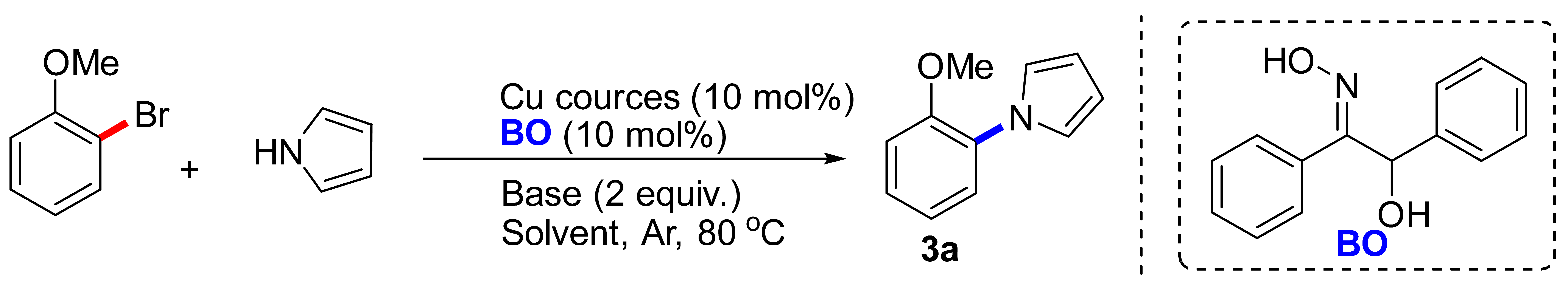
Abstract
We first reported the new application of a translate metal chelating ligand α-benzoin oxime for improving Cu-catalyzed C-N coupling reactions. The system could catalyse coupling reactions of (hetero)aryl halides with a wide of nucleophiles (e.g., azoles, piperidine, pyrrolidine and amino acids) in moderate to excellent yields. The protocol allows rapid access to the most common scaffolds found in FDA-approved pharmaceuticals.
1. Introduction
N-Arylated compounds are ubiquitous synthons in numerous natural products and functional molecules [1,2]. Particularly, their most important function is as structural fragment for FDA-approved pharmaceuticals (Figure 1), for example, antibacterial agents (Ofloxacin [3] and Pipemidic acid [4]), antidiabetic drug (Repaglinide [5]), nonsteroidal anti-inflammatory drug (Celecoxib [6]), antihypertensive drug (Prazosin [7]), etc. Although C-N bond-formation reactions between (hetero)aryl halides and N-nucleophiles are well known, including the SNAr reactions [8], classical Ullmann-type coupling reactions [9,10,11], and Buchwald-Hartwig reactions [12,13,14]. Amongst Pd-catalyzed C-N coupling reaction has been confirmed as a very useful method to build aromatic amines or arylazoles [15,16,17,18]. However, considering to the cost and toxicity of both palladium catalyst and auxiliary phosphine ligands, the arylation of N-nucleophiles with (hetero)aryl halides to form new C-N bonds still remains a significant opportunity. In recent years, Cu-catalyzed Ullmann-type couplings have attracted more and more attention due to the inexpensive catalyst and low toxicity. Although many commercially available or novel designed ligands have been developed for promoting copper-catalyzed couplings of aryl halides with N-nucleophiles, few of them are effective with less inactivated heterocyclic aryl chlorides. Besides, an easy-removal catalyst system is also very important for the environment.
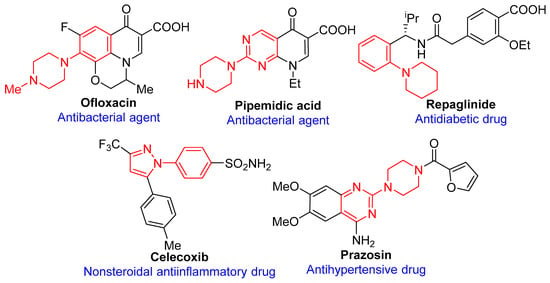
Figure 1.
Chemical structures of selected pharmaceuticals containing the N-arylated core.
During the past few years, Ma and co-workers have reported that the oxalic diamide ligands are powerful ligands for the copper-catalyzed couplings [19,20,21]. In the presence of ligands, the reaction temperature and catalyst loading could be significantly decreased while the yields were increased [22,23,24]. Following these studies, a number of bidentate ligands were reported for the synthesis of N-arylated compounds.
On the other hand, α-benzoin oxime (BO) is a common ligand, which is usually applied to inspect and measure copper, molybdenum, and tungsten [25,26], and also as a chelating agent for extracting antimony, vanadium, tungsten [27]. However, the use of BO in improving the Cu-catalyzed Ullmann style reactions is never reported. Herein, it was found that BO could be used in the direct couplings of the (hetero)aryl halides with N-nucleophiles. The reaction allowed rapid access to N-arylated compounds, the most common scaffolds found in FDA-approved pharmaceuticals. These reactions were occurred at mild temperature (80 °C), with employing (hetero)aryl halides, nucleophiles (e.g., azoles, piperidine, pyrrolidine and amino acids) and inexpensive catalysts, and affording high yields. Importantly, this process was general with respect to both the (hetero)aryl halides and nucleophiles, including the use of secondary amines and amino acids.
2. Results and Discussions
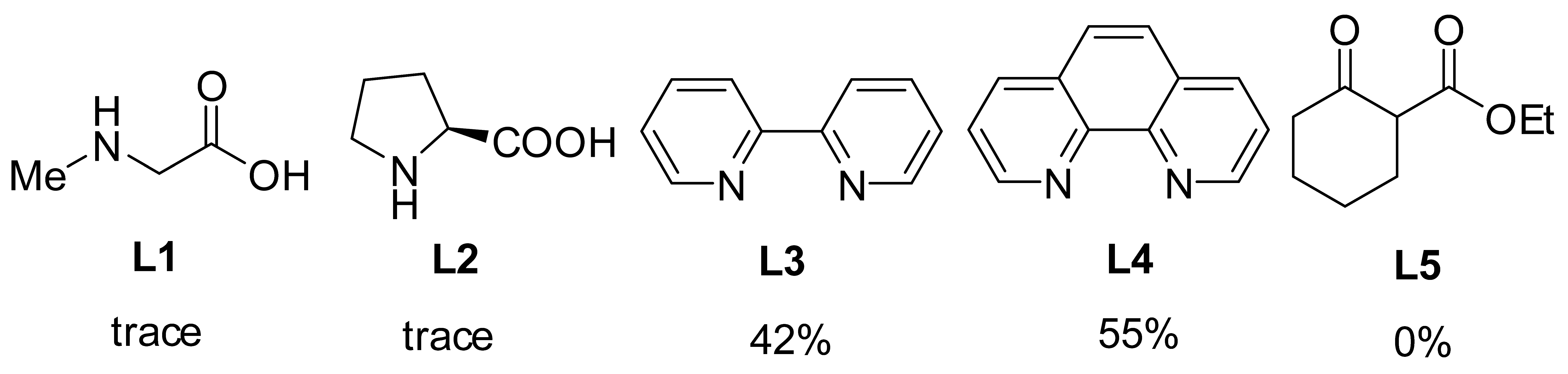
To initiate our studies, 2-bromoanisole was treated with pyrrole in the presence of 0.28 mol CuI in DMF. Regrettably, coupling product 3a was obtained in 12% yield, along with unreacted starting material (Table 1, entry 1). The use of Cu powder and Cu(OTf)2 as catalyst in DMF delivered 3a in low yields (entries 2, 3). Consistently, Cu(II) gluconate and Cu2(OH)2CO3 did not provide the desired product (entries 4, 5), only a trace of 3a was observed. In the event, 10 mol% Cu(OAc)2 enabled the coupling of 2-bromoanisole and pyrrole to provide the desired product in 53% yield (entry 6). Attempts to improve the yield through changing the base were successful, with K3PO4 proving to be optimal in terms of yield (entries 6–11). Furthermore, with dioxane, toluene, DCE, H2O as solvents (entries 12–16), the yield was lower than that in DMF. Fortunately, DMSO gave significantly better results, and 3a was obtained in a much-improved 90% yield. Meanwhile, lowering the temperature to 60 °C decreased the yield and led incomplete conversion (entry 17). A significant amount of coupling product was observed when using BO as ligand, comparing with the corresponding control experiments (entries 12 and 18). The scalability of this protocol was also tested through synthesis of the 3a on a 4.4 g scale (entry 19). Additionally, the BO-promoted C-N reaction was also carried out under air, 3a was obtained in 61% yield, indicating that this catalytic system was sensitive with air (entry 20). Finally, we checked other ligands (including N,N-, N,O-, O,O-type bidentate ligands) that were applied in Cu-catalyzed N-arylation reactions, and discovered that only L3 and L4 could furnish 3a in moderate yields under standard condition. It was reasoned that these reported ligands may be effective when chelating with Cu(I).

