Anti-Acetylcholinesterase Activities of Mono-Herbal Extracts and Exhibited Synergistic Effects of the Phytoconstituents: A Biochemical and Computational Study
Abstract
1. Introduction
2. Results and Discussion
2.1. Determination of Acetylcholinesterase Inhibitory Activity (Screening)
2.2. IC50 Determination of the Extracts/Components and the Positive Control
2.3. HPLC Analysis of the Methanolic Extract of Tinospora cordifolia
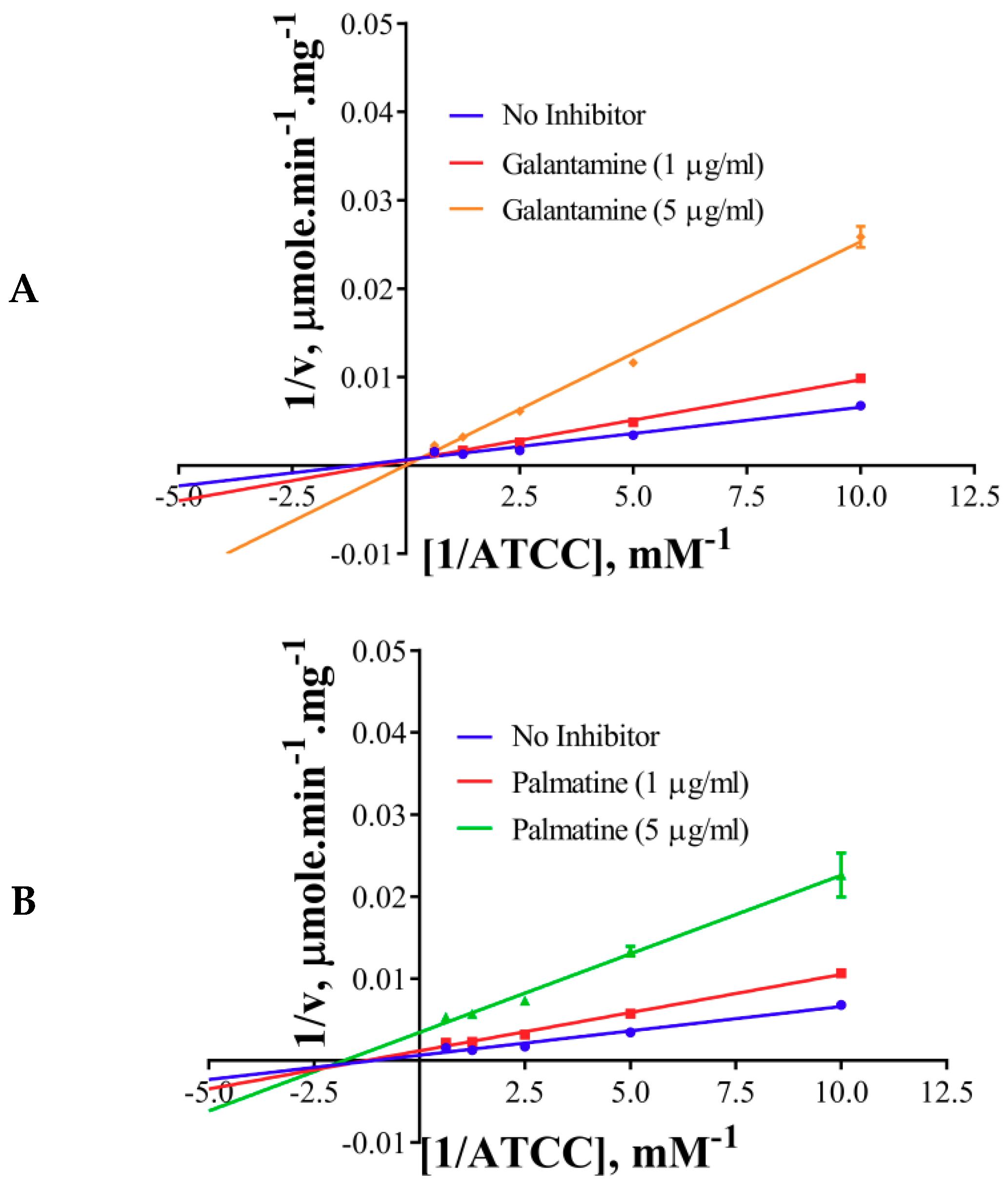
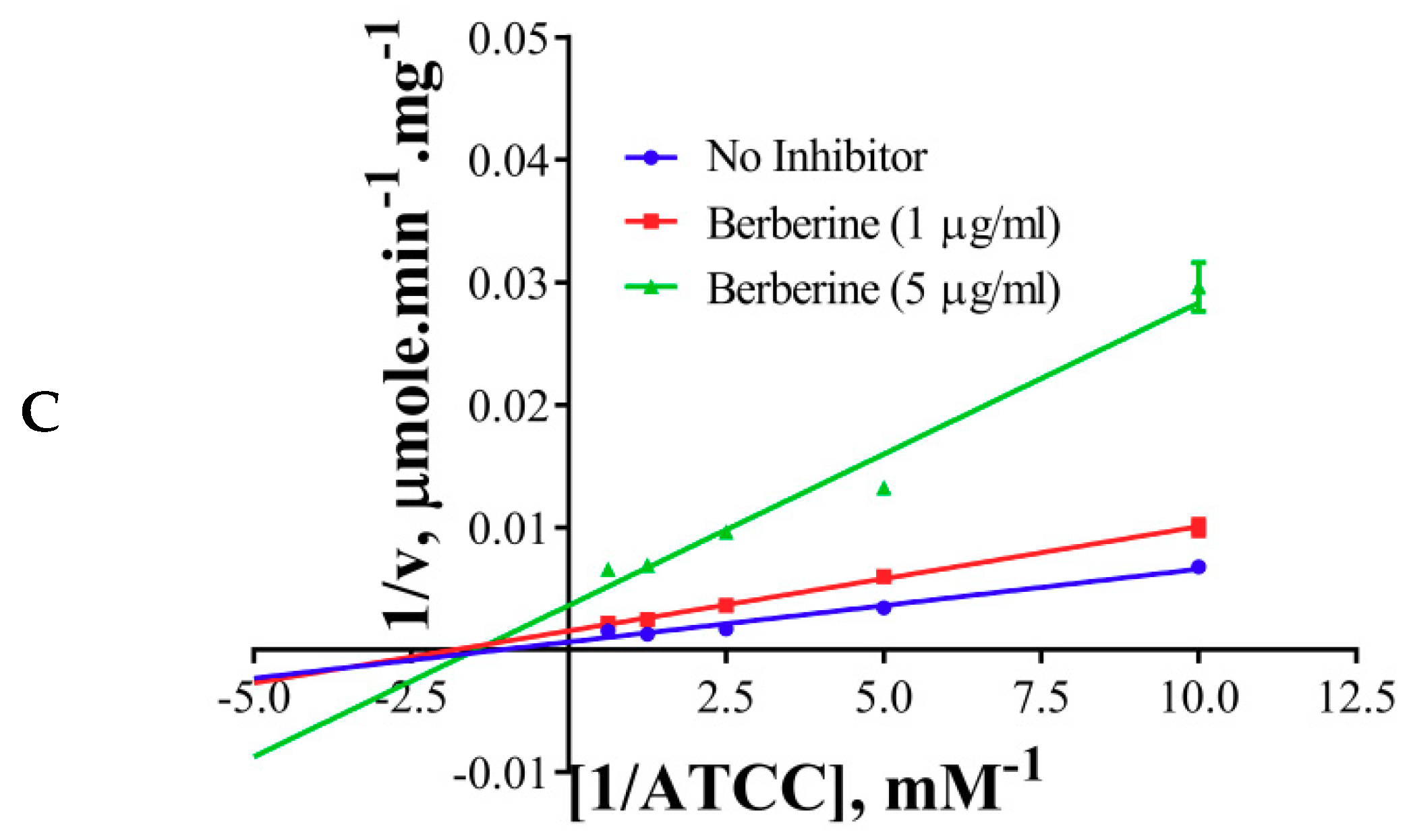
2.4. Inhibition Kinetics
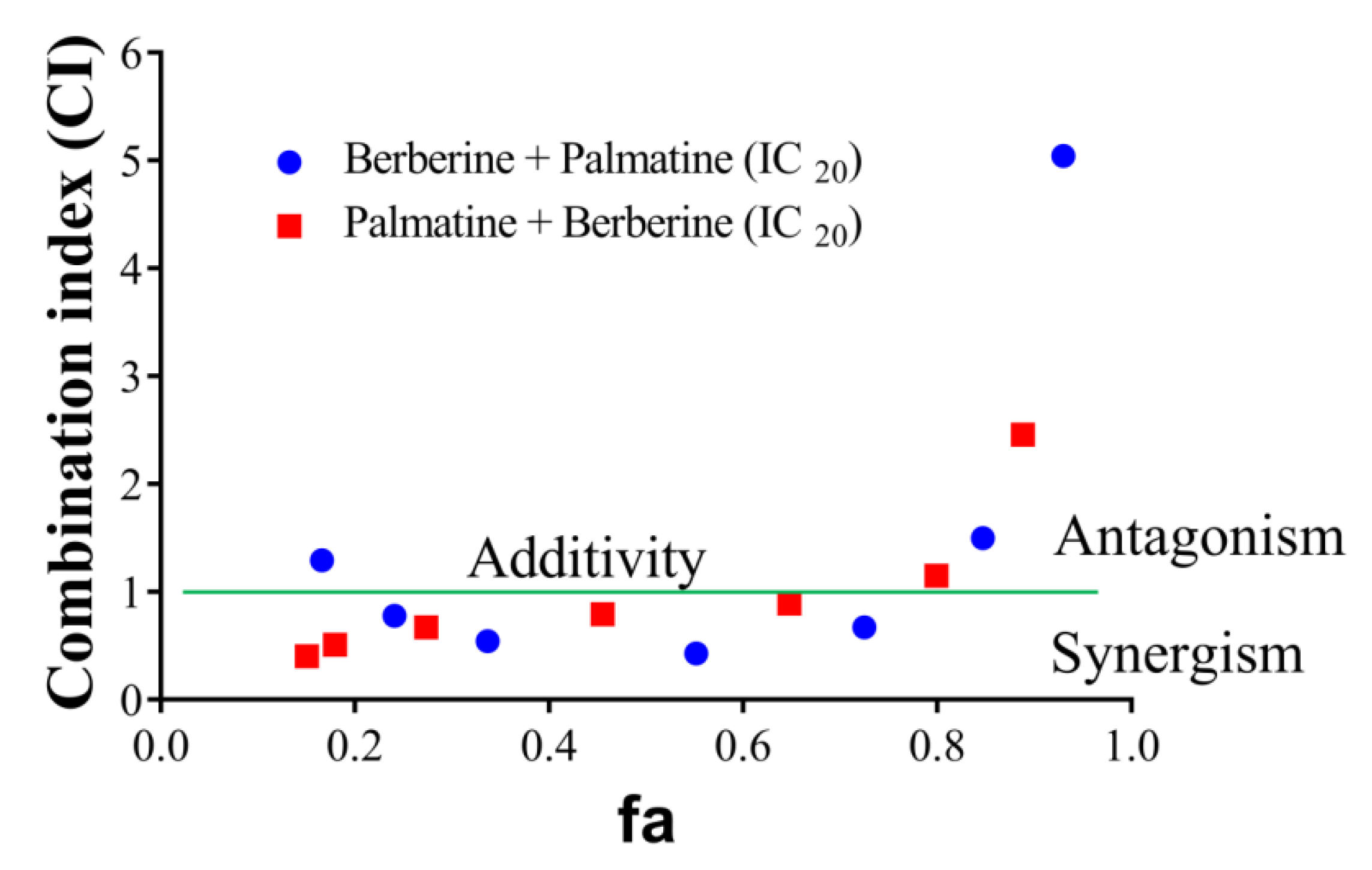
2.5. Evaluation of Synergistic Effects
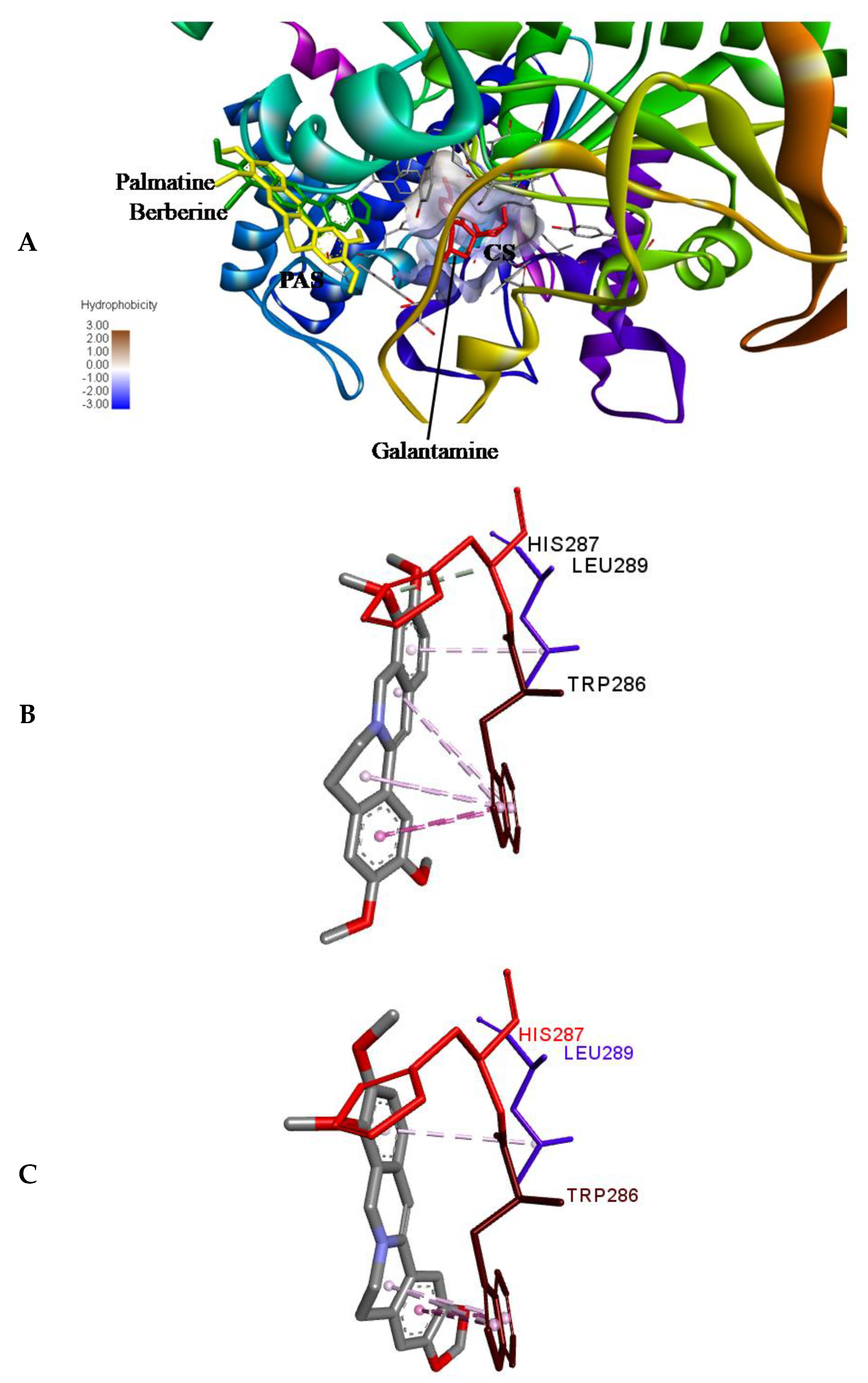
2.6. Molecular Docking
3. Materials and Methods
3.1. Plant Materials, Chemicals and Reagents
3.2. Preparation of Extracts
3.3. Determination of Acetylcholinesterase Inhibitory Activity (Screening)
3.4. IC50 Determination of the Extracts/Components and the Positive Control
3.5. HPLC Analysis of the Methanolic Extract of Tinospora cordifolia
3.6. Inhibition Kinetics
3.7. Evaluation of Synergistic Effects
3.8. Molecular Docking
3.9. Statistical Analyses and Graphics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Blennow, K.; de Leon, M.J.; Zetterberg, H. Alzheimer’s disease. Lancet 2006, 368, 387–403. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, A. A review on Alzheimer’s disease pathophysiology and its management: An update. Pharm. Rep. 2015, 67, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J. The cell biology of beta-amyloid precursor protein and presenilin in Alzheimer’s disease. Trends Cell Biol. 1998, 8, 447–453. [Google Scholar] [CrossRef]
- Davies, P.; Maloney, A.J. Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet 1976, 2, 1403. [Google Scholar] [CrossRef]
