Abstract
Phytochemical investigation of the whole plant of Tradescantia albiflora Kunth led to the isolation and characterization of a butanolide, rosmarinosin B (1), that was isolated from natural sources for the first time, a new butenolide, 5-O-acetyl bracteanolide A (2), and a new apocarotenoid, 2β-hydroxyisololiolide (11), together with 25 known compounds (compounds 3–10 and 12–28). The structures of the new compounds were elucidated by analysis of their spectroscopic data, including MS, 1D, and 2D NMR experiments, and comparison with literature data of known compounds. Furthermore, four butenolides 4a–4d were synthesized as novel derivatives of bracteanolide A. The isolates and the synthesized derivatives were evaluated for their preliminary anti-inflammatory activity against lipopolysaccharide (LPS)-stimulated nitric oxide (NO) production in RAW 264.7 cells. Among them, the synthesized butenolide derivative n-butyl bracteanolide A (4d) showed enhanced NO inhibitory activity compared to the original compound, with an IC50 value of 4.32 ± 0.09 μg/mL.
1. Introduction
Tradescantia albiflora Kunth (Commelinaceae) is native to tropical rainforests. It has been used as a traditional medicine for treating hyperuricemia and gout in Taiwan. Previous research described the inhibitory activity against xanthine oxidase (XO), which plays a central role in metabolic disorders such as hyperuricemia and gout, of the methanol extract and compounds isolated from the leaves of T. albiflora [1]. However, none of the isolated compounds showed significant inhibitory activity.
Our continuing investigation on the bioactive compounds from T. albiflora has now led to the extraction, purification, and structural elucidation of three new naturally occurring compounds, together with 25 known compounds. The butenolide bracteanolide A (4) was the most abundant compound among the isolates, and it has been reported to show inhibitory ability against lipopolysaccharide (LPS)-stimulated nitric oxide (NO) production in RAW 264.7 cells. This inhibition was associated with its selective suppression on inducible NO synthase (iNOS) induction [2], indicating its potential to treat inflammatory diseases caused by NO production. For this reason, bracteanolide A (4) was used as a starting material for the preparation of butenolide derivatives. In addition, the isolates and four newly synthesized derivatives were evaluated for their preliminary anti-inflammatory activity against LPS-stimulated NO production in RAW 264.7 cells.
2. Results and Discussion
Phytochemical investigation of the whole plants of T. albiflora Kunth led to the isolation and characterization of three new compounds and 25 known compounds, which were identified by comparison with literature spectroscopic data and determined as 4-(3’,4’-dihydroxyphenyl)furan-2(5H)-one (3) [3], bracteanolide A (4) [2], bracteanolide B (5) [2], methyl 3,4-dihydroxybenzoate (6) [4], hydroxytyrosol (7) [5], 1-(3,4-dihydroxyphenyl)-2-hydroxyethan-1-one (8) [5], (±)-tradescantin (9) [3], tricin (10) [6], isololiolide (12) [7], loliolide (13) [8], (3R)-3-hydroxy-β-ionone (14) [9], (6R,7E,9R)-9-hydroxy-4,7-megastigmadien-3-one (15) [10], (E)-3,5,5-trimethyl-4-(3-oxobut-1-en-1-yl)cyclohex-2-enone (16) [11], (S)-dehydrovomifoliol (17) [12], N-trans-feruloyltyramine (18) [13], N-trans-feruloyl-3-methoxytyramine (19) [13], sitosterol (20) [14], stigmasterol (21) [14], 7-ketositosterol (22) [15], 7-ketostigmasterol (23) [15], 7β-hydroxysitosterol (24) [15], schottenol (25) [14], ergosterol peroxide (26) [16], 24,25-dihydrocimicifugenol (27) [17], 3-epicyclomusalenol (28) [17] (Figure 1).
