Syntheses, Characterization, and Antioxidant Evaluation of Cu2+, Mn2+, and Fe3+ Complexes with a 14 Membered EDTA-Derived Macrocycle
Abstract
1. Introduction
2. Results and Discussion
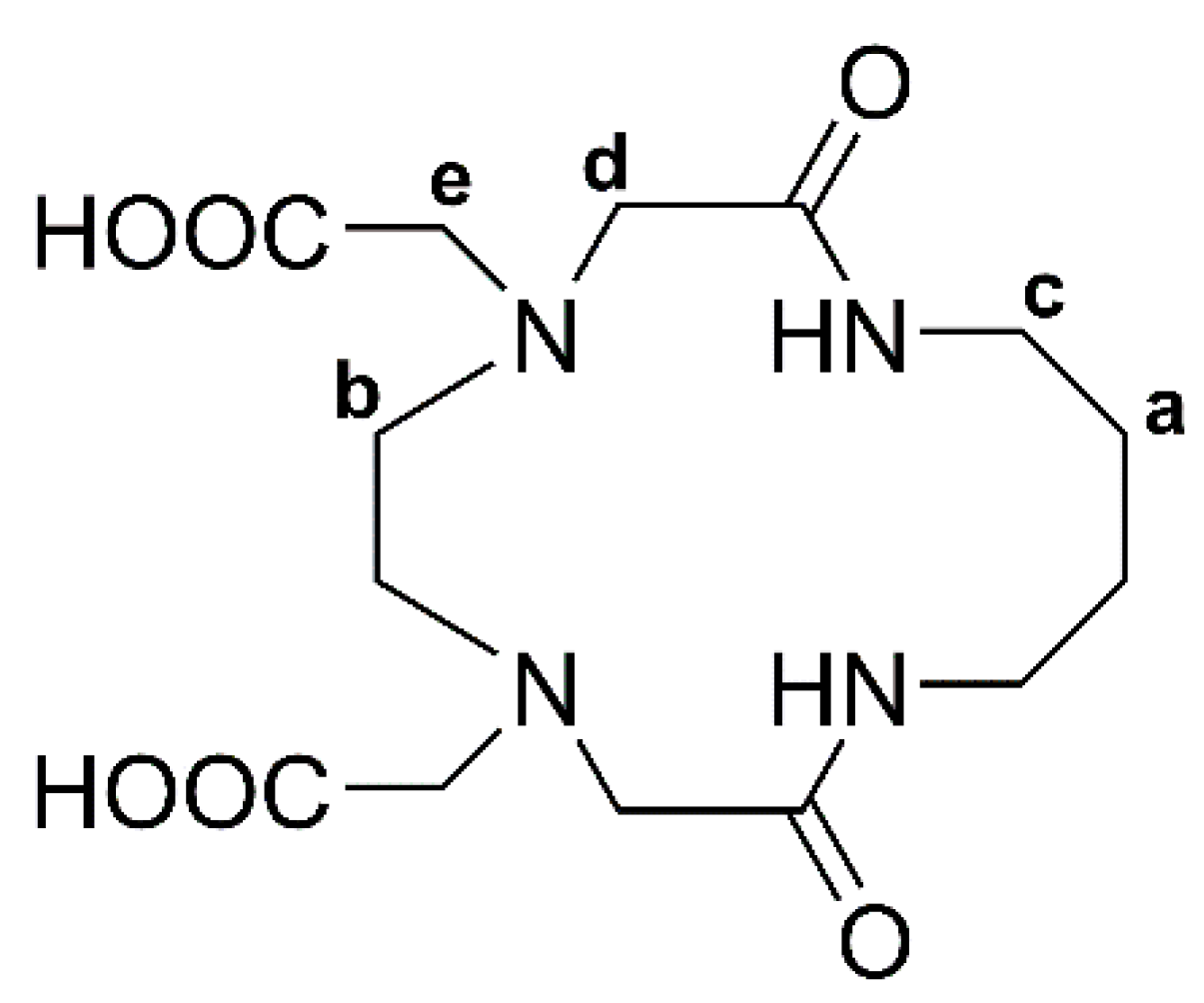
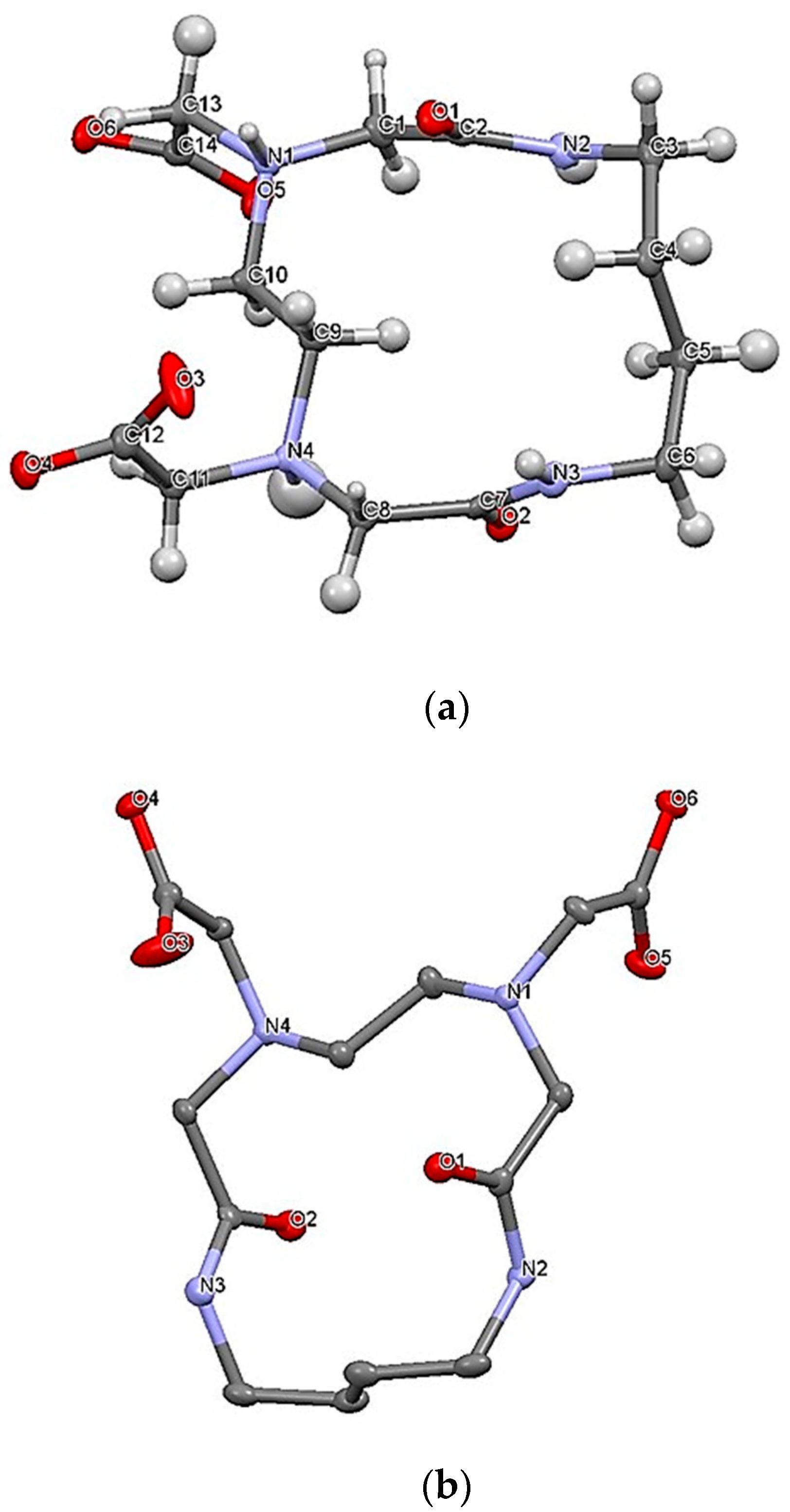
2.1. X-ray Crystal Analysis of H2L14
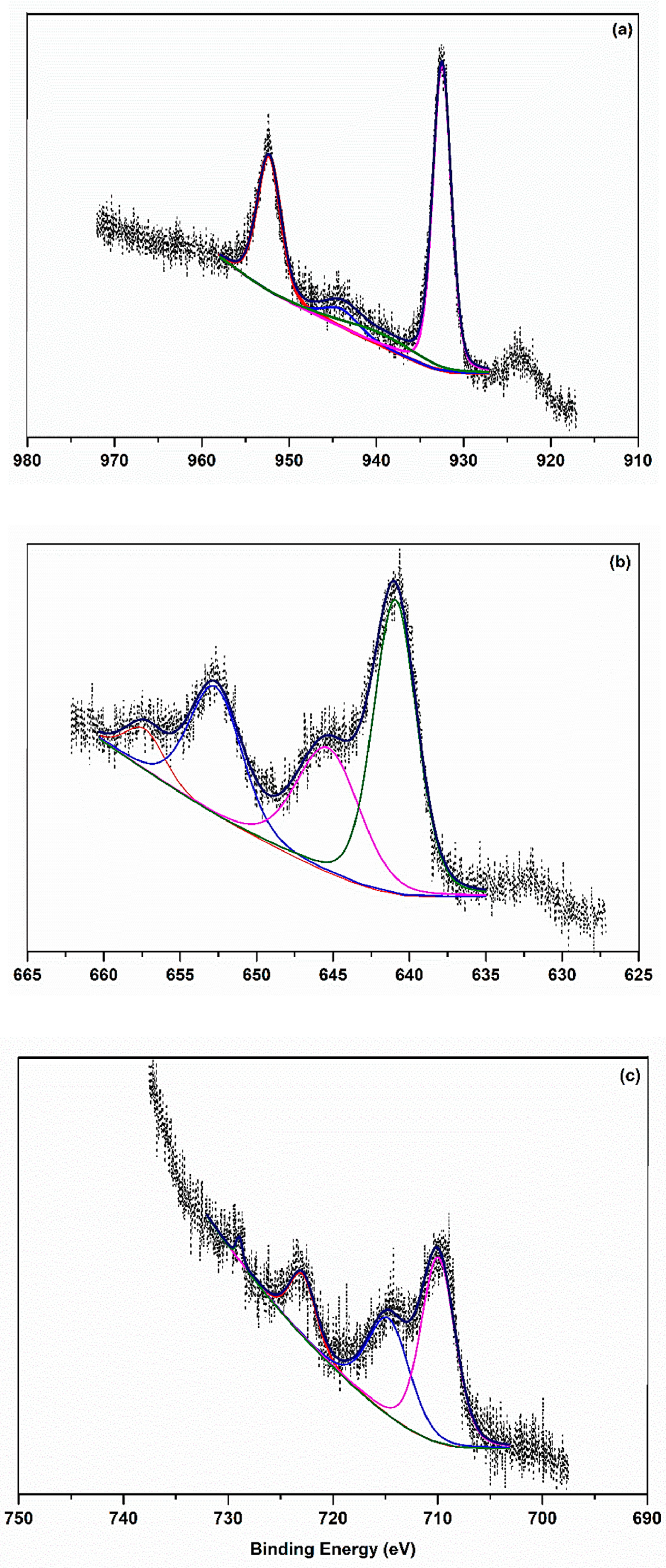
2.2. Characterization of Cu2+, Mn2+, and Fe3+ Complexes
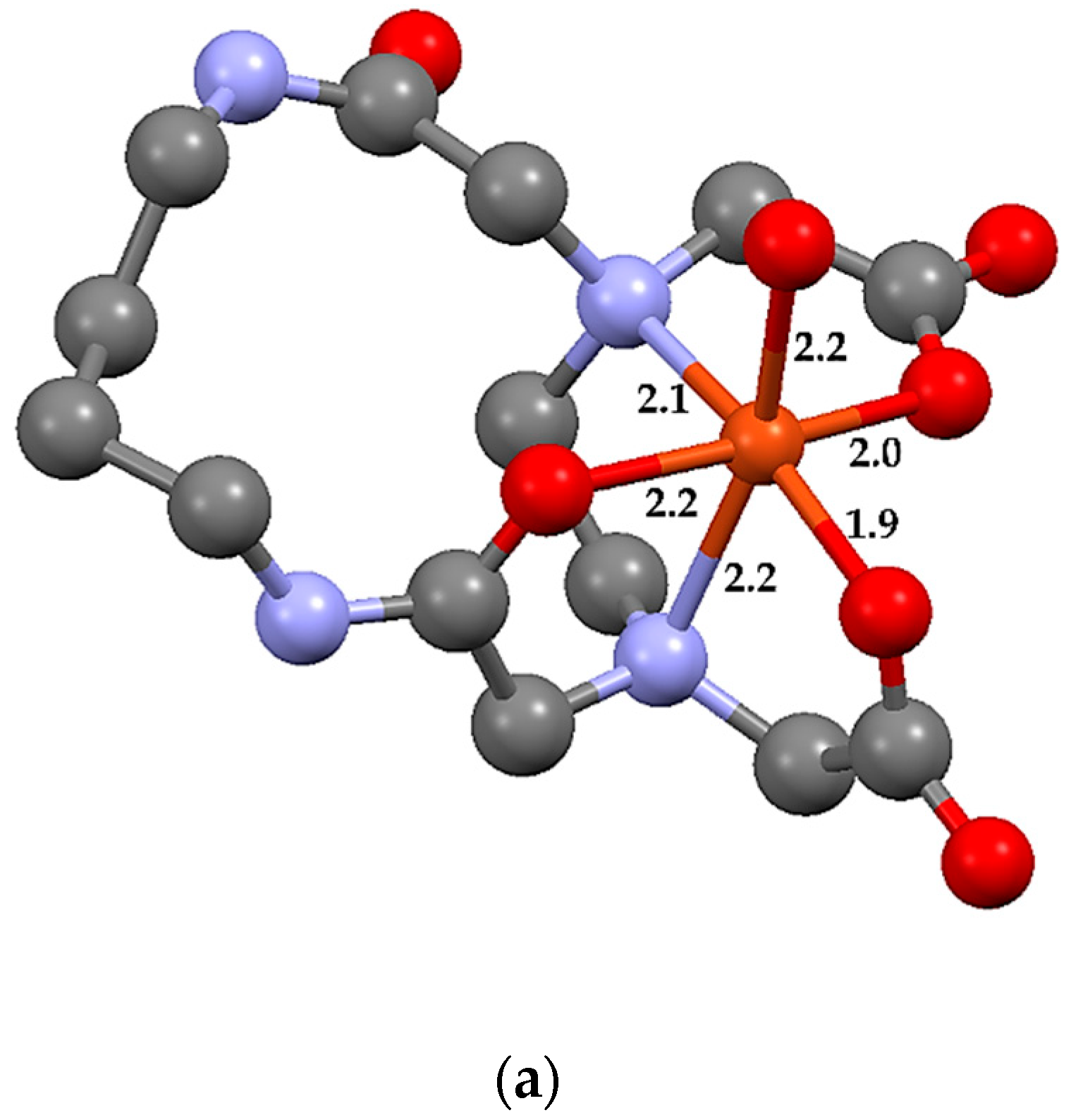
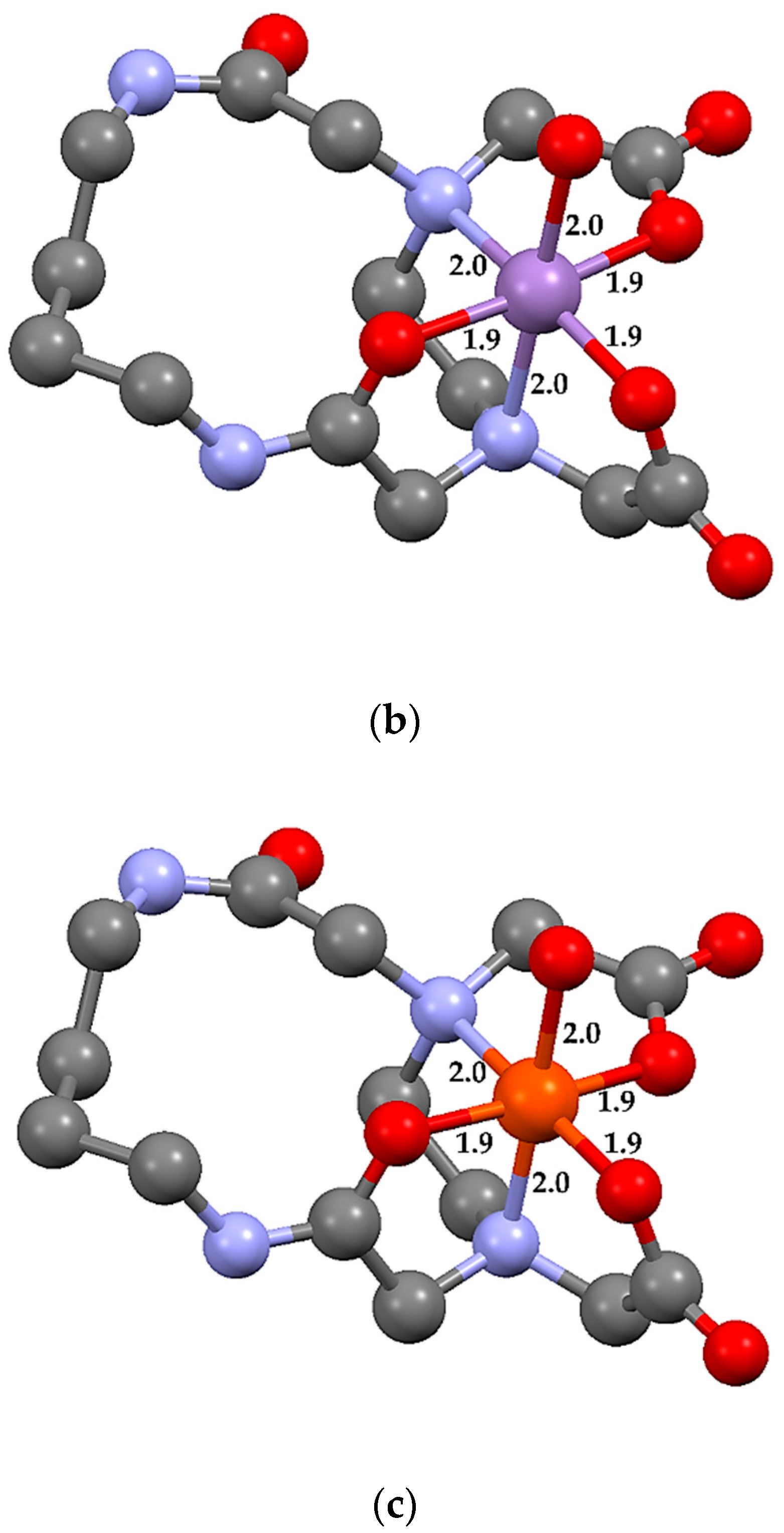
2.3. Theoretical Calculations
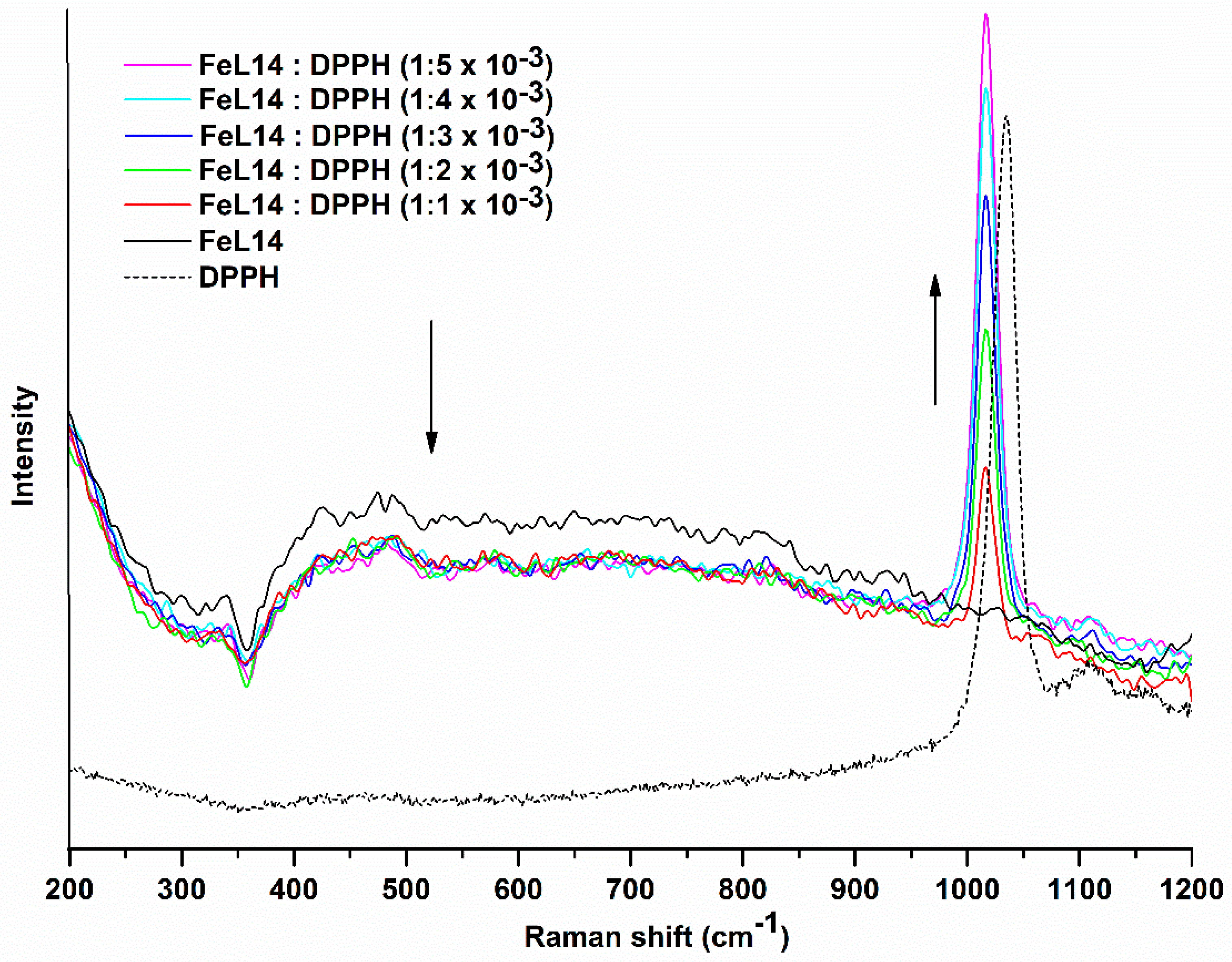
2.4. Chemical Antioxidant Assays
3. Materials and Methods
3.1. Synthesis and X-ray Crystal Analysis of H2L14
3.2. Syntheses of Cu2+, Mn2+, and Fe3+ Complexes
