(R)-2-Phenyl-4,5-Dihydrothiazole-4-Carboxamide Derivatives Containing a Diacylhydrazine Group: Synthesis, Biological Evaluation, and SARs
Abstract
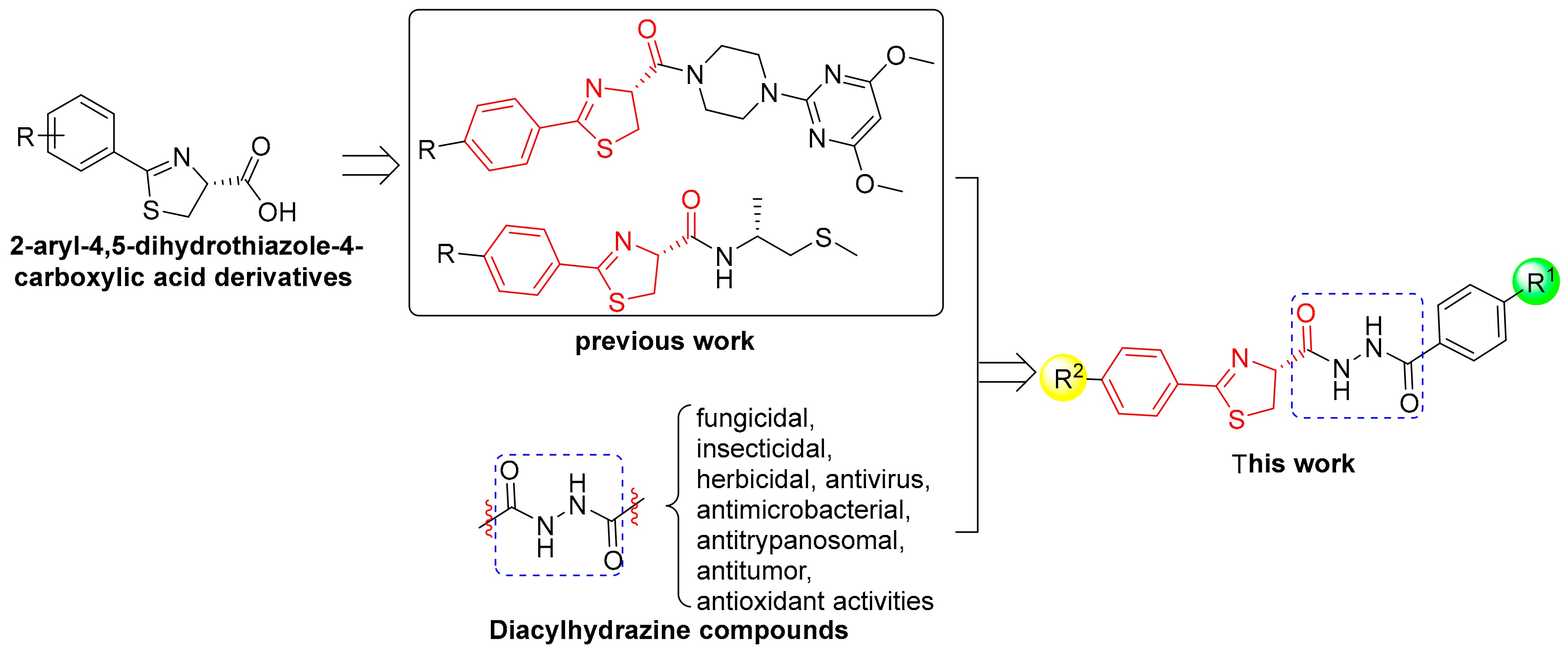
1. Introduction
2. Results and Discussion
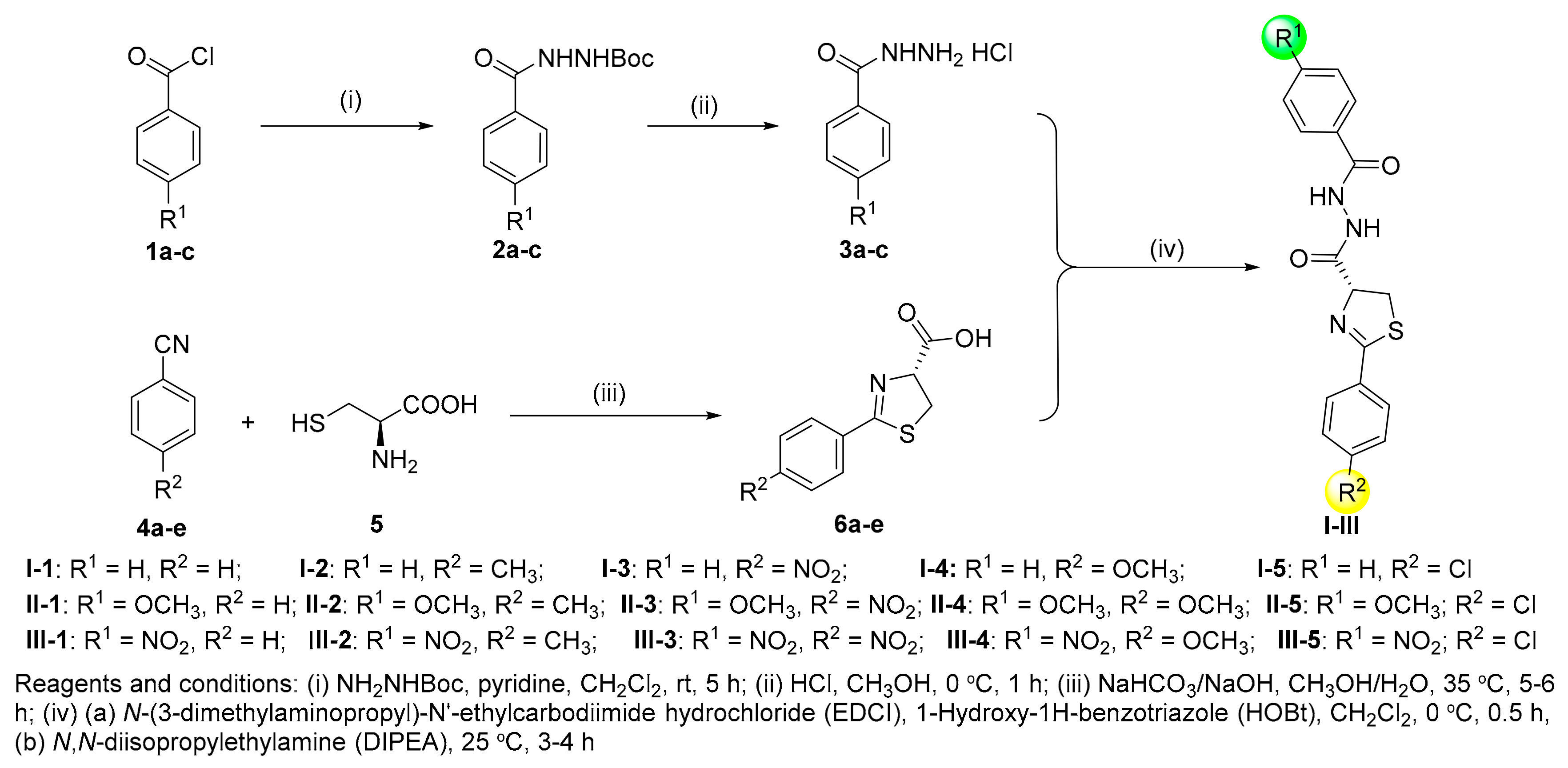
2.1. Chemistry
2.2. Biological Activity and Structure Activity Relationships
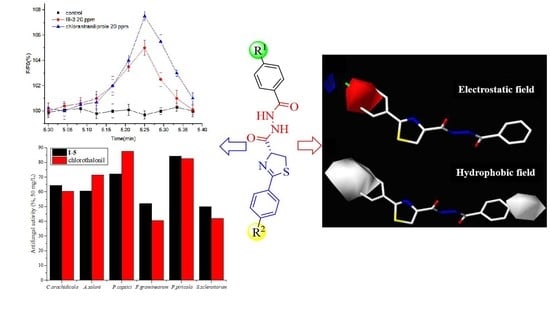
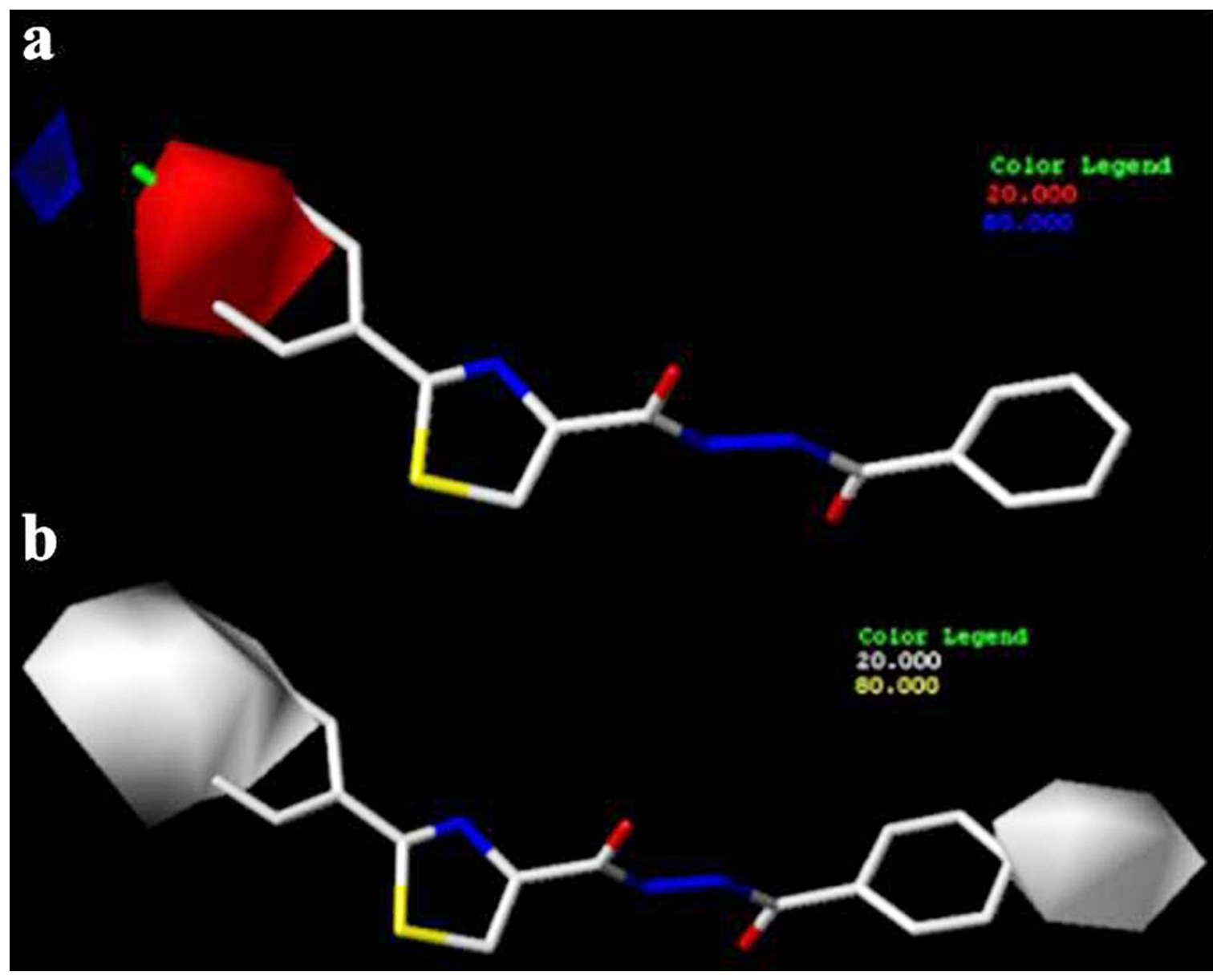
2.2.1. Antifungal Activity and 3D-QSAR
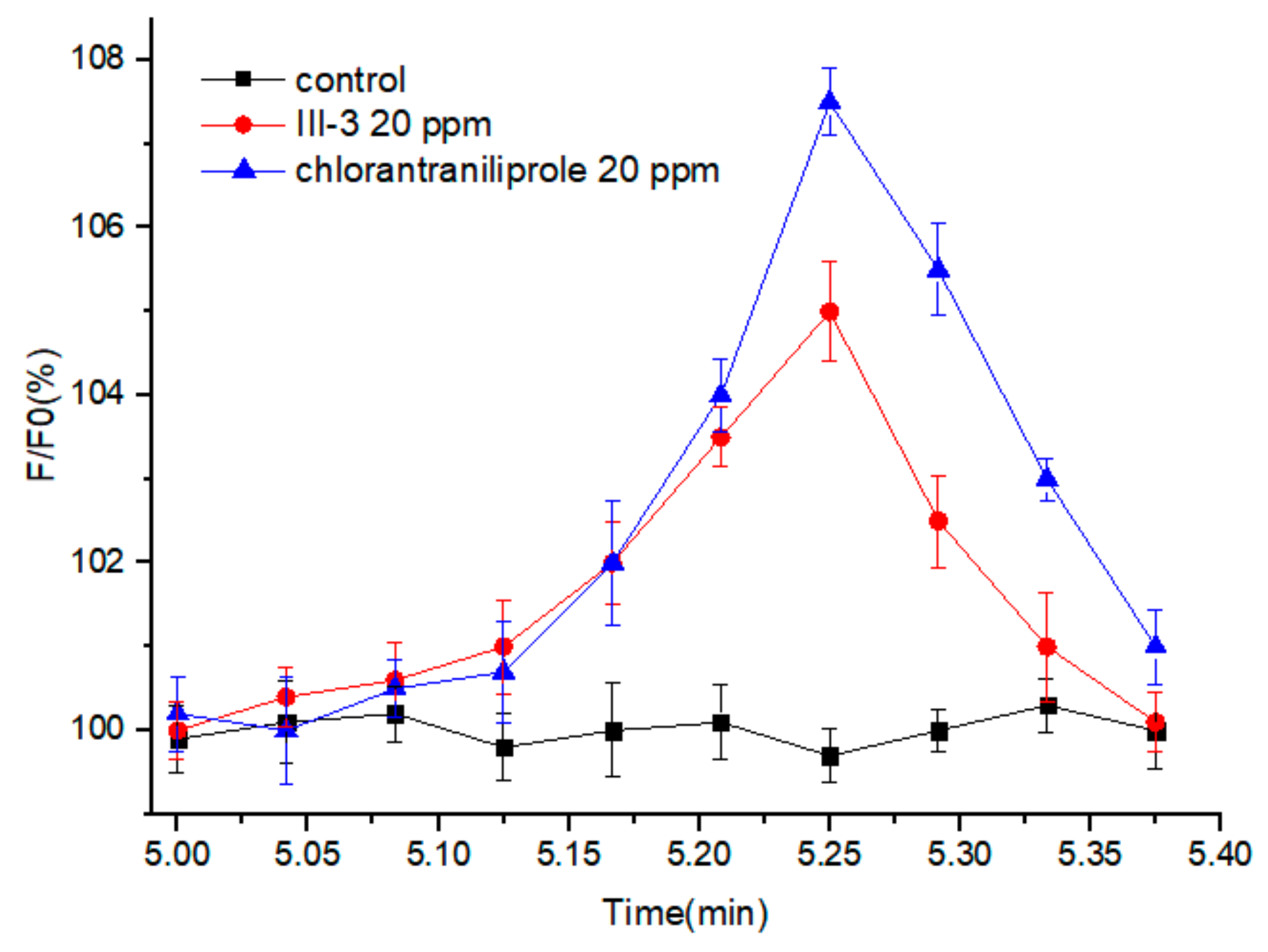
2.2.2. Insecticidal Activity against Mythimna separata and Calcium Imaging
3. Materials and Methods
3.1. Materials
3.2. General Synthetic Procedure for Title Compounds I–III
3.3. Biological Assay
3.3.1. Fungicidal Activity
3.3.2. Insecticidal Activity against Mythimna separata
3.3.3. D-QSAR Calculation Methods
3.4. Calcium Imaging Experiment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food Security: The Challenge of Feeding 9 Billion People. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Ray, D.K.; Mueller, N.D.; West, P.C.; Foley, J.A. Yield trends are insufficient to double global crop production by 2050. PLoS ONE 2013, 8, e66428. [Google Scholar] [CrossRef] [PubMed]
- Kuck, K.H.; Leadbeater, A.; Gisi, U. FRAC Mode of Action Classification and Resistance to Fungicides; Wiley-VCH: Weinheim, Germany, 2012; pp. 539–557. [Google Scholar]
- Tejero, R.; Gutierrez, B.; Lopez, D.; Lopez-Fabal, F.; Gomez-Garces, J.L.; Fernandez-Garcia, M. Copolymers of acrylonitrile with quaternizable thiazole and triazole side-chain methacrylates as potent antimicrobial and hemocompatible systems. Acta Biomater. 2015, 25, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Levy, S.B.; Marshall, B. Antibacterial resistance worldwide: Causes, challenges and responses. Nat. Med. 2004, 10, S122–S129. [Google Scholar] [CrossRef]
