Abstract
A one-pot route to 2-alkyl and 2-aryl-4H-benzo[d][1,3]oxazin-4-ones (also known as 4H-3,1-benzoxazin-4-ones) has been developed and studied. The method involves the reaction of aryl-substituted anthranilic acids with orthoesters in ethanol catalyzed by acetic acid. Additionally, we have also investigated the reaction under microwave conditions. Not all of the substrates were successful in yielding the target heterocycles as some of the reactions failed to undergo the final elimination. This process led to the isolation of (±)-2-alkyl/aryl-2-ethoxy-1,2-dihydro-4H-benzo[d][1,3]oxazin-4-ones. The formation of the dihydro analogs correlated with the electron density on the aromatic ring: Electron-donating groups favored the 4H- benzo[d][1,3]oxazin-4-ones, while electron-withdrawing groups tended to favor the dihydro product. Substituting a pyridine ring for the benzene ring in the substrate acid suppressed the reaction.
1. Introduction
We recently reported the high-yield synthesis of quinazolin-4(3H)-ones by reaction of 2-aminobenzamides with orthoesters in ethanol promoted by acetic acid [1]. These heterocycles have received considerable attention as they have demonstrated diverse biological activities. With the success of this previous study, we sought to evaluate a similar strategy for the synthesis of 2-alkyl and 2-aryl-4H-benzo[d][1,3]oxazin-4-ones (also known as 4H-3,1-benzoxazin-4-ones). These compounds are less stable, and thus have been less well studied. An early account noted that several substituted structures related to compound 1 exhibited hypolipidemic activity [2]. Other benzoxazinones demonstrated potential as protease inhibitors. In particular, 4H-benzo[d][1,3]oxazin-4-one 2 was shown to have substrate inhibitory activity towards the serine protease human leukocyte elastase which is the presumed tissue degenerating agent in the pathogenesis of several diseases [3]. Two additional studies revealed that 3 and 4 interfered with the action of intramembrane serine proteases known as rhomboids that are important in the progression of diseases such as cancer, diabetes, invasion of apicomplexan parasites, and Parkinson’s disease [4,5]. Thus, access to these drug candidates would allow further study of the etiology of these diseases and could lead to new drugs to treat them. Structures 1–4 are shown in Figure 1. Apart from investigations of their potential use as drugs, 4H-benzo[d][1,3]oxazin-4-ones have also been used as precursors to new heterocycles [6] as well as interesting peptide based oligomers [7].
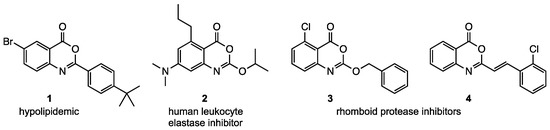
Figure 1.
4H-Benzo[d][1,3]oxazin-4-ones with potential drug activity.
Classic approaches to 4H-benzo[d][1,3]oxazin-4-ones involved reaction of anthranilic acid with two equivalents of benzoyl chloride in pyridine to afford >90% of 2-aryl-4H-benzo[d][1,3]oxazin-4-ones [2]. Similarly, treatment of substituted anthranilic acids with one equivalent of various acid chlorides to give the 2-amidobenzoic acid, followed by boiling in acetic anhydride, also afforded these products in good yields [8,9]. A later synthesis of 4H-benzo[d][1,3]oxazin-4-ones described a high-yield palladium-catalyzed cyclocarbonylation of o-iodoanilines with acid chlorides [10]. Furthermore, three oxidative approaches to these aryl-fused heterocycles have also been reported. In the first example, copper was used to promote the conversion of 2-alkynylanilines to 2-aryl-4H-benzo[d][1,3]oxazin-4-ones using base conditions under oxygen atmosphere [11]. Another approach utilized oxone in nitromethane at 100 °C to accomplish the oxidation of 2-arylindoles to these same targets. A third oxidative procedure involved the water assisted oxidation of 2-arylindoles to 2-aryl-4H-benzo[d][1,3]oxazin-4-ones using (diacetoxyiodo)benzene [12]. Finally, a method related to the current procedure was reported for a small series of compounds substituted by phenyl at C2. This procedure utilized microwave energy to promote the formation of 2-phenyl-4H-benzo[d][1,3]oxazin-4-ones from four anthranilic acids and triethyl orthobenzoate [13]. The yields varied in this reaction, but no problems were noted. In the current study, we have attempted to expand the scope of this transformation, examining the reaction of additional aryl-substituted anthranilic acids with four orthoesters. The results of our study are detailed below.
2. Results and Discussion
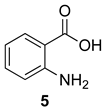
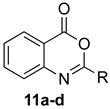
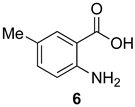
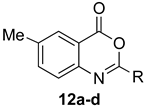
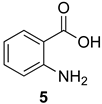
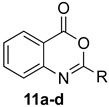
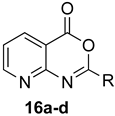
Scheme 1 depicts the focus and scope of the current study. We have explored the synthesis of a range of 4H-benzo[d][1,3]oxazin-4-ones from the reaction of six anthranilic acids with a range of commercially available orthoesters (Table 1 and Table 2). The reaction was run under two sets of conditions: one under thermal conditions promoted by acid, and the other under microwave conditions. The acid promoted conditions involved heating anthranilic acid (1 equivalent) with the orthoester (4.5 equivalents) in the presence of AcOH (2.6 equivalents), neat, at 100 °C, for 4–48 h. The microwave reaction was run using anthranilic acid (1 equivalent) and the orthoester (2.0–2.7 equivalents), neat, without AcOH, at 100 °C (400 W), for 0.75–3 h. Upon cooling, the products from both procedures crystallized from the crude reaction mixtures and were purified by trituration from 5% pentane in ether.
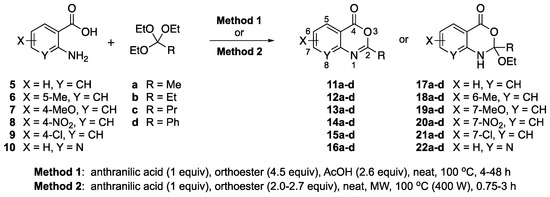
Scheme 1.
Formation of 4H-benzo[d][1,3]oxazin-4-ones and their dihydro analogs from anthranilic acids and orthoesters.

Table 1.
Formation of 4H-benzo[d][1,3]oxazin-4-ones.

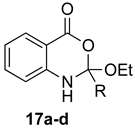
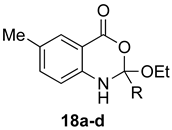
Table 2.
