New Trends in Biopolymer-Based Membranes for Pervaporation
Abstract
1. Introduction
2. Biopolymers: The Promising Materials in Membrane Preparation for Pervaporation Operations
2.1. Chitosan-Based Membranes
2.2. Sodium Alginate-Based Membranes
2.3. Other Biopolymer Membranes
3. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Am | Membrane area |
| β | separation factor |
| CNs | g-C3N4 nanosheets |
| CS | Chitosan |
| D | Diffusivity |
| EG | Ethylene glycol |
| GA | Glutaraldehyde |
| GO | Graphene oxide |
| J | Permeate flux |
| JA | Permeate flux of the compound A |
| K+MMT | Potassium montmorillonite |
| mA | Mass of the molecule A |
| MeOH | Methanol |
| MTBE | Methyl tert-butyl ether |
| MMM | Mixed matrix membrane |
| MOF | Metal-organic framework |
| PEG | Poly(ethylene glycol) |
| PHB | Polyhydroxybutyrate |
| PLA | Polylactic acid |
| POSS | Polyoctahedral oligomeric silsesquioxanes |
| PV | Pervaporation |
| PVA | Polyvinyl alcohol |
| PVMR | Pervaporation membrane reactor |
| PAN | Polyacrylonitrile |
| PVDF | Polyvinylidene fluoride |
| PDMS | Polydimethylsiloxane |
| POMS | Polyoctylmethyl siloxane |
| P | Permeability |
| S | Solubility |
| SA | Sodium alginate |
| SPVA | sulfonated polyvinyl alcohol |
References
- Galiano, F.; Briceño, K.; Marino, T.; Molino, A.; Christensen, K.V.; Figoli, A. Advances in biopolymer-based membrane preparation and applications. J. Membr. Sci. 2018, 564, 562–586. [Google Scholar] [CrossRef]
- Honarkar, H.; Barikani, M. Applications of biopolymers I: Chitosan. Monatsh Chem. 2009, 140, 1403–1420. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Galiano, F.; Fíla, V.; Drioli, E.; Figoli, A. Mixed matrix membranes (MMMs) for ethanol purification through pervaporation: Current state of the art. Rev. Chem. Eng. 2018. [Google Scholar] [CrossRef]
- Ong, Y.K.; Shi, G.M.; Le, N.L.; Tang, Y.P.; Zuo, J.; Nunes, S.P.; Chung, T.-S. Recent membrane development for pervaporation processes. Prog. Polym. Sci. 2016, 57, 1–31. [Google Scholar] [CrossRef]
- Chen, T.-H.; Huang, Y.-H. Dehydration of waste cutting oil using a pervaporation process. J. Taiwan Inst. Chem. Eng. 2018, 82, 75–79. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Galiano, F.; De La Iglesia, Ó.; Fíla, V.; Téllez, C.; Coronas, J.; Figoli, A. Graphene oxide – Filled polyimide membranes in pervaporative separation of azeotropic methanol–MTBE mixtures. Sep. Purif. Technol. 2019, 224, 265–272. [Google Scholar] [CrossRef]
- Kopeć, R.; Meller, M.; Kujawski, W.; Kujawa, J. Polyamide-6 based pervaporation membranes for organic–organic separation. Sep. Purif. Technol. 2013, 110, 63–73. [Google Scholar] [CrossRef]
- De La Iglesia, Ó; Sorribas, S.; Almendro, E.; Zornoza, B.; Téllez, C.; Coronas, J.; Pedraza, O.D.L.I.; Ariso, C.T. Metal-organic framework MIL-101(Cr) based mixed matrix membranes for esterification of ethanol and acetic acid in a membrane reactor. Renew. Energy 2016, 88, 12–19. [Google Scholar] [CrossRef][Green Version]
- Li, G.; Mo, X.; Wang, Y.; Chan, C.; Chan, K.C. All 3D-Printed Superhydrophobic/Oleophilic Membrane for Robotic Oil Recycling. Adv. Mater. Interfaces 2019, 6. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Buera-González, J.; De La Iglesia, Ó; Galiano, F.; Fíla, V.; Malankowska, M.; Rubio, C.; Figoli, A.; Téllez, C.; Coronas, J. Towards the dehydration of ethanol using pervaporation cross-linked poly(vinyl alcohol)/graphene oxide membranes. J. Membr. Sci. 2019, 582, 423–434. [Google Scholar]