Table 1.
Identification of reaction condition a.
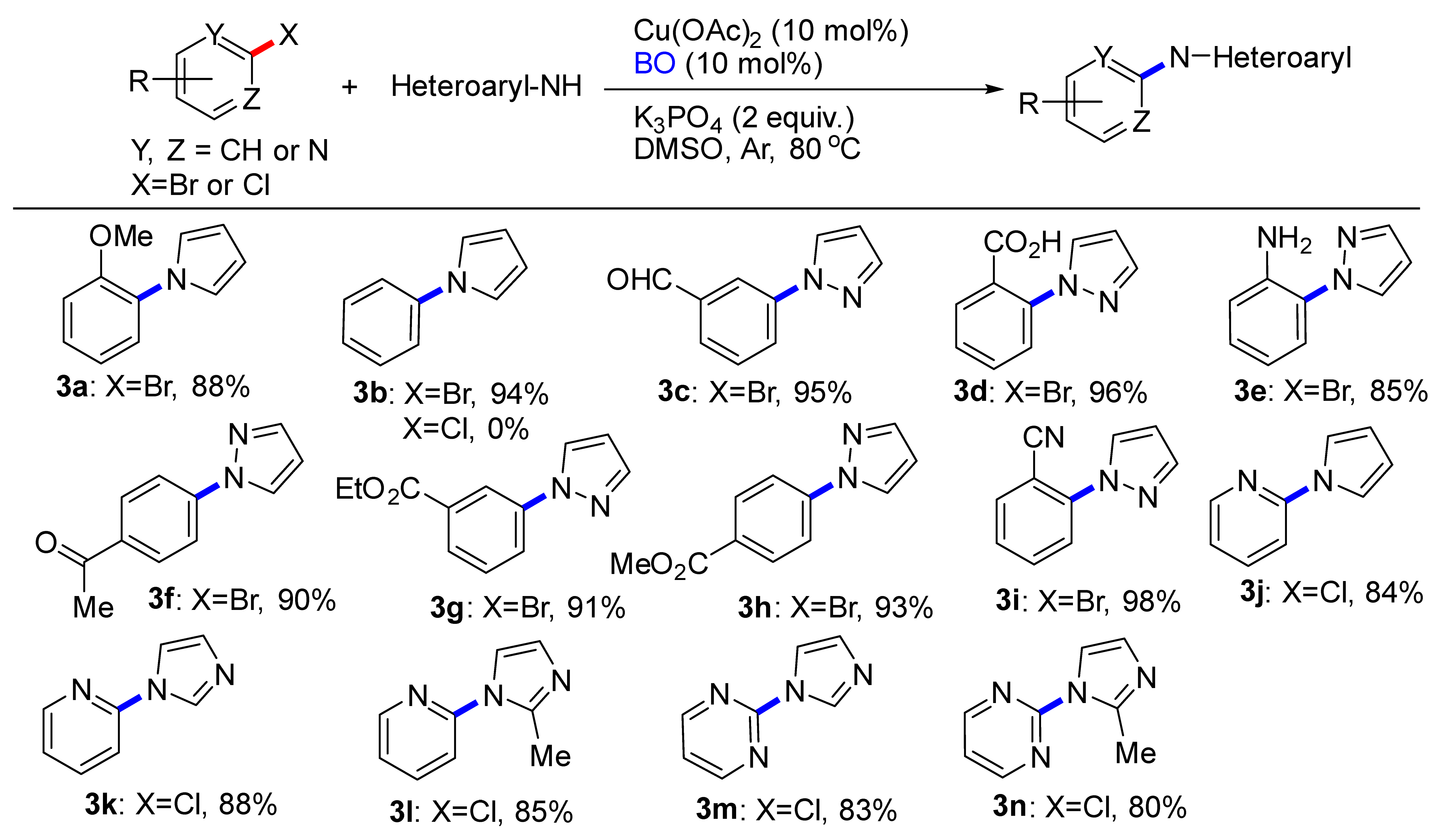
With optimized conditions in hand, we set out to evaluate the scope of (hetero)aryl halides that would participate in this transformation (Table 2). The reaction tolerated a diverse array of functional groups on the (hetero)aryl halides, including methoxy (3a), aldehyde (3c), carboxyl (3d), amino (3e), ketone (3f), ester (3g, 3h), cyano (3i). Electronic properties of the (hetero)aryl halides were evaluated by introducing electron-withdrawing and electron-donating groups on the aryl moiety. Although electron-poor (hetero)aryl halides (e.g., 3c, 3d, 3i) underwent coupling faster than electron rich ones (3a, 3e), the desired products were successfully obtained in all cases. Unfortunately, chlorobenzene couldn’t provide the product 3b. By changing which heteroaryl chlorides were employed, couplings were smoothly proceeded, although the yield was decreased. A more sterically encumbered 2-methylimidazole also reacted without incidents (3l, 3n).

Table 2.
C-N Coupling reactions of substituted aryl compounds with pyrrole or azoles a,b.
