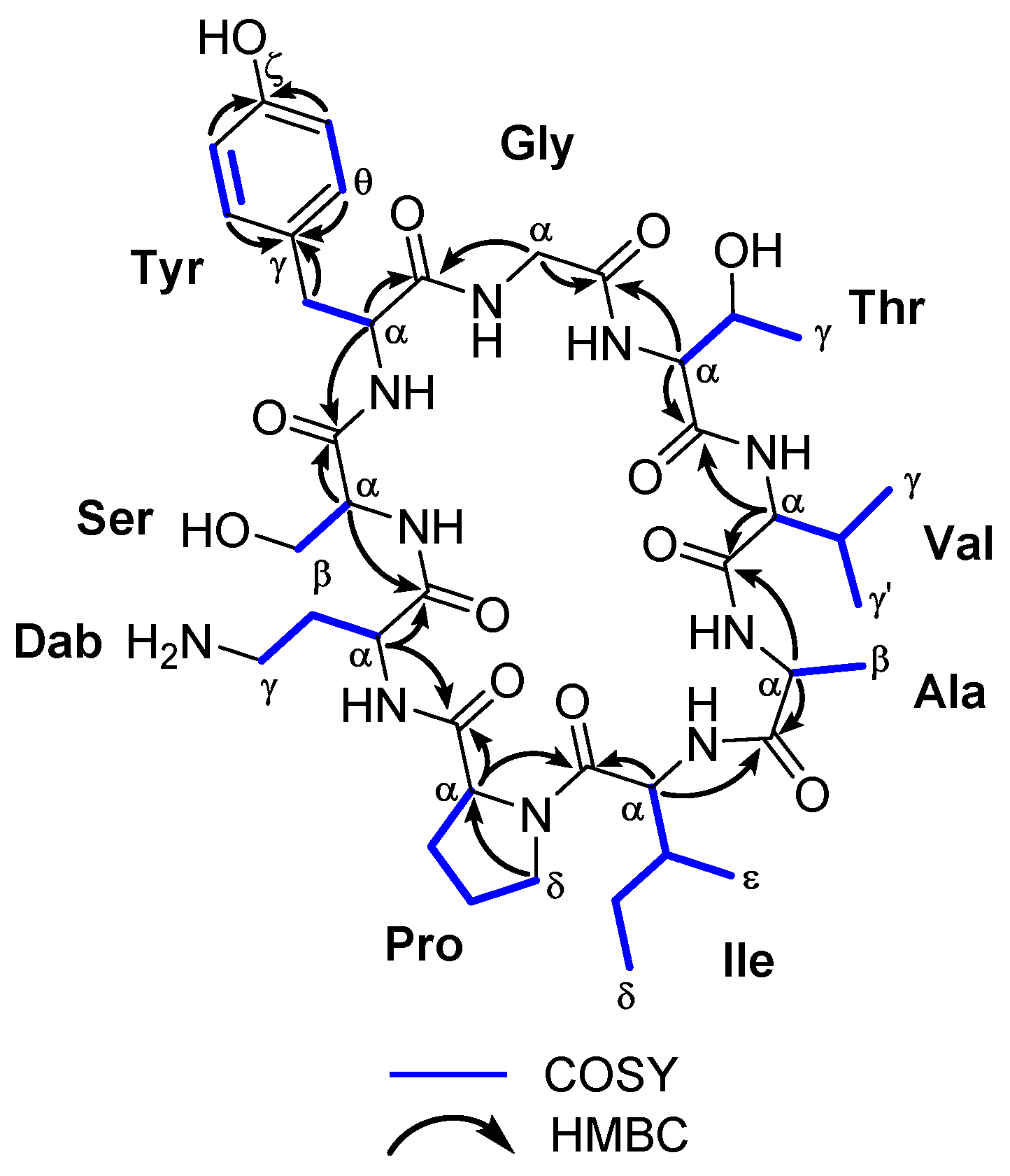
Sclerin (1) was isolated as an optically active powder − 3 (c 0.13, MeOH). Its molecular formula was deduced by ESI-HRMS as C41H64N10O12 (m/z 888.4695; calculated 888.4705 for C41H64N10O12, [M]+). The peptide nature of 1 was initially proposed by the high N content, together with the absorption of amino group at 3320 cm−1 and amide carbonyl group at 1653 cm−1 in the IR spectrum. The 1H- and 13C-NMR data recorded for sclerin A (1) in CD3OD allowed us to establish the presence of six CH3, nine CH2, fifteen CH, and eleven unprotonated carbons, nine of them carbonyl groups, suggesting that 1 should be a nonapeptide (Table 1). Careful analysis of 1H-1H COSY and TOCSY spectra of 1, revealed the existence ten 1H-1H spin systems belonging to nine amino acid units. The proton assignments of the non-essential amino acid, 2,4-diaminobutanoic acid (Dab), was started from H-α (δH 4.26, dd, J = 3.1, 10.1 Hz), which was coupled with H2-β (δH 1.95/2.18), and these sequentially to both H2-γ (δH 2.70/2.92). In the case of serine residue, the characteristic A2B system between H-α (δH 3.61) and H2-β (δH 3.70) was observed. For the tyrosine residue, two-spin systems were determined, H-α (δH 5.08) with H2-β (δH 2.78/3.59) and H-δ/H-θ (δH 7.08 d, J = 7.7 Hz) with H-ε/H-η (δH 6.79, d, J = 7.7 Hz). The 1H-1H spin coupling between geminal protons H2-α (δH 3.84/4.15, d, J = 17.3 Hz) was indicative of the presence of a glycine residue. The proton assignments of the next residue, threonine, was properly started from H3-γ (δH 1.12, d, J = 6.2 Hz), which is coupled with methine H-β (δH 4.53, dq, J = 2.3, 6.2 Hz), and this sequentially connected with the proton of methine H-α (δH 4.82, d, J = 2.3 Hz). The geminal coupling between the methyl groups H3-γ (δH 1.02, d, J = 6.5 Hz) and H3-γ’ (δH 0.91, d, J = 6.8 Hz), were coupled with H-β (δH 1.95), and this sequentially to H-α (δH 3.61) allowed for establishing the presence of a valine residue. For the alanine residue, the typical AX3 