Abstract
Sterols are widely distributed in nature from lipids in organisms to sediments. As a conventional method, extraction and derivatization with TMS have been applied for sterol analysis, requiring a long preparation time for gas chromatography–mass spectrometry analysis. In this study, for sterol analysis, thermochemolysis using tetramethylammonium hydroxide (TMAH) was applied. This method performs hydrolysis and methylation simultaneously; thus, free and ether-bonding sterols can be analyzed as sterol methyl ethers in a relatively short time period. A sediment sample from a tideland (the Yatsu tideland, Japan) was analyzed using the TMAH method, and we detected more than 10 sterols, which include cholest-5-en-3β-ol (cholesterol), 24-ethylcholest-5-en-3β-ol (sitosterol), 24-methylcholesta-5,22E-3β-ol (brassicasterol), 24-ethylcholesta-5,24(28)Z-dien-3β-ol (isofucosterol), 4α,23,24-trimethyl-5α(H)-cholest-22E-en-3β- ol (dinosterol), and 5β(H)-cholestan-3β-ol (coprostanol). The detection of the various sterols can be attributed to multiple natural and artificial sources around the Yatsu tideland. In this paper, the mass spectra of these sterols are provided together with an interpretation of their fragmentation patterns. Additionally, the fecal pollution in the Yatsu tideland is discussed in the context of the detection of coprostanol.
1. Introduction
Sterols exist in the membranes of eukaryotic organisms, including microorganisms and terrestrial plants [1,2]. Thus, sterols have been detected in various natural environments, such as in lake and ocean waters and in sediments [3,4,5,6,7]. These natural sterols can be used to estimate ecological sources because sterol structures differ depending on the types of microorganisms [1,8]. Moreover, they have been used as environmental tracers, such as in degradation processes and for the reconstruction of paleoenvironments [9,10,11].
Sterols can be extracted with organic solvents, such as chloroform and methanol. A hydrolysis step is required if necessary. For sterol analysis by gas chromatography (GC)–mass spectrometry (MS), the extracted sterols must be derivatized to obtain enough volatility to pass through the GC. By derivatization, in the case of sterols, hydroxyl functional groups will be changed to more low-polarity functional groups. Typically, derivatization techniques are categorized into three types, namely, silylation, acylation, and alkylation. Although acylation is also used, silylation is most commonly used for derivatization.
Silylation is effective for various functional groups, including carboxylic acids, amides, and alcohols, and it is performed using silylation reagents, such as N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA) and N,O-bis(trimethylsilyl)acetamide (BSA). The mass spectra of sterols determined via these conventional derivatizations have been reported in earlier studies [12,13,14,15,16]; thus, the interpretation of the mass spectra of sterols has become common, which allows us to easily identify sterols. Sterol trimethylsilyl (TMS) ether spectra can be divided into two patterns: ions that are accompanied by the loss of the side chain (SC) and ions that are not accompanied by the loss of the side chain (Figure 1). The loss of the side chain has five main variations: SC, SC1, SC2, SC + C2H3, and SC + C3H5. SC, SC1, and SC occur via the cleavage of C17–C20, C20–C22, and C22–C23 bonds, respectively. SC + C2H3 represents the cleavage of the side chain followed by the cleavage of C13–C17 and C15–C16 bonds (C2H3) on the D-ring. SC + C3H5 is from the cleavage of SC and C13–C17 and C14–C15 bonds (C3H5). However, the ions that are not accompanied by the loss of the SC only have two main variations: [HO–Si(CH3)3]+ and [(CH3)3Si–O–C3H4]+. Sterols can be identified by these fragmentations because these fragmentation patterns are different for each sterol. Meanwhile, silylation is very sensitive to water, causing degradation of the derivatized functional groups. Additionally, because Si in TMS reagents can remain in the inlet of the GC, there is a possibility that the inlet of the GC will be contaminated. Moreover, sample preparation before derivatization (e.g., the extraction step) requires a relatively long time.
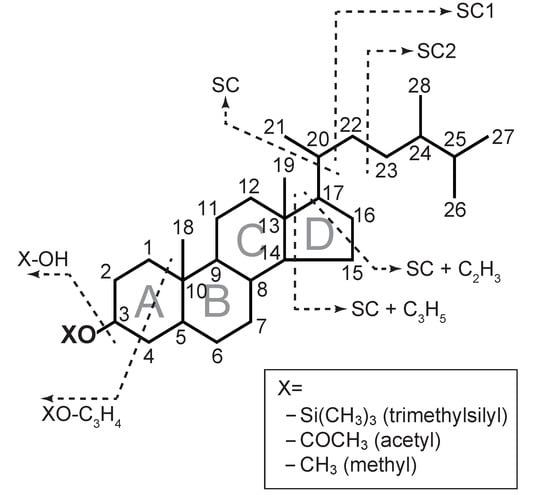
Figure 1.
Diagnostic fragmentation of the sterols. Each number represents the position of the carbons. X varied with derivatization, for example, –Si(CH3)3 in trimethylsilyl, –COCH3 in acetyl, and –CH3 in methyl ethers. The X of the original sterols is H (i.e., –OH). SC refers to the side chain.
Since it is a rapid method, thermochemolysis with tetramethylammonium hydroxide (TMAH) was applied for sterol analysis in this study. This method involves hydrolysis and methylation simultaneously, which allow the analysis of both ester and ether compounds and nonbonding compounds that have hydroxyl and carboxyl functional groups [17]. Thus, the TMAH method can provide various compounds, including lignin phenols, amino acids, sugars, and sterols, in one analysis in a relatively short time period [18,19,20,21,22,23]. Additionally, a recent study on the TMAH method extended the analysis to nucleobases [24]. Typically, thermochemolysis with TMAH includes using a pyrolyzer [25,26,27]. Several milligrams of the sample (the optimal weight is varied with the organic carbon content in the sample) was wrapped in a pyrofoil, and it was heated in a pyrolyzer that was directly connected to a GC–MS. Alternatively, thermochemolysis with TMAH can be carried out in a glass ampule [28]. Using this approach, thermochemolysis is performed on an ampule that contains a sample and the TMAH reagent that is sealed under vacuum in an oven.
Upon the TMAH reaction, sterols are provided as sterol methyl ethers. The application of the TMAH method for sterols can produce different types of sterols, including cholesterol, sitosterol, brassicasterol, and stanols [5,6,29]. The efficiency of methylation in sterols reaches >90% [29], indicating that sterols are able to be stably analyzed. Using the TMHA method, both free and bonding (e.g., cholesteryl ester) sterols can be analyzed (Figure 2). However, there are few reports on the interpretation of mass spectra of sterol methyl ethers, which could help detect them. Therefore, in this study, we provide the mass spectra of sterol ethers obtained using the TMAH method from a sediment sample and also provide an interpretation of these mass spectral patterns to show how to detect them.