- Craig, L.A.; Hong, N.S.; McDonald, R.J. Revisiting the cholinergic hypothesis in the development of Alzheimer’s disease. Neurosci. Biobehav. Rev. 2011, 35, 1397–1409. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Chowdhury, S. Kinetics of acetylcholinesterase inhibition by an aqueous extract of Cuminumcyminum seeds. Int. J. ApplSciBiotechnol. 2014, 2, 64–68. [Google Scholar]
- Kim, H.K.; Kim, M.; Kim, S.; Kim, M.; Chung, J.H. Effects of green tea polyphenol on cognitive and acetlycholinesterase activities. Biosci. Biotechnol. Biochem. 2004, 68, 1977–1979. [Google Scholar] [CrossRef] [PubMed]
- National Institute on Aging. Alzheimer’s disease medications fact sheet. National Institute of Health. 2015. Available online: https://www.nia.nih.gov (accessed on 23 September 2019).
- Allgaier, M.; Allgaier, C. An update on drug treatment options of Alzheimer’s disease. Front. Biosci. (Landmark Ed.) 2014, 19, 1345–1354. [Google Scholar] [CrossRef]
- Schneider, L.S.; Mangialasche, F.; Andreasen, N.; Feldman, H.; Giacobini, E.; Jones, R.; Mantua, V.; Mecocci, P.; Pani, L.; Winblad, B.; et al. Clinical trials and late-stage drug development for Alzheimer’s disease: An appraisal from 1984 to 2014. J. Intern. Med. 2014, 275, 251–283. [Google Scholar] [CrossRef]
- Nordberg, A.; Svensson, A.L. Cholinesterase inhibitors in the treatment of Alzheimer’s disease. Drug Saf. 1998, 19, 465–480. [Google Scholar] [CrossRef]
- Mehta, M.; Adem, A.; Sabbagh, M. New acetylcholinesterase inhibitors for Alzheimer’s disease. Int. J. Alzheimers Dis. 2012, 7, 28983. [Google Scholar] [CrossRef] [PubMed]
- Howes, M.J.R.; Houghton, P.J. Traditional medicine for memory enhancement. In Herbal Drugs: Ethnomedicine to Modern Medicine; Ramawat, K.G., Ed.; Springer Press: Berlin/Heidelberg, Germany, 2009; pp. 239–291. [Google Scholar]
- Gupta, G.L.; Rana, A.C. Withania somnifera(Ashwagandha): A review. Pharm. Rev. 2007, 1, 129. [Google Scholar]
