Ultrahigh Performance Liquid Chromatography–Electrospray Ionization Tandem Mass Spectrometry Method for Qualitative and Quantitative Analyses of Constituents of Corydalis bungeana Turcz Extract
Abstract
:1. Introduction
2. Results
2.1. Method Optimization
2.2. Qualitative Analysis
Fragmentation Regularity
2.3. Quantitative Analysis
2.3.1. Optimization of the Condition
2.3.2. Linearity
2.3.3. Precision and Accuracy
2.3.4. Repeatability and Stability
2.3.5. Recovery
2.3.6. Sample Detection
3. Materials and Methods
3.1. Reagents and Materials
3.2. Preparation of Standards
3.3. Sample Preparation
3.4. Instrumentation and Operation Conditions
3.4.1. Optimization of the UHPLC System
3.4.2. Optimization of MS System
3.5. Validation of UHPLC–ESI–MS/MS Quantitation
3.5.1. Preparation of the Standard Solutions
3.5.2. Calibration Curve and Limit of Detection
3.5.3. Precision and Accuracy
3.5.4. Recovery
3.5.5. Repeatability and Stability
3.6. Qualitative Analysis and Quantitative analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| C.bungeana | Corydalis bungeana Turcz |
| CE | collision energy |
| ESI+ | positive electrospray ionization |
| ESI− | negative electrospray ionization |
| I.S. | internal standard |
| LOD | limits of detection |
| LOQ | limits of quantification |
| MRM | multiple reaction monitoring |
| RDA | Retro-Diels–Alder |
| TCM | Traditional Chinese Medicine |
| UHPLC–ESI–MS/MS | ultrahigh performance liquid chromatography–electrospray ionization–tandem mass spectrometry |
| Z23 | 7’-(3’,4’-dihydroxy-phenyl)-N-[(4-methoxyphenyl)-ethyl]propenamide |
References
- Yang, C.; Zhang, C.; Wang, Z.; Tang, Z.; Kuang, H.; Kong, A.-N.T. Corynoline Isolated from Corydalis bungeana Turcz. Exhibits Anti-Inflammatory Effects via Modulation of Nfr2 and MAPKs. Molcules 2016, 21, 975. [Google Scholar] [CrossRef] [PubMed]
- China Pharmacopoeia Committee. Pharmacopoeia of the People’s Republic of China; China Chemical Industry Press: Beijing, China, 2015; p. 234. [Google Scholar]
- Niu, L.; Xie, Z.; Cai, T.; Wu, P.; Xue, P.; Chen, X. Preparative isolation of alkaloids from corydalis bungeana turcz. by high-speed counter-current chromatography using stepwise elution. J. Sep. Sci. 2015, 34, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.-T.; Chen, J.-Q.; Jiang, C.-H.; Song, J.; Li, D.-Y.; Zhang, H.; Jia, X.-B.; Tan, W.; Wang, S.-X.; Yang, Y.; et al. Corydalis bungeana Turcz. attenuates LPS-induced inflammatory responses via the suppression of NF-κB signaling pathway in vitro and in vivo. J. Ethnopharmacol. 