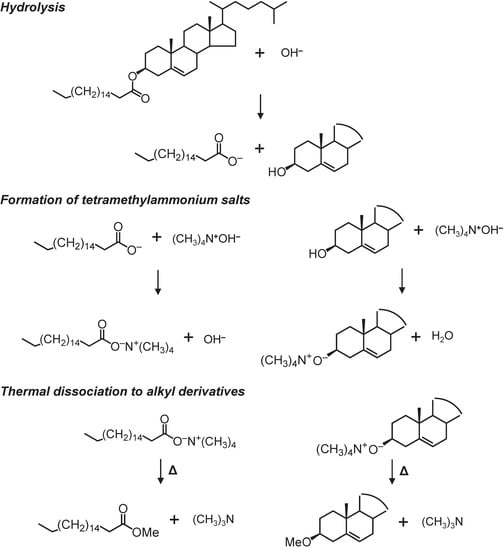
Figure 2.
Generation processes for sterol methyl ether from cholesteryl stearate via the tetramethylammonium hydroxide (TMAH) reaction. This figure was drawn based on Asperger et al. [29] and Challinor [17].
2. Materials and Methods
2.1. A Tideland Surface Sediment Sample
In this study, the Yatsu tideland, Japan, was selected as the research location (Figure 3). The Yatsu tideland is located in the innermost location of Tokyo Bay, and is surrounded by industrial and residential areas that have expanded through reclamation works. In 1993, for the conservation of ecosystems and landscapes in the natural environment, the Yatsu tideland was registered in the Ramsar Convention. Various organisms, such as lugworms, gobies, clams, sea lettuces, and migratory birds inhabit the Yatsu Tideland [30,31,32,33], although the area is small (40 hectares). Therefore, various organic compound inputs are expected. A sediment sample was taken from the edge of the Yatsu tideland using an Ekman–Birge grab (Figure 3). The sediment sample was freeze-dried immediately after sampling and then powdered well. The powdered sample was stored in a freezer at −20 °C until the sterols were analyzed.

Figure 3.
Pictures of the sampling point (Yatsu Tideland, Japan). (a) Picture of the sample. (b) State of the sampling place. The sample was taken from a bridge, as shown in the picture, using an Ekman–Birge grab.
2.2. Thermochemolysis with TMAH
Several milligrams of the sample were added into a 20-mL glass ampule with 150 µL (25% in methanol) of TMAH reagent (Sigma–Aldrich Co., St. Louis, MO, USA) and with nonadecanoic-d37 acid (99.1%, CDN isotopes Co., Pointe-Claire, QC, Canada) as an internal standard. The glass ampule was placed into a desiccator to dry the solvents for approximately 15 min. To remove the solvents more sufficiently, a 40 °C hot plate with a nitrogen stream was applied for approximately 10 min. After the solvents were dried, the glass ampule was sealed under vacuum conditions and was heated in an oven at 300 °C for 30 min. After the ampule was cooled to room temperature, the reacted products were extracted with ethyl acetate thrice. The solvent was removed once, and the extracted products were re-resolved with ethyl acetate to an accurate volume of 100 µL. Several microliters within the re-resolved products were injected into the GC–MS system.
2.3. GC–MS Conditions
An Agilent GC–MS (GC, 6890N; MS, 5973 inert) was used for this study. A fused silica capillary GC column for the DB-5 ms (inner diameter, 0.25 mm; column length, 30 m; film thickness, 0.25 µm) was installed on the GC system with helium gas (purity: >99.9 vol.%) flowing as the carrier gas. The spitless injection mode was employed. The injection room temperature was set to 310 °C. The setting for the oven was as follows: 60 °C (20 min), 310 °C (6 °C/min), 310 °C (20 min). The mass ion source and quadrupole were programmed at 230 °C and 150 °C, respectively. MS data were recorded using the full scanning mode from 50 to 650 Da (Dalton). Ionization was achieved using the electron impact mode at 70 eV.
2.4. Identification of Sterols
The mass spectrum of the sterols obtained using the TMAH method was interpreted by comparing it with reported mass spectra from previous studies on methyl ether derivatization [34] and TMS derivatization [12,13,14,15]. The National Institute of Standards and Technology (NIST)/ United States Environmental Protection Agency (EPA)/ National Institutes of Health (NIH) Mass Spectral Library (NIST 05) was also employed for the interpretation of the sterols. The epicoprostanol-to-coprostanol and coprostanol-to-cholesterol ratios were calculated from their concentrations, which were obtained by comparison with the internal standard.
3. Results and Discussion
3.1. Identified Sterols in the Samples
From the Yatsu sediment, seven types of sterols from three Δ5-sterols, four 5α(H)-stanols, two 5β(H)-stanols, one Δ5,22-sterol, two Δ22-sterols, two Δ5,24(28)-sterols, and one 4α-Me-Δ22-sterol were determined (Table 1 and Figure 4). These multiple differences indicate that there are various input sources present for the Yatsu tideland, which can result from the rich ecosystem and human-living area being adjacent to each other.

Table 1.
Sterols identified from the Yatsu surface sediment.
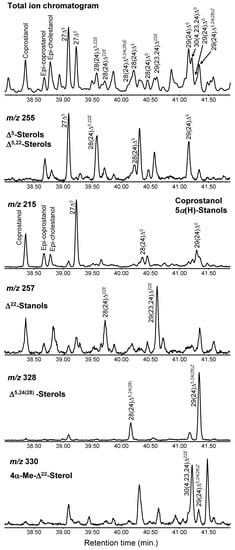
Figure 4.
Partial total ion chromatogram and extracted ion chromatograms (m/z 255, 215, 257, 328, and 330) focused on sterols (38.00–41.88 min) via thermochemolysis using tetramethylammonium hydroxide (TMAH). The symbols are listed in Table 1.
3.2. Δ5-Sterols
The compounds of cholest-5-en-3β-ol (cholesterol), 24-methyl-cholest-5-en-3β-ol (campesterol), and 24-ethylcholest-5-en-3β-ol (sitosterol) were detected from the tidal sediment, and they were grouped as Δ5-sterols (Figure 4 and Figure 5). C27 and C28 sterols, including cholesterol and campesterol, are widely present in plankton [1,8]. However, C29 sterols, such as sitosterol, are present in terrestrial plants [35,36]. Therefore, the distributions of C27, C28, and C29 sterols have been used for the estimation of sources in sedimentary sterols [8]. However, the results must be interpreted carefully, because C29 sterols are also found in phytoplankton [1].
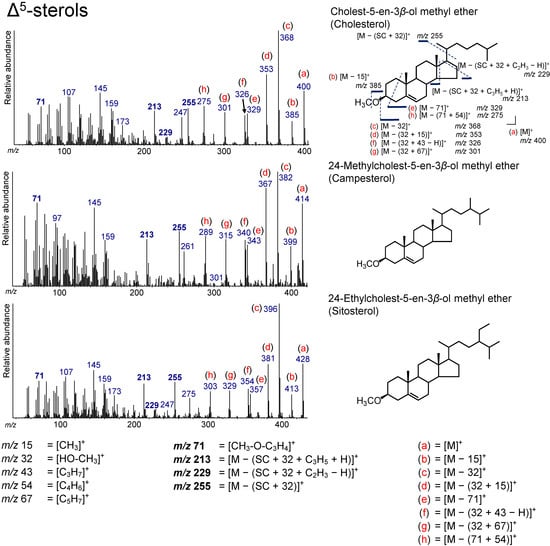
Figure 5.
Electron ionization (70 eV) mass spectrum of Δ5-sterol. Bold numbers indicate an ion that accompanies the cleavage of the side chain. (a)–(h) Ions that are not accompanied by the loss of the side chain or other groups.