Formation of (±)-1,2-dihydro-4H-benzo[d][1,3]oxazin-4-ones.
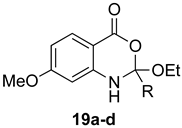
Contrary to what has been reported in related studies to date, we found that not all of the thermal reactions produced the target heterocycles cleanly. In several cases, (±)-2-substituted-1,2-dihydro-4H-benzo[d][1,3]oxazin-4-ones, resulting from ring closure without elimination of the final molecule of ethanol, were isolated. This led us to expand our study to evaluate microwave reactions in addition to the standard acid-promoted thermal reactions; however, both protocols afforded the intermediate dihydro compounds from specific substrates. In several cases, it was possible to isolate either product by modification of the procedure. For example, the reaction of anthranilic acid (5) with triethyl orthobenzoate (d) for 24 h under thermal conditions afforded dihydro product 17d, while extending the reaction to 48 h yielded 4H-benzo[d][1,3]oxazin-4-one 11d. In several other examples, however, it was not possible to induce the final elimination reaction.
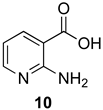
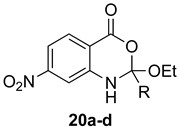
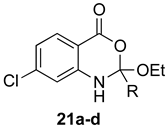
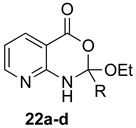
The outcome of the reaction appeared to correlate with the electron density in the aromatic ring of the anthranilic acid. For rings substituted with electron donating groups (OMe, Me) or hydrogen, the 4H-benzo[d][1,3]oxazin-4-one product predominated. More electron-poor aromatic rings bearing a second electron-withdrawing group on the aromatic ring (NO2 and Cl), in addition to the ortho carbonyl group, showed a tendency to give the 1,2-dihydro-4H-benzo[d][1,3]oxazin-4-one product. Changing the benzene ring of the anthranilic acid to an electron-deficient pyridine, as in 2-aminonicotinic acid (10), almost completely shut down the reaction.
In most cases, it was possible to isolate at least one of the products in pure form. The lone exception was the attempted conversion of 5 to 17c with triethyl orthobutyrate (c), which gave an inseparable mixture of the benzoxazinone and dihydro product under all conditions. Attempts to drive the reaction to the benzoxazinone using 5 equivalents of AcOH or spiking the reaction with H2SO4 failed to alter the outcome, while treatment with base (K2CO3, EtOH, molecular sieves) resulted in ring opening to the N-acylanthranilic acid. Finally, in attempted reactions of 2-aminonicotinic acid (10), triethyl orthoacetate (a) proceeded to give dihydro product 21a, but all others afforded only ethyl 2-aminonicotinate resulting from esterification of the substrate acid.
The presumed mechanism for the formation of 4H-benzo[d][1,3]oxazin-4-ones is shown in Scheme 2. The process involves initial protonation of the orthoester and loss of ethanol to afford the stabilized carbocation A. Attack on A by the anthranilic acid amino group, proton exchange and loss of a second molecule of ethanol would then yield iminium ion B. Subsequent closure of the acid oxygen on the iminium carbon and proton exchange would give ring closed dihydro product C. Finally, protonation of C to give D and loss of ethanol would then generate the desired heterocycle E.
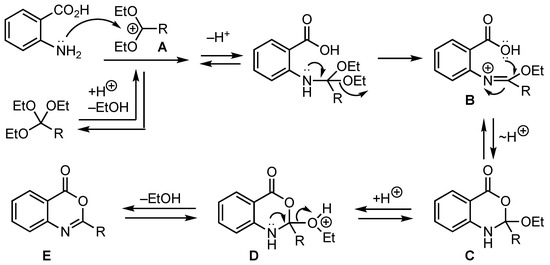
Scheme 2.
Presumed mechanism for formation of 4H-benzo[d][1,3]oxazin-4-ones.
Difficulty was noted in the final elimination of ethanol when the aromatic ring carried a second electron-withdrawing group (EWG) in addition to the adjacent ester carbonyl. For this elimination, the availability of the unshared electron pair on N1 and its ability to align antiperiplanar to the departing alcohol were assumed to be keys to the success of this elimination. The availability of these electrons, however, would be diminished in substrates with a strongly electron-deficient ring. Relatively little seems to be known about the necessity for the antiperiplanar alignment of the unshared pair for efficient elimination in this system. This anomeric effect has been extensively studied in acetals and hemiacetals [14], but much less so for their nitrogen analogs since elimination is normally facile. If achievable, conformation D1 would result in good overlap for elimination of ethanol (see Figure 2). Placement of the C2 ethoxy group in a pseudo-axial position for this elimination should be favorable since this group is considered to be sterically smaller than an alkyl group [15,16]. However, this conformation also maximizes overlap of the N1 electron pair with the aromatic ring as well as the ortho carbonyl, and this could slow the elimination if the aromatic ring bears an additional electron-withdrawing substituent. The alternative conformation D2 is not optimally aligned for elimination (see Figure 2). If the ring closure and protonation steps led to this conformation, conversion to D1 could occur, but would likely be sluggish since flexibility in this rigid system is allowed only at the saturated atoms of the benzoxazinone.
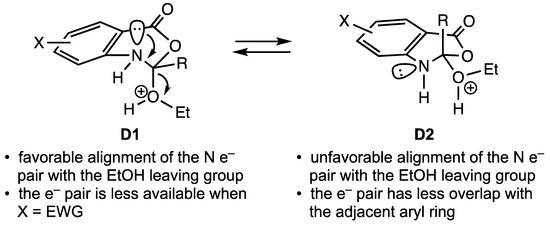
Figure 2.
Conformational options for intermediate D.
3. Experimental Section
3.1. General
All commercial reagents and solvents were used as received. Unless otherwise indicated, all reactions were carried out under dry N2 in oven-dried glassware. Reactions were monitored by thin layer chromatography (TLC) using silica gel GF plates (Analtech No. 21521, Newark, DE, USA). Microwave reactions were performed in 2–5 mL microwave tubes (Biotage No. 354624, Charlotte, NC, USA) at 100 °C using a Biotage Initiator 2.5 system (Charlotte, NC, USA) at a power of 400 W from a magneton at 2.45 GHz. Melting points were obtained on a MEL-TEMP apparatus (Laboratory Devices, Cambridge, MA, USA), and were uncorrected. FT-IR spectra were run using a Varian Scimitar FTS 800 spectrophotometer (Randolph, MA, USA) as thin films or nujol mulls on NaCl disks. 1H- and 13C-NMR spectra were measured using a Bruker Avance 400 system (Billerica, MA, USA) in the indicated solvents (Cambridge Isotope, Andover, MA, USA) at 400 MHz and 101 MHz, respectively, with (CH3)4Si as the internal standard; coupling constants (J) are given in Hz. Copies of NMR spectra for the products are included in the Supplementary Information. High-resolution mass spectra (HRMS-ESI) were obtained using a Thermo LTQ-Orbitrap XL mass spectrometer (Thermo Scientific, Waltham, MA, USA).