3.3. Physical Measurements
3.4. Geometry Optimization
3.5. Chemical Antioxidant Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Halliwell, B. The antioxidant paradox. The Lancet 2000, 355, 1179–1180. [Google Scholar] [CrossRef]
- Cao, G.-J.; Jiang, X.; Zhang, H.; Croley, T.R.; Yin, J.-J. Mimicking horseradish peroxidase and oxidase using ruthenium nanomaterials. RSC Adv. 2017, 7, 52210–52217. [Google Scholar] [CrossRef]
- Che, M.; Wang, R.; Li, X.; Wang, H.-Y.; Zheng, X.S. Expanding roles of superoxide dismutases in cell regulation and cancer. Drug Discov. Today 2016, 21, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Afanas’eva, I.B.; Ostrakhovitch, E.A.; Mikhal’chik, E.V.; Ibragimova, G.A.; Korkina, L.G. Enhancement of antioxidant and anti-inflammatory activities of bioflavonoid rutin by complexation with transition metals. Biochem. Pharmacol. 2001, 61, 677–684. [Google Scholar] [CrossRef]
- Salazar-Medina, A.J.; Sugich-Miranda, R.; Teran-Cabanillas, E.; Hernández, J.; González-Aguilar, G.A.; Rudiño-Piñera, E.; Sotelo-Mundo, R.R.; Velázquez-Contreras, E.F. Antioxidant capacity of two novel bioactive Fe (III)-Cyclophane complexes. Molecules 2013, 18, 1762–1774. [Google Scholar] [CrossRef] [PubMed]
- Sugich-Miranda, R.; Sotelo-Mundo, R.R.; Silva-Campa, E.; Hernández, J.; Gonzalez-Aguilar, G.A.; Velazquez-Contreras, E.F. Antioxidant capacity of binuclear Cu (II)-cyclophanes, insights from two synthetic bioactive molecules. J. Biochem. Mol. Toxic. 