A peak at m/z 71, a characteristic ion for Δ5-sterol, was observed for all Δ5-sterols, representing [CH3–O–C3H4]+. Peaks at m/z 255, 229, and 213 were due to an ion accompanying the loss of the SC (Figure 5). These peaks (m/z 71, 255, 229, and 213) did not change with the type of derivatization because these peaks appear together with the loss of a derivatized functional group at the C3 position. Therefore, these peaks have also been found in Δ5-sterol TMS derivatives [12,13,14]. The peak at m/z 255 is derived from the losses of the side chain and HO–CH3 from the Δ5-sterols ([M − (SC + 32)]+). In addition, the peak at m/z 229 results from the loss of C2H3 (without a hydrogen atom) from the D-ring ([M − (SC + 32 + C2H3 − H)]+). The peak at m/z 213 is from the loss of m/z 255 from the molecular ion of Δ5-sterols followed by the loss of C3H5 (with a hydrogen atom) on the D-ring ([M − (SC + 32 + C3H5 + H)]+). The peaks at m/z 255 and m/z 213 are clear in all the Δ5-sterols detected, although the peak at m/z 229 is weak for campesterol.
[M]+, [M − 15]+, [M − 32]+, [M − (32 + 15)]+, [M − 71]+, [M − (32 + 67)]+, [M − (71 + 54)]+, and [M − (71 + 82)]+ are all ions that were not accompanied by the loss of the SC (Figure 5). These ions were also confirmed from the fragmentation of sterol TMS derivatives, although their intensities in methyl ethers are relatively high than in TMS ethers. [M − 15]+ is produced by the loss of a methyl group from sterols. [M − 32]+ represents the loss of HO–CH3. The additional loss of a methyl group and C5H7 from [M − 32]+ yields [M − (32 + 15)]+ and [M − (32 + 67)]+, respectively. [M – 71]+ arises from the loss of CH3–O–C3H4. Additionally, [M − (71 + 54)]+ and [M − (71 + 82)]+ are generated by an additional loss of C4H6 on the A-ring or C6H10 on the A- and B-rings, respectively. [M − (32 + 43 − H)]+ can result from the loss of HO–CH3 from Δ5-sterols, followed by the loss of C3H7 (without a hydrogen atom) at the end of the SC (Figure 5).
3.3. 5α(H)-Stanols
Four 5α(H)-stanols, 5α(H)-cholestan-3α-ol (epi-cholestanol), 5α(H)-cholestan-3β-ol (cholestanol), 24-methyl-5α(H)-cholestan-3β-ol (campestanol), and 24-ethyl-5α(H)-cholestan-3β-ol (sitostanol) were determined (Figure 4 and Figure 6). Typically, 5α(H)-stanols are produced under anoxic conditions via bacterial conversion from sterols [7,37,38]. Thus, the ratio of 5α(H)-stanol/Δ5-sterol can be applied as a tracer for redox conditions in the field of organic geochemistry [5,6,39]. However, the 5α(H)-stanol/Δ5-sterol ratio could be large due to the preferential degradation of sterols in terrestrial organic matters, even under oxic conditions [35].
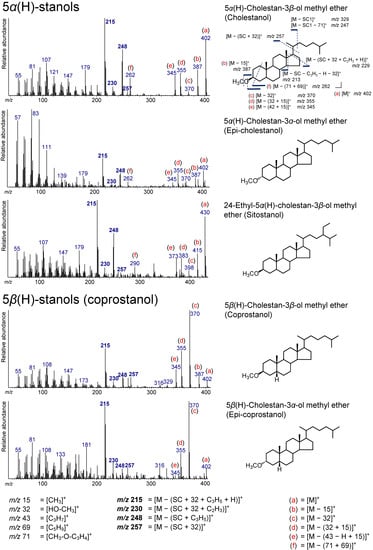
Figure 6.
Electron ionization mass spectra of 5α(H)-stanol and 5β(H)-stanol (coprostanol). Bold numbers indicate an ion that accompanies the cleavage of the side chain. (a)–(f) Ions that not accompanied by the loss of the side chain or other groups.
The peaks at m/z 257, 248, 230, and 215 for 5α(H)-stanols are due to an ion accompanying the loss of the SC in 5α(H)-stanols (Figure 6). The peak at m/z 248 is derived from the losses from the side chain and the D-ring from 5α(H)-stanols ([M – (SC + C3H5)]+). The ion at m/z 257 represents the losses of HO–CH3 and the side chain ([M – (SC + 32)]+), and the ion at m/z 230 is produced by the additional loss of the D-ring from the ion at m/z 257 ([M – (SC + 32 + C2H3)]+). The peak at m/z 215 is a base peak in 5α(H)-stanols and is due to the further loss of the D-ring (with a hydrogen atom) from the m/z 257 ion ([M – (SC + 32 + C3H5 + H)]+). As an ion that is not accompanied by the loss of the SC, for example, in cholestanol, peaks at m/z 402 ([M]+), 387 ([M − 15]+), 370 ([M − 32]+), and 355 ([M − (32 + 15)]+) were confirmed (Figure 6). The peaks appearing at m/z 345 and 262 in the spectrum of cholestanol were not found in the spectra of the TMS ethers (Figure 6). The peak at m/z 345 may originate from the loss of C3H7 from the SC and a methyl group ([M – (42 + 15)]+). Additionally, the peak at m/z 262 can be from the loss of CH3–O–C3H4 followed by the loss of C5H9 at the A- and B-rings ([M − (71 + 69)]+). Although campestanol was also confirmed, the spectrum is not shown in Figure 6, since it was ambiguous.
3.4. 5β(H)-Stanols (Coprostanol and Epicoprostanol)
From the sediment sample, 5β(H)-cholestan-3β-ol (coprostanol) and 5β(H)-cholestan-3α-ol (epicoprostanol) were determined (Figure 4 and Figure 6). Coprostanol can be produced through the reduction of cholesterol by bacteria within the gut of higher animals including humans [40,41], which is ubiquitously present in their fecal matter [42,43]. Therefore, coprostanol has been used as an indicator of fecal pollution and has been widely applied to environments [30,44,45,46,47,48,49].
The mass fragments that appeared for coprostanol and epicoprostanol were similar to those of the 5α(H)-stanols, implying that the same fragmentation as in 5α(H)-stanols occurs in 5β(H)-stanols (Figure 6). However, these intensities are considerably different from those of 5α(H)-stanols. As with 5α(H)-stanols, the peaks at m/z 257, 248, 230, and 215 were confirmed from coprostanol and epicoprostanol as an ion accompanying the cleavage of the SC (Figure 6). An intense peak at m/z 248 represents C3H5 in the D-ring according to the molecular weight followed by the loss of the side chain ([M – (SC + C3H5)]+). The peak at m/z 257 is derived from the loss of HO–CH3 and the side chain ([M – (32 + SC)]+), and the peak at m/z 230 is from an additional loss of C2H3 in the D-ring from the m/z 257 ion ([M – (SC + 32 + C2H3)]+). The peak at m/z 215 represents the loss of C3H5 (with molecular hydrogen) in the D-ring from the m/z 257 ion ([M − (SC + 32 + C3H5 + H)]+), which is a characteristic ion for 5α(H)- and 5β(H)-stanols [50,51,52]. Additionally, in the spectrum of coprostanol and epicoprostanol, the peaks at m/z 402 ([M]+), 387 ([M − 15]+), 370 ([M − 32]+), and 355 ([M − (32 + 15)]+) were confirmed to be due to ions that are not accompanied by the loss of the SC (Figure 6).