system between H3-β (δH 1.39, d, J = 7.4 Hz) and H-α (δH 4.13, q, J = 7.4 Hz) was assigned. The sec-butyl group of isoleucine residue was started from proton H-α (δH 4.28), that was correlated to methine proton H-β (δH 1.99), and this, in turn, with the methyl protons H3-ε (δH 0.65, d, J = 6.4 Hz) and the diastereotopic methylene H2-γ (δH 0.94/1.33); the diastereotopic methylene H2-γ were further correlated to H3-δ (δH 0.86, t, J = 7.3 Hz). Finally, the spin system in the proline residue was started from H-α (δH 4.48, t, J = 8.8 Hz), which was coupled with H2-β (δH 1.91/2.34), that connected, in turn, to H2-γ (δH 1.97/2.08). These were further correlated to H2-δ (δH 3.43/3.71). Long-range 1H-13C connectivity, extracted from the HMBC experiment data, allowed us to establish, unambiguously, the presence of nine amino acid residues in 1. Furthermore, key HMBC correlations between the carbonyl group of residue i with the α protons of residue i + 1 (H-6 and C-1, H-9 and C-5, H2-18 and C-8, H-20 and C-17, H-24 and C-19, H-29 and C-23, H-32 and C-28, H-38 and C-31, and H-2 and C-37) allowed us to determine the planar structure of 1, as shown in Figure 2 and Table 1 (see Supplementary Materials). The heteronuclear correlations were preferred with respect to the dipolar connectivities from the ROESY spectrum, because in small-sized cyclic peptides, conformational information can interfere with sequential information. Phytochemical studies on species of the genus Annona have demonstrated that this genus produce a remarkable variety of cyclopeptide derivatives with a great diversity of ring sizes. However, there are few examples of cyclononapeptides such as cherimolacyclopeptide F [11], cyclosquamosin E [12], cyclomontanin C [13] and sclerin (1). On the other hand, sclerin (1) shows the presence of an unnatural amino acid residue, l-2,4-diaminobutyric acid (Dab), observed in only a few cyclic polypeptides, such as the polymyxins A–E [14].

Table 1.
NMR data (CD3OD) for cyclononapeptide sclerin (1).