3.2. Representative Procedure under Thermal Conditions. Method 1
The anthranilic acid (1.0 mmol) and the orthoester (4.5 mmol) were placed in a 15-mL Chemglass screw-cap pressure tube (CG-1880-01, Vineland, NJ, USA). Glacial acetic acid (2.6 mmol) was added, N2 was introduced to the vessel and the cap was tightened. The vessel was heated at 100 °C under neat conditions for 4–48 h, and then cooled to room temperature. Upon cooling, the crude product crystallized from the reaction mixture and was purified by trituration from 5% ether in pentane.
3.3. Representative Procedure under Microwave Conditions. Method 2
A solution of the anthranilic acid (1.0 mmol) in the orthoester (0.45 mL, 2.0–2.7 equivalents) was prepared in a 5-mL microwave tube and stirred for 30 s prior to irradiation at the "high absorption" setting. The reaction was performed under N2 at 100 °C (400 W) for 0.75–3 h. Upon cooling, the crude product crystallized from the reaction mixture and was purified by trituration from 5% ether in pentane.
The following compounds were prepared:
2-Methyl-4H-benzo[d][1,3]oxazin-4-one (11a): 130 mg (81%, method 1) and 132 mg (82%, method 2) as white crystals, m.p. 79–80 °C (ref. [8] 80–81 °C); IR (thin film): 1700, 1624 cm−1; 1H-NMR (400 MHz, CDCl3): δ 8.29 (dd, 1H, J = 7.5, 1.6 Hz, ArH), 7.78 (ddd, 1H, J = 8.4, 7.1, 1.6 Hz, ArH), 7.69 (d, 1H, J = 8.4 Hz, ArH), 7.48 (ddd, 1H, J = 8.1, 7.1, 1.2 Hz, ArH), 2.60 (s, 3H, CH3); 13C-NMR (101 MHz, CDCl3): δ 164.1, 153.2, 149.4, 134.9, 127.0, 126.5, 126.3, 120.3, 22.2; HRMS (ESI): m/z [M + H]+ calcd. for C9H7NO2: 162.0555, found: 162.0553.
2-Ethyl-4H-benzo[d][1,3]oxazin-4-one (11b): 140 mg (80%, method 1) and 145 mg (83%, method 2) as white crystals, m.p. 83–84 °C (ref. [8] m.p. 85–86 oC); IR (thin film): 1750, 1645 cm−1; 1H-NMR (400 MHz, CDCl3): δ 8.20 (dd, 1H, J = 7.9, 1.5 Hz, ArH), 7.80 (ddd, 1H, J = 8.4, 7.5, 1.5 Hz, ArH), 7.57 (d, 1, J = 8.4 Hz, ArH), 7.50 (ddd, 1H, J = 8.2, 7.5, 1.2 Hz, ArH), 2.73 (q, 2H, J = 7.5 Hz, CH2CH3), 1.37 (t, 3H, J = 7.5 Hz, CH2CH3); 13C-NMR (101 MHz, CDCl3): δ 163.9, 159.9, 146.5, 136.5, 128.4, 128.1, 126.6, 116.9, 28.2, 10.3; HRMS (ESI): m/z [M + H]+ calcd. for C10H9NO2: 176.0712, found: 176.0713.
2-Phenyl-4H-benzo[d][1,3]oxazin-4-one (11d): 174 mg (78%, method 1) and 179 mg (80%, method 2) as off-white crystals, m.p. 121–122 °C (ref. [8] m.p. 123 °C); IR (thin film): 1762, 1615 cm−1; 1H-NMR (400 MHz, CDCl3): δ 8.34-8.30 (complex, 2H, ArH), 8.25 (dd, 1H, J = 7.9, 1.5, ArH), 7.84 (ddd, 1H, J = 8.1, 7.3, 1.5 Hz, ArH), 7.70 (d, J = 8.1 Hz, ArH), 7.62-7.48 (complex, 4H, ArH); 13C-NMR (101 MHz, CDCl3): δ 159.6, 157.1, 147.0, 136.6, 132.6, 130.3, 128.8, 128.6, 128.32, 128.26, 127.2, 117.0; HRMS (ESI): m/z [M + H]+ calcd. for C14H9NO2: 224.0712, found: 224.0709.
2,6-Dimethyl-4H-benzo[d][1,3]oxazin-4-one (12a): 119 mg (68%, method 2) as off-white crystals, m.p. 122–123 °C; IR (thin film): 1682, 1595 cm−1; 1H-NMR (400 MHz, CDCl3): δ 7.98 (s, 1H, ArH), 7.61 (d, 1H, J = 8.2 Hz, ArH), 7.44 (d, 1H, J = 8.2 Hz, ArH), 2.47 (s, 3H, CH3), 2.45 (s, 3H, CH3); 13C-NMR (101 MHz, CDCl3): δ 159.9, 159.4, 144.3, 138.5, 137.7, 128.0, 126.2, 116.3, 21.3, 21.2; HRMS (ESI): m/z [M + H]+ calcd. for C10H9NO2: 176.0712, found: 176.0709.
2-Ethyl-6-methyl-4H-benzo[d][1,3]oxazin-4-one (12b): 125 mg (66%, method 2) as white crystals, m.p. 103–104 °C; IR (thin film): 1686, 1596 cm−1; 1H-NMR (400 MHz, CDCl3): δ 7.98 (s, 1H, ArH), 7.60 (d, 1H, J = 8.2 Hz, ArH), 7.46 (d, 1H, J = 8.2 Hz, ArH), 2.71 (q, 2H, J = 7.5 Hz, CH2CH3), 2.47 (s, 3H, ArCH3), 1.36 (t, 3H, J = 7.5 Hz, CH2CH3); 13C-NMR (101 MHz, CDCl3): δ 163.1, 160.1, 144.3, 138.4, 137.7, 128.0, 126.4, 116.5, 26.1, 21.2, 10.3; HRMS (ESI): m/z [M + H]+ calcd. for C11H11NO2: 190.0868, found: 190.0865.