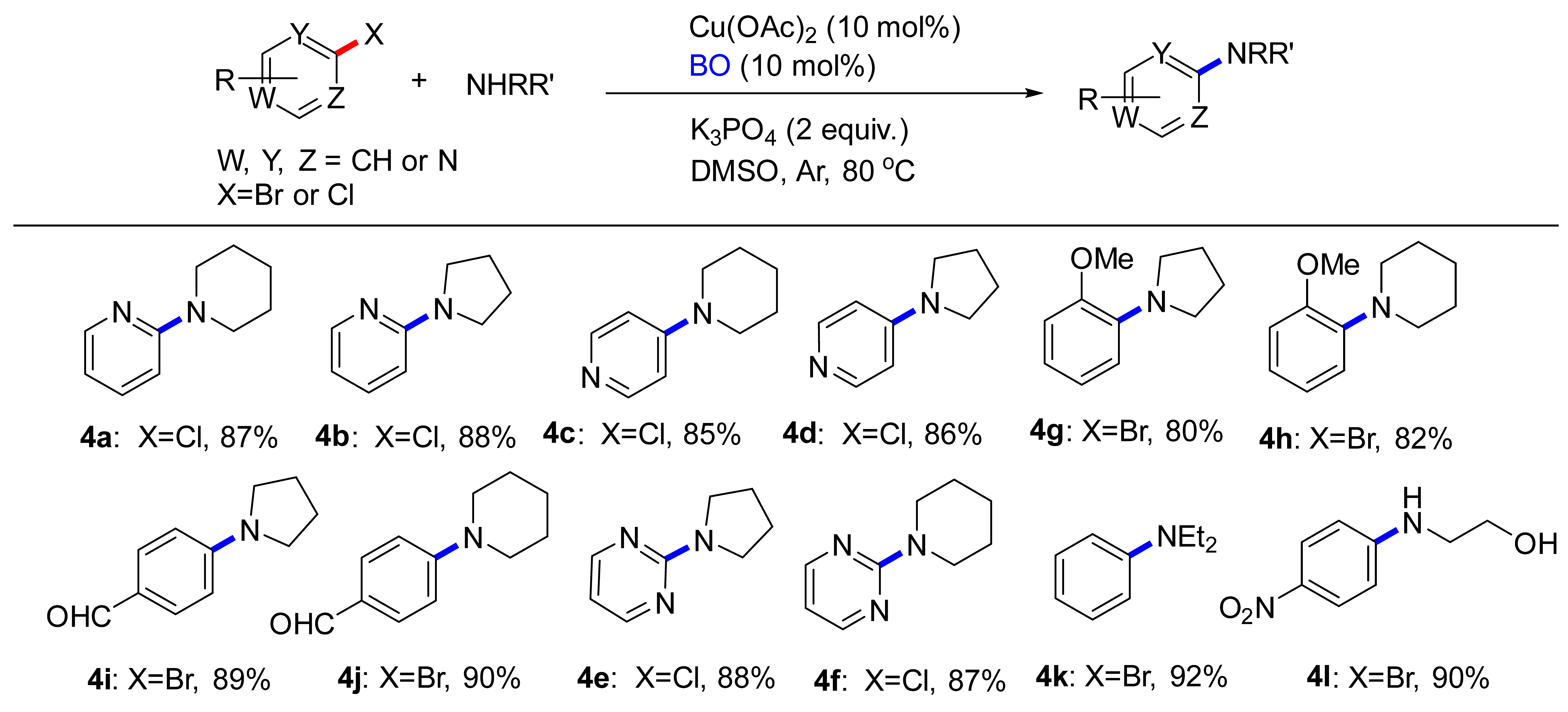
In order to explore the feasibility of this approach, cyclic secondary amines (piperidine and pyrrolidine), acyclic secondary amine (diethylamine), aliphatic primary amine (ethanolamine) were examined to achieve the desired coupling (Table 3). First, the reactions were carried out by using 2-chloropyridine as starting material. To our delight, the corresponding coupling product 4a and 4b were obtained in 87% and 88% yield, respectively. We also attempted the coupling of 4-chloropyridine to form 4c and 4d. In both cases, the products were obtained in good yields. Both electron-rich (4g) and electron-poor (4i) aryl bromides participated equally well in the reaction. The reaction was effective in the presence of unprotected polar functional groups such as alcohol. It was encouraged that the ethanolamine substitute could provide 4l in 90% yield; the lower yield was due to incomplete conversion of the starting material.

Table 3.
C-N Coupling reactions of substituted aryl compounds with amines a,b.
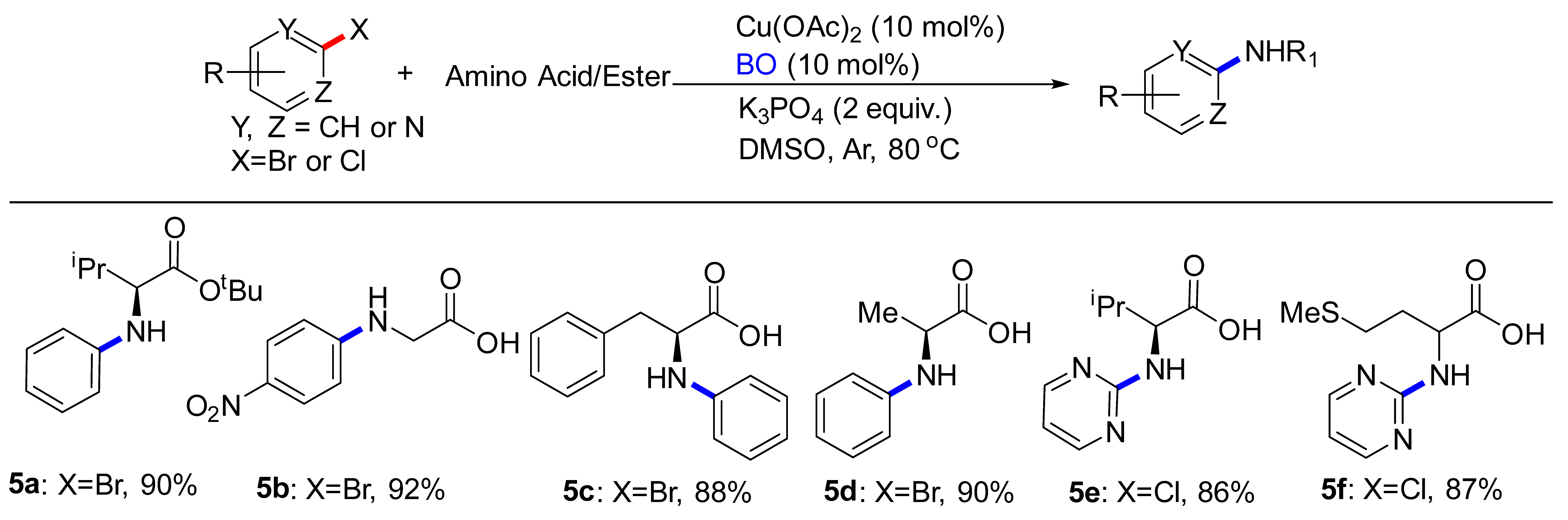
Although N-heterocycle electrophiles were the primary focus of this study, amino acids-based electrophiles were also evaluated (Table 4). Amino acids underwent coupling to afford corresponding products in moderate yields. Although the yields were modest, it was noted that these reactions were conducted under the conditions developed for the 2-bromoanisole with minimal reoptimization. Substrates bearing either electron-withdrawing (5b) or electron-donating groups (5c) on the (hetero)aryl halides coupled with high yields. Introduction of an ester group into amino acid was also tolerated (5a). Finally, we were pleased to find that our method was not limited to 2-chloropyrimidine. Using 2-chloropyrimidine as the substrate led to the formation of 5e, 5f in 86%, 87% yield, respectively.

Table 4.
C-N Coupling reactions of substituted aryl compounds with amino acids/esters a,b.
The ligand BO has been reported to be used as a metal chelating agent [28], which is a typical N,O-ligand. Thus, it was believed that the Cu-catalyzed couplings could process in a homogeneous manner due to the formation of Cu-benzoinoxime complex. Herein, it was proposed that the possible mechanism for the couplings might run via a prototypical Cu (I)/Cu (III) catalytic cycle. [29] As shown in Figure 2, the catalytic cycle initiated from a Cu complex (A). Then, coordinated copper species (B) were produced via oxidative addition of an ArX and A. Ligand exchange was subsequently occurred between A and N-heterocycles to form intermediate C, which could be converted to D in the presence of base. The N-arylazole was obtained by final reductive elimination of D.