- Davies, J.; Davies, D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef]
- Li, P.; Tian, P.Y.; Chen, Y.Z.; Song, X.P.; Xue, W.; Jin, L.H.; Hu, D.Y.; Yang, S.; Song, B.A. Novel bisthioether derivatives containing a 1,3,4-oxadiazole moiety: Design, synthesis, antibacterial and nematocidal activities. Pest Manag. Sci. 2018, 74, 844–852. [Google Scholar] [CrossRef]
- He, H.F.; Wang, W.; Zhou, Y.; Xia, Q.; Ren, Y.L.; Feng, J.T.; Peng, H.; He, H.W.; Feng, L.L. Rational design, synthesis and biological evaluation of 1,3,4-oxadiazole pyrimidine derivatives as novel pyruvate dehydrogenase complex E1 inhibitors. Bioorg. Med. Chem. 2016, 24, 1879–1888. [Google Scholar] [CrossRef]
- Bauer, A.; Bronstrupt, M. Industrial natural product chemistry for drug discovery and development. Nat. Prod. Rep. 2014, 31, 35–60. [Google Scholar] [CrossRef]
- Rodrigues, T.; Reker, D.; Schneider, P.; Schneider, G. Counting on natural products for drug design. Nat. Chem. 2016, 8, 531–541. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Elliot, G.T.; Kelly, K.F.; Bonna, R.L.; Wardlaw, T.R.; Burns, E.R. In vitro antiproliferative activity of 2’ -(2-hydroxyphenyl)-2’ -thiazoline-4’ -carboxylic acid and its methyl ester of L1210 and P388 murine neoplasms. Cancer Chemother. Pharmacol. 1988, 21, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Zamri, A.; Schalk, I.J.; Pattus, F.; Abdallah, M.A. Bacterial siderophores: Synthesis and biological activities of novel pyochelin analogues. Bioorg. Med. Chem. Lett. 2003, 13, 1147–1150. [Google Scholar] [CrossRef]