- Pal, A.; Bhushan, B.; Khanum, F. Therapeutic uses of Withania somnifera (Ashwagandha). In Recent Progress in Medicinal Plants (RPMP); Studium Press: New Delhi, India, 2012; Volume 34, pp. 97–118. [Google Scholar]
- Mukherjee, P.K.; Kumar, V.; Mal, M.; Houghton, P.J. Acetylcholinesterase inhibitors from plants. Phytomedicine 2007, 14, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.P.; Faraoni, M.B.; Castro, M.J.; Alza, N.P.; Cavallaro, V. Natural AChE inhibitors from plants and their contribution to Alzheimer’s disease therapy. Curr. Neuropharmacol. 2013, 11, 388–413. [Google Scholar] [CrossRef]
- Jung, M.; Park, M. Acetylcholinesterase inhibition by flavonoids from Agrimoniapilosa. Molecules 2007, 12, 2130–2139. [Google Scholar] [CrossRef]
- Hung, T.M.; Na, M.; Dat, N.T.; Ngoc, T.M.; Youn, U.; Kim, H.J.; Min, B.S.; Lee, J.; Bae, K. Cholinesterase inhibitory and anti-amnesic activity of alkaloids from Corydalisturts chaninovii. J. Ethnopharmacol. 2008, 119, 74–80. [Google Scholar] [CrossRef]
- Houghton, P.J.; Ren, Y.; Howes, M.J. Acetylcholinesterase inhibitors from plants and fungi. Nat. Prod. Rep. 2006, 23, 181–199. [Google Scholar] [CrossRef]
- Bolognesi, M.L.; Matera, R.; Minarini, A.; Rosini, M.; Melchiorre, C. Alzheimer’s disease: Newapproaches to drug discovery. Curr. Opin. Chem. Biol. 2009, 13, 303–308. [Google Scholar] [CrossRef]
- Loy, C.; Schneider, L. Galantamine for Alzheimer’s disease and mild cognitive impairment. Cochrane Database Syst. Rev. 2006. [Google Scholar] [CrossRef]
- Scott, L.J.; Goa, K.L. Galantamine: A review of its use in Alzheimer’s disease. Drugs 2000, 60, 1095–1122. [Google Scholar] [CrossRef]
- Harvey, A.L. The pharmacology of galanthamine and its analogues. Pharm. Ther. 1995, 68, 113–128. [Google Scholar] [CrossRef]