6-Methyl-2-phenyl-4H-benzo[d][1,3]oxazin-4-one (12d): 202 mg (85%, method 2) as off-white crystals, m.p. 144–145 °C; IR (thin film): 1752, 1607 cm−1; 1H-NMR (400 MHz, CDCl3): δ 8.29 (d, 2H, J = 8.2 Hz, ArH), 8.04 (s, 1H, ArH), 7.66-7.47 (complex, 5H, ArH), 2.49 (s, 3H, ArCH3); 13C-NMR (101 MHz, CDCl3): δ 159.8, 156.4, 144.8, 138.7, 137.8, 132.4, 130.4, 128.7, 128.19, 128.17, 127.0, 116.7, 21.2; HRMS (ESI): m/z [M + H]+ for C15H11NO2: 238.0868, found: 238.0870.
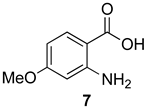
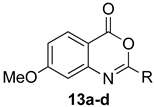
7-Methoxy-2-methyl-4H-benzo[d][1,3]oxazin-4-one (13a): 166 mg (87%, method 1) and 168 mg (88%, method 2) as light yellow crystals, m.p. 119–120 °C; IR (nujol): 2830, 1747, 1610 cm−1; 1H-NMR (400 MHz, DMSO-d6): δ 8.00 (d, 1H, J = 8.8 Hz, ArH), 7.14 (dd, 1H, J = 8.8, 2.5 Hz, ArH), 7.05 (d, 1H, J = 2.5 Hz, ArH), 3.91 (s, 3H, OCH3), 2.38 (s, 3H, CH3); 13C-NMR (101 MHz, DMSO-d6): δ 166.3, 161.5, 159.2, 149.0, 130.2, 117.1, 109.5, 109.1, 56.5, 21.5; HRMS (ESI): m/z [M + H]+ calcd. for C10H9NO3: 192.0661, found: 192.0659.
2-Ethyl-7-methoxy-4H-benzo[d][1,3]oxazin-4-one (13b): 176 mg (86%, method 1) and 181 mg (88%, method 2) as light yellow crystals, m.p. 89–90 °C; IR (nujol): 2840, 1767, 1644, 1609 cm−1; 1H-NMR (400 MHz, DMSO-d6): δ 7.99 (d, 1H, J = 8.8 Hz, ArH) 7.14 (dd, 1H, J = 8.8, 2.5 Hz, ArH), 7.07 (d, 1H, J = 2.5 Hz, ArH), 3.92 (s, 3H, OCH3), 2.68 (q, 2H, J = 7.5 Hz, CH2CH3), 1.24 (t, 3H, J = 7.5 Hz, CH2CH3); 13C-NMR (101 MHz, DMSO-d6): δ 166.3, 164.9, 159.2, 149.0, 130.1, 117.3, 109.7, 109.1, 56.6, 27.7, 10.2; HRMS (ESI): m/z [M + H]+ calcd. for C11H11NO3: 206.0817, found: 206.0817.
7-Methoxy-2-propyl-4H-benzo[d][1,3]oxazin-4-one (13c): 189 mg (86%, method 1) and 191 mg (87%, method 2) as light yellow crystals, m.p. 77–78 °C; IR (nujol): 2841, 1766, 1643, 1609 cm−1; 1H-NMR (400 MHz, DMSO-d6): δ 8.00 (d, 1H, J = 8.8 Hz, ArH), 7.14 (dd, 1H, J = 8.8, 2.5 Hz, ArH), 7.07 (d, 1H, J = 2.5 Hz, ArH), 3.92 (s, 3H, OCH3), 2.63 (t, 2H, J = 7.4 Hz, CH2CH2CH3), 1.75 (sextet, 2H, J = 7.4 Hz, CH2CH2CH3); 0.98 (t, 3H, J = 7.4 Hz, CH2CH2CH3); 13C-NMR (101 MHz, DMSO-d6): δ 166.3, 163.9, 159.2, 148.9, 130.1, 117.3, 109.7, 109.1, 56.6, 36.2, 19.2, 13.9; HRMS (ESI): m/z [M + H]+ calcd. for C12H13NO3: 220.0974, found: 220.0971.
7-Methoxy-2-phenyl-4H-benzo[d][1,3]oxazin-4-one (13d): 210 mg (83%, method 1) and 215 mg (85%, method 2) as light yellow crystals, m.p. 149–150 °C; IR (nujol): 2842, 1744, 1607 cm−1; 1H-NMR (400 MHz, DMSO-d6): δ 8.17 (d, 2H, J = 8.1 Hz, ArH), 8.03 (d, 1H, J = 9.4 Hz, ArH), 7.67 (d, 1H, J = 7.3 Hz, ArH), 7.59 (t, 2H, J = 8.1 Hz, ArH), 7.15 (m, 2H, ArH), 3.94 (s, 3H, OCH3); 13C-NMR (101 MHz, DMSO-d6): δ 166.4, 158.8, 157.6, 149.2, 133.2, 130.5, 130.3, 129.4, 128.3, 117.6, 109.9, 109.6, 56.6; HRMS (ESI): m/z [M + H]+ calcd. for C15H11NO3: 254.0817, found: 254.0814.
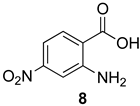
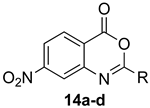
7-Nitro-2-phenyl-4H-benzo[d][1,3]oxazin-4-one (14d): 244 mg (91%, method 2) as light orange crystals, m.p. 175–176 °C; IR (thin film): 1757, 1629, 1608, 1530, 1349 cm−1; 1H-NMR (400 MHz, CDCl3): δ 8.52 (d, 1H, J = 2.2 Hz, ArH), 8.41 (d, 1H, J = 8.6 Hz, ArH), 8.33 (d, 2H, J = 7.4 Hz, ArH), 8.29 (dd, 1H, J = 8.6, 2.2 Hz, ArH), 7.64 (t, 1H, J = 7.4 Hz, ArH), 7.55 (t, 2H, J = 7.4 Hz, ArH); 13C-NMR (101 MHz, CDCl3): δ 159.0, 157.9, 152.9, 148.1, 133.6, 130.4, 129.3, 129.0, 128.7, 122.5, 122.0, 121.3; HRMS (ESI): m/z [M + H]+ calcd. for C14H8N2O4: 269.0562, found: 269.0558.