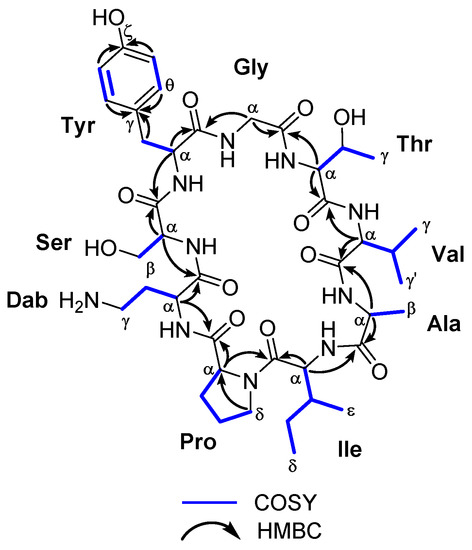
Figure 2.
Key COSY and HMBC correlations observed for the cyclononapeptide sclerin (1).
The next cyclopeptide, cyclo(–Pro1–Gly2–Leu3–Ser4–Ala5–Val6–Thr7–) (2), was isolated as an optically active powder, − 4 (c 0.31, MeOH). The molecular formula of 2, C28H47N7O9, was established by HRESIMS analysis, where its sodiated molecular ion was observed at m/z 648.3339 (calculated 648.3333 for C28H47N7O9Na, [M + Na]+). Analysis of the 1H- and 13C-NMR spectra of 2 indicated the presence of six CH3, six CH2, and nine CH, as well as seven quaternary carbonyl groups. Detailed analysis of 2D NMR spectroscopy (1H-1H COSY, HSQC in CD3OD, and HMBC in CD3OD and CD3OH) suggested that the structure of 2 was identical in all to cyclosenegalin A, reported by Wélé et al., and isolated from the methanol extract of the seeds of A. senegalensis (see Supplementary Materials). [15]
The absolute stereochemistry of the amino acid residues for compounds 1 and 2 were established by Marfey’s method [8]. The acid hydrolysate of sclerin (1) and cyclosenegalin A (2) was derivatized, with Nα-(2,4-dinitro-5-fluorophenyl)-l-alaninamide (l-FDLA). The retention times of these FDAA amino acid derivatives were established by HPLC monitoring with UV absorption at 340 nm. All FDAA derivatives were identified based on a comparison of their retention times in HPLC with authentic amino acid standards. The absolute stereochemistry of all the amino acid residues of 1 and 2 were identified as l. Thus, the absolute configurations of 1 and 2 can be assigned as 2S, 6S, 9S, 20S, 21R, 20S, 24S, 29S, 32S, 33S, 38S and 2S, 9S, 15S, 18S, 21S, 26S, 27R, respectively.
The in vitro cytotoxic activity of sclerin (1) and cyclosenegalin A (2) was assessed by XTT assay, using the prostate cancer cell line DU-145 [16,17]. As shown in Table 2, sclerin (1) and cyclosenegalin A (2) were able to inhibit cell proliferation of the human prostate cancer at μM concentration.

Table 2.
In vitro growth inhibitory activity for compounds sclerin (1) and cyclosenegalin A (2) against human prostate carcinoma cell line DU-145.
3. Materials and Methods
3.1. General Experiment Procedures
Optical rotation was determined on a PerkinElmer 241 polarimeter (Waltham, MA, USA), using a sodium lamp operating at 589 nm. The IR spectrum was measured on a Bruker IFS55 spectrometer (Billerica, MA, USA), using a chloroform solution to place a film of the compounds on the NaCl disk. NMR spectra were performed on Bruker AVANCE 600 MHz instruments at 298 K, and coupling constants are given in Hz. NMR experiments, COSY, HSQC, and HMBC, were acquired using standard pulse sequences. 3JH,H values were measured from 1D 1H-NMR. NMR data were processed using Topspin and MestReNova software (v 11.01, Santiago de Compostela, Spain). Mass spectra were recorded on a VG AutoSpec FISON spectrometer (Danvers, MA, USA). HPLC (High performance liquid chromatography) separations were carried out with an LKB 2248 system (LKB-Producter AB, Bromma, Sweden) that was equipped with a photodiode array detector. All of the solvents used were HPLC-grade. HPLC chromatography was monitored by TLC, performed on AL Si gel Merck 60 F254 (Kenilworth, NJ, USA). TLC plates were visualized by UV light (365 nm) and phosphomolybdic acid solution 10 wt% in ethanol.