2010, 24, 379–383. [Google Scholar] [CrossRef]
- Inoue, M.B.; Navarro, R.E.; Landín, I.O.; López, D.M.; Inoue, M.; Fernando, Q. Electronic spectra and structures of Cu 2+ and Ni 2+ complexes with dioxotetraazacycloalkanediacetates and their diester derivatives. Inorg. Chim. Acta 1998, 269, 224–228. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Nesbitt, H.; Banerjee, D. Interpretation of XPS Mn (2p) spectra of Mn oxyhydroxides and constraints on the mechanism of MnO 2 precipitation. Am. Mineral. 1998, 83, 305–315. [Google Scholar] [CrossRef]
- Carlson, T.A. Satellite structure in the photoelectron spectra of transition metal compounds ionized in the K shell of the metal ion. Faraday Discuss. Chem. Soc. 1975, 60, 30–36. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Sarma, D.; Vasudevan, S.; Hegde, M. Study of transition metal oxides by photoelectron spectroscopy. Proc. R. Soc. Lond. A 1979, 367, 239–252. [Google Scholar] [CrossRef]
- Watts, J.F.; Wolstenholme, J. An introduction to surface analysis by XPS and AES; Wiley-VCH: Weinheim, Germany, 2003; p. 224. ISBN 0-470-84713-1. [Google Scholar]
- Biesinger, M.C.; Lau, L.W.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V., Cu and Zn. Appl. Sur. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Brisk, M.A.; Baker, A. Shake-up satellites in X-ray photoelectron spectroscopy. J. Electron Spectrosc. 1975, 7, 197–213. [Google Scholar] [CrossRef]
- Salazar-Medina, A.; Alday, E.; Carreño, A.; Hernandez, J.; González-Aguilar, G.; Velázquez, C.; Velázquez-Contreras, E. Cellular Redox State Modifications Induced by Bioactive Fe (III)-Cyclophane Complexes Approaching to Selective Therapy Drug Design. Med. Chem. (Los Angeles) 2017, 7, 208–212. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C: Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; Streek, J.v.d. Mercury: Visualization and analysis of crystal structures. J. Appl. Crystallogr. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision, B.01, Gaussian, Inc.: Wallingford, CT, USA, 2009; citeulike-article-id:9096580.