- Choi, J.H.; Jegal, J.; Kim, W.N.; Choi, H.S. Incorporation of Multiwalled Carbon Nanotubes into Poly(vinyl alcohol) Membranes for Use in the Pervaporation of Water/Ethanol Mixtures. J. Appl. Polym. Sci. 2008, 111, 2186–2193. [Google Scholar] [CrossRef]
- Amirilargani, M.; Sadatnia, B. Poly(vinyl alcohol)/zeolitic imidazolate frameworks (ZIF-8) mixed matrix membranes for pervaporation dehydration of isopropanol. J. Membr. Sci. 2014, 469, 1–10. [Google Scholar] [CrossRef]
- Okumuş, E.; Gürkan, T.; Yılmaz, L.; Yilmaz, L. Effect of fabrication and process parameters on the morphology and performance of a PAN-based zeolite-filled pervaporation membrane. J. Membr. Sci. 2003, 223, 23–38. [Google Scholar] [CrossRef]
- Sukitpaneenit, P.; Chung, T.-S.; Chung, N.T.-S. PVDF/Nanosilica Dual-Layer Hollow Fibers with Enhanced Selectivity and Flux as Novel Membranes for Ethanol Recovery. Ind. Eng. Chem. Res. 2012, 51, 978–993. [Google Scholar] [CrossRef]
- Yadav, A.; Lind, M.L.; Ma, X.; Lin, Y.S. Nanocomposite Silicalite-1/Polydimethylsiloxane Membranes for Pervaporation of Ethanol from Dilute Aqueous Solutions. Ind. Eng. Chem. Res. 2013, 52, 5207–5212. [Google Scholar] [CrossRef]
- Li, Y.; Wee, L.H.; Martens, J.A.; Vankelecom, I.F.J. ZIF-71 as a potential filler to prepare pervaporation membranes for bio-alcohol recovery. J. Mater. Chem. A 2014, 2, 10034–10040. [Google Scholar] [CrossRef]
- Rom, A.; Friedl, A. Investigation of pervaporation performance of POMS membrane during separation of butanol from water and the effect of added acetone and ethanol. Sep. Purif. Technol. 2016, 170, 40–48. [Google Scholar] [CrossRef]
- Ray, S. Separation of organic mixtures by pervaporation using crosslinked rubber membranes. J. Membr. Sci. 2006, 270, 132–145. [Google Scholar] [CrossRef]
- Wang, N.; Ji, S.; Li, J.; Zhang, R.; Zhang, G. Poly(vinyl alcohol)–graphene oxide nanohybrid “pore-filling” membrane for pervaporation of toluene/n-heptane mixtures. J. Membr. Sci. 2014, 455, 113–120. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Galiano, F.; Fíla, V.; Drioli, E.; Figoli, A. Matrimid®5218 dense membrane for the separation of azeotropic MeOH-MTBE mixtures by pervaporation. Sep. Purif. Technol. 2018, 199, 27–36. [Google Scholar] [CrossRef]
- Baker, R.W.; Wijmans, J.; Huang, Y. Permeability, permeance and selectivity: A preferred way of reporting pervaporation performance data. J. Membr. Sci. 2010, 348, 346–352. [Google Scholar] [CrossRef]
- Luis, P.; Van Der Bruggen, B. The driving force as key element to evaluate the pervaporation performance of multicomponent mixtures. Sep. Purif. Technol. 2015, 148, 94–102. [Google Scholar] [CrossRef]
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Castro-Muñoz, R. Pervaporation: The emerging technique for extracting aroma compounds from food systems. J. Food Eng. 2019, 253, 27–39. [Google Scholar] [CrossRef]
- Galiano, F.; Castro-Muñoz, R.; Mancuso, R.; Gabriele, B.; Figoli, A. Membrane Technology in Catalytic Carbonylation Reactions. Catal. 2019, 9, 614. [Google Scholar] [CrossRef]