2016, 194, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.-B.; Zhang, Y.-H.; Zhao, B.-J.; Li, C.; Tian, G.; Niu, B.; Qi, H.; Feng, L.; Shao, J.-G. Screening for anti-inflammatory components from Corydalis bungeana Turcz. based on macrophage binding combined with HPLC. BMC Complement. Altern. Med. 2015, 15, 987. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Du, Y.; Wang, X.; Cao, H.; Lin, B.; Liu, Y. Hexahydroben-zophenanthridine alkaloids from corydalis bungeana turcz. and their anti-inflammatory activity. Bioorganic Med. Chem. Lett. 2018, 28, 2265–2269. [Google Scholar] [CrossRef]
- Wang, X.; Geng, Y.; Li, F.; Shi, X.; Liu, J. Large-scale separation of alkaloids from corydalis decumbens by ph-zone-refining counter-current chromatography. J. Chromatogr. A 2012, 17, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Huang, K.L.; Zeng, J.G.; Li, S.; She, J.M.; Li, G.; Zhang, L. Optimization of microwave-assisted extraction of protopine and allocryptop-ine from stems of Macleaya cordata (Willd) R. Br. using response surface methodology. J. Sep. Sci. 2010, 33, 2160–2167. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Song, M.; Zhu, G.; Xi, X.; Li, K.; Wu, C.; Huang, L. Corynoline attenuates LPS-induced acute lung injury in mice by activating Nrf2. Int. Immunopharmacol. 2017, 48, 96. [Google Scholar] [CrossRef]
- Huang, T.; Xiao, Y.; Yi, L.; Li, L.; Wang, M.; Tian, C.; Ma, H.; He, K.; Wang, Y.; Han, B.; et al. Coptisine from Rhizoma Coptidis Suppresses HCT-116 Cells-related Tumor Growth in vitro and in vivo. Sci. Rep. 2017, 7, 38524. [Google Scholar] [CrossRef]
- Liu, B.; Su, K.; Wang, J.; Wang, J.; Xin, Z.; Li, F.; Fu, Y. Corynoline Exhibits Anti-inflammatory Effects in Lipopolysaccharide (LPS)-Stimulated Human Umbilical Vein Endothelial Cells through Activating Nrf2. Inflammation 2018, 41, 1640–1647. [Google Scholar] [CrossRef]
- Liu, R.; Zheng, L.; Cheng, M.; Wu, Y.; Gu, P.; Liu, Y.; Ma, P.; Ding, L. Simultaneous determination of corynoline and acetylcorynoline in human urine by LC–MS/MS and its application to a urinary excretion study. J. Chromatogr. B 2016, 1014, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Cai, J.; Lin, C.; Ma, J.; Wang, X. Gradient elution liquid chromatography mass spectrometry determination of acetylcorynoline in rat plasma and its application to a pharmacokinetic study. Xenobiotica 2014, 44, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Gu, P.; Wang, L.; Cheng, M.; Wu, Y.; Zheng, L.; Liu, Y.; Ding, L. Study