The detection of coprostanol from sediment suggests that the Yatsu tideland is affected by some fecal pollution. This might be because industrial and residential areas were expanded by reclamation works, and they have enhanced the environmental load on the Yatsu tideland. A biplot of the coprostanol/cholesterol ratio with the epicoprostanol/coprostanol ratio in the sediment is shown in Figure 7. Epicoprostanol is typically converted from coprostanol via a bacterial reaction during sewage treatment processes, and therefore is observed from treated or old sewage samples [53]. Thus, a high epicoprostanol/coprostanol ratio is observed from “old or treated” sewage samples [54]. In our Yatsu tideland sediment sample, the epicoprostanol/coprostanol ratio shows approximately 0.4, indicating that the source is a mixture of new and old. The ratio of coprostanol/cholesterol can estimate the degree of fecal contamination [55,56,57]. According to a proposal by Grimalt and Albaigés [56], when the coprostanol/cholesterol ratio is greater than 0.2 in an environmental sample, the environment can be considered to have fecal contamination. The coprostanol/cholesterol ratio in our sediment sample was approximately 0.5 (Figure 7), which is over the value of the definition. Therefore, the Yatsu tideland is an environment that is affected by fecal pollution according to this definition. However, the value is not higher as with large contaminated areas (Figure 7). Additionally, our data is limited to one site. Thus, a more accurate investigation of the polluted area of the Yatsu tideland requires that different sample areas be analyzed.
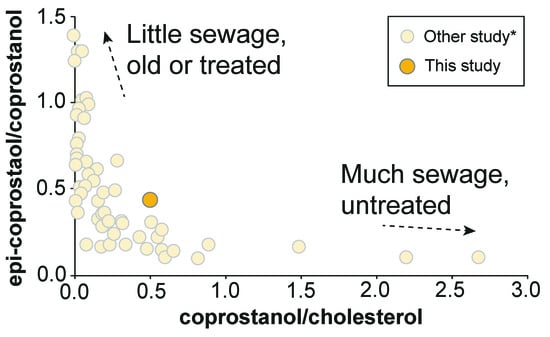
Figure 7.
A biplot of the coprostanol/cholesterol ratio with the epicoprostanol/coprostanol ratio for the Yatsu sediment. Our data is plotted on a redrawn figure from Mudge and Seguel [54] and Mudge and Ball [55].
Of note, coprostanol analysis via the TMAH method has methodological advantages because the process can be performed quickly with a small amount of solvent. These advantages can result in the treatment of a large number of samples and the reduction of solvents during analysis.
3.5. Δ5,22-Sterol (Brassicasterol)
A Δ5,22-sterol, 24-methylcholesta-5,22E-dien-3β-ol (brassicasterol), was determined from the sediment (Figure 4 and Figure 8). Brassicasterol is present in diatoms as a major sterol [58,59,60]. In particular, pennate diatoms have a high relative abundance of brassicasterol (>60% of sterols) [61]. However, this sterol cannot be a specific biomarker for diatoms because the sterol is present in other algal groups [61].
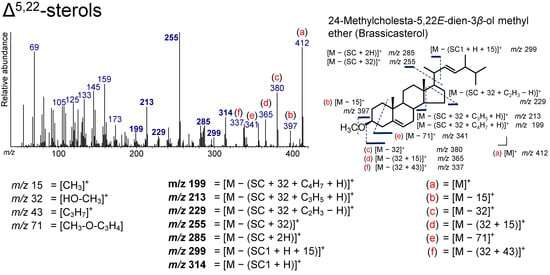
Figure 8.
Electron ionization mass spectrum of 24-methylcholesta-5,22E-dien-3β-ol methyl ether (brassicasterol). Bold numbers indicate an ion accompanying the cleavage of the side chain. (a)–(f) Ions that are not accompanied by the loss of the side chain or other groups.
The peaks at m/z 314 ([M − (SC1 + H)]+), 299 ([M − (SC1 + H + 15)]+), 285 ([M − (SC + 2H)]+), 255 ([M − (SC + 32)]+), 229 ([M − (SC + 32 + C2H3 − H)]+), 213 ([M − (SC + 32 + C3H5 + H)]+,), and 199 ([M − (SC + 32 + C4H7 + H)]+) in the brassicasterol are due to ions that accompany the cleavage of the SC (Figure 8). These ions are present in the spectra of Δ5-sterols and Δ22-sterols as well. Additionally, the peaks at m/z 255 and 213 were also found in the TMS derivative, because these ions do not include a derivatized functional group [12,62]. However, peaks at m/z 412 ([M]+), 397 ([M − 15]+), 380 ([M − 32]+), 365 ([M − (32 + 15)]+), 341 ([M − 71]+), and 337 ([M − (32 + 43)]+) were found to result not by the loss of the SC, and they are also observed for Δ5-sterols and Δ22-sterols (Figure 8). The peak at m/z 125 represents the molecular weight of its SC. These ions are important for the determination of Δ5,22-sterols.
3.6. Δ22-Stanols
Two Δ22-stanols, 24-methyl-5α(H)-cholest-22E-en-3β-ol (brassicastanol) and 23,24-dimethyl-5α(H)-cholest-22E-en-3β-ol, were determined from the sediment (Figure 4 and Figure 9). As with 5α(H)-sterols, Δ22-stanols can be produced from the bacterial conversion from Δ5,22-sterols. Indeed, a sterol analysis of particle matter at the oxic–anoxic boundary in the Black Sea implied that the brassicastanol/brassicasterol ratio increases at the anoxic water layer [7]. Additionally, a continuous record of the brassicastanol/brassicasterol ratio from California late Quaternary sediments has shown historical changes in redox conditions over 40 kyr [5].
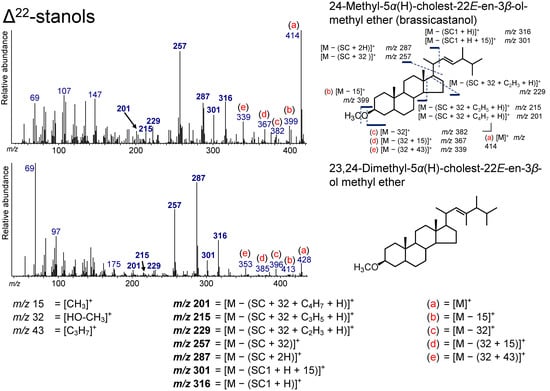
Figure 9.
Electron ionization mass spectrum of Δ22-stanol. Bold numbers indicate an ion that accompanies the cleavage of the side chain. (a)–(e) Ions that are not accompanied by the loss of the side chain or other groups.