- Jia, Z.; Wu, G. Metal-organic frameworks based mixed matrix membranes for pervaporation. Microporous Mesoporous Mater. 2016, 235, 151–159. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; De La Iglesia, Ó; Fíla, V.; Téllez, C.; Coronas, J. Pervaporation-Assisted Esterification Reactions by Means of Mixed Matrix Membranes. Ind. Eng. Chem. Res. 2018, 57, 15998–16011. [Google Scholar]
- Crespo, J.; Brazinha, C. 1-Fundamentals of pervaporation. In Pervaporation, Vapour Permeation and Membrane Distillation; Woodhead Publishing: Oxford, UK, 2015; pp. 1–17. [Google Scholar]
- Wijmans, J.; Baker, R. The solution-diffusion model: A review. J. Membr. Sci. 1995, 107, 1–21. [Google Scholar] [CrossRef]
- Wee, S.-L.; Tye, C.-T.; Bhatia, S. Membrane separation process—Pervaporation through zeolite membrane. Sep. Purif. Technol. 2008, 63, 500–516. [Google Scholar] [CrossRef]
- Kujawski, W.; Krajewski, S.R. Sweeping gas pervaporation with hollow-fiber ion-exchange membranes. Desalination 2004, 162, 129–135. [Google Scholar] [CrossRef]
- Santoro, S.; Galiano, F.; Jansen, J.C.; Figoli, A. Strategy for scale-up of SBS pervaporation membranes for ethanol recovery from diluted aqueous solutions. Sep. Purif. Technol. 2017, 176, 252–261. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Galiano, F.; Figoli, A. Chemical and bio-chemical reactions assisted by pervaporation technology. Crit. Rev. Biotechnol. 2019, 39, 884–903. [Google Scholar] [CrossRef] [PubMed]
- Wynn, N. Pervaporation comes of age. Chem. Eng. Prog. 2001, 97, 66–72. [Google Scholar]
- Fontalvo, J.; Cuellar, P.; Timmer, J.M.K.; Vorstman, M.A.G.; Wijers, J.G.; Keurentjes, J.T.F. Comparing Pervaporation and Vapor Permeation Hybrid Distillation Processes. Ind. Eng. Chem. Res. 2005, 44, 5259–5266. [Google Scholar] [CrossRef]
- Jyoti, G.; Keshav, A.; Anandkumar, J. Review on Pervaporation: Theory, Membrane Performance, and Application to Intensification of Esterification Reaction. J. Eng. 2015, 2015, 1–24. [Google Scholar] [CrossRef]
- Chovau, S.; Degrauwe, D.; Van Der Bruggen, B. Critical analysis of techno-economic estimates for the production cost of lignocellulosic bio-ethanol. Renew. Sustain. Energy Rev. 2013, 26, 307–321. [Google Scholar] [CrossRef]
- Shao, P.; Huang, R. Polymeric membrane pervaporation. J. Membr. Sci. 2007, 287, 162–179. [Google Scholar] [CrossRef]
- Peng, P.; Shi, B.; Lan, Y. A Review of Membrane Materials for Ethanol Recovery by Pervaporation. Sep. Sci. Technol. 2011, 46, 234–246. [Google Scholar] [CrossRef]
- Gontarek, E.; Macedonio, F.; Militano, F.; Giorno, L.; Lieder, M.; Politano, A.; Drioli, E.; Gugliuzza, A. Adsorption-assisted transport of water vapour in super-hydrophobic membranes filled with multilayer graphene platelets. Nanoscale 2019, 11, 11521–11529. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Fíla, V.; Dung, C.T. Mixed Matrix Membranes Based on PIMs for Gas Permeation: Principles, Synthesis, and Current Status. Chem. Eng. Commun. 2017, 204. [Google Scholar] [CrossRef]
- Chen, F.; Lu, Y.; Liu, X.; Song, J.; He, G.; Tiwari, M.K.; Carmalt, C.J.; Parkin, I.P. Table Salt as a Template to Prepare Reusable Porous PVDF-MWCNT Foam for Separation of Immiscible Oils/Organic Solvents and Corrosive Aqueous Solutions. Adv. Funct. Mater. 2017, 27, 1702926. [Google Scholar] [CrossRef]