- Rao, R.V.; Descamps, O.; John, V.; Bredesen, D.E. Ayurvedic medicinal plants for Alzheimer’s disease: A review. Alzheimer’s Res. 2012, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Main, A.R.; Hastings, F.L. Carbamylation and binding constants for the inhibition of acetylcholinesterase by physostigmine (eserine). Science 1966, 154, 400–402. [Google Scholar] [CrossRef] [PubMed]
- Mathew, M.; Subramanian, S. In vitro screening for anti-cholinesterase and antioxidant activity of methanolic extracts of ayurvedic medicinal plants used for cognitive disorders. PLoS ONE 2014, 9, e86804. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, D.; Kaur Dogra, A.; Tahrani, A.; Herrmann, F.; Wink, M. Extracts from traditional Chinese medicinal plants inhibit acetylcholinesterase, a known Alzheimer’s disease target. Molecules 2016, 21, 1161. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Kumar, K.H.; Bhushan, B.; Saharan, V. Ashwagandha Root Extract Inhibits Acetylcholine Esterase, Protein Modification and Ameliorates H2O2-Induced Oxidative Stress in Rat Lymphocytes. Pharm. J. 2017, 9. [Google Scholar] [CrossRef]
- Long, J.; Song, J.; Zhong, L.; Liao, Y.; Liu, L.; Li, X. Palmatine: A Review of its Pharmacology, Toxicity and Pharmacokinetics. Biochimie 2019, 162, 176–184. [Google Scholar] [CrossRef]
- Ye, M.; Fu, S.; Pi, R.; He, F. Neuropharmacological and pharmacokinetic properties of berberine: A review of recent research. J. Pharm. Pharm. 2009, 61, 831–837. [Google Scholar] [CrossRef]
- Abd, A.E.W.; Ghareeb, D.A.; Sarhan, E.E.; Abu-Serie, M.M.; El, M.D. In vitro biological assessment of Berberis vulgaris and its active constituent, berberine: Antioxidants, anti-acetylcholinesterase, anti-diabetic and anticancer effects. BMC Complement. Altern. Med. 2013, 13, 218. [Google Scholar]