- Chai, J.-D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. J. Chem. Phys. 1985, 82, 299–310. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J. Chem. Phys. 1985, 82, 270–283. [Google Scholar] [CrossRef]
- Cancès, E.; Mennucci, B.; Tomasi, J. A new integral equation formalism for the polarizable continuum model: Theoretical background and applications to isotropic and anisotropic dielectrics. J. Chem. Phys. 1997, 107, 3032–3041. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Bio. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Tech. 1995, 28, 25–30. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds H2L14, CuL14, MnL14 and FeL14 are available from the authors. |

| H2O | NO3 | –CH2COO– | Metal % | ||
|---|---|---|---|---|---|
| Compound | Moisture 1 | Coordinated 1 | Counter Ion 1 | Pendant Groups 1 | Experimental/Theoretical |
| H2L14 | n/a | n/a | n/a | 19.5/286 | n/a |
| 19.6/386 | |||||
| CuL14·3H2O | 4.2/71 | 3.9/168 | n/a | 23.6/255 | 14.4/13.8 |
| 5.1/114 | |||||
| MnL14·H2O | n/a | 4.6/197 | n/a | n/a | 13.4/13.2 |
| FeL14·NO3·3H2O | 7.6/117 | 4.0/182 | 12.0/245 | 18.5/405 | 12.1/10.9 |
| Compound | 2p3/2 | 2p3/2 (sat) | 2p1/2 | 2p1/2 (sat) | Peak Differences 2p3/2−2p1/2 |
|---|---|---|---|---|---|
| CuL14 | 932.5 | 939.5 | 952.3 | 944.0 | 19.8 |
| MnL14 | 640.9 | 645.3 | 652.6 | 657.3 | 11.7 |
| FeL14 | 710.0 | 714.7 | 722.9 | 728.9 | 12.9 |
| Assignment | H2L14 | CuL14·3H2O | MnL14·H2O | FeL14·3H2O | ||||
|---|---|---|---|---|---|---|---|---|
| FTIR | Raman | FTIR | Raman | FTIR | Raman | FTIR | Raman | |
| N–H ν | 3348 vs | 3351 m | 3318 m | 3317 vw | 3229 s | 3238 vw | 3236 s | 3238 vw |
| C–H ν | 2933 s | 2937 vs | 2941 m | 2931 vs | 2935 s | 2937 vs | 2941 m | 2943 m |
| C=O ν | 1719 s | 1723 w | - | - | 1739 shd | 1758 w | - | 1772 w |
| O–C ν | 1237 s 1285 s | 1206 m | 1316 m | 1288 m 1322 m | 1279 s 1328 s | 1302 m | 1292 shd 1243 shd | 1295 m |
| C=O ν (amide I) | 1661 s | 1644 m | 1655 s | 1651 w | 1673 s | 1651 w | 1642 s | 1625 s |
| C=O δ (amide II) | 1533 s | 1534 w | 1564 s | 1584 m | 1576 s | 1550 vw | 1576 shd | 1537 shd |
| C–N ν | 1150 m | 1159 w | 1312 m | 1104 w | 1113 m | 1127 w | 1097 w | 1117 w |
| CO2–M ν | - | - | 1655 s | 1651 w | 1619 vs | 1651 w | 1570 m | 1618 m |
| M–N ν | - | - | 493 w | 468 m | 451 w | 462 m | 463 vw | 482 w |
| H–O–H ν cw | - | - | 3414 m | 3324 w | 3476 shd | - | 3457 shd | 3331 w |
| M–OH2 ω | - | - | 560 w | 569 w | 524 m | 576 w | 590 w | 562 vw |