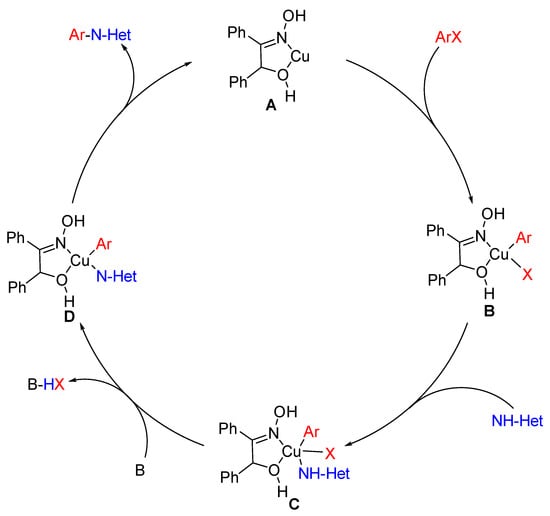
Figure 2.
Proposed mechanism for the couplings of (hetero)aryl halides with N-containing heterocycles. NH-Het represented N-hetero nucleophiles; X was bromine or chloride.
3. Materials and Methods
All of the starting materials, reagents, and solvents are commercially available and used without further purification. Melting points were determined with a X-4 apparatus (Beijing Taike Instrument Co., Ltd., Beijing, China) and were uncorrected. The nuclear magnetic resonance (NMR) spectra were recorded on a Bruker (Bruker Technology Co., Ltd., Karlsruhe, Germany) 400 MHz spectrometer in CDCl3 or DMSO-d6 using tetramethylsilane (TMS) as an internal standard. Electrospray ionization mass spectrometry (MS (ESI)) analyses were recorded in an Agilent 1100 Series MSD Trap SL (Santa Clara, CA, USA). The reactions were monitored by thin-layer chromatography (TLC: HG/T2354-92, GF254), and compounds were visualized on TLC with UV light (Gongyi Yuhua Instrument Co., Ltd, Zhengzhou, China).
General Procedure for Catalytic Experiments
To a solution of (hetero)aryl halide (2.81 mmol), N-nucleophile (3.37 mmol), BO (0.28 mmol), K3PO4 (5.62 mmol) in DMSO (4 mL), were added Cu(OAc)2 (0.28 mmol). The flask was evacuated and backfilled with argon for three times. The resulting suspension was heated in a 80 °C oil bath with stirring for the indicated time. The reactor was cooled to r.t., the flask was opened to air and the reaction mixture was poured into water (20 mL), extracted with ethyl acetate (20 mL × 3), and organic layer was washed with water (20 mL × 2) and once with brine (25 mL), dried over magnesium sulfate and concentrated in vacuo. The product was purified by column chromatography on silica gel using petroleum ether and ethyl acetate as eluent.
1-(2-Methoxyphenyl)-1H-pyrrole (3a) [30]: colorless oil (0.43 g, 88%). 1H-NMR (400 MHz, CDCl3) δ (ppm): 7.30–7.23 (2H, m), 7.03–6.98 (4H, m), 6.30 (2H, t, J = 2.2 Hz), 3.82 (3H, s). MS (ESI) m/z: 174.11 [M + H]+, see Supplementary Materials.
1-Phenyl-1H-pyrrole (3b) [31]: white solid (0.38 g, 94%). m.p. 60–62 C. 1H-NMR (400 MHz, CDCl3) δ (ppm): 7.43–7.37 (4H, m), 7.25–7.21 (1H, m), 7.08 (2H, t, J = 2.2 Hz), 6.34 (2H, t, J = 2.2 Hz). MS (ESI) m/z: 144.04 [M + H]+.
3-(1H-Pyrazol-1-yl)benzaldehyde (3c) [32]: off white solid (0.46 g, 95%), m.p. 28–30 °C. 1H-NMR (400 MHz, CDCl3) δ (ppm): 10.08 (1H, s), 8.19 (1H, t, J = 1.8 Hz), 8.05–8.02 (2H, m), 7.81–7.76 (2H, m), 7.64 (1H, t, J = 7.9 Hz), 6.52 (1H, t, J = 2.1 Hz). MS (ESI) m/z: 173.08 [M + H]+.
2-(1H-Pyrazol-1-yl)benzoic acid (3d) [33]: white solid (0.51 g, 96%), m.p. 128–129 °C. 1H-NMR (400 MHz, CDCl3) δ (ppm): 11.40 (1H, br), 8.05–8.02 (1H, dd, J = 7.8 Hz, 1.2 Hz), 7.76–7.74 (2H, m), 7.62–7.58 (1H, m), 7.49–7.40 (2H, m), 6.48 (1H, s). MS (ESI) m/z: 189.06 [M + H]+.
2-(1H-Pyrazol-1-yl)aniline (3e) [34]: brown oil (0.38 g, 85%). 1H-NMR (400 MHz, CDCl3) δ (ppm): 7.74–7.71 (2H, m), 7.19–7.13 (2H, m), 6.85–6.76 (2H, m), 6.44 (1H, t, J = 2.0 Hz), 4.63 (2H, br). MS (ESI) m/z: 160.09 [M + H]+.
1-(4-(1H-Pyrazol-1-yl)phenyl)ethan-1-one (3f) [35]: yellow oil (0.47 g, 90%). 1H-NMR (400 MHz, CDCl3) δ (ppm): 8.08–8.06 (2H, m), 8.02 (1H, d, J = 2.5 Hz), 7.83–7.81 (2H, m), 7.78 (1H, d, J = 1.4 Hz), 6.53 (1H, t, J = 2.0 Hz), 2.63 (3H, s). MS (ESI) m/z: 187.09 [M + H]+.
Ethyl 3-(1H-pyrazol-1-yl)benzoate (3g) [36]: yellow liquid (0.55 g, 91%). 1H-NMR (400 MHz, CDCl3) δ (ppm): 8.31 (1H, t, J = 1.8 Hz), 7.99 (1H, d, J = 2.4 Hz), 7.97–7.93 (2H, m), 7.74 (1H, d, J = 1.6 Hz), 7.53 (1H, t, J = 8.0 Hz), 6.49 (1H, t, J = 2.0 Hz), 4.44–4.38 (2H, q, J = 14.3 Hz, 7.2 Hz), 1.41 (3H, t, J = 7.1 Hz). MS (ESI) m/z: 217.10 [M + H]+.
Methyl 4-(1H-pyrazol-1-yl)benzoate (3h) [37]: white solid (0.53 g, 93%), m.p. 103–105 °C. 1H-NMR (400 MHz, CDCl3) δ (ppm): 8.15–8.12 (2H, m), 8.00 (1H, d, J = 2.4 Hz), 7.80–7.77 (2H, m), 7.76 (1H, d, J = 1.4 Hz), 6.51 (1H, t, J = 2.1 Hz), 3.93 (3H, s). MS (ESI) m/z: 203.11 [M + H]+.