- Bonesi, M.; Loizzo, M.R.; Conforti, F.; Passalacqua, N.G.; Saab, A.; Menichini, F.; Tundis, R. Berberis aetnensis and B. libanotica: A comparative study on the chemical composition, inhibitory effect on key enzymes linked to Alzheimer’s disease and antioxidant activity. J. Pharm. Pharm. 2013, 65, 1726–1735. [Google Scholar] [CrossRef]
- Su, T.; Xie, S.; Wei, H.; Yan, J.; Huang, L.; Li, X. Synthesis and biological evaluation of berberine–thiophenyl hybrids as multi-functional agents: Inhibition of acetylcholinesterase, butyrylcholinesterase, and Aβ aggregation and antioxidant activity. Bioorganic Med. Chem. 2013, 21, 5830–5840. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.; Moore, S.W. The Peripheral Anionic Site of Acetylcholinesterase: Structure, Functions and Potential Role in Rational Drug Design. Curr. Pharm. 2006, 12, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharm. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Riddles, P.W.; Blakeley, R.L.; Zerner, B. Reassessment of Ellman’s reagent. Methods Enzym. 1983, 91, 49–60. [Google Scholar]
- Chou, T.C.; Talalay, P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enzym. Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef]
- Chou, T.C.; Martin, N. CompuSyn for Drug Combinations: PC Software and User’s Guide: A Computer Program for Quantitation of Synergism and Antagonism in Drug Combinations, and the Determination of IC50 and ED50 and LD50 Values; ComboSyn, Inc.: Paramus, NJ, USA, 2005. [Google Scholar]
- DeLano, W.L. Pymol: An open-source molecular graphics tool. Ccp4 Newsl. Protein Cryst. 2002, 40, 82–92. [Google Scholar]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An open chemical tool box. J. Cheminformatics 2011, 3, 33. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDock Tools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Dassault Systèmes BIOVIA, Release 2017; Discovery Studio Modeling Environment, Dassault Systèmes: San Diego, CA, USA, 2016.