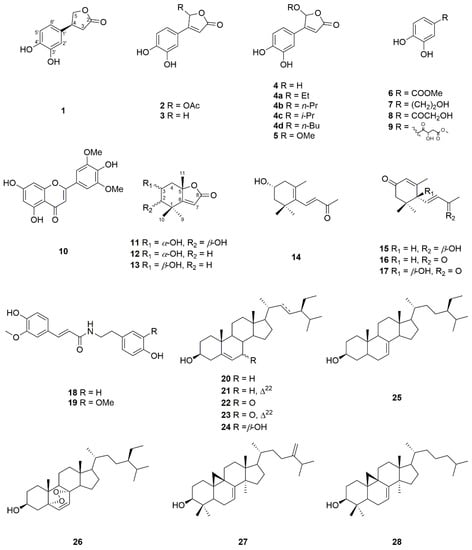
Figure 1.
The chemical structures of compounds 1–28.
Compound 1 was obtained as a colorless amorphous solid with − 13.5, and its high resolution electrospray ionization mass spectrometry (HRESIMS) data determined the molecular formula as C10H10O4 (m/z 217.0464, assigned as C10H10O4Na) indicating six degrees of unsaturation. The IR spectrum displayed the presence of hydroxyl (3312 cm−1), γ-lactone (1758 cm−1), and aromatic (1607, 1526 cm−1) functionalities.
The 1H-NMR spectrum (Table 1) displayed signals characteristic of a trisubstituted benzene ring indicated by an ABX-pattern for three aromatic protons [δH 6.73 (d, J = 8.4 Hz), 6.61 (dd, J = 8.4, 2.0 Hz), and 6.71 (d, J = 2.0 Hz)] and a butanolide moiety deduced from the following spectroscopic data: one pair of oxymethylene protons [δH 4.62 (t, J = 8.0 Hz) and 4.20 (t, J = 8.0 Hz)], one pair of lactone methylene protons [δH 2.86 (dd, J = 17.4, 8.6 Hz) and 2.62 (dd, J = 17.4, 8.9 Hz)], and one methine proton (δH 3.68, m), together with the IR peak at νmax 1758 cm−1. The HMBC correlations from H-3a, H-3b, H-4, H-5a, and H-5b to C-1’ (δC 133.0) indicated that the butanolide functionality was attached on C-1’. This assignment was confirmed by the deshielded signal of benzylic proton at δH 3.68 (H-4). According to the overall specific rotation, 1D, and 2D NMR, compound 1 was determined as rosmarinosin B with a 3R configuration, which was previously obtained as an artificial compound by gamma irradiation-assisted degradation of rosmarinic acid and exhibited moderately enhanced anti-adipogenic properties in 3T3-L1 cells than the original compound [18]. It was isolated from the natural sources for the first time.

Table 1.
1H- and 13C-NMR spectral data of compounds 1 and 2 (δ in ppm, J in Hz).
Compound 2 was obtained as a colorless amorphous solid with + 2.5, and its HRESIMS data determined the molecular formula as C12H10O6 (m/z 249.0371, assigned as C12H9O6), indicating eight degrees of unsaturation. The IR spectrum displayed the presence of hydroxyl (3470, 3169 cm−1), γ-lactone (1757 cm−1), ester (1728 cm−1), and aromatic (1609, 1516 cm−1) functionalities.