2-(1H-Pyrazol-1-yl)benzonitrile (3i) [38]: yellow oil (0.47 g, 98%). 1H-NMR (400 MHz, CDCl3) δ (ppm): 8.15 (1H, d, J = 2.5 Hz), 7.81–7.79 (3H, m), 7.77 (1H, d, J = 1.3 Hz), 7.72–7.68 (1H, m), 6.55 (1H, t, J = 2.1 Hz). MS (ESI) m/z: 170.07 [M + H]+.
2-(1H-Pyrrol-1-yl)pyridine (3j) [39]: colorless oil (0.34 g, 84%). 1H-NMR (400 MHz, CDCl3) δ (ppm): 8.43–8.42 (1H, m), 7.75–7.71 (1H, m), 7.52 (2H, t, J = 2.3 Hz), 7.32 (1H, d, J = 8.3 Hz), 7.11–7.08 (1H, m), 6.36 (2H, t, J = 2.3 Hz). MS (ESI) m/z: 145.04 [M + H]+.
2-(1H-Imidazol-1-yl)pyridine (3k) [40]: white solid (0.36 g, 88%), m.p. 40–41 °C. 1H-NMR (400 MHz, CDCl3) δ (ppm): 8.50–8.48 (1H, m), 8.35 (1H, s), 7.85–7.80 (1H, m), 7.65 (1H, t, J = 1.3 Hz), 7.37–7.35 (1H, m), 7.27–7.23 (1H, m), 7.20 (1H, s). MS (ESI) m/z: 146.08 [M + H]+.
2-(2-Methyl-1H-imidazol-1-yl)pyridine (3l) [41]: colorless oil (0.38 g, 85%). 1H-NMR (400 MHz, CDCl3) δ (ppm): 8.56–8.54 (1H, m), 7.86–7.82 (1H, m), 7.32–7.29 (2H, m), 7.28 (1H, d, J = 1.5 Hz), 7.02 (1H, d, J = 1.5 Hz), 7.02 (1H, d, J = 1.4 Hz), 2.59 (3H, s). MS (ESI) m/z: 160.09 [M + H]+.
2-(1H-Imidazol-1-yl)pyrimidine (3m) [42]: white solid (0.34 g, 83%), m.p. 120–122 °C. 1H-NMR (400 MHz, CDCl3) δ (ppm): 8.71 (2H, d, J = 4.8 Hz), 8.64 (1H, s), 7.90 (1H, s), 7.21 (1H, t, J = 4.8 Hz), 7.18 (1H, s). MS (ESI) m/z: 147.06 [M + H]+.
2-(2-Methyl-1H-imidazol-1-yl)pyrimidine (3n) [43]: white solid (0.36 g, 80%), m.p. 90–92 °C.1H-NMR (400 MHz, CDCl3) δ (ppm): 8.72 (2H, d, J = 4.8 Hz), 7.86 (1H, d, J = 1.4 Hz), 7.18 (1H, t, J = 4.8 Hz), 6.97 (1H, d, J = 1.3 Hz), 2.82 (3H, s). MS (ESI) m/z: 161.10 [M + H]+.
2-(Piperidin-1-yl)pyridine (4a) [44]: colorless oil (0.40 g, 87%). 1H-NMR (400 MHz, CDCl3) δ (ppm): 8.16–8.15 (1H, m), 7.44–7.39 (1H, m), 6.63 (1H, d, J = 8.6 Hz), 6.54–6.51 (1H, m), 3.52 (4H, d, J = 4.9 Hz), 1.62 (6H, s). MS (ESI) m/z: 163.13 [M + H]+.
2-(Pyrrolidin-1-yl)pyridine (4b) [45]: colorless oil (0.37 g, 88%). 1H-NMR (400 MHz, CDCl3) δ (ppm): 8.16–8.14 (1H, m), 7.44–7.39 (1H, m), 6.51–6.48 (1H, m), 6.35 (1H, d, J = 8.5 Hz), 3.46–3.43 (4H, m), 2.02–1.98 (4H, m). MS (ESI) m/z: 149.12 [M + H]+.
4-(Piperidin-1-yl)pyridine (4c) [46]: colorless oil (0.39 g, 85%). 1H-NMR (400 MHz, CDCl3) δ (ppm): 8.22–8.20 (2H, q, J = 5.1 Hz, 1.6 Hz), 6.62–6.61 (2H, q, J = 5.1 Hz, 1.6 Hz), 3.31 (4H, d, J = 4.9 Hz), 1.63 (6H, s). MS (ESI) m/z: 163.13 [M + H]+.
4-(Pyrrolidin-1-yl)pyridine (4d) [44]: colorless oil (0.36 g, 86%). 1H-NMR (400 MHz, CDCl3) δ (ppm): 8.18 (2H, d, J = 4.9 Hz), 6.36 (2H, d, J = 4.9 Hz), 3.30–3.27 (4H, m), 2.03–1.99 (4H, m). MS (ESI) m/z: 149.09 [M + H]+.
2-(Pyrrolidin-1-yl)pyrimidine (4e) [47]: colorless oil (0.37 g, 88%). 1H-NMR (400 MHz, CDCl3) δ (ppm): 8.32 (2H, d, J = 4.8 Hz), 6.45 (1H, t, J = 4.8 Hz), 3.59–3.56 (4H, m), 2.02–1.98 (4H, m). MS (ESI) m/z: 150.11 [M + H]+.
2-(Piperidin-1-yl)pyrimidine (4f) [47]: colorless oil (0.40 g, 87%). 1H-NMR (400 MHz, CDCl3) δ (ppm): 8.30 (2H, t, J = 5.6 Hz), 6.42 (1H, t, J = 5.6 Hz), 3.81–3.78 (4H, m), 1.72–1.59 (6H, m). MS (ESI) m/z: 164.14 [M + H]+.
1-(2-Methoxyphenyl)pyrrolidine (4g) [48]: colorless oil (0.40 g, 80%). 1H-NMR (400 MHz, CDCl3) δ (ppm): 6.90–6.81 (4H, m), 3.83 (3H, s), 3.30–3.27 (4H, m), 1.95–1.91 (4H, m). MS (ESI) m/z: 178.16 [M + H]+.
1-(2-Methoxyphenyl)piperidine (4h) [49]: colorless oil (0.44 g, 82%). 1H-NMR (400 MHz, CDCl3) δ (ppm): 6.99–6.84 (4H, m), 3.86 (3H, s), 2.99–2.97 (4H, m), 1.78–1.54 (6H, m). MS (ESI) m/z: 192.17 [M + H]+.
4-(Pyrrolidin-1-yl)benzaldehyde (4i) [50]: white solid (0.44 g, 89%), m.p. 83–85 °C.1H-NMR (400 MHz, CDCl3) δ (ppm): 9.72 (1H, s), 7.73 (2H, d, J = 8.8 Hz), 6.58 (2H, d, J = 8.8 Hz), 3.40–3.37 (4H, m), 2.08–2.02 (4H, m). MS (ESI) m/z: 176.13 [M + H]+.
4-(Piperidin-1-yl)benzaldehyde (4j) [51]: white solid (0.48 g, 90%). m.p. 63–64 °C. 1H-NMR (400 MHz, CDCl3) δ (ppm): 9.75 (1H, s), 7.75–7.71 (2H, m), 6.91 (2H, d, J = 8.9 Hz), 3.41–3.40 (4H, m), 1.68 (6H, s). MS (ESI) m/z: 190.16 [M + H]+.