on the pharmacokinetic profiles of corynoline and its potential interaction in traditional Chinese medicine formula Shuanghua Baihe tablets in rats by LC–MS/MS. J. Pharm. Biomed. Anal. 2016, 117, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.B.; Rai, D.K.; Brunton, N.P. Optimisation and validation of ultra-high performance liquid chromatographic-tandem mass spectrometry method for qualitative and quantitative analysis of potato steroidal alkaloids. J. Chromatogr. B 2015, 997, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wu, L.J.; Yang, C.J. Research progress on chemical structure and biological activity of isoquinoline alkaloids in Corydalis plants. Pract. Pharm. Clin. Remedies 2016, 19, 371–380. [Google Scholar]
- Wang, X.; Dong, H.; Shu, X.; Zheng, Z.; Yang, B.; Huang, L. Large-Scale Separation of Alkaloids from Corydalis bungeana Turcz. by pH-Zone-Refining Counter-Current Chromatography. Molcules 2012, 17, 14968–14974. [Google Scholar] [CrossRef]
- Qing, Z.X.; Huang, J.L.; Yang, X.Y.; Liu, J.H.; Cao, H.L.; Xiang, F.; Cheng, P.; Zeng, J.G. Anticancer and Reversing Multidrug Resistance Activities of Natural Isoquinoline Alkaloids and their Structure-activity Relationship. Curr. Med. Chem. 2018, 25, 5088–5114. [Google Scholar] [CrossRef]
- Yu, F.U.; Liang, J.; Bai, Y.; Zhuo-Ma, D.; Bai, B.R.; Ding, L.S. Tandem mass spectrometry analysis of total alkaloids from corydalis hendersonii. J. Chin. Mass Spectrom. Soc. 2010, 2, 98–102. [Google Scholar]
- Qi, L.W.; Chen, C.Y.; Li, P. Structural characterization and identification of iridoid glycosides, saponins, phenolic acids and flavonoids in flos lonicerae japonicae by a fast liquid chromatography method with diode-array detection and time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. Rcm 2010, 23, 3227–3242. [Google Scholar] [CrossRef]
- Moshafi, M.H.; Sharififar, F.; Dehghan, G.R.; Ameri, A. Bioassay screening of the essential oil and various extracts of fruits of heracleum persicum desf. and rhizomes of zingiber officinale rosc. using brine shrimp cytotoxicity assay. Iran. J. Pharm. Res. 2009, 8, 373–384. [Google Scholar]
- Soušek, J.; Guédon, D.; Adam, T.; Bochořáková, H.; Táborská, E.; Válka, I. Alkaloids and organic acids content of eight fumaria species. Phytochem. Anal. 2015, 10, 6–11. [Google Scholar] [CrossRef]