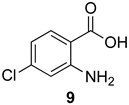
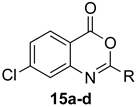
7-Chloro-2-methyl-4H-benzo[d][1,3]oxazin-4-one (15a): 166 mg (85%, method 2) as white crystals, m.p. 149–150 °C; IR (thin film): 1762, 1696, 1642, 1596 cm−1; 1H-NMR (400 MHz, CDCl3): δ 8.11 (d, 1H, J = 8.4 Hz, ArH), 7.54 (d, 1H, J = 2.0 Hz, ArH), 7.46 (dd, 1H, J = 8.4, 2.0 Hz, ArH), 2.47 (s, 3H CH3); 13C-NMR (101 MHz, CDCl3): δ 161.6, 158.9, 147.5, 142.9, 129.7, 128.8, 126.3, 115.1, 21.4; HRMS (ESI): m/z [M + H]+ calcd. for C9H635ClNO2: 196.0165, found: 196.0166.
7-Chloro-2-phenyl-4H-benzo[d][1,3]oxazin-4-one (15d): 237 mg (92%, method 2) as off-white crystals, m.p. 188–189 °C; IR (thin film): 1760, 1619, 1596 cm−1; 1H-NMR (400 MHz, CDCl3): δ 8.31 (d, 2H, J = 7.4 Hz, ArH), 8.17 (d, 1H, J = 8.4 Hz, ArH), 7.70 (d, 1H, J = 2.0 Hz, ArH), 7.60 (tt, 1H, J = 7.4, 1.4 Hz, ArH), 7.52 (t, 2H, J = 7.4 Hz, ArH), 7.48 (dd, 1H, J = 8.4, 2.0 Hz, ArH); 13C-NMR (101 MHz, CDCl3): δ 158.8, 158.3, 148.1, 143.0, 133.0, 129.9, 129.86, 128.83, 128.79, 128.5, 127.0, 115.4; HRMS (ESI): m/z [M + H]+ calcd. for C14H835ClNO2: 258.0322, found: 258.0317.
(±)-2-Ethoxy-2-phenyl-1,2-dihydro-4H-benzo[d][1,3]oxazin-4-one (17d): 232 mg (86%, method 1) as white crystals, m.p. 92–93 °C; IR (thin film): 3254, 1668, 1595 cm−1; 1H-NMR (400 MHz, CDCl3): δ 12.09 (br s, 1H, NH), 8.94 (dd, 1H, J = 8.5, 1.1 Hz), ArH), 8.1 (dd, 1H, J = 8.0, 1.7 Hz, ArH), 8.06 (dd, 2H, J = 7.8, 1.5 Hz, ArH), 7.64-7.49 (complex, 4H, ArH), 7.13 (td, 1H, J = 7.4, 1.2 Hz, ArH), 4.43 (q, 2H, J = 7.1 Hz, OCH2CH3), 1.43 (t, 3H, J = 7.1 Hz, OCH2CH3); 13C-NMR (101 MHz, CDCl3): δ 168.6, 165.4, 141.9, 134.9, 134.7, 131.9, 130.9, 128.8, 127.4, 122.6, 120.5, 115.5, 61.5, 14.2; HRMS (ESI): m/z [M + H]+ calcd. for C16H15NO2: 254.1181, found: 254.1177.
(±)-2-Ethoxy-2,6-dimethyl-1,2-dihydro-4H-benzo[d][1,3]oxazin-4-one (18a): 195 mg (88%, method 1) as white crystals, m.p. 110–111 °C; IR (thin film): 3266, 1677, 1595 cm−1; 1H-NMR (400 MHz, CDCl3): δ 10.97 (br s, 1H, NH), 8.57 (d, 1H, J = 8.6 Hz, ArH), 7.83 (s, 1H, ArH), 7.34 (d, 1H, J = 8.6 Hz, ArH), 4.37 (q, 2H, J = 7.1 Hz, OCH2CH3), 2.33 (s, 3H, ArCH3), 2.22 (s, 3H, CH3), 1.42 (t, 3H, J = 7.1 Hz, OCH2CH3); 13C-NMR (101 MHz, CDCl3): δ 168.9, 168.4, 139.2, 135.3, 131.9, 130.8, 120.3, 115.0, 61.3, 25.5, 20.7, 14.2; HRMS (ESI): m/z [M + H]+ calcd. for C12H15NO3: 222.1130, found: 222.1128.
(±)2-Ethoxy-2-ethyl-6-methyl-1,2-dihydro-4H-benzo[d][1,3]oxazin-4-one (18b): 205 mg (87%, method 1) as white crystals, m.p. 78–79 °C; IR (thin film): 3261, 1677, 1596 cm−1; 1H-NMR (400 MHz, CDCl3): δ 11.0 (br s, 1H, NH), 8.62 (d, 1H, J = 8.6 Hz, ArH), 7.83 (s, 1H, ArH), 7.34 (d, 1H, J = 8.6 Hz, ArH), 4.37 (q, 2H, J = 7.0 Hz, OCH2CH3), 2.46 (q, 2H, J = 7.5 Hz, CH2CH3), 2.33 (s, 3H, ArCH3), 1.42 (t, 3H, J = 7.0 Hz, OCH2CH3), 1.27 (t, 3H, J = 7.5 Hz, CH2CH3); 13C-NMR (101 MHz, CDCl3): δ 172.7, 168.4, 139.4, 135.3, 131.7, 130.8, 120.3, 114.9, 61.3, 31.7, 20.7, 14.2, 9.7; HRMS (ESI): m/z [M + H]+ calcd. for C13H17NO3: 236.1287, found: 236.1284.
(±)-2-Ethoxy-6-methyl-2-propyl-1,2-dihydro-4H-benzo[d][1,3]oxazin-4-one (18c): 219 mg (88%, method 1) and 222 mg (89%, method 2) as off-white crystals, m.p. 39–40 °C; IR (thin film): 3265, 1682, 1599 cm−1; 1H-NMR (400 MHz, CDCl3): δ 10.98 (br s, 1H, NH), 8.62 (d, 1H, J = 8.6 Hz, ArH), 7.83 (s, 1H, ArH), 7.35 (d, 1H, J = 8.6 Hz, ArH), 4.38 (q, 2H, J = 7.1 Hz, OCH2CH3), 2.41 (q, 2H, J = 7.5 Hz, CH2CH2CH3), 2.33 (s, 3H, ArCH3), 1.78 (sextet, 2H, J = 7.5 Hz, CH2CH2CH3), 1.42 (t, 3H, J = 7.1 Hz, OCH2CH3), 1.01 (t, 3H, J = 7.5 Hz, CH2CH2CH3); 13C-NMR (101 MHz, CDCl3): δ 171.9, 168.4, 139.3, 135.3, 131.7, 130.8, 120.3, 114.9, 61.3, 40.6, 20.7, 19.0, 14.2, 13.8; HRMS (ESI): m/z [M + H]+ calcd. for C14H19NO3: 250.1443, found: 250.1441.