- Zimmermann, J.; Reifler, F.; Fortunato, G.; Gerhardt, L.; Seeger, S. A Simple, One-Step Approach to Durable and Robust Superhydrophobic Textiles. Adv. Funct. Mater. 2008, 18, 3662–3669. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, F.; Pan, F.; Zhang, M.; Yang, X.; Li, P.; Jiang, Z.; Zhang, P.; Cao, X.; Wang, B. Enhanced pervaporation dehydration performance of ultrathin hybrid membrane by incorporating bioinspired multifunctional modifier and TiCl4 into chitosan. J. Membr. Sci. 2013, 446, 395–404. [Google Scholar] [CrossRef]
- Zhang, Q.G.; Hu, W.W.; Liu, Q.L.; Zhu, A.M. Chitosan/polyvinylpyrrolidone-silica hybrid membranes for pervaporation separation of methanol/ethylene glycol azeotrope. J. Appl. Polym. Sci. 2013, 129, 3178–3184. [Google Scholar] [CrossRef]
- Wang, M.; Xing, R.; Wu, H.; Pan, F.; Zhang, J.; Ding, H.; Jiang, Z. Nanocomposite membranes based on alginate matrix and high loading of pegylated POSS for pervaporation dehydration. J. Membr. Sci. 2017, 538, 86–95. [Google Scholar] [CrossRef]
- Zhang, Q.G.; Han, G.L.; Hu, W.W.; Zhu, A.M.; Liu, Q.L. Pervaporation of Methanol–Ethylene Glycol Mixture over Organic–Inorganic Hybrid Membranes. Ind. Eng. Chem. Res. 2013, 52, 7541–7549. [Google Scholar] [CrossRef]
- Wang, M.; Pan, F.; Yang, L.; Song, Y.; Wu, H.; Cheng, X.; Liu, G.; Yang, H.; Wang, H.; Jiang, Z.; et al. Graphene oxide quantum dots incorporated nanocomposite membranes with high water flux for pervaporative dehydration. J. Membr. Sci. 2018, 563, 903–913. [Google Scholar] [CrossRef]
- Kang, C.-H.; Lin, Y.-F.; Huang, Y.-S.; Tung, K.-L.; Chang, K.-S.; Chen, J.-T.; Hung, W.-S.; Lee, K.-R.; Lai, J.-Y. Synthesis of ZIF-7/chitosan mixed-matrix membranes with improved separation performance of water/ethanol mixtures. J. Membr. Sci. 2013, 438, 105–111. [Google Scholar] [CrossRef]
- Casado-Coterillo, C.; Andrés, F.; Téllez, C.; Coronas, J.; Irabien, Á. Synthesis and Characterization of ETS-10/Chitosan Nanocomposite Membranes for Pervaporation. Sep. Sci. Technol. 2014, 49, 1903–1909. [Google Scholar] [CrossRef]
- Asghari, M.; Sheikh, M.; Afsari, M.; Dehghani, M. Molecular simulation and experimental investigation of temperature effect on chitosan-nanosilica supported mixed matrix membranes for dehydration of ethanol via pervaporation. J. Mol. Liq. 2017, 246, 7–16. [Google Scholar] [CrossRef]
- Cao, K.; Jiang, Z.; Zhang, X.; Zhang, Y.; Zhao, J.; Xing, R.; Yang, S.; Gao, C.; Pan, F. Highly water-selective hybrid membrane by incorporating g-C3N4 nanosheets into polymer matrix. J. Membr. Sci. 2015, 490, 72–83. [Google Scholar] [CrossRef]
- Fazlifard, S.; Mohammadi, T.; Bakhtiari, O. Chitosan/ZIF-8 Mixed-Matrix Membranes for Pervaporation Dehydration of Isopropanol. Chem. Eng. Technol. 2017, 40, 648–655. [Google Scholar] [CrossRef]
- Gao, C.; Zhang, M.; Jiang, Z.; Liao, J.; Xie, X.; Huang, T.; Zhao, J.; Bai, J.; Pan, F. Preparation of a highly water-selective membrane for dehydration of acetone by incorporating potassium montmorillonite to construct ionized water channel. Chem. Eng. Sci. 2014, 135, 461–471. [Google Scholar] [CrossRef]