- Pattenden, G.; Thom, S.M. Naturally occurring linear fused thiazoline-thiazole containing metabolites: Total synthesis of (–)-didehydromirabazole A, a cytotoxic alkaloid from blue-green algae. J. Chem. Soc. Perk. Trans. 1993, 14, 1629–1636. [Google Scholar] [CrossRef]
- Gududuru, V.; Hurh, E.; Dalton, J.T.; Miller, D.D. SAR studies of 2-arylthiazolidine-4-carboxylic acid amides: A novel class of cytotoxic agents for prostate cancer. J. Med. Chem. 2005, 48, 2584–2588. [Google Scholar] [CrossRef] [PubMed]
- Guan, A.Y.; Liu, C.L.; Li, Z.N.; Zhang, M.X.; Si, N.G. The discovery of fungicide coumoxystrobin. Agrochemicals 2011, 50, 90–92. [Google Scholar]
- Mersey, B.G.; Hall, J.C.; Anderson, D.M.; Swanton, C.J. Factors affecting the herbicidal activity of glufosinate-ammonium: Absorption, translocation, and metabolism in barley and green foxtail. Pestic. Biochem. Phys. 1990, 37, 90–98. [Google Scholar] [CrossRef]
- Miyazawa, M.; Yoshio, K.; Ishikawa, Y.; Kameoka, H. Insecticidal alkaloid against Drosophila melanogaster from tubers of Corydalis Bulbosa. Nat. Prod. Lett. 1996, 8, 299–302. [Google Scholar] [CrossRef]
- Miyazawa, M.; Yoshio, K.; Ishikawa, Y.; Kameoka, H. Insecticidal alkaloids against Drosophila melanogaster from Nuphar japonicum DC. J. Agric. Food Chem. 1998, 46, 1059–1063. [Google Scholar] [CrossRef]
- Yi, F.; Zou, C.H.; Hu, Q.B.; Hu, M.Y. The Joint Action of Destruxins and Botanical Insecticides (Rotenone, Azadirachtin and Paeonolum) against the Cotton Aphid, Aphis gossypii Glover. Molecules 2012, 17, 7533–7542. [Google Scholar] [CrossRef]
- Hummer, R.W.; Kenaga, E.E. Structural and Insecticidal Relationships in Rotenone, Methoxychlor, and DDT. Science 1951, 113, 653–655. [Google Scholar] [CrossRef]