- Deng, X.; Zhao, L.; Fang, T.; Xiong, Y.; Ogutu, C.; Yang, D.; Vimolmangkang, S.; Liu, Y.; Han, Y. Investigation of benzylisoquinoline alkaloid biosynthetic pathway and its transcriptional regulation in lotus. Hortic Res. 2018, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, C.J.; Zhong, M.L.; Jiang, X.C.; Liu, G.F.; Pharmacy, S.O. Chemical constituents from corydalis bungeana turcz. Nat. Prod. Res. Dev. 2013, 25, 1665–1668. [Google Scholar]
Sample Availability: Samples of the compounds 7’-(3’,4’-dihydroxyphenyl)-N-[(4-methoxyphenyl)-ethyl] propena-mide, coptisine, berberrubine, sanguinarine, worenine, berberine, jateorhizine, columbamine, palmatine, protopine, tetrahydropalmatine, corynoline, 8-oxocorynoline, acetylcorynoline are available from the authors. |
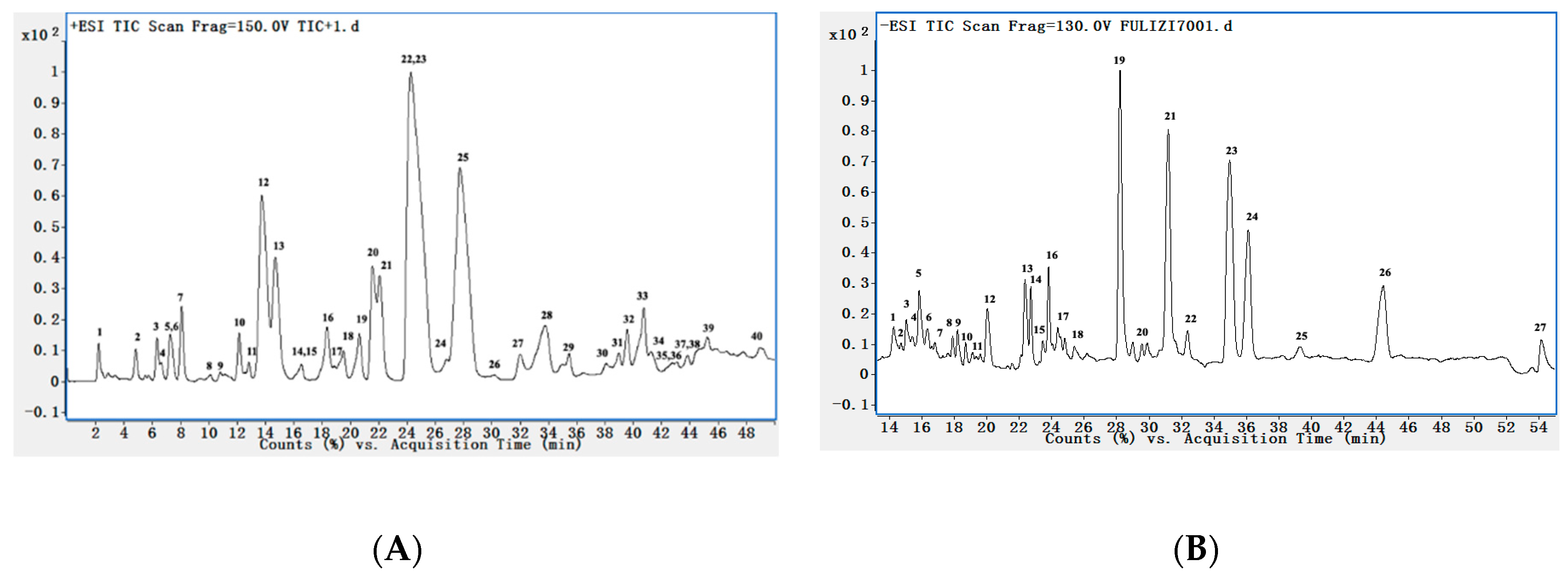
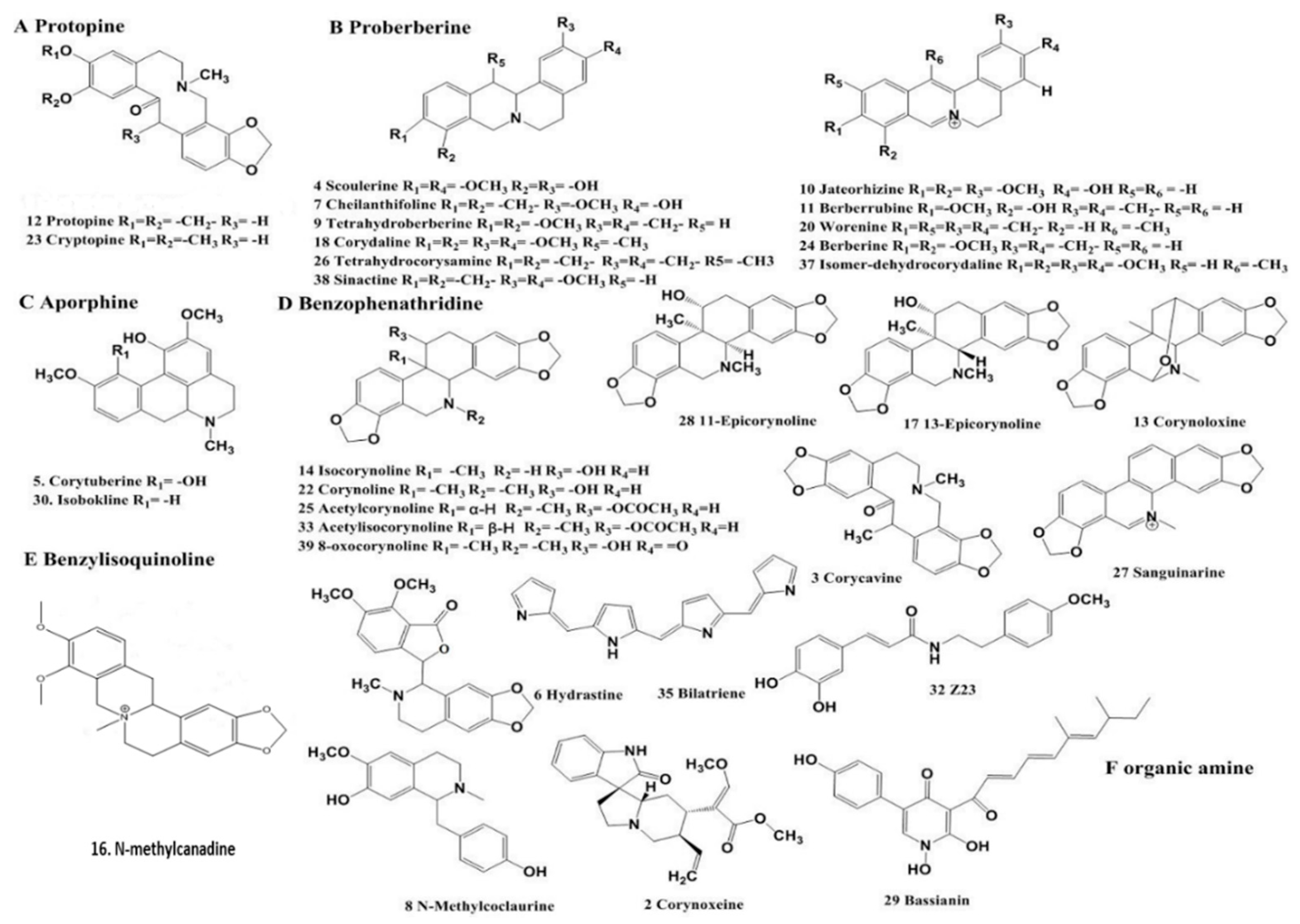
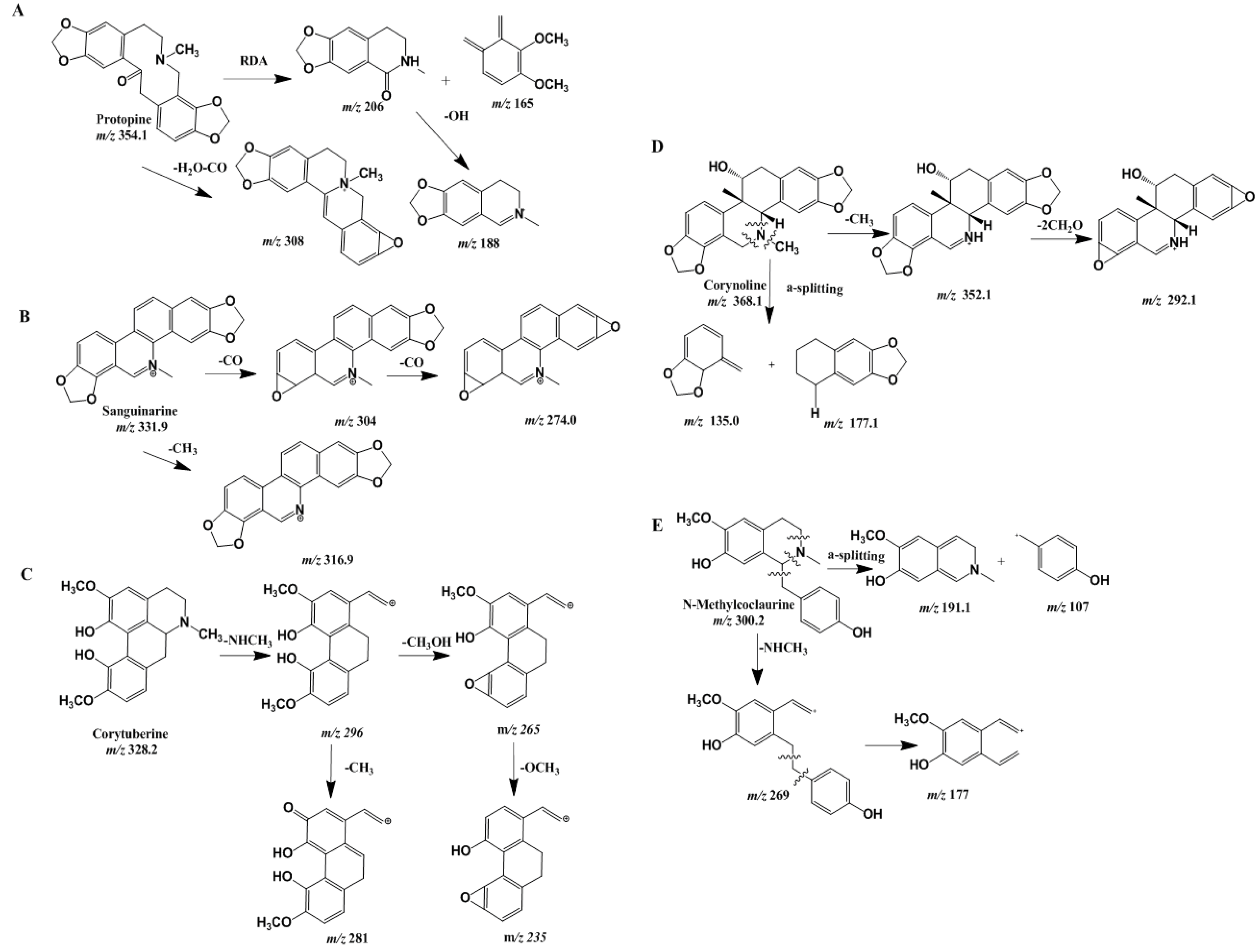
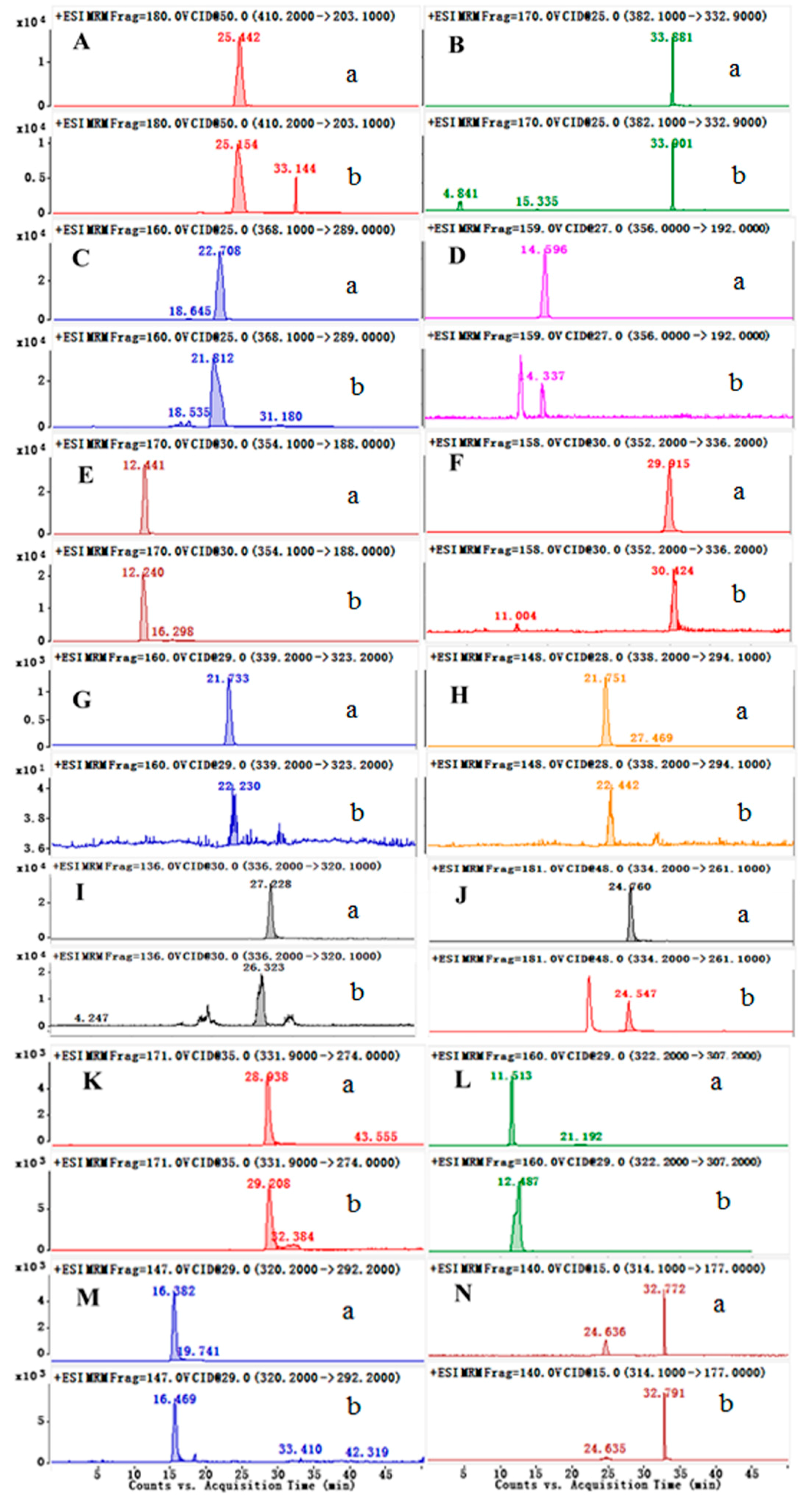
| No. | Tr (min) | Calcd Mass [M − H]+ | MS/MS Fragments m/z | Tentative Identification |
|---|---|---|---|---|
| 1 | 2.1 | 301 [M + X]+ | Unknown | |
| 2 | 4.9 | 382 [M]+ | 178; 265; 222; 162; 207 | Corynoxeine |
| 3 | 6.4 | 368 | 277; 190; 90; 44; 307; | Corycavine |
| 4 | 6.6 | 328 | 178; 150; 264 | Scoulerine |
| 5 | 7.4 | 328 | 296; 235; 281 | Corytuberine |
| 6 | 7.5 | 384 | 364; 303; 58 | Hydrastine |
| 7 | 8.1 | 326 | 178; 151; 91; 311; 283 | Cheilanthifoline |
| 8 | 10.1 | 300 | 191; 177; 107 | N-Methylcoclaurine |
| 9 | 10.9 | 340 | 208;114; | Tetrahydroberberine |
| 10 | 12.2 | 338 | 322; 294 | Jateorhizine |
| 11 | 12.7 | 323 | 307; 279; 250; 197 | Berberrubine |
| 12 | 13.9 | 354 | 188; 149; 247; 320; 91 | Protopine |
| 13 | 14.8 | 366 | 317; 206; 146 | Corynoloxine |
| 14 | 16.6 | 354 | 188; 149; 247; 249 | Isocorynoline |
| 15 | 16.5 | 426 | Unknown | Lythranidine |
| 16 | 18.4 | 354 [M]+ | 190; 247;1 88; 174; 166 | N-methylcanadine |
| 17 | 18.8 | 368 | 176; 289; 319; 174 | 13-epicorynoline |
| 18 | 19.4 | 369 [M]+ | 174; 274; 246; 259 | Corydaline |
| 19 | 20.3 | 368 [M + X]+ | Unknown | |
| 20 | 21.6 | 334 [M]+ | 261; 291; 2333; 332; 147 | Worenine |
| 21 | 22.0 | 408 | Unknown | Erysothiopine |
| 22 | 24.3 | 368 | 289; 261; 177; 319; 231; 135 | Corynoline |
| 23 | 25.1 | 369 [M]+ | 336; 190; 188; 158; 181 | Coryptopine |
| 24 | 26.8 | 336 [M]+ | 320; 292 | Berberine |
| 25 | 27.9 | 410 | 204; 246; 162; 332 | Acetylcorynoline |
| 26 | 30.1 | 338 | 277; 163 | Tetrahydrocorysamine |
| 27 | 32.0 | 331 [M]+ | 317; 274; 246; 218; 189 | Sanguinarine |
| 28 | 33.5 | 368 | 289; 261; 177 | 11-Epicorynoline |
| 29 | 34.8 | 396 | 114; 209; 114 | Bassianin |
| 30 | 37.9 | 312 | 119 | Corytuberine |
| 31 | 39.1 | 394 | Unknown | Coryincine |