The 1H-NMR spectrum (Table 1) displayed signals characteristic of the presence of two hydroxyl groups attached on the benzene ring (δH 8.63, brs) as determined by D2O exchange experiment, a highly deshielded oxymethine (δH 7.40, s, H-5), a trisubstituted benzene ring indicated by an ABX-pattern for three aromatic protons [δH 7.15 (d, J = 2.1 Hz), 7.11 (dd, J = 8.3, 2.1 Hz), and 6.94 (d, J = 8.3 Hz)], a conjugated olefinic proton (δH 6.49, s, H-3), and an acetyl group (δH 2.15, 3H, s). Additionally, twelve carbon signals were displayed in the 13C-NMR spectrum. The assignments of two ortho-hydroxyl groups attached on the benzene ring were confirmed by two deshielded signals of aromatic carbons at δC 150.7 and 146.8. The IR peak at νmax 1757 cm−1 for γ-lactone functionality together with the carbon signals at δC 171.0, 162.8, 112.8, and 93.5 indicated the presence of the oxygenated unsaturated butenolide moiety. This moiety was also deduced from the HSQC correlations from H-3 (δH 6.49 s) to C-3 (δC 112.8) and from H-5 (δH 7.40 s) to C-5 (δC 93.5). The HMBC correlation from H-5 (δH 7.40, s) to the acetyl carbon (δC 170.0) indicated that the acetoxyl group was attached on the C-5 in butenolide moiety. The overall 1D and 2D NMR data suggested the structural similarities between 2 and bracteanolide A (4), except that the hydroxy group on C-5 in 4 was replaced by the O-acetyl group. This assignment was also confirmed by a highly deshielded oxymethine signal (δH/δC 7.40/93.5). Compound 2 was consequently determined as new butenolide, named 5-O-acetyl bracteanolide A.
Compound 11 was obtained as a colorless oil with + 27.6, and its high resolution electron ionization mass spectrometry (HREIMS) peak at m/z 212.1043 determined the molecular formula as C11H16O4, indicating four degrees of unsaturation. The IR spectrum displayed the presence of hydroxyl (3406 cm−1) and lactone (1741 cm−1) functionalities.
The 1H-NMR spectrum (Table 2) indicated the presence of an olefinic proton signal at δH 5.78 (1H, s), two oxymethine signals at δH 3.80 and 3.04, a methylene signal at δH 2.38 and 1.43, and three methyl signals at δH 1.58, 1.33, and 1.18 (each 3H, s). 13C-NMR and DEPT experiments revealed the presence of 11 carbon signals, indicating a lactone carbon at δC 170.7, a pair of conjugated carbon at δC 180.3 and 114.0, one oxygenated quaternary carbon at δC 85.3, two oxymethines at δC 81.7 and 67.9, a methylene at δC 43.6, and three methyls at δC 25.7, 25.2, and 18.7.

Table 2.
1H- and 13C-NMR spectral data of compound 11 (δ in ppm, J in Hz) in acetone-d6.
An additional hydroxy group was assigned at C-2 (δC 81.7) of 11 by comparing the NMR data of 12. The planar structure of 11 was confirmed by HMBC correlations shown in Figure 2. The di-axial orientations of H-2 and H-3 were deduced from the coupling constant of 9.3 Hz between them. The NOESY correlations between H-2 and Me-9; H-3 and Me-11; Me-10 and Me-11 established the relative configuration of 11. Thus, compound 11 was determined as a new apocarotenoid, named 2β-hydroxyepiloliolide.
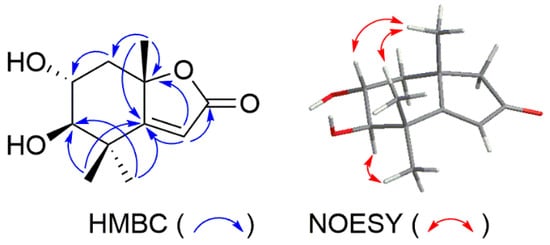
Figure 2.
Selected HMBC and NOESY correlations of compound 11.
In this study, the phytochemical investigation on the bioactive compounds from T. albiflora led to the isolation of 28 compounds including a butanolide rosmarinosin B (1) and four butenolides, 5-O-acetyl bracteanolide A (2), 4-(3’,4’-dihydroxyphenyl)furan-2(5H)-one (3), bracteanolide A (4), and bracteanolide B (5). Among the isolates, the butanolide bracteanolide A (4) was highly abundant in this plant (1.31 mg/g extract). It has been reported to exhibit inhibitory activity against LPS-activated NO production in RAW 264.7 cells by suppressing iNOS expression selectively [2], which is the potential target for the treatment of the inflammatory diseases caused by NO production. In order to screen the naturally anti-inflammatory butenolides and their related derivatives, four new butenolide derivatives 4a–4d were synthesized by modification from bracteanolide A (4) at C-4, focusing on changing the hydroxy group into alkoxy groups (Scheme 1). Then, the isolated compounds 2–8 and four new butenolide derivatives 4a–4d were evaluated for their preliminary anti-inflammatory activity against NO production in RAW 264.7 cells, a reliable indicator in investigating inflammatory activity [19].