- Vats, S. Larvicidal activity and in vitro regulation of rotenoids from Cassia tora L. 3 Biotech 2018, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Rimando, A.M.; Duke, S.O. Natural products for pest management. In Natural Products for Pest Management; Rimando, A.M., Duke, S.O., Eds.; American Chemical Society: Washington, DC, USA, 2006; pp. 2–21. [Google Scholar]
- Liu, J.B.; Li, Y.X.; Chen, Y.W.; Hua, X.W.; Wan, Y.Y.; Wei, W.; Song, H.B.; Yu, S.J.; Zhang, X.; Li, Z.M. Design, synthesis, antifungal activities and SARs of (R)-2-aryl-4,5-dihydrothiazole-4-carboxylic acid derivatives. Chin. J. Chem. 2015, 33, 1269–1275. [Google Scholar] [CrossRef]
- Liu, J.B.; Li, F.Y.; Wang, Y.H.; Zhang, H.X.; Dong, J.Y.; Sun, P.W.; Li, Y.X.; Li, Z.M. Synthesis, biological activities and 3D-QSAR studies of (R)-2-phenyl-4,5-dihydrothiazole-4-carboxamide derivatives containing a sulfur ether moiety. Chin. Chem. Lett. 2019, 30, 668–671. [Google Scholar] [CrossRef]
- Tan, C.X.; Shen, D.L.; Weng, J.Q.; Sun, N.B.; Ou, X.M. Synthesis and biological activity of 1-pyrazole formyl -2-aryl hydrazide compounds. Chin. J. Pestic. Sci. 2006, 8, 363. [Google Scholar]
- Liu, X.; Zhang, L.; Tan, J.G.; Xu, H.H. Design and synthesis of N-alkyl-N’ -substituted 2,4-dioxo-3,4-dihydropyrimidin-1-diacylhydrazine derivatives as ecdysone receptor agonist. Bioorg. Med. Chem. 2013, 21, 4687–4697. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Wei, W.; Zhu, L.L.; Li, Y.X.; Li, Z.M. Synthesis and insecticidal activity study of novel anthranilic diamides analogs containing a diacylhydrazine bridge as effective Ca2+ modulators. Chem. Biol. Drug Des. 2018, 92, 1914–1919. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Li, Y.S.; Weng, J.Q.; Tan, C.X. Synthesis and herbicidal activity of some new diacylhydrazine derivatives fluoride-containing pyrazolyl moiety. Chin. J. Org. Chem. 2011, 31, 1295–1299. [Google Scholar]