| 32 | 39.6 | 314 | 289; 177; 261; 231; 135; 79 | Z23 |
| 33 | 40.7 | 410 | 394; 349; 334; 321; 190; 176 | Acetylisocorynoline |
| 34 | 41.2 | 344[M + X]+ | Unknown | |
| 35 | 42.6 | 299 | 295; 99 | Bilatriene |
| 36 | 43.1 | 395[M + X]+ | Unknown | |
| 37 | 43.8 | 366[M]+ | 350; 334; 308; 292 | Isomerdehydrocorydaline |
| 38 | 44.8 | 340 | 113 | Sinactine |
| 39 | 45.3 | 382 | 333;275;247;189;135 | 8-oxocorynoline |
| 40 | 49.2 | 181[M + X]+ | Unknown |
| No | TR (min) | Calcd mass [M − H]+ | MS/MS Fragments m/z | Tentative Identification |
|---|---|---|---|---|
| 2 | 14.8 | 329 | 279; 223; 212; 194 | Tianshic acid |
| 5 | 15.9 | 942 | 923; 733 | Soysaponin 1 |
| 6 | 16.5 | 795 [M+HCOO−]− | 615; 113; 119 | Dipsacussaponin L |
| 7 | 17.3 | 311 | 223; 57 | Caftaric acid |
| 8 | 18.0 | 577 | 299 | Acacia-7-o-β-d-apiose-(1→2)-β-d-glucose |
| 16 | 23.9 | 295 | 277; 195; 171; 183 | Tanshinone II A |
| 19 | 28.2 | 277 | 127;59 | Linolenic acid |
| 20 | 29.6 | 339 | 177; 133; 105 | Aesculin |
| 21 | 31.3 | 279 | Isolinolic acid | |
| 22 | 32.4 | 253 | 223; 195 | Daidzein |
| 23 | 34.9 | 255 | Bupleurynol | |
| 24 | 35.9 | 281 | 236; 203; 174; 150 | Gloeosteretriol |
| Compound | Linear Range (ng/mL) | Linearity Equation | R2 | LOQ (ng/mL) |
|---|---|---|---|---|
| Acetylcorynoline | 13.00–3250 | Y = 18.47X + 829.27 | 0.9992 | 13.00 |
| 8-oxocorynoline | 11.60–2900 | Y = 1.66X + 57.44 | 0.9994 | 11.60 |
| Corynoline | 10.22–2555 | Y = 60.43X + 1730.90 | 0.9994 | 10.22 |
| Tetrahydropalmatine | 10.14–2535 | Y = 54.83X + 2275.20 | 0.9991 | 10.14 |
| Protopine | 9.74–2435 | Y = 33.34X + 1766.50 | 0.9991 | 9.74 |
| Palmatine | 11.30–2825 | Y = 58.96X + 1811.50 | 0.9992 | 11.30 |
| Columbamine | 11.15–2788 | Y = 15.74X + 943.69 | 0.9991 | 11.15 |
| Jateorhizine | 10.00–2500 | Y = 45.28X + 1907.30 | 0.9992 | 10.00 |
| Berberine | 10.80–2700 | Y = 13.67X + 478.40 | 0.9995 | 10.80 |
| Worenine | 11.10–2775 | Y = 5.64X + 230.93 | 0.9993 | 11.10 |
| Sanguinarine | 10.00–2500 | Y = 14.54X + 500.13 | 0.9992 | 10.00 |
| Berberrubine | 11.05–2763 | Y = 86.92X + 4687.20 | 0.9991 | 11.05 |
| Coptisine | 10.00–2500 | Y = 16.42X + 712.74 | 0.9994 | 10.00 |
| Z23 | 10.68–2670 | Y = 1.67X + 44.10 | 0.9996 | 10.68 |
| Compounds | Nominal Mass Concentration (ng/mL) | Observed Mass Concentration (ng/mL) | Accuracy (bias %) | Intra-Day Precision (RSD %) | Inter-Day Precision (RSD %) |
|---|---|---|---|---|---|
| Acetylcorynoline | 13.00 | 13.00 ± 0.80 | 0.11 | 2.42 | 1.41 |