The peaks at m/z 316, 301, 287, 257, 229, 215, and 201 are due to ions that accompany the cleavage of the SC (Figure 9). The peak at m/z 316 results from the loss of the side chain (SC1) with a hydrogen atom from Δ22-stanols ([M – (SC1 + H)]+), and the additional loss of a methyl group yields the ion at m/z 301 ([M – (SC1 + H + 15]+). The peak at m/z 287 originates from the loss of the side chain (with two hydrogen atoms) from Δ22-stanols ([M – (SC + 2H)]+). An intense peak at m/z 257 is derived from the loss of HO–CH3 and the side chain from Δ22-stanols ([M – (32 + SC)]+). This ion, m/z 257, is a characteristic ion that is used for the determination of Δ22-sterols, as in the case of TMS derivatization [51,63,64], and it shows that the sterol skeleton is saturated (no double bond on the C5–C6 position) as with 5α(H)-stanols [51]. The peaks at m/z 229, 215, and 201 can arise from the loss of C2H3 (with a hydrogen atom) at the D-ring ([M – (32 + SC + C2H3 + H)]+), the loss of C3H5 (with a hydrogen atom) at the D-ring ([M – (32 + SC + C3H5 + H)]+), and C4H7 (with a hydrogen atom) at the D- and C-rings from the ion at m/z 257 ([M – (32 + SC + C4H7 + H)]+). Ions [M]+, [M − 15]+, [M − 32]+, [M − (32 + 15)]+, and [M − (32 + 43)]+ were confirmed to be not accompanied by the loss of the SC (Figure 9). [M − (32 + 43)]+ can arise from the loss of HO–CH3 from Δ22-stanols, which is followed by the loss of C3H7 on the end of the SC.
3.7. Δ5,24(28)-Sterols
Two Δ5,24(28)-sterols, 24-methylcholesta-5,24(28)-dien-3β-ol (24-methylenecholesterol) and 24-ethyl-cholesta-5,24(28)Z-dien-3β-ol (isofucosterol), were determined from the sediment (Figure 3 and Figure 9). 24-methylenecholesterol is present in some microalgae, such as diatoms [1], which are found in various aquatic environments including in the Arctic and Antarctic oceans [65,66,67]. Isofucosterol has been observed in green microalgae as a major sterol [1].
The spectra of both Δ5,24(28)-sterols show peaks at m/z 328, 313, 296, 285, 281, 255, 243, 229, and 213 (Figure 10). Of these ions, the peaks at m/z 328, 313, 285, and 243 were also confirmed in the spectra of the Δ24(28)-sterols. The ion at m/z 328 arises from the loss of the side chain (SC2) with a hydrogen atom and is also a characteristic ion for Δ5,24(28)-sterols. The peaks at m/z 255, 253, 229, and 213 are also visible in the fragmentation pattern of other sterols, such as Δ24(28)-sterols and Δ5-sterols. The peak at m/z 296 results from the losses of the side chain (SC1) and HO–CH3 from Δ5,24(28)-sterols ([M − (SC1 + 32)]+). The additional elimination of a methyl group yields m/z 281 ([M − (SC1 + 32 + 15)]+). Ions [M]+, [M − 15]+, [M − 32]+, and [M − (32 + 15)]+ were confirmed to be ions that are not accompanied by the loss of the SC, which are also present for other sterols, including Δ5-sterols (Figure 10).
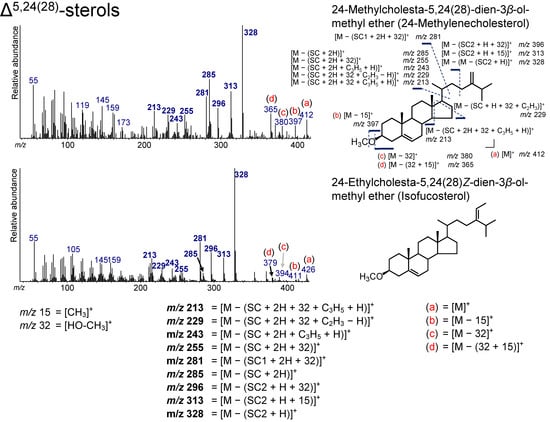
Figure 10.
Electron ionization mass spectrum of Δ5,24(28)-sterol. Bold numbers indicate an ion that accompanies the cleavage of the side chain. (a)–(d) Ions that are not accompanied by the loss of the side chain.
3.8. 4α-Me-Δ22 Sterol
A 4α-Me-Δ22 sterol, 4α,23,24-trimethyl-5α(H)-cholest-22E-en-3β-ol (dinosterol), was identified from the sediment (Figure 4 and Figure 11). Sterols having a methyl functional group at the C4 position, including dinosterol, are abundant in dinoflagellates [68,69,70]. Therefore, dinosterol has been used as a unique biomarker for dinoflagellates [71,72].
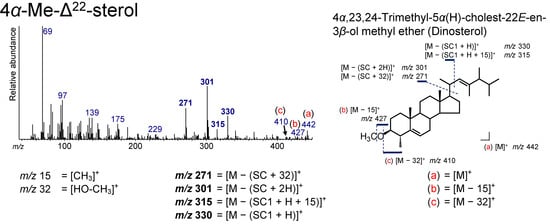
Figure 11.
Electron ionization mass spectrum of 4α,23,24-trimethyl-5α(H)- cholest-22E-en-3β-ol methyl ether (dinosterol). Bold numbers indicate an ion that accompanies the cleavage of the side chain. (a)–(c) Ions that are not accompanied by the loss of the side chain.
In the spectrum of dinosterol, [M]+, [M − 15]+, [M − 32]+, m/z 330, 315, 301, 271, 245, and 229 were confirmed, which can be interpreted by the 14 Da (CH3) higher shift of the corresponding fragmentation patterns in the spectrum of 23,24-dimethyl-5α(H)-cholest-22E-en-3β-ol methyl ether (Figure 11).
3.9. Identification of Sterols from the Extracted Ion Chromatograms
Determining each sterol from the GC–MS results can be effectively achieved from the extracted ion chromatograms of each sterol. The ion at m/z 255 is useful to determine Δ5-, Δ5,22-, and Δ5,24(28)-sterols. Ion chromatograms with m/z 215, 257, 328, and 330 represent 5α- and 5β-stanols, Δ22-stanols, Δ5,24(28)-sterols, and 4α-Me-Δ22-sterols, respectively (Figure 4).
The retention time in the GC–MS results is also important for determining sterols. For example, coprostanol and cholestanol, isomers of the H atom at the C5 position (i.e., 5β(H) or 5α(H)), elute at substantially different times even though the structures are similar (Figure 4). The retention time of coprostanol is earlier than that of cholestanol. Additionally, 5α(H)-stanols and Δ22-stanols were obtained immediately after their corresponding Δ5-sterols and Δ5,22-stanols, which is useful for the determination of these compounds (Figure 4). Additionally, the number of double bonds and their positions (C22 and/or C5 positions) appear at different retention times; for example, retention times become longer in the order of 28(24)Δ5,22E (brassicasterol), 28(24)Δ22E (brassicastanol), and 28(24)Δ5 (campesterol) (Figure 4).