Sample Availability: Samples used in the study are available at Patanjali Research Institute, Patanjali Research Foundation Trust, Haridwar, India. |
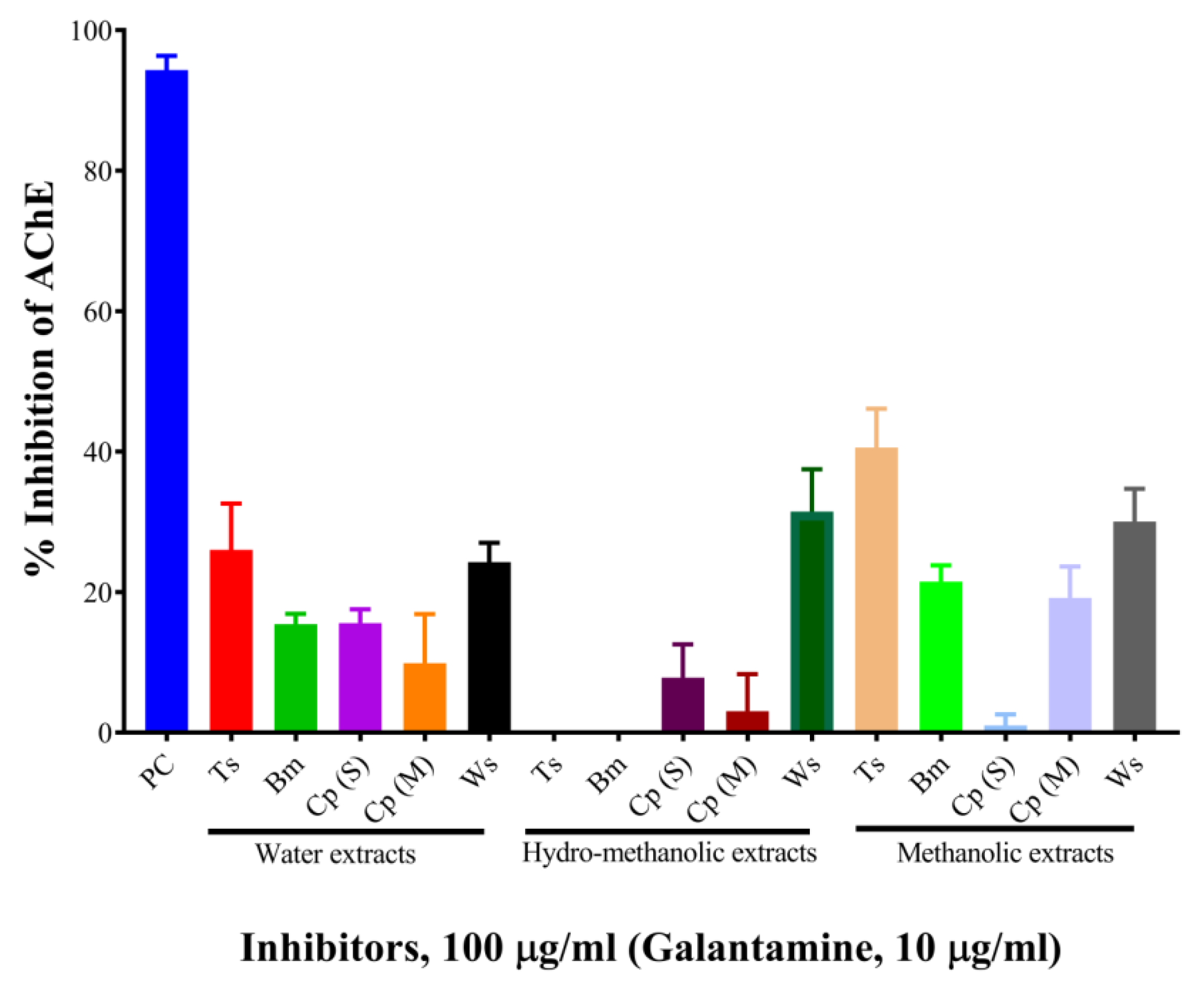
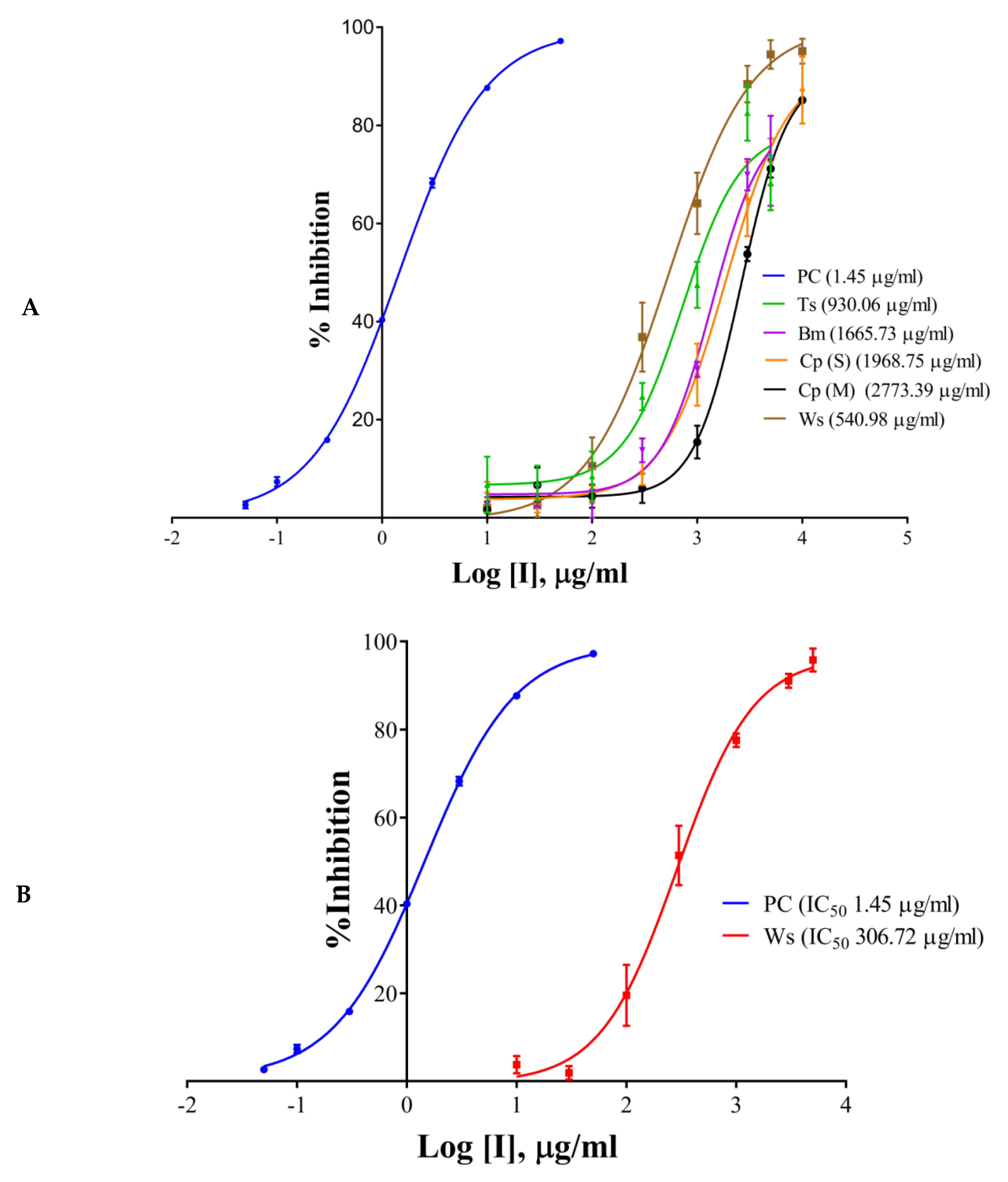
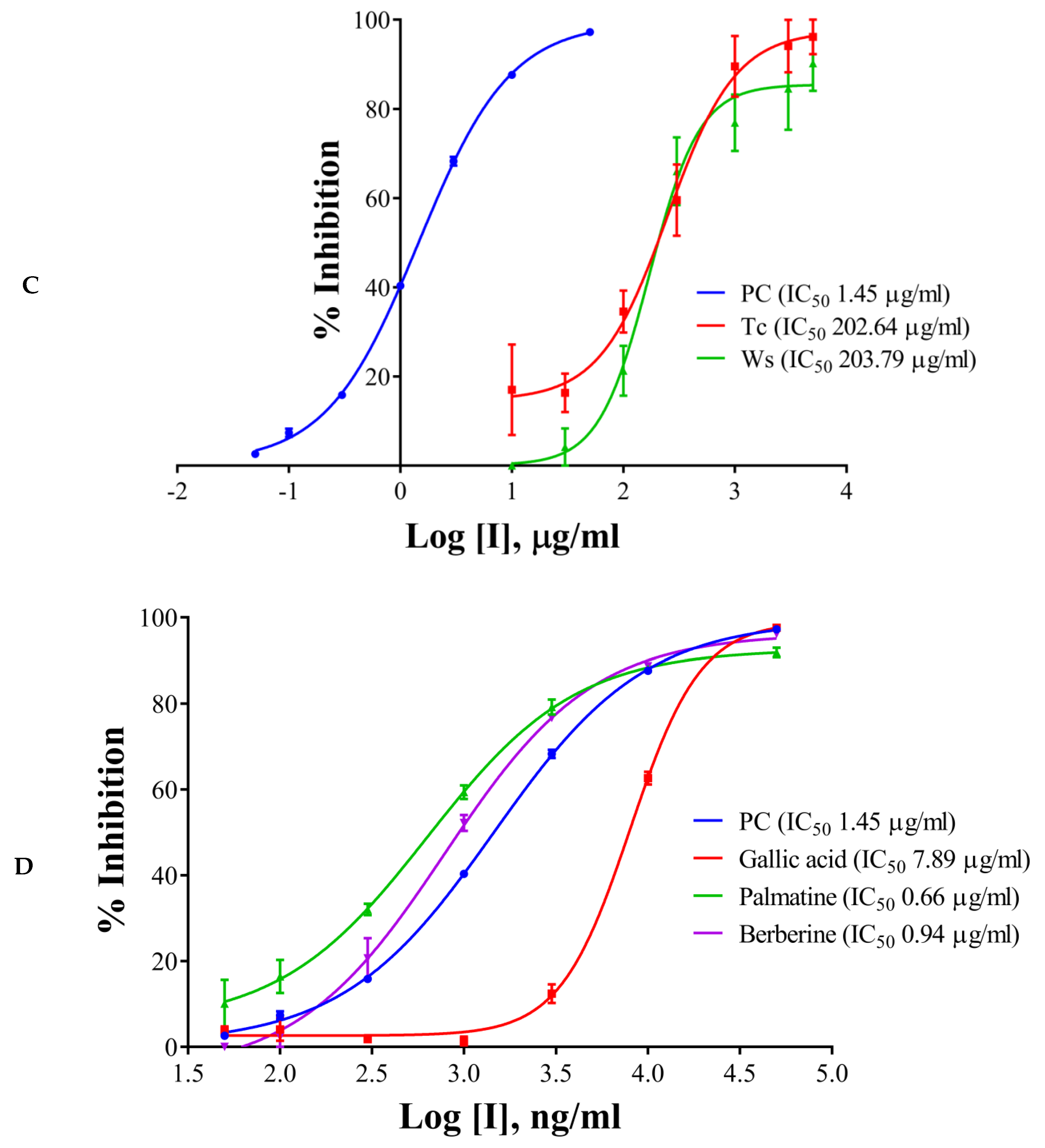

| Inhibitors | % Inhibition at 100 µg/mL | IC50, µg/mL | ||||
|---|---|---|---|---|---|---|
| A | HM | M | A | HM | M | |
| T. cordifolia (Giloy) | 26.01 | 0.00 | 40.58 | 930.06 | ND | 202.64 |
| B. monnieri (Brahmi) | 15.42 | 0.00 | 21.49 | 1665.73 | ND | ND |
| C. pluricaulis (Shankhpushpi) | 15.57 | 7.83 | 1.00 | 1968.75 | ND | ND |
| C. paniculatus (Malkagni) | 9.88 | 3.04 | 19.17 | 2773.39 | ND | ND |
| W. somnifera (Ashwagandha) | 24.26 | 31.47 | 30.03 | 540.98 | 306.72 | 203.79 |
| Galantamine hydrobromide (at 10 µg/mL) | 94.33 | - | - | 1.45 | ND | ND |
| S.N. | Compound | Quantity, mg/g |
|---|---|---|