- Jin, Y.X.; Tan, Z.W.; He, M.Z.; Tian, B.H.; Tang, S.X.; Hewlett, I.; Yang, M. SAR and molecular mechanism study of novel acylhydrazone compounds targeting HIV-1 CA. Bioorg. Med. Chem. 2010, 18, 2135–2140. [Google Scholar] [CrossRef]
- Canales, E.; Carlson, J.S.; Appleby, T.; Fenaux, M.; Lee, J.; Tian, Y.; Tirunagari, N.; Wong, M.; Watkins, W.J. Tri-substituted acylhydrazines as tertiary amide bioisosteres: HCV NS5B polymerase inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 4288–4292. [Google Scholar] [CrossRef]
- Carvalho, S.A.; Feitosa, L.O.; Soares, M.; Costa, T.E.M.M.; Henriques, M.G.; Salomão, K.; Castro, S.L.; Kaiser, M.; Brun, R.; Wardell, J.L.; et al. Design and synthesis of new (E)-cinnamic N-acylhydrazones as potent antitrypanosomal agents. Eur. J. Med. Chem. 2012, 54, 512–521. [Google Scholar] [CrossRef]
- Liu, Z.J.; Wu, S.S.; Wang, Y.; Li, R.J.; Wang, J.; Wang, L.H.; Zhao, Y.F.; Gong, P. Design, synthesis and biological evaluation of novel thieno [3,2-d]pyrimidine derivatives possessing diaryl semicarbazone scaffolds as potent antitumor agents. Eur. J. Med. Chem. 2014, 87, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Mihailović, N.; Marković, V.; Matić, I.Z.; Stanisavljević, N.S.; Jovanović, Ž.S.; Trifunović, S.; Joksović, L. Synthesis and antioxidant activity of 1,3,4-oxadiazoles and their diacylhydrazine precursors derived from phenolic acids. RSC Adv. 2017, 7, 8550–8560. [Google Scholar] [CrossRef]