(±)-2-Ethoxy-6-methyl-2-phenyl-1,2-dihydro-4H-benzo[d][1,3]oxazin-4-one (18d): 249 mg (88%, method 1) as white crystals, m.p. 117–118 °C; IR (thin film): 3249, 1662, 1602 cm−1; 1H-NMR (400 MHz, CDCl3): δ 11.98 (br s, 1H, NH), 8.82 (d, 1H, J = 8.5 Hz, ArH), 8.05 (d, 2H, J = 6.8 Hz, ArH), 7.89 (s, 1H, ArH), 7.58-7.47 (complex, 3H), 7.42 (d, 1H, J = 8.5 Hz, ArH), 4.42 (q, 2H, J = 7.1 Hz, OCH2CH3), 3.36 (s, 3H, ArCH3), 1.44 (q, 3H, J = 7.1 Hz, OCH2CH3); 13C-NMR (101 MHz, CDCl3): δ 168.9, 165.5, 139.5, 135.4, 135.1, 132.1, 131.8, 131.0, 128.8, 127.3, 120.4, 115.4, 61.5, 20.8, 14.3; HRMS (ESI): m/z [M + H]+ calcd. for C17H17NO3: 284.1287, found: 284.1283.
(±)-2-Ethoxy-2-methyl-7-nitro-1,2-dihydro-4H-benzo[d][1,3]oxazin-4-one (20a): 212 mg (84%, method 1) and 219 mg (87%, method 2) as light tan crystals, m.p. 108–109 °C; IR (thin film): 3270, 1702, 1604, 1538, 1350 cm−1; 1H-NMR (400 MHz, CDCl3): δ 11.16 (br s, 1H, NH), 9.60 (d, 1H, J = 2.4 Hz, ArH), 8.20 (d, 1H, J = 8.8 Hz, ArH), 7.87 (dd, 1H, J = 8.8, 2.4 Hz, ArH), 4.45 (q, 2H, J = 7.1 Hz, OCH2CH3), 2.28 (s, 3H, CH3), 1.46 (t, 3H, J = 7.1 Hz, OCH2CH3); 13C-NMR (101 MHz, CDCl3): δ 169.2, 166.9, 151.2, 142.2, 131.9, 119.3, 116.4, 115.2, 62.5, 25.5, 14.1; HRMS (ESI): m/z [M + H]+ calcd. for C11H12N2O5: 253.0825, found: 253.0824.
(±)-2-Ethoxy-2-ethyl-7-nitro-1,2-dihydro-4H-benzo[d][1,3]oxazin-4-one (20b): 229 mg (86%, method 1) and 234 mg (88%, method 2) as light tan crystals, m.p. 74–75 °C; IR (thin film): 3273, 1694, 1600, 1538, 1349 cm−1; 1H-NMR (400 MHz, CDCl3): δ 11.18 (br s, 1H, NH), 9.65 (d, 1H, J = 2.4 Hz, ArH), 8.20 (d, 1H, J = 8.8 Hz, ArH), 7.86 (dd, 1H, J = 8.8, 2.4 Hz, ArH), 4.45 (q, 2H, J = 7.1 Hz, OCH2CH3), 2.53 (q, 2H, J = 7.5 Hz, CH2CH3), 1.46 (t, 3H, J = 7.1 Hz, OCH2CH3), 1.30 (t, 3H, J = 7.5 Hz, CH2CH3); 13C-NMR (101 MHz, CDCl3): δ 173.1, 166.9, 151.2, 142.5, 131.9, 119.3, 116.3, 115.3, 62.4, 31.6, 14.1, 9.3; HRMS (ESI): m/z [M + H]+ calcd. for C12H14N2O5: 267.0981, found: 267.0977.
(±)-2-Ethoxy-2-propyl-7-nitro-1,2-dihydro-4H-benzo[d][1,3]oxazin-4-one (20c): 238 mg (85%, method 1) and 245 mg (88%, method 2) as light tan crystals, m.p. 66–67 °C; IR (thin film): 3270, 1697, 1604, 1538, 1349 cm−1; 1H-NMR (400 MHz, CDCl3): δ 11.17 (br s, 1H, NH), 9.65 (d, 1H, J = 2.4 Hz, ArH), 8.20 (d, 1H, J = 8.8 Hz, ArH), 7.86 (dd, 1H, J = 8.8, 2.4 Hz, ArH), 4.45 (q, 2H, J = 7.1 Hz, OCH2CH3), 2.47 (q, 2H, J = 7.4 Hz, CH2CH2CH3), 1.81 (sextet, 2H, J = 7.4 Hz, CH2CH2CH3), 1.46 (t, 3H, J = 7.1 Hz, OCH2CH3), 1.04 (t, 3H, J = 7.4 Hz, CH2CH2CH3); 13C-NMR (101 MHz, CDCl3): δ 172.3, 166.9, 151.2, 142.5, 131.9, 119.3, 116.3, 115.3, 62.4, 40.4, 18.8, 14.1, 13.7; HRMS (ESI): m/z [M + H]+ calcd. for C13H16N2O5: 281.1138, found: 281.1140.
(±)-2-Ethoxy-7-nitro-2-phenyl-1,2-dihydro-4H-benzo[d][1,3]oxazin-4-one (20d): 283 mg (90%, method 1) as off-white crystals, m.p. 180–181 °C; IR (thin film): 3257, 1673, 1651, 1600, 1538, 1346 cm−1; 1H-NMR (400 MHz, CDCl3): δ 11.62 (br s, 1H, NH), 9.31 (d, 1H, J = 2.4 Hz, ArH), 8.20 (d, 1H, J = 8.8 Hz, ArH), 8.03 (dd, 1H, J = 8.8, 2.4 Hz, ArH), 7.97 (d, 2H, J = 7.4 Hz, ArH, 7.69 (t, 1H, J = 7.4 Hz, ArH), 7.61 (t, 2H, J = 7.4 Hz, ArH), 4.38 (q, 2H, J = 7.1 Hz, OCH2CH3), 1.33 (t, 3H, J = 7.1 Hz, OCH2CH3); 13C-NMR (101 MHz, CDCl3): δ 166.7, 165.7, 150.6, 141.0, 134.1, 133.1, 132.7, 129.5, 127.7, 1239, 118.2, 116.0, 62.6, 14.3; HRMS (ESI): m/z [M + H]+ calcd. for C16H14N2O5: 315.0981, found: 315.0975.