4. Conclusions
A tideland surface sediment from the Yatsu tideland, Japan, was analyzed using thermochemolysis with tetramethylammonium hydroxide (TMAH) for sterols. From the sediment, 10 sterols, including Δ5-sterols, 5α(H)-sterols, Δ5,22-sterols, Δ22-sterols, Δ5,24(28)-sterols, 4α-Me-Δ22-sterols, and coprostanol, were determined. The Yatsu tideland sediment sample enabled the determination of these various sterols, because the Yatsu tideland is located where rich ecosystems and human-living areas are adjacent to each other. The MS results of these sterols helped determine them. Characteristic mass fragments at m/z 255, m/z 215, m/z 257, and m/z 330 were useful to detect Δ5-type sterols (i.e., Δ5-sterols, Δ5,22-sterols, and Δ5,24(28)-sterols), 5α(H)-sterols, 5β(H)-sterols, Δ22-sterols, and 4α-Me-Δ22-sterols from the extracted mass chromatograms. Additionally, some fecal pollution in the Yatsu tideland was suggested by the detection of coprostanol. The TMAH method can treat samples in a relatively short time with a small amount of sample and then provide sterol methyl ethers. Thus, this method is suitable for studies that analyze many samples. The provided sterol spectra in this study, including other such studies, will help determine the kinds of sterols present in a sample using the GC–MS result.
Author Contributions
Conceptualization, M.N. and S.Y.; Investigation, M.N., Y.Y., N.Y., and K.T.; Resources, M.N., N.Y., and K.T.; Data Curation, M.N., Y.Y., and S.Y.; Writing – Original Draft Preparation, M.N. and S.Y.; Writing – Review and Editing, Y.Y., N.Y., K.T., and S.Y.; Visualization, M.N.; Supervision, M.N. and S.Y.; Funding Acquisition, M.N.
Founding
The research was financially supported by the Sasakawa Scientific Research Grant (2018) from The Japan Science Society.
Acknowledgments
The authors thank the laboratory members of our university, and would like to thank MARUZEN-YUSHODO Co., Ltd. (https://kw.maruzen.co.jp/kousei-honyaku/) for the English language editing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Volkman, J.K. Sterols in microorganisms. Appl. Microbiol. Biotechnol. 2003, 60, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Volkman, J.K. A review of sterol markers for marine and terrigenous organic matter. Org. Geochem. 1986, 9, 83–99. [Google Scholar] [CrossRef]
- Wu, P.; Bi, R.; Duan, S.; Jin, H.; Chen, J.; Hao, Q.; Cai, Y.; Mao, X.; Zhao, M. Spatiotemporal variations of phytoplankton in the East China Sea and the Yellow Sea revealed by lipid biomarkers. J. Geophys. Res. 2016, 121, 109–125. [Google Scholar] [CrossRef]
- Bechtel, A.; Schubert, C.J. Biogeochemistry of particulate organic matter from lakes of different trophic levels in Switzerland. Org. Geochem. 2009, 40, 441–454. [Google Scholar] [CrossRef]
- Nakakuni, M.; Dairiki, C.; Kaur, G.; Yamamoto, S. Stanol to sterol ratios in late Quaternary sediments from southern California: An indicator for continuous variability of the oxygen minimum zone. Org. Geochem. 2017, 111, 126–135. [Google Scholar] [CrossRef]
- Nakakuni, M.; Kitano, J.; Uemura, H.; Yamamoto, S. Modern sediment records of stanol to sterol ratios in Lake Suigetsu, Japan: An indicator of variable lacustrine redox conditions. Org. Geochem. 2018, 119, 59–71. [Google Scholar] [CrossRef]
- Wakeham, S.G. Reduction of stenols to stanols in particulate matter at oxic-anoxic boundaries in sea water. Nature 1989, 342, 787–790. [Google Scholar] [CrossRef]
- Huang, W.Y.; Meinschein, W.G. Sterols as ecological indicators. Geochim. Et Cosmochim. Acta 1979, 43, 739–745. [Google Scholar] [CrossRef]
- Rontani, J.F.; Charriere, B.; Petit, M.; Vaultier, F.; Heipieper, H.J.; Link, H.; Chaillou, G.; Sempéré, R. Degradation state of organic matter in surface sediments from the Southern Beaufort Sea: A lipid approach. Biogeosciences 2012, 9, 3513–3530. [Google Scholar] [CrossRef]
- Rontani, J.F.; Vaultier, F.; Bonin, P. Biotic and abiotic degradation of marine and terrestrial higher plant material in intertidal surface sediments from Arcachon Bay (France): A lipid approach. Mar. Chem. 2014, 158, 69–79. [Google Scholar] [CrossRef]
- Rontani, J.F.; Charrière, B.; Sempéré, R.; Doxaran, D.; Vaultier, F.; Vonk, J.E.; Volkman, J.K. Degradation of sterols and terrigenous organic matter in waters of the Mackenzie Shelf, Canadian Arctic. Org. Geochem. 2014, 75, 61–73. [Google Scholar] [CrossRef]
- Huang, W.Y.; Meinschein, W.G. Sterols in sediments from Baffin Bay. Texas. Geochim. Et Cosmochim. Acta 1978, 42, 1391–1396. [Google Scholar] [CrossRef]
- Smith, D.J.; Eglinton, G.; Morris, R.J.; Poutanen, E.L. Aspects of the steroid geochemistry of a recent diatomaceous sediment from the Namibian Shelf. Oceanol. Acta 1982, 5, 365–378. [Google Scholar]
- Smith, D.J.; Eglinton, G.; Morris, R.J.; Poutanen, E.L. Aspects of the steroid geochemistry of an interfacial sediment from the Peruvian upwelling. Oceanol. Acta 1983, 6, 211–219. [Google Scholar]
- Volkman, J.K.; Kearney, P.; Jeffrey, S.W. A new source of 4-methyl sterols and 5α(H)-stanols in sediments: Prymnesiophyte microalgae of the genus Pavlova. Org. Geochem. 1990, 15, 489–497. [Google Scholar] [CrossRef]
- Junker, J.; Chong, I.; Kamp, F.; Steiner, H.; Giera, M.; Müller, C.; Bracher, F. Comparison of strategies for the determination of sterol sulfates via GC–MS leading to a novel deconjugation-derivatization protocol. Molecules 2019, 24, 2353. [Google Scholar] [CrossRef] [PubMed]
- Challinor, J.M. Review: The development and applications of thermally assisted hydrolysis and methylation reactions. J. Anal. Appl. Pyrolysis 2001, 61, 3–34. [Google Scholar] [CrossRef]
- Challinor, J.M. Characterization of wood by pyrolysis derivatisation-gas chromatography/mass spectrometry. J. Anal. Appl. Pyrolysis 1995, 34, 93–107. [Google Scholar] [CrossRef]
- Kuroda, K.; Suzuki, A. Analysis of cinnamic acids in rice (Oryza sativa) by simultaneous pyrolysis-methylation-gas chromatography. Mokuzai Gakkaishi 1995, 41, 851–857. [Google Scholar]