- Cui, Z.N.; Zhang, L.; Huang, J.; Yang, X.L.; Ling, Y. Synthesis and Bioactivity of Novel N,N′-Diacylhydrazine Derivatives Containing Furan (III). Chin. J. Chem. 2010, 28, 1257–1266. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, R.R.; Wu, H.K.; Liu, X.H.; Xu, T.M. The synthesis of 6-(tert-butyl)-8-fluoro-2,3-dimethylquinoline carbonate derivatives and their antifungal activity against Pyricularia oryzae. Front. Chem. Sci. Eng. 2019, 13, 369–376. [Google Scholar] [CrossRef]
- Liu, J.B.; Li, F.Y.; Li, Y.X.; Zhang, X.L.; Hua, X.W.; Xiong, L.X.; Li, Z.M. Synthesis, insecticidal evaluation and 3D-QSAR study of novel anthranilic diamide derivatives as potential ryanodine receptor modulators. Pest Manag. Sci. 2019, 75, 1034–1044. [Google Scholar] [CrossRef]
- Liu, J.B.; Li, F.Y.; Dong, J.Y.; Li, Y.X.; Zhang, X.L.; Wang, Y.H.; Xiong, L.X.; Li, Z.M. Anthranilic diamides derivatives as potential ryanodine receptor modulators: Synthesis, biological evaluation and structure activity relationship. Bioorg. Med. Chem. 2018, 26, 3541–3550. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds I–III are available from the authors. |
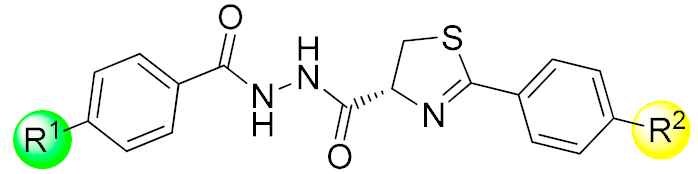 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Compd. | R1 | R2 | Antifungal Activity, Inhibition Rate (%, 50 mg/L) | |||||
| CA1 | AS | PC | FG | PP | SS | |||
| I-1 | H | H | 61.2 | 56.7 | 71.5 | 50.5 | 75.2 | 50.7 |
| I-2 | H | CH3 | 55.1 | 43.8 | 64.2 | 42.4 | 72.5 | 46.9 |
| I-3 | H | NO2 | 48.3 | 37.3 | 52.7 | 35.3 | 61.9 | 35.8 |
| I-4 | H | OCH3 | 50.8 | 37.4 | 50.2 | 35.8 | 63.2 | 19.5 |
| I-5 | H | Cl | 64.5 | 60.4 | 72.4 | 52.3 | 84.3 | 49.8 |
| II-1 | OCH3 | H | 48.5 | 42.2 | 63.6 | 35.6 | 73.8 | 45.2 |
| II-2 | OCH3 | CH3 | 35.7 | 40.3 | 57.1 | 35.2 | 71.6 | 30.8 |
| II-3 | OCH3 | NO2 | 28.2 | 34.2 | 44.3 | 18.7 | 54.3 | 34.8 |