- Li, Q.; Liu, Q.; Zhao, J.; Hua, Y.; Sun, J.; Duan, J.; Jin, W. High efficient water/ethanol separation by a mixed matrix membrane incorporating MOF filler with high water adsorption capacity. J. Membr. Sci. 2017, 544, 68–78. [Google Scholar] [CrossRef]
- Lin, Y.-F.; Wu, C.-Y.; Liu, T.-Y.; Lin, K.-Y.A.; Tung, K.-L.; Chung, T.-W. Synthesis of mesoporous SiO 2 xerogel/chitosan mixed-matrix membranes for butanol dehydration. J. Ind. Eng. Chem. 2018, 57, 297–303. [Google Scholar] [CrossRef]
- Premakshi, H.; Ramesh, K.; Kariduraganavar, M. Modification of crosslinked chitosan membrane using NaY zeolite for pervaporation separation of water–isopropanol mixtures. Chem. Eng. Res. Des. 2015, 94, 32–43. [Google Scholar] [CrossRef]
- Song, Y.; Jiang, Z.; Gao, B.; Wang, H.; Wang, M.; He, Z.; Cao, X.; Pan, F. Embedding hydrophobic MoS 2 nanosheets within hydrophilic sodium alginate membrane for enhanced ethanol dehydration. Chem. Eng. Sci. 2018, 185, 231–242. [Google Scholar] [CrossRef]
- Premakshi, H.G.; Sajjan, A.M.; Kariduraganavar, M.Y. Development of pervaporation membranes using chitosan and titanium glycine-N,N-dimethylphosphonate for dehydration of isopropanol. J. Mater. Chem. A 2015, 3, 3952–3961. [Google Scholar] [CrossRef]
- Galiano, F.; Ghanim, A.H.; Rashid, K.T.; Marino, T.; Simone, S.; Alsalhy, Q.F.; Figoli, A. Preparation and characterization of green polylactic acid (PLA) membranes for organic/organic separation by pervaporation. Clean Technol. Environ. Policy 2018, 21, 109–120. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, G.; Ye, H.; Jin, W.; Cui, Z. Two-dimensional MXene incorporated chitosan mixed-matrix membranes for efficient solvent dehydration. J. Membr. Sci. 2018, 563, 625–632. [Google Scholar] [CrossRef]
- Vinu, M.; Raja, D.S.; Jiang, Y.-C.; Liu, T.-Y.; Xie, Y.-Y.; Lin, Y.-F.; Yang, C.-C.; Lin, C.-H.; AlShehri, S.M.; Ahamad, T.; et al. Effects of structural crystallinity and defects in microporous Al-MOF filled chitosan mixed matrix membranes for pervaporation of water/ethanol mixtures. J. Taiwan Inst. Chem. Eng. 2018, 83, 143–151. [Google Scholar] [CrossRef]
- Shen, J.; Chu, Y.; Ruan, H.; Wu, L.; Gao, C.; Van der Bruggen, B. Pervaporation of benzene/cyclohexane mixtures through mixed matrix membranes of chitosan and Ag+/carbon nanotubes. J. Memb. Sci. 2014, 462, 160–169. [Google Scholar] [CrossRef]
- Wang, L.; Liao, J.; Nie, T.; Cao, K.; Jiang, Z.; Gao, C.; Zhang, M.; Pan, F.; Zhao, J.; Zhan, L. Pervaporation dehydration of an acetone/water mixture by hybrid membranes incorporated with sulfonated carbon molecular sieves. RSC Adv. 2016, 6, 55272–55281. [Google Scholar]
- Hung, W.-S.; Chang, S.-M.; Lecaros, R.L.G.; Ji, Y.-L.; An, Q.-F.; Hu, C.-C.; Lee, K.-R.; Lai, J.-Y. Fabrication of hydrothermally reduced graphene oxide/chitosan composite membranes with a lamellar structure on methanol dehydration. Carbon 2017, 117, 112–119. [Google Scholar] [CrossRef]
- Langari, S.; Saljoughi, E.; Mousavi, S.M. Chitosan/polyvinyl alcohol/amino functionalized multiwalled carbon nanotube pervaporation membranes: Synthesis, characterization, and performance. Polym. Adv. Technol. 2018, 29, 84–94. [Google Scholar] [CrossRef]