- Hatcher, P.G.; Nanny, M.A.; Minard, R.D.; Dible, S.D.; Carson, D.M. Comparison of two thermochemolytic methods for the analysis of lignin in decomposing gymnosperm wood: The CuO oxidation method and the method of thermochemolysis with tetramethylammonium hydroxide (TMAH). Org. Geochem. 1995, 23, 881–888. [Google Scholar] [CrossRef]
- Fabbri, D.; Helleur, R. Characterization of the tetramethylammonium hydroxide thermochemolysis products of carbohydrates. J. Anal. Appl. Pyrolysis 1999, 49, 277–293. [Google Scholar] [CrossRef]
- Gallois, N.; Templier, J.; Derenne, S. Pyrolysis-gas chromatography–mass spectrometry of the 20 protein amino acids in the presence of TMAH. J. Anal. Appl. Pyrolysis 2007, 80, 216–230. [Google Scholar] [CrossRef]
- Zang, X.; Brown, J.C.; van Heemst, J.D.H.; Palumbo, A.; Hatcher, P.G. Characterization of amino acids and proteinaceous materials using online tetramethylammonium hydroxide (TMAH) thermochemolysis and gas chromatography-mass spectrometry technique. J. Anal. Appl. Pyrolysis 2001, 61, 181–193. [Google Scholar] [CrossRef]
- He, Y.; Buch, A.; Morisson, M.; Szopa, C.; Freissinet, C.; Williams, A.; Millan, M.; Guzman, M.; Navarro-Gonzalez, R.; Bonnet, J.Y.; et al. Application of TMAH thermochemolysis to the detection of nucleobases: Application to the MOMA and SAM space experiment. Talanta 2019, 204, 802–811. [Google Scholar] [CrossRef]
- Martin, F.; Gonzalez-Vila, F.J.; del Rio, J.C.; Verdejo, T. Pyrolysis derivatization of humic substances 1. Pyrolysis of fulvic acids in the presence of tetramethylammonium hydroxide. J. Anal. Appl. Pyrolysis 1994, 28, 71–80. [Google Scholar] [CrossRef]
- Hatcher, P.G.; Clifford, D.J. Flash pyrolysis and in situ methylation of humic acids from soil. Org. Geochem. 1994, 21, 1081–1092. [Google Scholar] [CrossRef]
- Clifford, D.J.; Carson, D.M.; McKinney, D.E.; Bortiatynski, J.M.; Hatcher, P.G. A new rapid technique for the characterization of lignin in vascular plants: Thermochemolysis with tetramethylammonium hydroxide (TMAH). Org. Geochem. 1995, 23, 169–175. [Google Scholar] [CrossRef]
- McKinney, D.E.; Carson, D.M.; Clifford, D.J.; Minard, R.D.; Hatcher, P.G. Off-line thermochemolysis versus flash pyrolysis for the in-situ methylation of lignin: Is pyrolysis necessary? J. Anal. Appl. Pyrolysis 1995, 34, 41–46. [Google Scholar] [CrossRef]
- Asperger, A.; Engewald, W.; Fabian, G. Thermally assisted hydrolysis and methylation – a simple and rapid online derivatization method for the gas chromatographic analysis of natural waxes. J. Anal. Appl. Pyrolysis 2001, 61, 91–109. [Google Scholar] [CrossRef]
- Isobe, K.O.; Tarao, M.; Chiem, N.H.; Minh, L.Y.; Takada, H. Effect of environmental factors on the relationship between concentrations of coprostanol and decal indicator bacteria in tropical (Mekong Delta) and temperate (Tokyo) freshwaters. Appl. Environ. Microbiol. 2004, 70, 814–821. [Google Scholar] [CrossRef]
- Kamata, M.; Kanai, Y.; Ueta, M.; Narusue, M.; Kurosawa, R.; Koita, M.; Fukui, K.; Tsukamoto, Y.; Kaji, K.; Kaneko, T. Assessment of the amount of benthos by substratum conditions. Strix 1996, 14, 201–203. [Google Scholar]
- Arao, K.; Motai, K.; Shibahara, T.; Furota, T. Ichthyofauna of Yatsu Tidal Flat in the inner Tokyo Bay. Nat. Hist. Rep. Kanagawa 2019, 40, 41–48. [Google Scholar]
- Murakami, K.; Inoue-Kohama, A. Water environmental condition assessment of inflow rivers of Yatsu tidal flat using biotic indicator. J. Jpn. Soc. Civ. Eng. Ser. B3 (Ocean Eng.) 2015, 71, 844–849. [Google Scholar]
- Idler, D.R.; Safe, L.M.; Safe, S.H. Mass spectrometric studies of methyl ether derivatives of sterols. Steroids 1970, 16, 251–262. [Google Scholar] [CrossRef]
- Nishimura, M. Origin of stanols in young lacustrine sediments. Nature 1977, 270, 711–712. [Google Scholar] [CrossRef]
- Nishimura, M.; Koyama, T. The occurrence of stanols in various living organisms and the behavior of sterols in contemporary sediments. Geochim. Et Cosmochim. Acta 1977, 41, 379–385. [Google Scholar] [CrossRef]
- Rosenfeld, R.S.; Hellman, L. Reduction and esterification of cholesterol and sitosterol by homogenates of feces. J. Lipid Res. 1971, 12, 192–197. [Google Scholar]
- Eyssen, H.J.; Parmentier, G.G.; Compernolle, F.C.; de Pauw, G.; Piessens-Denef, M. Biohydrogenation of sterols by eubacterium ATCC 21,408—Nova Species. Eur. J. Biochem. 1973, 36, 411–421. [Google Scholar] [CrossRef]
- Rinna, J.; Warning, B.; Meyers, P.A.; Brumsack, H.J.; Rullkötter, J. Combined organic and inorganic geochemical reconstruction of paleodepositional conditions of a Pliocene sapropel from the eastern Mediterranean Sea. Geochim. Et Cosmochim. Acta 2002, 66, 1969–1986. [Google Scholar] [CrossRef]
- Antharam, V.C.; McEwen, D.C.; Garrett, T.J.; Dossey, A.T.; Li, E.C.; Kozlov, A.N.; Mesbah, Z.; Wang, G.P. An integrated metabolomic and microbiome analysis identified specific gut microbiota associated with fecal cholesterol and coprostanol in Clostridium difficile infection. PLoS ONE 2016, 11, 1–23. [Google Scholar] [CrossRef]
- Kriaa, A.; Bourgin, M.; Mkaouar, H.; Jablaoui, A.; Akermi, N.; Soussou, S.; Maguin, E.; Rhimi, M. Microbial reduction of cholesterol to coprostanol: An old concept and new insights. Catalysts 2019, 9, 167. [Google Scholar] [CrossRef]
- Férézou, J.; Gouffier, E.; Coste, T.; Chevallier, F. Daily elimination of fecal neutral sterols by humans. Digestion 1978, 18, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Leeming, R.; Ball, A.; Ashbolt, N.; Nichols, P. Using faecal sterols from humans and animals to distinguish faecal pollution in receiving waters. Water Res. 1996, 30, 2893–2900. [Google Scholar] [CrossRef]
- Hatcher, P.G.; McGillivary, P.A. Sewage contamination in the New York Bight. Coprostanol as an indicator. Environ. Sci. Technol. 1979, 13, 1225–1229. [Google Scholar] [CrossRef]