| II-4 | OCH3 | OCH3 | 33.5 | 40.1 | 43.9 | 23.6 | 50.4 | 13.4 |
| II-5 | OCH3 | Cl | 47.1 | 53.4 | 65.8 | 36.3 | 76.7 | 43.6 |
| III-1 | NO2 | H | 49.9 | 55.6 | 60.5 | 26.2 | 67.3 | 36.7 |
| III-2 | NO2 | CH3 | 36.2 | 50.3 | 50.3 | 14.9 | 63.4 | 30.2 |
| III-3 | NO2 | NO2 | 33.8 | 30.2 | 42.6 | 9.8 | 51.1 | 27.7 |
| III-4 | NO2 | OCH3 | 36.3 | 47.3 | 45.8 | 13.0 | 52.5 | 14.9 |
| III-5 | NO2 | Cl | 51.6 | 54.4 | 60.6 | 35.7 | 69.2 | 33.4 |
| 6a | -- | H | 24.8 | 25.9 | 33.7 | 8.3 | 43.5 | 10.3 |
| chlorothalonil | 60.3 | 71.5 | 87.6 | 40.5 | 82.5 | 42.0 | ||
| carbendazim | 12.1 | 38.3 | 31.4 | 99.1 | 45.6 | 97.7 | ||
| Compd. | Fungus | EC50 | Compd. | Fungus | EC50 |
|---|---|---|---|---|---|
| I-5 | Cercospora arachidicola | 15.6 | chlorothalonil | Cercospora arachidicola | 15.5 |
| Alternaria solani | 21.5 | Alternaria solani | 17.9 | ||
| Phytophthora capsici | 9.3 | Phytophthora capsici | 5.0 | ||
| Fusarium graminearum | 8.5 | Fusarium graminearum | 8.1 | ||
| Physalospora piricola | 6.1 | Physalospora piricola | 6.6 | ||
| Sclerotinia sclerotiorum | 2.4 | Sclerotinia sclerotiorum | 1.3 |
| Compd. | EC50 | pEC50 a | pEC50 b | Compd. | EC50 | pEC50 a | pEC50 b |
|---|---|---|---|---|---|---|---|
| I-1c | 9.6 | 2.02 | 2.13 | II-5 | 9.7 | 2.01 | 1.97 |
| I-2 | 12.3 | 1.91 | 1.94 | III-1 | 15.2 | 1.82 | 1.87 |
| I-3c | 16.2 | 1.79 | 1.78 | III-2 | 16.7 | 1.78 | 1.84 |
| I-4 | 19.0 | 1.72 | 1.74 | III-3c | 21.2 | 1.67 | 1.76 |
| I-5 | 6.1 | 2.21 | 2.24 | III-4 | 20.5 | 1.69 | 1.64 |
| II-1 | 9.8 | 2.01 | 1.97 | III-5 | 11.3 | 1.95 | 2.02 |
| II-2c | 12.5 | 1.90 | 1.99 | Chlorothalonil | 6.6 | - | - |
| II-3 | 20.1 | 1.70 | 1.68 | Carbendazim | 32.3 | - | - |
| II-4c | 21.3 | 1.67 | 1.60 |