N,N-Diethylaniline (4k) [52]: yellow liquid (0.39 g, 92%). 1H-NMR (400 MHz, CDCl3) δ (ppm): 7.23–7.18 (2H, m), 6.69–6.61 (3H, m), 3.37–3.32 (4H, q, J = 14.1 Hz, 7.0 Hz), 1.15 (6H, t, J = 7.1 Hz). MS (ESI) m/z: 150.12 [M + H]+.
2-((4-Nitrophenyl)amino)ethan-1-ol (4l) [53]: yellow solid (0.46 g, 90%). m.p. 110–111 °C.1H-NMR (400 MHz, DMSO-d6) δ (ppm): 8.05 (2H, d, J = 9.2 Hz), 7.35 (1H, t, J = 5.0 Hz), 6.73 (2H, d, J = 9.2 Hz), 4.86 (1H, t, J = 5.4 Hz), 3.65–3.61 (2H, q, J = 11.2 Hz, 5.6 Hz), 3.31–3.27 (2H, q, J = 11.4 Hz, 5.7 Hz). MS (ESI) m/z: 183.08 [M + H]+.
tert-Butyl phenyl-D-valinate (5a) [54]: white solid (0.63 g, 90%), m.p. 64–66 °C. 1H-NMR (400 MHz, CDCl3) δ (ppm): 7.17–7.13 (2H, m), 6.72–6.69 (1H, m), 6.64–6.62 (2H, m), 4.12 (1H, br), 3.75 (1H, d, J = 5.3 Hz), 2.15–2.04 (1H, m), 1.42 (9H, s), 1.05–1.01 (6H, m). MS (ESI) m/z: 250.16 [M + H]+.
(4-Nitrophenyl)glycine (5b) [55]: brown solid (0.51 g, 92%), 224–226 °C. 1H-NMR (400 MHz, DMSO-d6) δ (ppm): 8.02 (2H, d, J = 9.0 Hz), 7.44 (1H, t, J = 5.6 Hz), 6.66 (2H, d, J = 9.1 Hz), 3.98 (2H, d, J = 6.0 Hz). MS (ESI) m/z: 197.08 [M + H]+.
Phenyl-D-phenylalanine (5c) [56]: white solid (0.60 g, 88%), m.p. 173–176 °C .1H-NMR (400 MHz, CDCl3) δ (ppm): 7.33–7.17 (7H, m), 6.80 (1H, t, J = 7.3 Hz), 6.62 (2H, d, J = 7.8 Hz), 4.32 (1H, t, J = 5.8 Hz), 3.30–3.10 (2H, m). MS (ESI) m/z: 242.11 [M + H]+.
Phenyl-L-alanine (5d) [57]: white solid (0.42 g, 90%), m.p. 133–135 °C. 1H-NMR (400 MHz, DMSO-d6) δ (ppm): 7.06 (2H, t, J = 7.8 Hz), 6.56–6.53 (3H, m), 3.95–3.89 (1H, q, J = 14.0 Hz, 7.0 Hz), 1.37 (3H, d, J = 7.0 Hz). MS (ESI) m/z: 166.07 [M + H]+.
Pyrimidin-2-yl-D-valine (5e): white solid (0.47 g, 86%), 113–115 °C. 1H-NMR (400 MHz, CDCl3) δ (ppm): 11.24 (1H, br), 8.25 (2H, s), 7.22 (1H, d, J = 8.1 Hz), 6.55 (1H, t, J = 4.9 Hz), 4.62–4.59 (1H, q, J = 13.1 Hz, 5.0 Hz), 2.37–2.29 (1H, m), 1.06 (6H, t, J = 7.2 Hz). MS (ESI) m/z: 196.16 [M + H]+, 218.11 [M + H]+. 13 C-NMR (100 MHz, CDCl3) δ (ppm): 176.0, 161.2, 110.4, 59.4, 31.0, 18.9, 18.2.
Pyrimidin-2-ylmethionine (5f): white solid (0.56 g, 87%). 1H-NMR (400 MHz, CDCl3) δ (ppm): 13.32 (1H, br), 8.29 (2H, br), 7.92 (1H, d, J = 6.6 Hz), 6.62 (1H, t, J = 4.9 Hz), 4.88–4.84 (1H, q, J = 12.2 Hz, 6.1 Hz), 2.70–2.64 (2H, m), 2.37–2.22 (2H, m), 2.11 (3H, s). MS (ESI) m/z: 228.13 [M + H]+. 13 C-NMR (100 MHz, CDCl3) δ (ppm): 175.8, 160.3, 110.3, 53.7, 31.9, 30.0, 15.4.
4. Conclusions
In summary, a highly effective coupling reaction has been developed for the preparation of N-aryl compounds. This transformation occurs with good to excellent yields. A variety of substituted (hetero)aryl halides can be used as electrophiles, and azoles, piperidine, pyrrolidine, and amino acids, etc. function as nucleophiles. The key to this discovery was the identification of benzoin oxime ligand that can promote the (hetero)aryl halides to the corresponding N-arylation compounds. Efforts to apply our Cu-based system to other catalytic reactions and to expand the scope of the N-nucleophiles to other classes of nucleophiles are currently underway in our laboratory.
Supplementary Materials
The following are available online at https://www.mdpi.com/1420-3049/24/22/4177/s1. Copies of 1H NMR and MS for known compounds and copies of 13C NMR for new compounds.
Author Contributions
Conceptualization, C.Y.; Methodology, C.Y. and L.Z.; Software, C.Y.; Formal Analysis, Y.Z. and L.Z.; Writing-Original Draft Preparation, C.Y.; Writing-Review and Editing, C.Y. and Y.Z.; Project Administration, C.Y.