- Pierce, R.H.; Brown, R.C. Coprostanol distribution from sewage discharge into Sarasota Bay, Florida. Bull. Environ. Contam. Toxicol. 1984, 32, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.W.; Wun, C.K.; Litsky, W.; Dutka, B.J. Coprostanol as an indicator of fecal pollution. C R C Crit. Rev. Environ. Control 1982, 12, 91–112. [Google Scholar] [CrossRef]
- Rada, J.P.A.; Duarte, A.C.; Pato, P.; Cachada, A.; Carreira, R.S. Sewage contamination of sediments from two Portuguese Atlantic coastal systems, revealed by fecal sterols. Mar. Pollut. Bull. 2016, 103, 319–324. [Google Scholar] [CrossRef]
- Lyons, B.P.; Devlin, M.J.; Abdul Hamid, S.A.; Al-Otiabi, A.F.; Al-Enezi, M.; Massoud, M.S.; Al-Zaidan, A.S.; Smith, A.J.; Morris, S.; Bersuder, P.; et al. Microbial water quality and sedimentary faecal sterols as markers of sewage contamination in Kuwait. Mar. Pollut. Bull. 2015, 100, 689–698. [Google Scholar] [CrossRef]
- Frena, M.; Bataglion, G.A.; Tonietto, A.E.; Eberlin, M.N.; Alexandre, M.R.; Madureira, L.A.S. Assessment of anthropogenic contamination with sterol markers in surface sediments of a tropical estuary (Itajaí-Açu, Brazil). Sci. Total Environ. 2016, 544, 432–438. [Google Scholar] [CrossRef]
- Brooks, C.J.W.; Horning, E.C.; Young, J.S. Characterization of sterols by gas chromatography-mass spectrometry of the trimethylsilyl ethers. Lipids 1968, 3, 391–402. [Google Scholar] [CrossRef]
- Ballantine, J.A.; Roberts, J.C.; Morris, R.J. Marine sterols. III—the sterol compositions of oceanic jellyfish. The use of gas chromatographic mass spectrometric techniques to identify unresolved components. Biol. Mass Spectrom. 1976, 3, 14–20. [Google Scholar] [CrossRef] [PubMed]
- de Leeuw, J.W.; Rijpstra, W.I.C.; Schenck, P.A.; Volkman, J.K. Free, esterified and residual bound sterols in Black Sea Unit I sediments. Geochim. Et Cosmochim. Acta 1983, 47, 455–465. [Google Scholar] [CrossRef]
- McCalley, D.V.; Cooke, M.; Nickless, G. Effect of sewage treatment on faecal sterols. Water Res. 1981, 15, 1019–1025. [Google Scholar] [CrossRef]
- Mudge, S.M.; Seguel, C.G. Organic contamination of San Vicente Bay, Chile. Mar. Pollut. Bull. 1999, 38, 1011–1021. [Google Scholar] [CrossRef]
- Mudge, S.M.; Ball, A.S. Sewage. In Environmental Forensics; Morrison, R.D., Murphy, B.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 36–53. [Google Scholar]
- Grimalt, J.O.; Albaigés, J. Characterization of the depositional environments of the Ebro Delta (western Mediterranean) by the study of sedimentary lipid markers. Mar. Geol. 1990, 95, 207–224. [Google Scholar] [CrossRef]
- Gilpin, B.J.; Gregor, J.E.; Savill, M.G. Identification of the source of faecal pollution in contaminated rivers. Water Sci. Technol. 2002, 46, 9–15. [Google Scholar] [CrossRef]
- Barrett, S.M.; Volkman, J.K.; Dunstan, G.A.; LeRoi, J.M. Sterols of 14 species of marine diatoms (Bacillariophyta). J. Phycol. 1995, 31, 360–369. [Google Scholar] [CrossRef]
- Orcutt, D.M.; Patterson, G.W. Sterol, fatty acid and elemental composition of diatoms grown in chemically defined media. Comp. Biochem. Physiol. Part B: Comp. Biochem. 1975, 50, 579–583. [Google Scholar] [CrossRef]
- Gladu, P.K.; Patterson, G.W.; Wikfors, G.H.; Chitwood, D.J.; Lusby, W.R. Sterols of some diatoms. Phytochemistry 1991, 30, 2301–2303. [Google Scholar] [CrossRef]
- Rampen, S.W.; Abbas, B.A.; Schouten, S.; Sinninghe Damsté, J.S. A comprehensive study of sterols in marine diatoms (Bacillariophyta): Implications for their use as tracers for diatom productivity. Limnol. Oceanogr. 2010, 55, 91–105. [Google Scholar] [CrossRef]
- Huang, W.Y.; Meinschein, W.G. Sterols as source indicators of organic materials in sediments. Geochim. Et Cosmochim. Acta 1976, 40, 323–330. [Google Scholar]
- Wyllie, S.G.; Djerassi, C. Mass spectrometry in structural and stereochemical problems. CXLVI. Mass spectrometric fragmentations typical of sterols wit unsaturated side chains. J. Org. Chem. 1968, 33, 305–313. [Google Scholar] [CrossRef]
- Volkman, J.K.; Gllan, F.T.; Johns, R.B.; Eglinton, G. Sources of neutral lipids in a temperate intertidal sediment. Geochim. Et Cosmochim. Acta 1981, 45, 1817–1828. [Google Scholar] [CrossRef]
- Rontani, J.F.; Belt, S.T.; Brown, T.A.; Amiraux, R.; Gosselin, M.; Vaultier, F.; Mundy, C.J. Monitoring abiotic degradation in sinking versus suspended Arctic sea ice algae during a spring ice melt using specific lipid oxidation tracers. Org. Geochem. 2016, 98, 82–97. [Google Scholar] [CrossRef]
- Smik, L.; Belt, S.T.; Lieser, J.L.; Armand, L.K.; Leventer, A. Distributions of highly branched isoprenoid alkenes and other algal lipids in surface waters from East Antarctica: Further insights for biomarker-based paleo sea-ice reconstruction. Org. Geochem. 2016, 95, 71–80. [Google Scholar] [CrossRef]
- Rontani, J.F.; Belt, S.T.; Amiraux, R. Biotic and abiotic degradation of the sea ice diatom biomarker IP25 and selected algal sterols in near-surface Arctic sediments. Org. Geochem. 2018, 118, 73–88. [Google Scholar] [CrossRef]
- Withers, N. Dinoflagellate sterols. In Marine Natural Products. Scheuer, P.J., Ed.; Academic Press: Cambridge, MA, USA, 1983; pp. 87–130. [Google Scholar]
- Janouškovec, J.; Gavelis, G.S.; Burki, F.; Dinh, D.; Bachvaroff, T.R.; Gornik, S.G.; Bright, K.J.; Imanian, B.; Strom, S.L.; Delwiche, C.F.; et al. Major transitions in dinoflagellate evolution unveiled by phylotranscriptomics. Proc. Natl. Acad. Sci. 2017, 114, 171–180. [Google Scholar] [CrossRef]
- Piretti, M.V.; Pagliuca, G.; Boni, L.; Pistocchi, R.; Diamante, M.; Gazzotti, T. Investigation of 4-methyl sterols from cultured dinoflagellate algal strains. J. Phycol. 1997, 33, 61–67. [Google Scholar] [CrossRef]
- Robinson, N.; Eglinton, G.; Brassell, S.C.; Cranwell, P.A. Dinoflagellate origin for sedimentary 4α-methylsteroids and 5α-stanols. Nature 1984, 308, 439–442. [Google Scholar] [CrossRef]
- Boon, J.J.; Rijpstra, W.I.C.; De Lange, F.; De Leeuw, J.W.; Yoshioka, M.; Shimizu, Y. Black Sea sterol—A molecular fossil for dinoflagellate blooms. Nature 1979, 277, 125–127. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).