| Compd. | Larvicidal Activity (%) at Concn of (mg/L) | Compd. | Larvicidal Activity (%) at Concn of (mg/L) | ||||
|---|---|---|---|---|---|---|---|
| 200 mg/L | 50 mg/L | 10 mg/L | 200 mg/L | 50 mg/L | 10 mg/L | ||
| I-1 | 100 | 83 | 50 | II-4 | 100 | 83 | 23 |
| I-2 | 100 | 77 | 43 | II-5 | 100 | 90 | 53 |
| I-3 | 100 | 100 | 67 | III-1 | 100 | 100 | 53 |
| I-4 | 100 | 77 | 33 | III-2 | 100 | 100 | 57 |
| I-5 | 100 | 87 | 57 | III-3 | 100 | 100 | 77 |
| II-1 | 100 | 83 | 43 | III-4 | 100 | 87 | 43 |
| II-2 | 100 | 100 | 47 | III-5 | 100 | 77 | 37 |
| II-3 | 100 | 100 | 57 | Chlo 1 | 100 | 100 | 100 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.-Y.; Liu, J.-B.; Gong, J.-N.; Li, G. (R)-2-Phenyl-4,5-Dihydrothiazole-4-Carboxamide Derivatives Containing a Diacylhydrazine Group: Synthesis, Biological Evaluation, and SARs. Molecules 2019, 24, 4440. https://doi.org/10.3390/molecules24244440
Li F-Y, Liu J-B, Gong J-N, Li G. (R)-2-Phenyl-4,5-Dihydrothiazole-4-Carboxamide Derivatives Containing a Diacylhydrazine Group: Synthesis, Biological Evaluation, and SARs. Molecules. 2019; 24(24):4440. https://doi.org/10.3390/molecules24244440
Chicago/Turabian StyleLi, Feng-Yun, Jing-Bo Liu, Jia-Ning Gong, and Gen Li. 2019. "(R)-2-Phenyl-4,5-Dihydrothiazole-4-Carboxamide Derivatives Containing a Diacylhydrazine Group: Synthesis, Biological Evaluation, and SARs" Molecules 24, no. 24: 4440. https://doi.org/10.3390/molecules24244440
APA StyleLi, F.-Y., Liu, J.-B., Gong, J.-N., & Li, G. (2019). (R)-2-Phenyl-4,5-Dihydrothiazole-4-Carboxamide Derivatives Containing a Diacylhydrazine Group: Synthesis, Biological Evaluation, and SARs. Molecules, 24(24), 4440. https://doi.org/10.3390/molecules24244440