| Bond Type | CuL14·H2O | MnL14·H2O | FeL14·H2O | NiL14·H2O 1 |
|---|---|---|---|---|
| D M–O(CO) | 2.0 1.9 | 1.9 1.9 | 2.0 2.0 | 2.0 2.1 |
| D M–O(CN) | 2.1 | 1.9 | 2.1 | 2.1 |
| D M–OW | 2.2 | 2.0 | 2.1 | 2.0 |
| D M–N | 2.2 2.1 | 2.0 2.0 | 2.1 2.2 | 2.1 2.2 |
| <O(CO)–M–O(CO) | 92.7 | 90.5 | 91.7 | 92.5 |
| <O(CO)–M–O(CN) | 173.2 92.9 | 174.7 94.5 | 174.2 94.1 | 1709 95.1 |
| <O(CO)–M–OW | 101.5 98.5 | 90.1 94.0 | 84.3 96.3 | 93.1 98.1 |
| <O(CO)–M–N | 98.2 83.9 81.4 166.5 | 92.1 84.2 85.5 172.7 | 97.1 82.1 82.7 165.9 | 93.9 81.4 80.5 164.3 |
| <N–M–O(CN) | 78.7 89.8 | 86.6 90.6 | 82.6 92.1 | 82.5 90.0 |
| <N–M–OW | 160.3 95.0 | 177.7 91.2 | 178.2 95.7 | 172.9 96.8 |
| <N–M–N | 86.1 | 89.6 | 85.5 | 85.4 |
| ABTS | DPPH | |||
|---|---|---|---|---|
| Inhibitory % | IC50 | Inhibitory % | IC50 | |
| H2L14 | 33 ± 0.8 | n/a | 2 ± 0.6 | n/a |
| CuL14 | 21 ± 3.2 | n/a | 4 ± 15 | n/a |
| MnL14 | 8 ± 1.6 | n/a | 9 ± 1.5 | n/a |
| FeL14 | 63 ± 1.9 | 153 ± 6 | 52 ± 2.4 | 193 ± 5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soberanes, Y.; Navarro, R.E.; Inoue, M.; Velázquez-Contreras, E.F.; Beltran Torres, M.; Lugo, G.; Sotelo-Mundo, R.R.; Salazar-Medina, A.J. Syntheses, Characterization, and Antioxidant Evaluation of Cu2+, Mn2+, and Fe3+ Complexes with a 14 Membered EDTA-Derived Macrocycle. Molecules 2019, 24, 3556. https://doi.org/10.3390/molecules24193556
Soberanes Y, Navarro RE, Inoue M, Velázquez-Contreras EF, Beltran Torres M, Lugo G, Sotelo-Mundo RR, Salazar-Medina AJ. Syntheses, Characterization, and Antioxidant Evaluation of Cu2+, Mn2+, and Fe3+ Complexes with a 14 Membered EDTA-Derived Macrocycle. Molecules. 2019; 24(19):3556. https://doi.org/10.3390/molecules24193556
Chicago/Turabian StyleSoberanes, Yedith, Rosa Elena Navarro, Motomichi Inoue, Enrique F. Velázquez-Contreras, Melissa Beltran Torres, Gustavo Lugo, Rogerio R. Sotelo-Mundo, and Alex J. Salazar-Medina. 2019. "Syntheses, Characterization, and Antioxidant Evaluation of Cu2+, Mn2+, and Fe3+ Complexes with a 14 Membered EDTA-Derived Macrocycle" Molecules 24, no. 19: 3556. https://doi.org/10.3390/molecules24193556
APA StyleSoberanes, Y., Navarro, R. E., Inoue, M., Velázquez-Contreras, E. F., Beltran Torres, M., Lugo, G., Sotelo-Mundo, R. R., & Salazar-Medina, A. J. (2019). Syntheses, Characterization, and Antioxidant Evaluation of Cu2+, Mn2+, and Fe3+ Complexes with a 14 Membered EDTA-Derived Macrocycle. Molecules, 24(19), 3556. https://doi.org/10.3390/molecules24193556