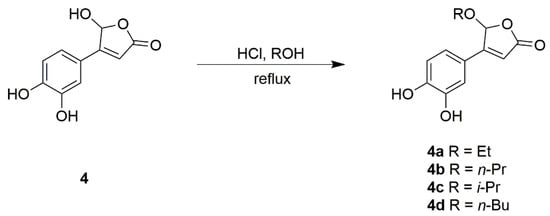
Scheme 1.
Synthesis of butenolide derivatives 4a–4d.
All compounds evaluated displayed lower anti-inflammatory activity than the positive control dexamethasone (Table 3), which has been reported to decrease iNOS-dependent NO production [20]. Dexamethasone is a highly effective anti-inflammatory and immunosuppressant corticosteroid. Unfortunately, the long-term use may cause serious systemic side effects, ranging from weight gain, diabetes, hypertension, immunosuppression, psychological disturbances, fragile skin, muscle weakness, osteoporosis, and Cushing’s syndrome [21].

Table 3.
Inhibitory effect of compounds 2–8 and 4a–4d against NO production in LPS-stimulated RAW 264.7 cells.
As shown in Table 3 and Figure S22, compounds 4, 4b, 4d, 6, and 7 showed inhibitory potential against NO production, which was not associated with their cytotoxicity against RAW 264.7 cells (Figure S23). The results suggested that the disappearance of the hydroxy group (3) or the presence of methyl (5), ethyl (4a), i-propyl (4c), and acetyl groups (2) resulted in a decreased activity; the presence of n-propyl group did not affect the activity. The data also revealed that the catechol group might have contributed to the activity even if the result of compound 8 was unsatisfactory (the IC50 value was above 50 µg/mL). Among compounds evaluated, compound 4d with an n-butyl group showed enhanced anti-inflammatory activity (IC50 value of 4.32 ± 0.09 μg/mL) compared to the original compound (Table 3).
3. Materials and Methods
3.1. General
Optical rotations were measured with a DIP-1000 Polarimeter (JASCO, Tokyo, Japan). UV spectra were recorded on a Heλios Beta UV-Visible spectrophotometer (Thermo Scientific Inc., Waltham, MA, USA). Infrared spectra were acquired on a Nicolet MAGNA-IR 500 spectrophotometer (Thermo Scientific Inc., Waltham, MA, USA). The NMR experiments were performed on DMX-400 and DMX-500 MHz NMR spectrometers (Bruker, Bremen, Germany). HREIMS and HRESIMS spectra were generated with SX-102A (JEOL, Tokyo, Japan) and maXis impact mass spectrometers (Bruker Daltonics, Bremen, Germany), respectively. Column chromatography was performed on Silica gel 60 (40–63 µm, Merck, Darmstadt, Germany), high performance liquid chromatography (HPLC) was performed using Keystone Spherisorb silica (5 µm, 250 × 10 mm), and thin-layer chromatography (TLC) was performed on silica gel 60 F254 plates (200 µm, Merck).
3.2. Plant Material
The greenhouse-grown plant material was obtained from Dr. T.-F.K. (Department of Post-Baccalaureate Veterinary Medicine, Asia University). A voucher specimen (TAIF-PLANT-199332) has been retained at the Herbarium of Taiwan Forestry Research Institute, Taipei, Taiwan.