| 325 | 312.5 ± 3.90 | −3.83 | 4.21 | 1.83 | |
| 1625 | 1495.3 ± 62.40 | −3.90 | 1.24 | 3.82 | |
| 8-oxocorynoline | 11.60 | 10.60 ± 0.60 | −4.80 | 2.70 | 1.90 |
| 290 | 261.8 ± 6.90 | −0.60 | 1.20 | 1.20 | |
| 1450 | 1462.7 ± 40.00 | 0.92 | 2.73 | 1.32 | |
| Corynoline | 10.22 | 10.60 ± 1.00 | 4.01 | 4.43 | 3.74 |
| 255.5 | 261.8 ± 6.90 | 2.54 | 2.62 | 2.62 | |
| 1277.5 | 1231.3 ± 77.20 | −3.63 | 1.33 | 1.71 | |
| Tetrahydropalmatine | 10.14 | 11.25 ± 0.30 | 4.73 | 2.82 | 3.70 |
| 253.5 | 275.4 ± 6.50 | 3.60 | 2.43 | 2.62 | |
| 1267.5 | 1235.4 ± 3.10 | −2.54 | 0.30 | 1.72 | |
| Protopine | 9.74 | 10.80 ± 1.60 | 4.71 | 4.80 | 2.41 |
| 243.5 | 250.8 ± 2.40 | 3.03 | 1.02 | 1.23 | |
| 1217.5 | 1320.5 ± 26.80 | 3.51 | 2.03 | 4.34 | |
| Palmatine | 11.30 | 11.20 ± 1.40 | −1.02 | 4.60 | 3.63 |
| 282.5 | 285.7 ± 9.70 | 1.12 | 3.44 | 1.82 | |
| 1412.5 | 1331.6 ± 5.40 | −1.72 | 0.40 | 3.13 | |
| Columbamine | 11.15 | 10.80 ± 0.50 | −2.82 | 1.13 | 3.63 |
| 278.8 | 273.6 ± 5.50 | −1.85 | 2.00 | 0.76 | |
| 1393.8 | 1355.7 ± 30.60 | −2.70 | 2.28 | 3.10 | |
| Jateorhizine | 10.00 | 11.60 ± 0.20 | 4.68 | 1.60 | 4.97 |
| 250 | 244.6 ± 3.40 | −2.22 | 1.37 | 1.12 | |
| 1250 | 1282.2 ± 13.80 | 2.55 | 1.10 | 4.78 | |
| Berberine | 10.80 | 10.14 ± 0.50 | −1.13 | 1.22 | 3.57 |
| 270 | 268.9 ± 9.40 | −0.43 | 3.48 | 0.79 | |
| 1350 | 1342.5 ± 27.20 | −0.57 | 2.03 | 3.12 | |
| Worenine | 11.10 | 10.70 ± 0.90 | −4.02 | 3.78 | 3.56 |
| 277.5 | 273.9 ± 12.00 | −1.34 | 4.40 | 0.78 | |
| 1387.5 | 1364.6 ± 13.00 | −1.60 | 1.04 | 3.13 | |
| Sanguinarine | 10.00 | 11.60 ± 2.30 | 4.32 | 4.27 | 4.89 |
| 250 | 252.1 ± 11.30 | 0.99 | 4.55 | 4.90 | |
| 1250 | 1226.8 ± 15.50 | −1.89 | 1.30 | 3.45 | |
| Berberrubine | 11.05 | 11.70 ± 0.80 | 2.30 | 3.12 | 3.56 |
| 276.25 | 276.1 ± 6.60 | −0.01 | 2.42 | 0.78 | |
| 1381.2 | 1398.3 ± 6.30 | 1.23 | 0.45 | 3.13 | |
| Coptisine | 10.00 | 13.20 ± 2.50 | 3.23 | 3.70 | 4.83 |
| 250 | 266.3 ± 9.10 | 1.45 | 3.43 | 0.78 | |
| 1250 | 1222.9 ± 24.20 | −2.20 | 2.00 | 2.13 | |
| Z23 | 10.68 | 11.90 ± 2.20 | 1.10 | 1.78 | 3.56 |
| 267 | 256.8 ± 6.80 | −3.78 | 2.60 | 0.78 | |
| 1335 | 1336.5 ± 55.50 | 1.23 | 4.22 | 3.10 |
| Compounds | Original (ng) | Addition (ng) | Detection (ng) | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|
| Acetylcorynoline | 163 | 130.0 | 293.65 | 100.50 | 2.43 |
| 162.5 | 320.89 | 97.16 | 3.25 | ||
| 195.0 | 359.02 | 100.52 | 3.73 | ||
| 8-oxocorynoline | 231.62 | 184.0 | 421.30 | 103.08 | 4.35 |
| 230.0 | 466.21 | 101.99 | 2.20 | ||
| 276.0 | 513.62 | 102.17 | 3.24 | ||