3.2. Plant Material
The seeds of A. scleroderma were collected from municipality of Ignacio de la Llave, Veracruz, (México) during May 2015, and identified by taxonomists in the Institute for Biological Research at Veracruz University. After collection, the vegetable material was dried at room temperature for one week and then triturated using a steel blender.
3.3. Extraction and Isolation
The seeds of A. scleroderma (700.3 g) were extracted with MeOH (4 × 3 L × 3 h) at room temperature and the solvent removed in vacuo to give a brownish viscous oil (ASS-1 17.5 g). The methanolic extract was first fractioned for liquid–liquid extraction using Kupchan method [8,9]. The ethyl acetate fraction (ASS-1C; 512.6 mg) was chromatographed over medium pressure chromatography Lobar LiChroprep-RP18, eluted with MeOH/H2O (6:4) at 4 mL/min flow. Fractions collected between 66 and 78 mL, and 79 and 84 mL, were pooled together (ASS-1C7 and ASS-1C8, 11.2 and 9.9 mg, respectively). Final purification of both fractions (ASS1C7 and 1C8) was performed on HPLC equipped with a μ-BondapakTM C-18 (1.9 Ø × 15 cm) column, using H2O/MeOH (7:3) as mobile phase, to afford pure sclerin (1) (1.3 mg) and cyclosenegalin A (2) (3.1 mg).
Sclerin (1). Amorphous white solid; − 3 (c 0.13, MeOD); IRνmax (MeOH) 3320, 2960, 1731, 1653, 1622, 1524 cm−1; HR-ESI–MS m/z 888.4695 [M]+ (calcd 888.4705 for C41H64N10O12); NMR data 1H (600 MHz, MeOD) and 13C (125 MHz, CD3OD); see Table 1.
Cyclosenegalin A (2). Amorphous white solid; − 4 (c 0.31, MeOH); IRνmax (MeOH) 3310, 2930, 1650, 1620 cm−1; HR-ESI-MS m/z 648.339 [M + Na]+ (calcd 648.3333 for C28H47N7O9); NMR data 1H (600 MHz, CD3OD) and 13C (125 MHz, CD3OD); see Supporting Information.
3.4. Marfey’s Analysis
Sclerin (1) (200 μg) and cyclosenegalin A (2) (200 μg) were hydrolyzed in 200 μL 6 M HCl at 50 °C for 18 h. After, the residual HCl was removed in vacuo, and then 100 μL of an acetone solution containing 0.1 M of NaHCO3 and 25 μg of 1-fluoro-2,4-dinitrophenyl-5-l-alaninamide (l-FDAA) was added to the residue. The solution mixture was heated at 75 °C for 4 h. Next, the reaction mixture was cooled, neutralized with 2 M HCl (50 µL) and dissolved in MeOH (200 μL). About 10 µL of each solution of FDLA derivatives was analyzed by HPLC. On the other hand, authentic standards of l-Dab, l-Pro, l-Ile, l-Leu, l-Ala, l-Val, l-Thr, l-Tyr, l-Ser (Sigma-Aldrich, St Louis, MO, USA) were treated with l-FDAA, as described above. The l-FDAA derivative of l-amino acid standard were analyzed by HPLC–UV, and the retention times of l-Dab (2.6), l-Pro (4.2), l-Ile (20.1), l-Leu (5.5), l-Ala (4.7), l-Val (9.8), l-Thr (3.0), l-Tyr (6.4), l-Ser (4.6) were compared with the Marfey’s derivative of 1 and 2. HPLC conditions: a 5 μM column X-Terra MS C-18 (150 × 3.0 mm) maintained at 25 °C was eluted at 1 mL/min with 40% MeOH/H2O containing 0.01% HCOOH for 25 min.
3.5. Cell Culture
DU-145 (human prostate cancer) was maintained in culture medium containing 10% (v/v) heat-inactivated fetal bovine serum (FBS), 2 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37 °C in air with 95% humidity and 5% CO2. Cells were periodically tested for Mycoplasma infection using the MycoAlert© Mycoplasma detection kit (Lonza, Basel, Switzerland), as well as the Venor©GeM Advance Mycoplasma PCR detection Kit (Minerva Biolabs, Berlin, Germany), and found to be negative.