Funding
This research was funded by Liaoning Provincial Natural Science Foundation of China, grant number “2019-BS-102”; College Students Innovation Training Program of Liaoning Province, grant number “201910160017” and “The APC was funded by Liaoning Provincial Natural Science Foundation of China”.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Roughley, S.D.; Jordan, A.M. The medicinal chemist’s toolbox: An analysis of reactions used in the pursuit of drug candidates. J. Med. Chem. 2011, 54, 3451–3479. [Google Scholar] [CrossRef] [PubMed]
- Enders, D.; Niemeier, O.; Henseler, A. Organocatalysis by N-heterocyclic carbenes. Chem. Rev. 2007, 107, 5606–5655. [Google Scholar] [CrossRef] [PubMed]
- Olcay, E.; Beytemur, O.; Kaleagasioglu, F.; Gulmez, T.; Mutlu, Z.; Olgac, V. Oral toxicity of pefloxacin, norfloxacin, ofloxacin and ciprofloxacin: Comparison of biomechanical and histopathological effects on Achilles tendon in rats. J. Toxicol. Sci. 2011, 36, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Iacovino, R.; Rapuano, F.; Caso, J.V.; Russo, A.; Lavorgna, M.; Russo, C.; Isidori, M.; Russo, L.; Malgieri, G.; Isernia, C. β-Cyclodextrin inclusion complex to improve physicochemical properties of pipemidic acid: Characterization and bioactivity evaluation. Int. J. Mol. Sci. 2013, 14, 13022–13041. [Google Scholar] [CrossRef]
- Owens, D.R. Repaglinide-prandial glucose regulator: A new class of oral antidiabetic drugs. Diabet. Med. 1998, 15, S28–S36. [Google Scholar] [CrossRef]
- Liu, C.; Liu, L.; Zhu, H.; Sheng, J.; Wu, X.; He, X.; Tian, D.; Liao, J.; Li, P. Celecoxib alleviates nonalcoholic fatty liver disease by restoring autophagic flux. Sci. Rep. 2018, 8, 4108. [Google Scholar] [CrossRef]
- Yu, C.; Zhu, C.; Xu, S.; Cao, X.; Wu, G. Selective MT2 melatonin receptor antagonist blocks melatonin-induced antinociception in rats. Neurosci. Lett. 2000, 282, 161–164. [Google Scholar] [CrossRef]
- Kwan, E.E.; Zeng, Y.; Besser, H.A.; Jacobsen, E.N. Concerted nucleophilic aromatic substitutions. Nat. Chem. 2018, 10, 917–923. [Google Scholar] [CrossRef]
- Monnier, F.; Taillefer, M. Catalytic C-C, C-N, and C-O Ullmann-type coupling reactions. Angew. Chem. Int. Ed. 2009, 48, 6954–6971. [Google Scholar] [CrossRef]
- Ma, D.; Cai, Q. Copper/amino acid catalyzed cross-couplings of aryl and vinyl halides with nucleophiles. Acc. Chem. Res. 2008, 41, 1450–1460. [Google Scholar] [CrossRef]
- Surry, D.S.; Buchwald, S.L. Diamine ligands in copper-catalyzed reactions. Chem. Sci. 2010, 1, 13–31. [Google Scholar] [CrossRef]
- Hartwig, J.F. Carbon-heteroatom bond-forming reductive eliminations of amines, ethers, and sulfides. Acc. Chem. Res. 1998, 31, 852–860. [Google Scholar] [CrossRef]
- Hartwig, J.F. Transition metal catalyzed synthesis of arylamines and aryl ethers from aryl halides and triflates: Scope and mechanism. Angew. Chem. Int. Ed. 1998, 37, 2046–2067. [Google Scholar]
- Wolfe, J.P.; Wagaw, S.; Marcoux, J.F.; Buchwald, S.L. Rational development of practical catalysts for aromatic carbon-nitrogen bond formation. Acc. Chem. Res. 1998, 31, 805–818. [Google Scholar] [CrossRef]
- Avila-Sorrosa, A.; Estudiante-Negrete, F.; Hernandez-Ortega, S.; Toscano, R.A.; Morales-Morales, D. Buchwald-Hartwig C-N cross coupling reactions catalyzed by a pseudo-pincer Pd(II) compound. Inorg. Chim. Acta. 2010, 363, 1262–1268. [Google Scholar] [CrossRef]
- Guram, A.S.; Rennels, R.A.; Buchwald, S.L. A simple catalytic method for the conversion of aryl bromides to arylamines. Angew. Chem. Int. Ed. 1995, 34, 1348–1350. [Google Scholar] [CrossRef]
- Louie, J.; Hartwig, J.F. Palladium-catalyzed synthesis of arylamines from aryl halides. Mechanistic studies lead to coupling in the absence of tin reagents. Tetrahedron Lett. 2010, 36, 3609–3612. [Google Scholar]
- Yang, B.H.; Buchwald, S.L. Palladium-catalyzed amination of aryl halides and sulfonates. J. Organomet. Chem. 1999, 576, 125–146. [Google Scholar] [CrossRef]
- Zhou, W.; Fan, M.; Yin, J.; Jiang, Y.; Ma, D. CuI/oxalic diamide catalyzed coupling reaction of (hetero)aryl chlorides and amines. J. Am. Chem. Soc. 2015, 137, 11942–11945. [Google Scholar] [CrossRef]
- Fan, M.; Zhou, W.; Jiang, Y.; Ma, D. CuI/oxalamide catalyzed couplings of (hetero)aryl chlorides and phenols for diaryl ether formation. Angew. Chem. Int. Ed. 2016, 55, 6211–6215. [Google Scholar] [CrossRef]
- Xia, S.; Gan, L.; Wang, K.; Li, Z.; Ma, D. Copper-catalyzed hydroxylation of (hetero)aryl halides under mild conditions. J. Am. Chem. Soc. 2016, 138, 13493–13496. [Google Scholar] [CrossRef]
- Pawar, G.G.; Wu, H.; De, S.; Ma, D. Copper(I) oxide/n,n’-bis[(2-furyl)methyl]oxalamide-catalyzed coupling of (hetero)aryl halides and nitrogen heterocycles at low catalytic loading. Adv. Synth. Catal. 2017, 359, 1631–1636. [Google Scholar] [CrossRef]
- Gao, J.; Bhunia, S.; Wang, K.; Gan, L.; Xia, S.; Ma, D. Discovery of N-(naphthalen-1-yl)-n′-alkyl oxalamide ligands enables Cu-catalyzed aryl amination with high turnovers. Org. Lett. 2017, 19, 2809–2812. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Jiang, Y.; Zhang, L.; Guo, Y.; Ma, D. Oxalic diamides and tert-butoxide: Two types of ligands enabling practical access to alkyl aryl ethers via Cu-catalyzed coupling reaction. J. Am. Chem. Soc. 2019, 141, 3541–3549. [Google Scholar] [CrossRef] [PubMed]
- Korob, R.O.; Cohen, I.M.; Agatiello, O.E. Tungsten and molybdenum co-precipitation by α-benzoin oxime for activation analysis of tungsten: Use of molybdenum as tracer. J. Radioanal. Chem. 1976, 34, 329–333. [Google Scholar] [CrossRef]
- Gao, Z.; Siow, K.S. Adsorptive stripping voltammetric determination of traces of molybdenum in natural water in the presence of α-benzoinoxime. Mikrochimica Acta. 1996, 124, 211–218. [Google Scholar] [CrossRef]
- Fan, F.; Bai, J.; Fan, F.; Yin, X.; Wang, Y.; Tian, W.; Wu, X.; Qin, Z. Solvent extraction of uranium from aqueous solutions by α-benzoinoxime. J. Radioanal. Nucl. Ch. 2014, 300, 1039–1043. [Google Scholar] [CrossRef]
- Joshi, S.R.; Habib, S.I. Synthesis, characterization and antimicrobial evaluation of benzoinoxime transition metal complexes. J. Chem. Pharm. Res. 2014, 6, 1085–1088. [Google Scholar]
- Ribas, X.; Güell, I. Cu(I)/Cu(III) catalytic cycle involved in Ullmann-type cross-coupling reactions. Pure Appl. Chem. 2014, 86, 345–360. [Google Scholar] [CrossRef]