- Kudasheva, A.; Sorribas, S.; Zornoza, B.; Téllez, C.; Coronas, J. Pervaporation of water/ethanol mixtures through polyimide based mixed matrix membranes containing ZIF-8, ordered mesoporous silica and ZIF-8-silica core-shell spheres. J. Chem. Technol. Biotechnol. 2015, 90, 669–677. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H. Poly(vinyl alcohol)/ZIF-8 NH2 Mixed Matrix Membranes for Ethanol Dehydration via Pervaporation. AIChE J. 2016, 62, 1728–1739. [Google Scholar] [CrossRef]
- Dharupaneedi, S.P.; Anjanapura, R.V.; Han, J.M.; Aminabhavi, T.M. Functionalized Graphene Sheets Embedded in Chitosan Nanocomposite Membranes for Ethanol and Isopropanol Dehydration via Pervaporation. Ind. Eng. Chem. Res. 2014, 53, 14474–14484. [Google Scholar] [CrossRef]
- Pandey, R.P.; Shahi, V.K. Functionalized silica–chitosan hybrid membrane for dehydration of ethanol/water azeotrope: Effect of cross-linking on structure and performance. J. Membr. Sci. 2013, 444, 116–126. [Google Scholar] [CrossRef]
- Nair, R.R.; Wu, H.A.; Jayaram, P.N.; Grigorieva, I.V.; Geim, A.K. Unimpeded Permeation of Water Through Helium-Leak-Tight Graphene-Based Membranes. Science 2012, 335, 442–444. [Google Scholar] [CrossRef]
- Huang, K.; Liu, G.; Lou, Y.; Dong, Z.; Shen, J.; Jin, W. A Graphene Oxide Membrane with Highly Selective Molecular Separation of Aqueous Organic Solution. Angew. Chem. Int. Ed. 2014, 53, 6929–6932. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Fíla, V. Effect of the ZIF-8 Distribution in Mixed-Matrix Membranes Based on Matrimid® 5218-PEG on CO 2 Separation. Chem. Eng. Technol. 2019, 42, 744–752. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Martin-Gil, V.; Ahmad, M.Z.; Fíla, V. Matrimid® 5218 in preparation of membranes for gas separation: Current state-of-the-art, Chem. Eng. Commun. 2018, 205, 161–196. [Google Scholar] [CrossRef]
- Goh, P.; Ismail, A.; Sanip, S.; Ng, B.; Aziz, M. Recent advances of inorganic fillers in mixed matrix membrane for gas separation. Sep. Purif. Technol. 2011, 81, 243–264. [Google Scholar] [CrossRef]
- Castro-Muñoz, R. Pervaporation-based membrane processes for the production of non-alcoholic beverages. J. Food Sci. Technol. 2019, 56, 2333–2344. [Google Scholar] [CrossRef] [PubMed]
- Ceia, T.; Silva, A.; Ribeiro, C.; Pinto, J.V.; Casimiro, M.H.; Ramos, A.M.; Vital, J.; Ribeiro, C. PVA composite catalytic membranes for hyacinth flavour synthesis in a pervaporation membrane reactor. Catal. Today 2014, 236, 98–107. [Google Scholar] [CrossRef]
- Penkova, A.; Polotskaya, G.; Toikka, A. Pervaporation composite membranes for ethyl acetate production. Chem. Eng. Process. Process. Intensif. 2015, 87, 81–87. [Google Scholar] [CrossRef]
- Bo, S.; Zhang, L.; Han, S.; Li, Y.; Li, W.; Xing, W. Fabrication of bilayer catalytic composite membrane PVA-SA/SPVA and application for ethyl acetate synthesis. J. Membr. Sci. 2018, 563, 10–21. [Google Scholar] [CrossRef]
- Meireles, I.T.; Portugal, C.; Alves, V.D.; Crespo, J.G.; Coelhoso, I.M. Impact of biopolymer purification on the structural characteristics and transport performance of composite polysaccharide membranes for pervaporation. J. Membr. Sci. 2015, 493, 179–187. [Google Scholar] [CrossRef]
- Meireles, I.T.; Huertas, R.M.; Torres, C.A.; Coelhoso, I.M.; Crespo, J.G. Development and characterisation of hybrid polysaccharide membranes for dehydration processes. Carbohydr. Polym. 2018, 191, 216–224. [Google Scholar] [CrossRef]