(±)-7-Chloro-2-ethoxy-2-methyl-1,2-dihydro-4H-benzo[d][1,3]oxazin-4-one (21a): 203 mg (84%, method 1) as white crystals, m.p. 75–76 °C; IR (thin film): 3262, 1681, 1578 cm−1; 1H-NMR (400 MHz, CDCl3): δ 10.70 (br s, 1H, NH), 8.43 (d, 1H, J = 2.2 Hz, ArH), 7.92 (d, 1H, J = 8.6 Hz, ArH), 7.23 (dd, 1H, J = 8.6, 2.2 Hz, ArH), 4.83 (q, 2H, J = 7.1 Hz, OCH2CH3), 2.15 (s, 3H, CH3), 1.34 (t, 3H, J = 7.1 Hz, OCH2CH3); 13C-NMR (101 MHz, CDCl3): δ 169.1, 167.7, 142.4, 140.8, 131.8, 122.6, 120.1, 113.2, 61.6, 25.5, 14.2; HRMS (ESI): m/z [M + H]+ calcd. for C11H1235ClNO3: 242.0584, found: 242.0582.
(±)-7-Chloro-2-ethoxy-2-ethyl-1,2-dihydro-4H-benzo[d][1,3]oxazin-4-one (21b): 215 mg (84%, method 1) and 220 mg (86%, method 2) as white crystals, m.p. 69–70 °C; IR (thin film): 3238, 1685, 1581 cm−1; 1H-NMR (400 MHz, CDCl3): δ 11.17 (br s, 1H, NH), 8.86 (d, 1H, J = 2.2 Hz, ArH), 7.96 (d, 1H, J = 8.6 Hz, ArH), 7.04 (dd, 1H, J = 8.6, 2.2 Hz, ArH), 4.38 (q, 2H, J = 7.1 Hz, OCH2CH3), 2.48 (q, 2H, J = 7.5 Hz, CH2CH3), 1.41 (t, 3H, J = 7.1 Hz, OCH2CH3), 1.27 (t, 3H, J = 7.5 Hz, CH2CH3); 13C-NMR (101 MHz, CDCl3): δ 173.0, 167.8, 142.6, 140.9, 131.8, 122.5, 120.2, 113.2, 61.6, 31.7, 14.2, 9.5; HRMS (ESI): m/z [M + H]+ calcd. for C12H1435ClNO3: 256.0741, found: 256.0743.
(±)-7-Chloro-2-ethoxy-2-propyl-1,2-dihydro-4H-benzo[d][1,3]oxazin-4-one (21c): 224 mg (83%, method 1) and 227 mg (84%, method 2) as white semisolid, m.p. 25–26 °C; IR (thin film): 3280, 1691, 1581cm−1; 1H-NMR (400 MHz, CDCl3): δ 11.16 (br s, 1H, NH), 8.85 (s, 1H, ArH), 7.96 (d, 1H, J = 8.6 Hz, ArH), 7.04 (dd, 1H, J = 8.6, 2.2 Hz, ArH), 4.38 (q, 2H, J = 7.1 Hz, OCH2CH3), 2.42 (q, 2H, J = 7.4 Hz, CH2CH2CH3), 1.78 (sextet, 2H, J = 7.4 Hz, CH2CH2CH3), 1.42 (t, 3H, J = 7.1 Hz, OCH2CH3), 1.02 (t, 3H, J = 7.4 Hz, CH2CH2CH3); 13C-NMR (101 MHz, CDCl3): δ 172.2, 167.7, 142.5, 140.8, 131.8, 122.5, 120.2, 113.2, 61.6, 40.5, 18.9, 14.2, 13.2; HRMS (ESI): m/z [M + H]+ calcd. for C13H1635ClNO3: 270.0897, found: 270.0893.
(±)-7-Chloro-2-ethoxy-2-phenyl-1,2-dihydro-4H-benzo[d][1,3]oxazin-4-one (21d): 258 mg (85%, method 1) as white crystals, m.p. 128–129 °C; IR (thin film): 3241, 1673, 1585 cm−1; 1H-NMR (400 MHz, CDCl3): δ 12.13 (br s, 1H, NH), 9.05 (d, 1H, J = 2.1 Hz, ArH), 8.03 (overlapping signals, apparent t, 3H, J ≈ 8.4 Hz, ArH), 7.60-7.49 (complex, 3H, ArH), 7.08 (dd, 1H, J = 8.6, 2.1 Hz, ArH), 4.42 (q, 2H, J = 7.1 Hz, OCH2CH3), 1.43 (t, 3H, J = 7.1 Hz, OCH2CH3); 13C-NMR (101 MHz, CDCl3): δ 168.1, 165.7, 142.7, 141.0, 134.4, 132.2, 132.0, 128.9, 127.4, 122.8, 120.3, 113.6, 61.8, 14.2; HRMS (ESI): m/z [M + H]+ calcd. for C16H1435ClNO3: 304.0741, found: 304.0737.
(±)-2-Ethoxy-2-methyl-1,2-dihydro-4H-pyrido[2,3-d][1,3]oxazin-4-one (22a): 177 mg (85%, method 1) as white crystals, m.p. 55–56 °C; IR (thin film): 3270, 1680, 1589 cm−1; 1H-NMR (400 MHz, CDCl3): δ 10.79 (br s, 1H, NH), 8.59 (dd, 1H, J = 4.8, 2.0 Hz, PyH), 8.33 (dd, 1H, J = 7.9, 2.0 Hz, PyH), 7.06 (dd, 1H, J = 7.9, 4.8 Hz, PyH), 4.41 (q, 2H, J = 2.1 Hz, OCH2CH3), 2.42 (s, 3H, CH3), 1.42 (t, 3H, J = 7.1 Hz, OCH2CH3); 13C-NMR (101 MHz, CDCl3): δ 169.6, 166.6, 152.9, 152.5, 140.0, 118.0, 111.1, 62.0, 26.0, 14.2; HRMS (ESI): m/z [M + H]+ calcd. for C10H12N2O3: 209.0926, found: 209.0925.