| Corynoline | 2021.03 | 1616.7 | 3643.62 | 100.36 | 1.77 |
| 2020.9 | 4045.78 | 100.19 | 1.06 | ||
| 2425.0 | 4432.96 | 99.46 | 2.88 | ||
| Tetrahydropalmatine | 1034.79 | 828.3 | 1850.37 | 98.46 | 3.40 |
| 1035.4 | 2069.05 | 99.89 | 2.20 | ||
| 1242.0 | 2283.14 | 100.51 | 1.41 | ||
| Protopine | 3580.28 | 2864.0 | 6439.87 | 99.84 | 2.30 |
| 3580.0 | 7123.05 | 98.96 | 4.33 | ||
| 4296.0 | 7879.34 | 100.07 | 1.77 | ||
| Palmatine | 2103.25 | 1682.7 | 3780.40 | 99.67 | 2.98 |
| 2103.3 | 4215.15 | 100.40 | 3.21 | ||
| 2524.0 | 4662.71 | 101.40 | 3.82 | ||
| Columbamine | 15933.82 | 12746.9 | 28513.12 | 98.68 | 4.30 |
| 15933.7 | 31850.01 | 99.89 | 2.05 | ||
| 19120.5 | 35262.08 | 101.08 | 1.11 | ||
| Jateorhizine | 1296.32 | 1036.8 | 2326.84 | 99.39 | 2.88 |
| 1296.0 | 2607.07 | 101.38 | 3.27 | ||
| 1555.2 | 2893.62 | 102.70 | 4.21 | ||
| Berberine | 3243.26 | 2594.9 | 5656.75 | 93.00 | 2.51 |
| 3243.6 | 6374.04 | 96.52 | 3.47 | ||
| 3892.3 | 7223.13 | 102.25 | 3.16 | ||
| Worenine | 728.54 | 583.2 | 1326.20 | 102.48 | 2.54 |
| 729.1 | 1445.03 | 98.27 | 2.29 | ||
| 874.9 | 1615.78 | 101.41 | 4.06 | ||
| Sanguinarine | 8653.42 | 6923.4 | 15564.26 | 99.82 | 1.78 |
| 8654.3 | 17458.99 | 101.75 | 2.26 | ||
| 10385.2 | 19321.52 | 102.72 | 1.15 | ||
| Berberrubine | 3721.64 | 2977.6 | 6962.49 | 108.84 | 2.24 |
| 3722.0 | 7150.28 | 92.11 | 4.60 | ||
| 4466.4 | 8482.74 | 106.90 | 3.28 | ||
| Coptisine | 1698.23 | 1358.4 | 3033.27 | 98.28 | 3.16 |
| 1698.0 | 3379.35 | 99.01 | 2.32 | ||
| 2037.6 | 3742.09 | 100.31 | 1.94 | ||
| Z23 | 793.85 | 635.2 | 1443.83 | 102.32 | 2.86 |
| 794.1 | 1529.76 | 92.67 | 4.55 | ||
| 952.9 | 1772.56 | 102.70 | 1.50 |
| Sources | Compounds (%) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | I | J | K | L | M | N | Total | |
| 1 | 0.174 | 0.117 | 0.334 | 0.002 | 0.106 | 0.002 | 0.004 | 0.002 | 0.022 | 0.037 | 0.045 | 0.010 | 0.022 | 0.693 | 1.570 |
| 2 | 0.175 | 0.095 | 0.288 | 0.001 | 0.108 | 0.001 | ND | 0.001 | 0.002 | 0.001 | 0.002 | 0.001 | 0.002 | 0.213 | 0.883 |
| 3 | 0.122 | 0.054 | 0.181 | ND | 0.056 | 0.003 | 0.003 | ND | 0.004 | 0.004 | ND | 0.004 | 0.005 | 0.006 | 0.440 |
| 4 | 0.001 | 0.036 | 0.502 | 0.001 | 0.002 | ND | 0.001 | ND | 0.001 | ND | ND | 0.001 | 0.001 | 0.004 | 0.547 |
| 5 | 0.001 | 0.036 | 0.600 | 0.001 | 0.004 | 0.001 | 0.001 | 0.002 | 0.001 | 0.002 | 0.001 | 0.001 | 0.001 | 0.011 | 0.654 |
| 6 | 0.006 | 0.011 | 0.092 | ND | 0.024 | ND | ND | ND | 0.001 | 0.005 | ND | 0.001 | ND | 0.055 | 0.197 |