- Gimenes, M.L.; Liu, L.; Feng, X. Sericin/poly(vinyl alcohol) blend membranes for pervaporation separation of ethanol/water mixtures. J. Membr. Sci. 2007, 295, 71–79. [Google Scholar] [CrossRef]
- Bucci, D.; Tavares, L.; Sell, I. Biodegradation and physical evaluation of PHB packaging. Polym. Test. 2007, 26, 908–915. [Google Scholar] [CrossRef]
- Villegas, M.; Vidaurre, E.F.C.; Gottifredi, J.C. Sorption and pervaporation of methanol/water mixtures with poly(3-hydroxybutyrate) membranes. Chem. Eng. Res. Des. 2014, 94, 254–265. [Google Scholar] [CrossRef]
- Villegas, M.; Romero, A.I.; Parentis, M.L.; Vidaurre, E.F.C.; Gottifredi, J.C.; Romero, A.I.; Parentis, M.L. Acrylic acid plasma polymerized poly(3-hydroxybutyrate) membranes for methanol/MTBE separation by pervaporation. Chem. Eng. Res. Des. 2016, 109, 234–248. [Google Scholar] [CrossRef]
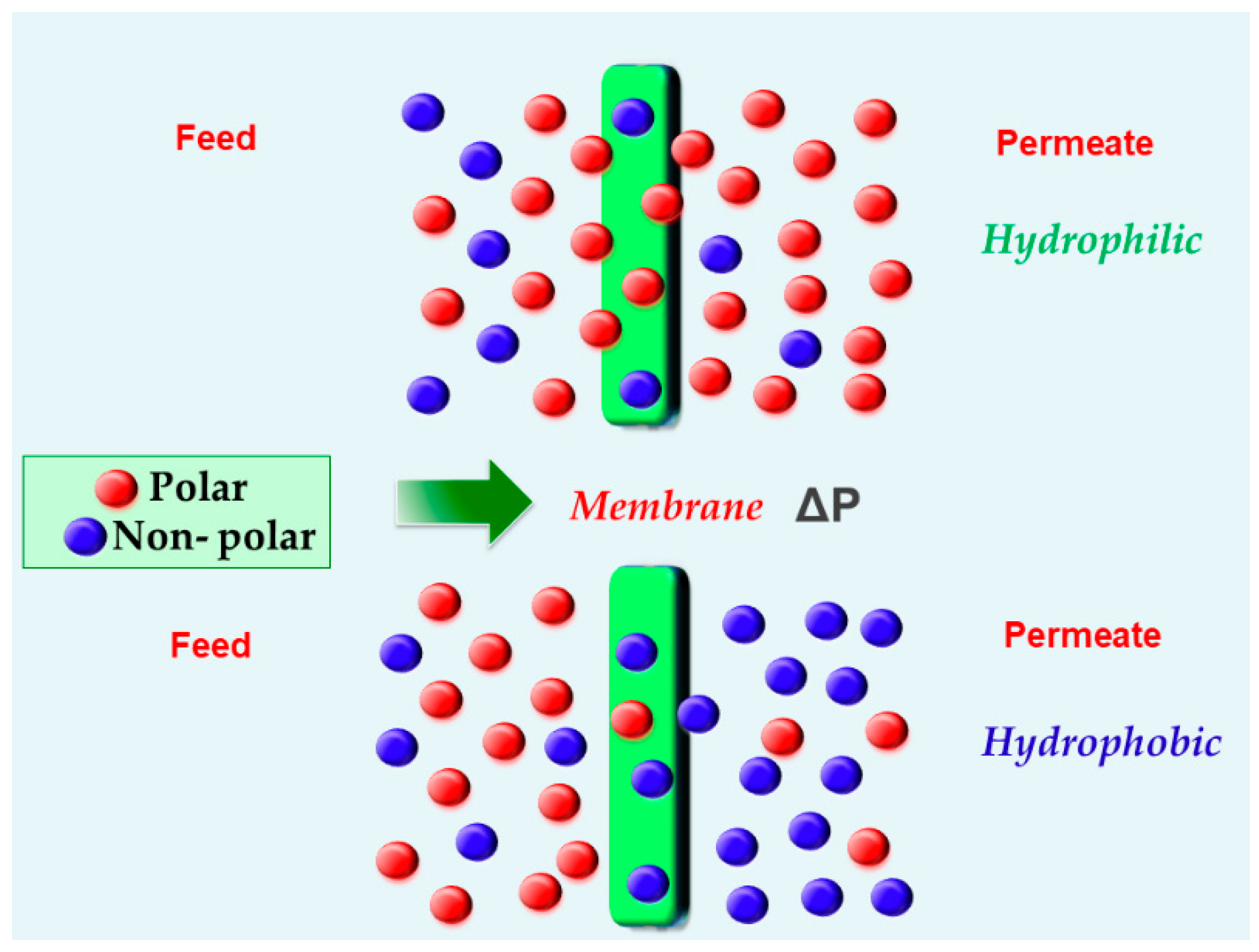
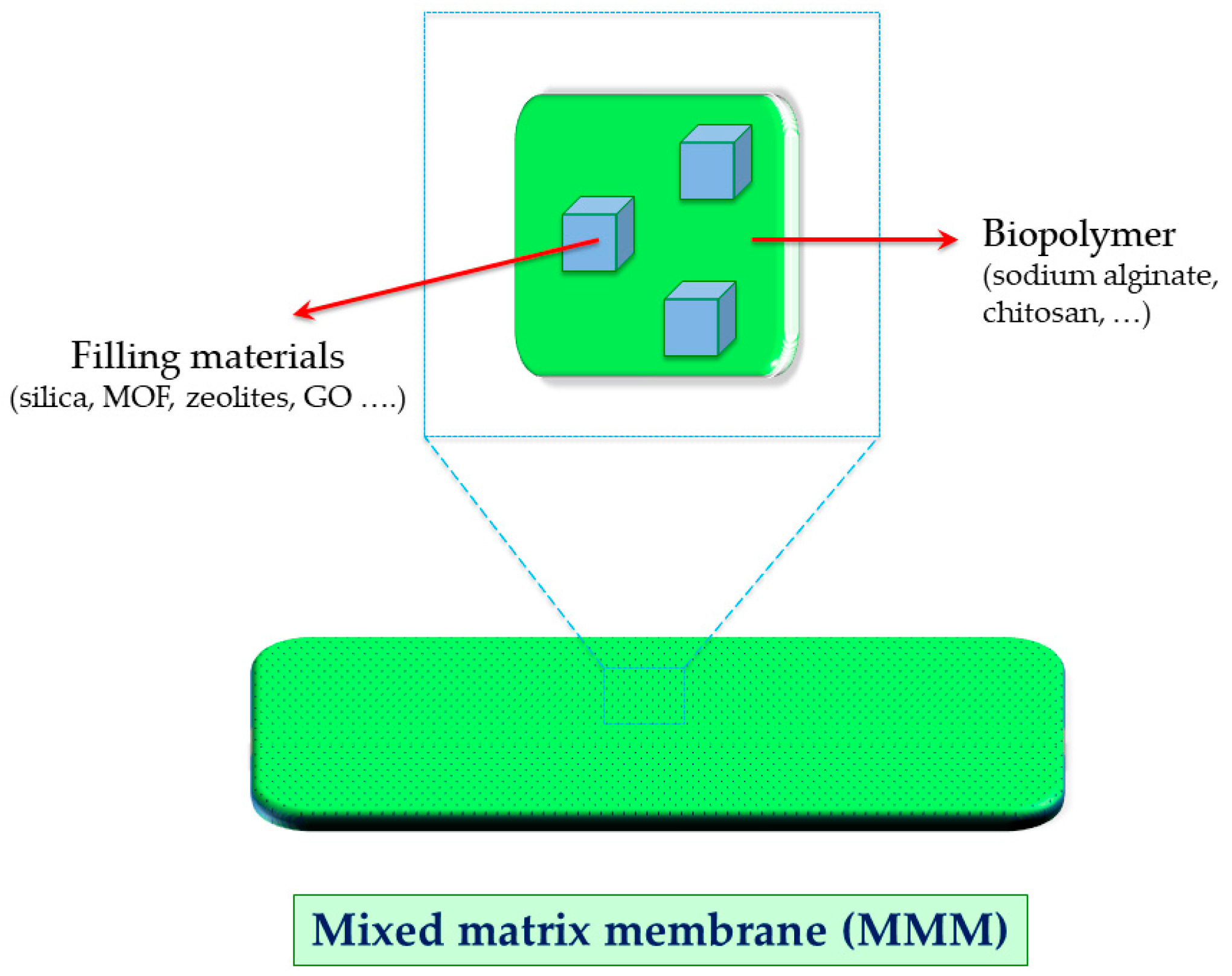
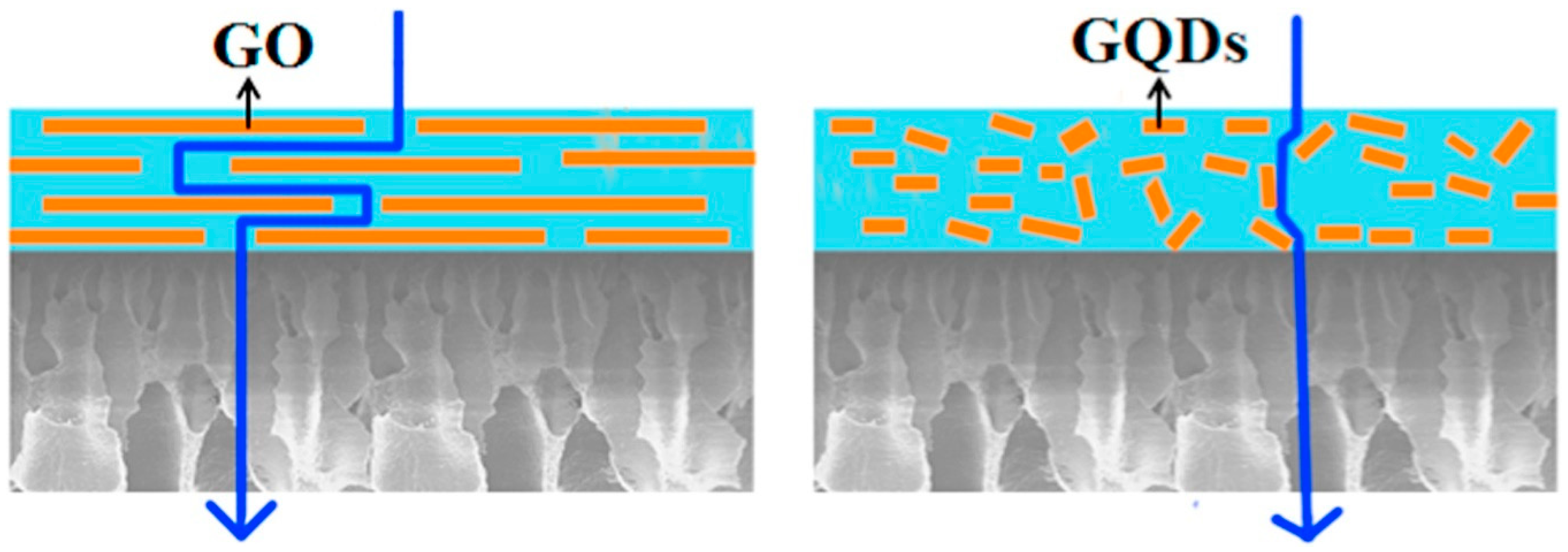
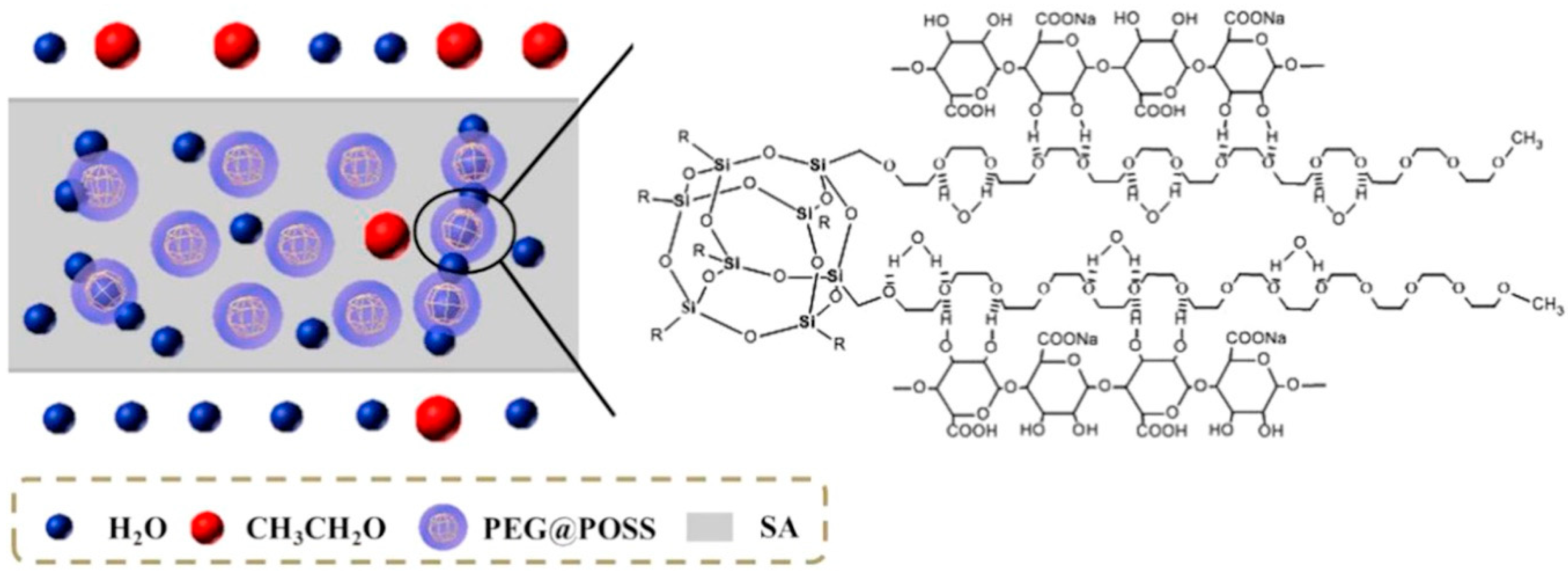
| Azeotropic Mixture | Type of Separation | Membrane Used | Membrane Type | Reference |
|---|---|---|---|---|
| Water/ethanol | Water-organic | Polyvinyl alcohol (PVA) | Hydrophilic | [10,11] |
| Water/isopropanol | Water-organic | Polyvinyl alcohol (PVA) | Hydrophilic | [12] |
| Ethanol/water | Organic-water | Polyacrylonitrile (PAN) | Hydrophilic | [13] |
| Ethanol/water | Organic-water | Polyvinylidene fluoride (PVDF) | Hydrophobic | [14] |
| Ethanol/water | Organic-water | Polydimethylsiloxane (PDMS) | Hydrophobic | [15,16] |
| Butanol/water | Organic-water | Polyoctylmethyl siloxane (POMS) | Hydrophobic | [17] |
| Toluene/methanol | Organic-organic | Poly(styrene-co-butadiene) rubber | Hydrophobic | [18] |
| Toluene/n-heptane | Organic-organic | Polyvinyl alcohol (PVA) | Hydrophilic | [19] |
| Methanol/MTBE | Organic-organic | Polyimide | Hydrophilic | [20] |
| Methanol/MTBE | Organic-organic | Polyamide-6 | Hydrophilic | [7] |
| Polymer Matrix: | Filler: | PV Separation: | Operating Conditions: | Performance Unfilled Membrane | Performance Filled Membrane | Reference: |
|---|---|---|---|---|---|---|
| CS/PVP | Silica | MeOH-EG | 60 °C, 6 wt.% MeOH, 10 mbar | J: 0.15 kg/m2 h β:500 | At 28.4 wt.% loading: J: 0.06 kg/m2 h β: 6000 | [45] |
| SA | PEG-POSS | Water-EtOH | 77 °C, 10 wt.% water, 3 mbar | J: 1.8 kg/m2 h β:900 | At 30 wt.% loading: J: 2.5 kg/m2 h β: 1077 | [46] |
| CS/PVP | BTEE | MeOH-EG | 60 °C, 6 wt.% MeOH, 10 mbar | J: 0.04 kg/m2 h β:500 | At 10.4 wt.% loading: J: 0.05 kg/m2 h β:6129 | [47] |
| CS | Titania | Water-EtOH | 77 °C, 10 wt.% water, 30 mbar | J: 1.500 kg/m2 h β:200 | At 14 wt.% loading: J: 1.403 kg/m2 h β: 730 | [44] |
| SA | GO dots | Water-EtOH | 76 °C, 10 wt.% water, 0 mbar | J: 1.500 kg/m2 h β:500 | At 2 wt.% loading: J: 2.4 kg/m2 h β: 1152 | [48] |
| CS | ZIF-7 | Water-EtOH | 25 °C, 10 wt.% water | J: 0.600 kg/m2 h β:148 | At 5 wt.% loading: J: 0.322 kg/m2 h β:2812 | [49] |
| CS | ETS-10 | Water-EtOH | 50 °C, 15 wt.% water, 2 mbar | J: 0.450 kg/m2 h β:47 | At 5 wt.% loading: J: 0.550 kg/m2 h β:30 | [50] |