4. Conclusions
We have studied the formation of 4H-benzo[d][1,3]oxazin-4-ones under thermal conditions with acetic acid as well as microwave conditions without acid. In most cases, the target heterocycle was formed in high yield. The reaction was performed with no solvent, and the desired products were isolated by crystallization and trituration. The microwave reaction proceeded much faster (0.75–3 h) than the thermal reactions (4–48 h) and did not require added acid. Thus, the microwave protocol represents the superior procedure. Stereoelectronic effects strongly impacted the outcome of the reaction. In addition to the benzoxazinone products, a dihydro intermediate resulting from failure to undergo the final elimination was observed in some substrates. The formation of the dihydro compound arose in reactants where the fused aromatic ring possessed a second electron-withdrawing group in addition to the conjugated carbonyl on the ortho carbon. This could deactivate the electron pair on N1 and slow the elimination process, even if the C2 ethoxy group is able to adopt the required pseudo-axial conformation. The formation of these dihydro systems provides access to a new substitution pattern in this family of compounds, and could yield additional candidates for biological testing. This finding coupled with the fact that orthoesters are easily prepared from nitriles [17,18] should provide a route to many members of this novel class of structures.
Supplementary Materials
The supplemental materials are available online.
Author Contributions
J.K.A-G. did the experimental work. R.A.B. directed the project, checked the data, and wrote the paper. Each of the authors read and approved the final version of the manuscript before submission.
Funding
This research was funded, in part, by the NSF MRI Program (BIR-9512269).
Acknowledgments
J.K.A-G. wishes to thank the Oklahoma State University (OSU) Department of Chemistry for a teaching assistantship. The authors are also grateful for support from the NSF (BIR-9512269), the Oklahoma State Regents for Higher Education, the W. M. Keck Foundation, and Conoco, Inc. that established the Oklahoma State-wide NMR facility. The authors also thank the OSU College of Arts and Sciences for funds that allowed the Chemistry Department to purchase a new 400 MHz NMR for this facility.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gavin, J.T.; Annor-Gyamfi, J.K.; Bunce, R.A. Quinazolin-4(3H)-ones and 5,6-dihydropyrimidin-4(3H)-ones from β-aminoamides and orthoesters. Molecules 2018, 23, 2925. [Google Scholar] [CrossRef] [PubMed]
- Fenton, G.; Newton, C.G.; Wyman, B.M.; Bagge, P.; Dron, D.I.; Riddell, D.; Jones, G.D. Hypolipidemic 2-[4-(1,1-dimethylethyl)phenyl-4H-3,1-benzoxazin-4-ones. Structure activity relationships of a novel series of high density lipoprotein elevators. J. Med. Chem. 1989, 32, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Krantz, A.; Spencer, R.W.; Tam, T.F.; Liak, T.J.; Copp, L.J.; Thomas, E.M.; Rafferty, S.P. Design and synthesis of 4H-3,1-benzoxazin-4-ones as potent alternate substrate inhibitors of human leukocyte elastase. J. Med. Chem. 1990, 33, 464–479. [Google Scholar] [CrossRef] [PubMed]
- Goel, P.; Jumpertz, T.; Tichá, A.; Ogorek, I.; Mikles, D.C.; Hubalek, M.; Piertrzik, C.U.; Strisovsky, K.; Schmidt, B.; Weggen, S. Discovery and validation of 2-styryl substituted benzoxazin-4-ones as a novel scaffold for rhomboid protease inhibitors. Bioorg. Med. Chem. Lett. 2018, 28, 1417–1422. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Barniol-Xicota, M.; Nguyen, M.T.N.; Ticha, A.; Strisovsky, K.; Verhelst, S.H.L. Benzoxazin-4-ones as novel, easily accessible inhibitors for rhomboid proteases. Bioorg. Med. Chem. Lett. 2018, 28, 1423–1427. [Google Scholar] [CrossRef] [PubMed]
- Madkour, H.M.F. Reactivity of 4H-3,1-benzoxazin-4-ones towards nitrogen and carbon nucleophilic reagents: Applications to the synthesis of new heterocycles. ARKIVOC 2004, 1, 36–54. [Google Scholar]
- Baravkar, S.B.; Roy, A.; Gawade, R.L.; Puranik, V.G.; Sanjayan, G.J. Nucleophilic ring opening of benzoxazinone by DBU: Some observations. Synth. Commun. 2014, 44, 2955–2960. [Google Scholar] [CrossRef]
- Zentmeyer, D.T.; Wagner, E.C. The so-called acylanthranils (3,1,4-benzoxazones). I. preparation; reactions with water, ammonia and aniline; structure. J. Org. Chem. 1949, 14, 967–981. [Google Scholar] [CrossRef]
- Bain, D.I.; Smalley, R.K. Synthesis of 2-substituted-4H-3,1-benzoxazin-4-ones. J. Chem. Soc. C 1968, 1593. [Google Scholar] [CrossRef]
- Larksarp, C.; Alper, H. A simple synthesis of 2-substituted 4H-3,1-benzoxazin-4-ones by palladium-catalyzed cyclocarbonylation of o-iodoanilines with acid chlorides. Org. Lett. 1999, 1, 1619–1622. [Google Scholar] [CrossRef]
- Yamashita, M.; Iida, A. One-pot approach to 2-arylbenzoxazinone derivatives from 1-alkynylanilines using copper mediated tandem reactions. Tetrahedron 2014, 70, 5746–5751. [Google Scholar] [CrossRef]
- Shang, X.-X.; Vu, H.-M.; Li, X.-Q. One-pot synthesis of 2-arylbenzoxazinones from 2-arylindoles with (diacetoxyiodo)benzene as the sole oxidant. Synthesis 2018, 50, 377–383. [Google Scholar]
- Plescia, S.; Raffa, D.; Plescia, F.; Casula, G.; Maggio, B.; Daidone, G.; Raimondi, M.V.; Cuismano, M.G.; Bombieri, G.; Meneghetti, F. Synthesis and biological evaluation of new indazole derivatives. ARKIVOC 2010, 10, 163–177. [Google Scholar]
- Deslongchamps, P. Stereoelectronic Effects in Organic Chemistry; Pergamon Press: Oxford, UK, 1983; pp. 4–53. [Google Scholar]
- Smith, M.B.; March, J. March’s Advanced Organic Chemistry, 6th ed.; Wiley-Interscience: New York, NY, USA, 2007; pp. 204–206. [Google Scholar]
- Kleinpeter, E.; Thielemann, J. Synthesis and conformational analysis of mono- and trans-1,4-dialkoxy substituted cyclohexanes-steric substituent/skeleton interactions. Tetrahedron 2007, 63, 9071–9081. [Google Scholar] [CrossRef]
- McElvain, S.N.; Nelson, J.W. The preparation of orthesters. J. Am. Chem. Soc. 1942, 64, 1825–1827. [Google Scholar] [CrossRef]
- Noe, M.; Perosa, A.; Selva, M. A flexible Pinner preparation of orthoesters: The model case of trimethylorthobenzoate. Green Chem. 2013, 15, 2252–2260. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).