| 1 | Gallic acid | 0.134 |
| 2 | Palmatine | 0.159 |
| 3 | Berberine | 0.022 |
| 4 | Vanillic acid | 0.494 |
| 5 | Ferulic acid | 0.205 |
| Inhibitor | Km, mM | Vmax, U/mg | Type of Inhibition |
|---|---|---|---|
| No inhibitor | 0.33 | 340.8 | NA |
| Galantamine | 1.87 | 360 | Competitive |
| Palmatine | 0.38 | 90.86 | Non-competitive |
| Berberine | 0.36 | 72.65 | Non-competitive |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balkrishna, A.; Pokhrel, S.; Tomer, M.; Verma, S.; Kumar, A.; Nain, P.; Gupta, A.; Varshney, A. Anti-Acetylcholinesterase Activities of Mono-Herbal Extracts and Exhibited Synergistic Effects of the Phytoconstituents: A Biochemical and Computational Study. Molecules 2019, 24, 4175. https://doi.org/10.3390/molecules24224175
Balkrishna A, Pokhrel S, Tomer M, Verma S, Kumar A, Nain P, Gupta A, Varshney A. Anti-Acetylcholinesterase Activities of Mono-Herbal Extracts and Exhibited Synergistic Effects of the Phytoconstituents: A Biochemical and Computational Study. Molecules. 2019; 24(22):4175. https://doi.org/10.3390/molecules24224175
Chicago/Turabian StyleBalkrishna, Acharya, Subarna Pokhrel, Meenu Tomer, Sudeep Verma, Ajay Kumar, Pradeep Nain, Abhishek Gupta, and Anurag Varshney. 2019. "Anti-Acetylcholinesterase Activities of Mono-Herbal Extracts and Exhibited Synergistic Effects of the Phytoconstituents: A Biochemical and Computational Study" Molecules 24, no. 22: 4175. https://doi.org/10.3390/molecules24224175
APA StyleBalkrishna, A., Pokhrel, S., Tomer, M., Verma, S., Kumar, A., Nain, P., Gupta, A., & Varshney, A. (2019). Anti-Acetylcholinesterase Activities of Mono-Herbal Extracts and Exhibited Synergistic Effects of the Phytoconstituents: A Biochemical and Computational Study. Molecules, 24(22), 4175. https://doi.org/10.3390/molecules24224175