| CS | TEOS | Water-EtOH | 30 °C, 50 wt.% water | - | At 10 wt.% loading: J: 0.720 kg/m2 h β:450 | [51] |
| SA | g-C3N4 nanosheets | Water-EtOH | 76 °C, 10 wt.% water, 3 mbar | J: 1.500 kg/m2 h β:500 | At 3 wt.% loading: J: 2.4 kg/m2 h β:1653 | [52] |
| CS | ZIF-8 | Water-IPA | 25 °C, 15 wt.% water, 0.05 mbar | J: 0.325 kg/m2 h β:800 | At 2.5 wt.% loading: J: 0.280 kg/m2 h β:800 | [53] |
| CS | K+MMT | Water-AC | 50 °C, 5 wt.% water, 0.03 mbar | J: 1.5 kg/m2 h β:250 | At 10 wt.% loading: J: 1.7 kg/m2 h β:2200 | [54] |
| CS | MOF-801 | Water-EtOH | 70 °C, 10 wt.% water, 3 mbar | J: 1.00 kg/m2 h β:700 | At 4.8 wt.% loading: J: 1.100 kg/m2 h β:2156 | [55] |
| CS | SiO2 xerogel | Water-BuOH | 25 °C, 10 wt.% water | J: 0.400 kg/m2 h β:500 | At 0.25 wt.% loading: J: 0.500 kg/m2 h β:1900 | [56] |
| CS | NaY | Water-IPA | 30 °C, 10 wt.% water, 10 mbar | J: 0.05 kg/m2 h β:2000 | At 40 wt.% loading: J: 0.113 kg/m2 h β:11,000 | [57] |
| SA | MoS2 | Water-EtOH | 77 °C, 10 wt.% water, 1 mbar | J: 1.2 kg/m2 h β:650 | At 2 wt.% loading: J: 1.83 kg/m2 h β:1229 | [58] |
| CS | TGDMP | Water-IPA | 30 °C, 10 wt.% water, 10 mbar | J: 0.35 kg/m2 h β:600 | At 1.2 wt.% loading: J: 0.737 kg/m2 h β:1050 | [59] |
| PLA | - | MeOH-MTBE | 40 °C, 14.3 wt.% MeOH, 6.1 mbar | J: 0.090 kg/m2 h β:75 | - | [60] |
| CS | MXene | Water-DMC | 50 °C, 2 wt.% water, 2 mbar | J: 1.0 kg/m2 h β:400 | At 3.0 wt.% loading: J: 1.4 kg/m2 h β:900 | [61] |
| CS | Al-MOF | Water-EtOH | 25 °C, 10wt.% water | J: 0.383 kg/m2 h β:240 | At 5 wt.% loading: J: 0.458 kg/m2 h β:2741 | [62] |
| CS | Ag+ grafted MWNTs | Ben-C-Hex | 20 °C, 50 wt.% benzene | J: 0.100 kg/m2 h β:4.5 | At 1.5 wt.% loading: J: 0.357 kg/m2 h β:7.89 | [63] |
| CS | S-CMS | Water-AC | 50 °C, 5 wt.% water, 0.03 mbar | J: 1.10 kg/m2 h β:480 | At 2 wt.% loading: J: 1.81 kg/m2 h β:832 | [64] |
| CS | r-GO | Water-MeOH | 30 °C, 10 wt.% water, 0.03 mbar | J: 0.230 kg/m2 h | At 1 wt.% loading: J: 340 kg/m2 h | [65] |
| CS/PVA | NH2- MWNTs | Water-IPA | 25 °C, 30 wt.% water, 24 mbar | J: 1.80 kg/m2 h β:5 | At 10 wt.% loading: J: 0.80 kg/m2 h β:99.5 | [66] |
| Mixed Matrix Membrane | Filler Loading: | J (kg m−2 h−1) | Separation Factor β) | Reference |
|---|---|---|---|---|
| Cross-linked PVA-filled GO | 1 wt.% | 0.137 | 263 | [10] |
| Polyimide-filled ZIF-8 | 12 wt.% | 0.260 | 300 | [67] |
| PVA-filled MWCNT | 5 wt.% | 0.080 | 500 | [11] |
| Polyimide-filled MSS-1 | 12 wt.% | 0.310 | 190 | [67] |
| Cross-linked PVA-filled ZIF-8-NH2 | 7.5 wt.% | 0.120 | 200 | [68] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro-Muñoz, R.; González-Valdez, J. New Trends in Biopolymer-Based Membranes for Pervaporation. Molecules 2019, 24, 3584. https://doi.org/10.3390/molecules24193584
Castro-Muñoz R, González-Valdez J. New Trends in Biopolymer-Based Membranes for Pervaporation. Molecules. 2019; 24(19):3584. https://doi.org/10.3390/molecules24193584
Chicago/Turabian StyleCastro-Muñoz, Roberto, and José González-Valdez. 2019. "New Trends in Biopolymer-Based Membranes for Pervaporation" Molecules 24, no. 19: 3584. https://doi.org/10.3390/molecules24193584
APA StyleCastro-Muñoz, R., & González-Valdez, J. (2019). New Trends in Biopolymer-Based Membranes for Pervaporation. Molecules, 24(19), 3584. https://doi.org/10.3390/molecules24193584