3.6. Cytotoxic Assay
The effect of the two compounds in the proliferation of human prostate cancer cell line was determined as previously described by using the XTT (sodium 3′-[1-(phenylaminocarbonyl)-3,4-tetrazolium]-bis(4-methoxy-6-nitro)benzene sulfonic acid hydrate) cell proliferation kit (Roche Molecular Biochemicals, Mannheim, Germany) as previously described [11,12]. Cells (5.0 × 103 in 100 μL) were incubated in RPMI-1640 culture medium containing 10% heat-inactivated FBS, in the absence and in the presence of the indicated compounds at a concentration range of 10−3 to 10−9 M, in 96-well flat-bottomed microtiter plates, and following 72 h of incubation at 37 °C in a humidified atmosphere of air/CO2 (19/1), the XTT assay was performed. Measurements were done in triplicate, and each experiment was repeated three times. The IC50 (50% inhibitory concentration) value, defined as the drug concentration required to cause 50% inhibition in the cellular proliferation with respect to the untreated controls, was determined for each compound.
4. Conclusions
In the present study, the structure of one new cyclopeptide, sclerin (1), together with the known metabolite, cyclosenegalin A (2), were unambiguously determined by the combined use of spectroscopic and Marfey’s method. Sclerin (1) contains nine amino acid residues, being, thus, one of the atypical examples of cyclic nonapeptide isolated of genus Annona. In addition, it is important to highlight that 1 possesses an unnatural amino acid residue, l-2,4-diaminobutyric acid (Dab) observed in few natural metabolites. The cytotoxic activity of these compounds, sclerin (1) and cyclosenegalin A (2), was tested against DU-145 human prostate cancer cell line, resulting in 1 IC50 27.3 ± 4.19 µM and 2 IC50 54.9 ± 2.35 µM.
Supplementary Materials
The following are available online, Scheme S1: Isolation procedure followed for compounds 1 and 2; Figure S1: 1H-NMR spectrum of sclerin (1) in D2O at 298 K, 600 MHz; Figure S2: 13C-NMR spectrum of sclerin (1) in D2O at 298 K, 150 MHz; Figure S3: COSY spectrum of sclerin (1) in D2O at 298 K, 600 MHz; Figure S4: HSQC spectrum of sclerin (1) in D2O at 298 K, 600 MHz; Figure S5: HMBC spectrum of sclerin (1) in D2O at 298 K, 600 MHz; Figure S6: HRMS spectrum of sclerin (1); Figure S7: 1H-NMR spectrum of cyclosenegalin A (2) in CD3OD at 298 K, 600 MHz; Figure S8: 13C-NMR spectrum of cyclosenegalin A (2) in CD3OD at 298 K, 150 MHz; Figure S9: COSY spectrum of cyclosenegalin A (2) in CD3OD at 298 K, 600 MHz; Figure S10: HSQC spectrum of cyclosenegalin A (2) in CD3OD at 298 K, 600 MHz; Figure S11: HMBC spectrum of cyclosenegalin A (2) in CD3OD at 298 K, 600 MHz; Figure S12: HMBC spectrum of cyclosenegalin A (2) in CD3OH at 298 K, 600 MHz; Figure S13: HRMS spectrum of cyclosenegalin A (2); Table S1. NMR data for sclerin (1) in D2O; Table S2. NMR data for cyclosenegalin A (2) in CD3OD.
Author Contributions
F.C.-P. and J.J.F. performed the majority of the experiments of structural elucidation, analyzed the data, and drafted the manuscript. F.C.-P., D.S.-L. and G.V.-A. performed the extraction and isolation of the pure compounds. G.V.-A. and F.C.-P. performed the Marfey’s method and analyzed the data. All authors read and approved the final manuscript.
Funding
This work was supported by the Secretary of Public Education of Mexico [SEP-PROMEP, grant number PROMEP/103.5/13/7135] and MAC/1.1.b./042, INTRREG (MAC-2014-2020) -BIOTRANSFER 2. D.S.L. thanks the CONACyT Foundation for a grant (573166).