- Reddy, V.P.; Kumar, A.V.; Rao, K.R. New strategy for the synthesis of N-aryl pyrroles: Cu-catalyzed C-N cross-coupling reaction of trans-4-hydroxy-l-proline with aryl halides. Tetrahedron Lett. 2011, 52, 777–780. [Google Scholar] [CrossRef]
- Taillefer, M.; Xia, N.; Ouali, A. Efficient iron/copper co-catalyzed arylation of nitrogen nucleophiles. Angew. Chem. Int. Ed. 2007, 46, 934–936. [Google Scholar] [CrossRef]
- Zhou, Q.; Du, F.; Chen, Y.; Fu, Y.; Sun, W.; Wu, Y.; Chen, G. l-(−)-Quebrachitol as a ligand for selective copper(0)-catalyzed N-arylation of nitrogen-containing heterocycles. J. Org. Chem. 2018, 84, 8160–8167. [Google Scholar] [CrossRef] [PubMed]
- Young, M.B.; Barrow, J.C.; Glass, K.L.; Lundell, G.F.; Newton, C.L.; Pellicore, J.M.; Rittle, K.E.; Selnick, H.G.; Stauffer, K.J.; Vacca, J.P.; et al. Discovery and evaluation of potent P1 aryl heterocycle-based thrombin inhibitors. J. Med. Chem. 2004, 47, 2995. [Google Scholar] [CrossRef] [PubMed]
- de La, H.A.; Diaz-Ortiz, A.; Elguero, J.; Martinez, L.J.; Moreno, A.; Sanchez-Migallon, A. Solvent-free preparation of tris-pyrazolyl-1,3,5-triazines. Tetrahedron 2001, 57, 4397–4403. [Google Scholar] [CrossRef]
- Li, L.; Zhu, L.; Chen, D.; Hu, X.; Wang, R. Use of acylhydrazine- and acylhydrazone-type ligands to promote CuI-catalyzed C-N cross-coupling reactions of aryl bromides with N-heterocycles. Eur. J. Org. Chem. 2011, 2692–2696. [Google Scholar] [CrossRef]
- Cristau, H.-J.; Cellier, P.P.; Spindler, J.-F.; Taillefer, M. Mild conditions for copper-catalyzed N-arylation of pyrazoles. Eur. J. Org. Chem. 2004, 695–709. [Google Scholar] [CrossRef]
- Taywade, A.; Chavan, S.; Ulhe, A.; Berad, B. Unique CuI-pyridine based ligands catalytic systems for N-arylation of indoles and other heterocycles. Synth. Commun. 2018, 48, 1443–1453. [Google Scholar] [CrossRef]
- Jia, X.F.; Yang, D.P.; Wang, W.H.; Luo, F.; Cheng, J. Chelation-assisted palladium-catalyzed cascade bromination/cyanation reaction of 2-arylpyridine and 1-arylpyrazole C-H bonds. J. Org. Chem. 2009, 74, 9470–9474. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, J. Unique chemoselective Clauson-Kass reaction of substituted aniline catalyzed by MgI2 etherate. Tetrahedron 2011, 67, 898–903. [Google Scholar] [CrossRef]
- Zhu, L.; Guo, P.; Li, G.; Lan, J.; Xie, R.; You, J. Simple copper salt-catalyzed N-arylation of nitrogen-containing heterocycles with aryl and heteroaryl halides. J. Org. Chem. 2007, 72, 8535–8538. [Google Scholar] [CrossRef]
- Liang, L.; Li, Z.; Zhou, X. Pyridine N-oxides as ligands in Cu-catalyzed N-arylation of imidazoles in water. Org. Lett. 2009, 11, 3294–3297. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, Y.; Zhu, L.; Lan, J.; Xie, R.; You, J. A concept of supported amino acid ionic liquids and their application in metal scavenging and heterogeneous catalysis. J. Am. Chem. Soc. 2007, 129, 13879–13886. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.X.; Guo, S.M.; Zhang, M.B.; Li, J.H. N-Arylations of nitrogen-containing heterocycles with aryl and heteroaryl halides using a copper(I) oxide nanoparticle/1,10-phenanthroline catalytic system. Synthesis 2008, 1707–1716. [Google Scholar] [CrossRef]
- Pedersen, E.B.; Carlsen, D. Phosphoramides; VIII. Phosphorus pentoxide/amine mixtures as reagents in a facile synthesis of dialkylaminopyridines. Synthesis 1978, 844–845. [Google Scholar] [CrossRef]
- Prokopcova, H.; Bergman, S.D.; Aelvoet, K.; Smout, V.; Herrebout, W.; Van der Veken, B.; Meerpoel, L.; Maes, B.U.W. C-2 Arylation of piperidines through directed transition-metal-catalyzed sp3 c-h activation. Chem-Eur. J. 2010, 16, 13063–13067. [Google Scholar] [CrossRef]
- Hatakeyama, T.; Yoshimoto, Y.; Ghorai, S.K.; Nakamura, M. Transition-metal-free electrophilic amination between aryl grignard reagents and N-chloroamines. Org. Lett. 2010, 12, 1516–1519. [Google Scholar] [CrossRef]
- Narayan, S.; Seelhammer, T.; Gawley, R.E. Microwave-assisted solvent-free amination of halo-(pyridine or pyrimidine) without transition metal catalyst. Tetrahedron Lett. 2004, 45, 757–759. [Google Scholar] [CrossRef]
- Shim, S.C.; Huh, K.T.; Park, W.H. A new and facile synthesis of N-substituted pyrrolidines from amines with aqueous succinaldehyde using tetracarbonylhydridoferrate, HFe(CO)4-, as a highly selective reducing agent. Tetrahedron 1986, 42, 259–263. [Google Scholar] [CrossRef]
- Lund, H.J. Electroanal. Some reactions of electrochemically prepared anions of DMF and DMSO. Chem. 2005, 584, 174–181. [Google Scholar]
- Fattorusso, C.; Campiani, G.; Kukreja, G.; Persico, M.; Butini, S.; Romano, M.P.; Altarelli, M.; Ros, S.; Brindisi, M.; Savini, L.; et al. Design, synthesis, and structure-activity relationship studies of 4-quinolinyl- and 9-acrydinylhydrazones as potent antimalarial agents. J. Med. Chem. 2008, 51, 1333–1343. [Google Scholar] [CrossRef]
- Abdel-Aziz, H.A.; Abdel-Wahab, B.F.; El-Sharief, M.A.M.S.; Abdulla, M.M. Synthesis and anti-arrhythmic activity of some piperidine-based 1,3-thiazole, 1,3,4-thiadiazole, and 1,3-thiazolo [2,3-c]-1,2,4-triazole derivatives. Mon. Fuer Chem. 2009, 140, 431–437. [Google Scholar] [CrossRef]
- Nacario, R.; Kotakonda, S.; Fouchard, D.M.D.; Tillekeratne, L.M.V.; Hudson, R.A. Reductive monoalkylation of aromatic and aliphatic nitro compounds and the corresponding amines with nitriles. Org. Lett. 2005, 7, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C.; Snyder, S.A.; Longbottom, D.A.; Nalbandian, A.Z.; Huang, X. New uses for the burgess reagent in chemical synthesis: Methods for the facile and stereoselective formation of sulfamidates, glycosylamines, and sulfamides. Chem-Eur. J. 2004, 10, 5581–5606. [Google Scholar] [CrossRef] [PubMed]
- King, S.M.; Buchwald, S.L. Development of a method for the N-arylation of amino acid esters with aryl triflates. Org. Lett. 2016, 18, 4128–4131. [Google Scholar] [CrossRef] [PubMed]
- Azarifar, D.; Bosra, H.G.; Zolfigol, M.-A.; Tajbaksh, M. Microwave-assisted synthesis of N-arylglycines: Improvement of sydnone synthesis. Heterocycles 2006, 68, 175–181. [Google Scholar] [CrossRef]
- Ma, F.; Xie, X.; Ding, L.; Gao, J.; Zhang, Z. Palladium-catalyzed coupling reaction of amino acids (esters) with aryl bromides and chlorides. Tetrahedron 2011, 67, 9405–9410. [Google Scholar] [CrossRef]
- Xu, H.; Wolf, C. Copper catalyzed coupling of aryl chlorides, bromides and iodides with amines and amides. Chem. Commun. 2009, 1715–1717. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).