| 7 | ND | 0.068 | 0.710 | 0.001 | 0.002 | 0.001 | 0.001 | 0.002 | 0.001 | 0.001 | 0.002 | 0.002 | 0.001 | 0.044 | 0.826 |
| 8 | 0.014 | 0.018 | 0.551 | 0.001 | 0.004 | ND | ND | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | ND | 0.002 | 0.592 |
| 9 | 0.205 | 0.132 | 0.733 | 0.001 | 0.099 | 0.003 | 0.018 | 0.001 | 0.008 | 0.038 | 0.067 | 0.019 | 0.010 | 0.431 | 1.763 |
| 10 | 0.007 | 0.009 | 0.430 | ND | 0.012 | ND | ND | 0.001 | 0.001 | 0.001 | 0.021 | 0.001 | ND | 0.042 | 0.524 |
| 11 | 0.258 | 0.193 | 0.792 | 0.001 | 0.121 | 0.003 | 0.005 | 0.002 | 0.081 | 0.041 | 0.054 | 0.048 | 0.028 | 0.328 | 1.954 |
| 12 | 0.003 | 0.001 | 0.649 | 0.001 | 0.008 | 0.001 | 0.001 | 0.002 | 0.001 | 0.002 | 0.019 | 0.003 | 0.001 | 0.180 | 0.866 |
| 13 | 0.029 | 0.004 | 0.402 | 0.001 | 0.044 | 0.001 | 0.001 | 0.002 | 0.002 | 0.021 | 0.026 | 0.006 | 0.001 | 0.190 | 0.724 |
| 14 | 0.002 | 0.016 | 0.580 | ND | 0.006 | ND | 0.001 | 0.001 | 0.003 | 0.001 | 0.025 | 0.002 | 0.001 | 0.079 | 0.712 |
| 15 | 0.041 | 0.009 | 0.404 | 0.001 | 0.049 | 0.001 | 0.001 | 0.001 | 0.006 | ND | 0.031 | 0.016 | 0.001 | 0.174 | 0.730 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, M.; Yang, C.; Yang, J.; Dong, H.; Liu, L.; Ren, Y.; Wang, Z. Ultrahigh Performance Liquid Chromatography–Electrospray Ionization Tandem Mass Spectrometry Method for Qualitative and Quantitative Analyses of Constituents of Corydalis bungeana Turcz Extract. Molecules 2019, 24, 3463. https://doi.org/10.3390/molecules24193463
Tian M, Yang C, Yang J, Dong H, Liu L, Ren Y, Wang Z. Ultrahigh Performance Liquid Chromatography–Electrospray Ionization Tandem Mass Spectrometry Method for Qualitative and Quantitative Analyses of Constituents of Corydalis bungeana Turcz Extract. Molecules. 2019; 24(19):3463. https://doi.org/10.3390/molecules24193463
Chicago/Turabian StyleTian, Miao, Chunjuan Yang, Jing Yang, Hongrui Dong, Lu Liu, Yixuan Ren, and Zhibin Wang. 2019. "Ultrahigh Performance Liquid Chromatography–Electrospray Ionization Tandem Mass Spectrometry Method for Qualitative and Quantitative Analyses of Constituents of Corydalis bungeana Turcz Extract" Molecules 24, no. 19: 3463. https://doi.org/10.3390/molecules24193463
APA StyleTian, M., Yang, C., Yang, J., Dong, H., Liu, L., Ren, Y., & Wang, Z. (2019). Ultrahigh Performance Liquid Chromatography–Electrospray Ionization Tandem Mass Spectrometry Method for Qualitative and Quantitative Analyses of Constituents of Corydalis bungeana Turcz Extract. Molecules, 24(19), 3463. https://doi.org/10.3390/molecules24193463