Acknowledgments
The authors express his thanks to Enrique Juarez-Aguilar who supported us in the cytotoxicity bioassays.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- McCloud, T.G. High Throughput Extraction of Plant, Marine and Fungal Specimens for Preservation of Biologically Active. Molecules 2010, 15, 4526–4563. [Google Scholar] [CrossRef] [PubMed]
- Ning-Hua, T.; Zhou, J. Plant Cyclopeptides. Chem. Rev. 2006, 106, 840–895. [Google Scholar]
- Wang, X.; Lin, M.; Xu, D.; Lai, D.; Zhou, L. Structural Diversity and Biological Activities of Fungal Cyclic Peptides, Excluding Cyclodipeptides. Molecules 2017, 22, 2069. [Google Scholar] [CrossRef] [PubMed]
- Sarabia, F.; Chammaa, S.; Ruiz, A.S.; Ortiz, L.M.; Herrera, F.J.L. Chemistry and Biology of Cyclic Depsipeptides of Medicinal and Biological Interest. Curr. Med. Chem. 2004, 11, 1309–1332. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef] [PubMed]
- Safford, W.E. Chelonocarpus, a new section of the genus Annona, with descriptions of Annona scleroderma and Annona testudinea. J. Wash. Acad. Sci. 1913, 3, 103–109. [Google Scholar]
- Bhushan, R.; Brückner, H. Marfey’s reagent for chiral amino acid analysis: A review. Amino Acids 2004, 27, 231–247. [Google Scholar] [CrossRef] [PubMed]
- Kupchan, S.M.; Tsou, G.; Sigel, C.W. Datiscacin, a novel cytotoxic cucurbitacin 20-acetate from Datisca glomerate. J. Org. Chem. 1973, 38, 1420–1421. [Google Scholar] [CrossRef] [PubMed]
- Vanwagenen, B.C.; Larsen, R.; Cardellina, J.H., II; Randazzo, D.; Lidert, Z.C.; Swithenbank, C. Ulosantoin, a Potent Insecticide from the Sponge UIosa ruetzler. J. Org. Chem. 1993, 58, 335–337. [Google Scholar] [CrossRef]
- Wélé, A.; Zhang, Y.; Brouard, J.P.; Pousset, J.L.; Bodo, B. Two cyclopeptides from the seeds of Annona cherimola. Phytochemistry 2005, 66, 2376–2380. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.L.; Hua, K.F.; Chuang, P.H.; Wu, S.H.; Wu, K.Y.; Chang, F.R.; Wu, Y.C. New cyclic peptides from the seeds of Annona squamosa L. and their anti-inflammatory activities. J. Agric. Food. Chem. 2008, 56, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Pei-Hsuan, C.; Pei-Wen, H.; Yu-Liang, Y.; Kuo-Feng, H.; Fang-Rong, C.; Jentaie, S.; Shih-Hsiung, W.; Yang-Chang, W. Cyclopeptides with Anti-inflammatory Activity from Seeds of Annona montana. J. Nat. Prod. 2008, 71, 1365–1370. [Google Scholar]
- Brown, P.; Dawson, M.J. A Perspective on the Next Generation of Antibacterial Agents Derived by Manipulation of Natural Products. In Progress in Medicinal Chemistry; Lawton, G., Witty, D.R., Eds.; Elsevier: Amsterdam, the Netherlands, 2015; Volume 54, pp. 135–184. [Google Scholar]
- Wélé, A.; Zhang, Y.; Caux, C.; Brouard, J.P.; Dubost, L.; Guette, C.; Pousset, J.L.; Badiane, M.; Bod, B. Isolation and structure of cyclosenegalins A and B, novel cyclopeptides from the seeds of Annona senegalensis. J. Chem. Soc. Perkin Trans. 1 2002, 23, 2712–2718. [Google Scholar] [CrossRef]
- Herrera-Sotero, M.; González-Cortés, F.; García-Galindo, H.; Juarez-Aguilar, E.; Rodríguez Dorantes, M.; Chávez-Servia, J.; Oliart-Ros, R.; Guzmán-Gerónimo, R. Anthocyanin Profile of Red Maize Native from Mixteco Race and Their Antiproliferative Activity on Cell Line DU145. In Flavonoids-From Biosynthesis to Human Health; Justino, J., Ed.; IntechOpen: London, UK, 2017; pp. 595–617. [Google Scholar]
- Cen-Pacheco, F.; Mollinedo, F.; Villa-Pulgarín, J.A.; Norte, M.; Fernández, J.J.; Hernández Daranas, A. Saiyacenols A and B: The key to solve the controversy about the configuration of aplysiols. Tetrahedron 2012, 68, 7275–7279. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).