3.3. Extraction and Isolation
Air-dried whole plant of T. albiflora (14.9 kg) was extracted twice with methanol (40 L) at room temperature for 7 days, and concentrated under vacuum. The methanol extract (1.5 kg) taken up in distilled water was fractionated successively with ethyl acetate and n-butanol to yield the corresponding solvent-soluble fractions. The ethyl acetate-soluble fraction (232.7 g) was subjected to silica gel column chromatography (2.0 kg, 70–230 mesh) using a gradient solvent system (n-hexane/ethyl acetate/methanol) as eluant to afford 10 fractions. Fr. 3 (35.2 g) was reseparated by silica gel column chromatography (n-hexane/acetone = 95/5) followed by semi-preparative normal phase HPLC to give compounds 25 (10.7 mg), 27 (7.6 mg), and 28 (7.4 mg). Fr. 4 (24.6 g) was reseparated by silica gel column chromatography (dichloromethane/acetone = 95/5) and recrystallization to obtain compounds 20/21 (740.2 mg) and 26 (8.8 mg). Fr. 5 (9.0 g) was reseparated by silica gel column chromatography using a gradient solvent system (dichloromethane/ethyl acetate) to afford 10 fractions. Fr. 5-2 was purified by normal phase HPLC (dichloromethane/acetone = 80/20) to give compounds 10 (8.1 mg), 12 (57.9 mg), 16 (9.0 mg), and 17 (11.0 mg). Fr. 5-4 was purified by normal phase HPLC (dichloromethane/acetone = 80/20) to obtain compounds 11 (4.7 mg), 14 (13.0 mg), and 22/23 (44.1 mg). Fr. 5-5 was purified by normal phase HPLC (n-hexane/acetone = 80/20) to obtain compound 24 (13.6 mg). Fr. 5-7 purified by normal phase HPLC (dichloromethane/ethyl acetate = 65/35) to obtain compound 9 (27.4 mg). Fr. 6 (18.4 g) was reseparated by silica gel column chromatography a gradient solvent system (dichloromethane/ethyl acetate) to afford 12 fractions. Fr. 6-2 was purified by normal phase HPLC (dichloromethane/ethyl acetate = 67/33) to obtain compounds 6 (8.8 mg) and 13 (30.1 mg). Fr. 6-3 was purified by normal phase HPLC (dichloromethane/ethyl acetate = 50/50) to obtain compound 15 (9.0 mg). Fr. 6-6 was purified by normal phase HPLC (n-hexane/acetone = 75/25) to obtain compounds 1 (5.1 mg) and 2 (10.3 mg). Fr. 6-7 was purified by normal phase HPLC (n-hexane/ethyl acetate = 30/70) to obtain compounds 3 (8.8 mg), 4 (1958.6 mg), and 5 (30.1 mg). Fr. 6-8 was purified by normal phase HPLC (dichloromethane/acetone = 75/25) to obtain compounds 7 (13.1 mg) and 8 (8.0 mg). Fr. 6-8 was purified by normal phase HPLC (dichloromethane/acetone = 70/30) to obtain compounds 18 (8.9 mg) and 19 (7.1 mg).
Rosmarinosin B (1): Colorless amorphous solid; − 13.5 (c 0.1, MeOH); UV (MeOH) λmax (log ε): 283 (2.54) nm; IR (KBr) νmax 3312, 2918, 2851, 1759, 1607, 1526, 1449, 1375, 1285, 1188, 1117, and 1015 cm−1; HRESIMS m/z 217.0464 [M + Na]+ (C10H10O4Na); 1H- and 13C-NMR: see Table 1.
5-O-Acetylbracteanolide A (2): Colorless amorphous solid + 2.5 (c 0.1, MeOH); UV (MeOH) λmax (log ε): 217 (4.08), 249 (4.02), 334.6 (4.19) nm; IR (KBr) νmax 3470, 3169, 2961, 2924, 1757, 1728, 1609, 1516, 1302, 1285, 1223, 1198, 1177, 1032, and 989 cm−1; HRESIMS m/z 249.0371 [M − H]− (C12H9O6); 1H- and 13C-NMR: see Table 1.
2β-Hydroxyisololiolide (11): Colorless oil; + 27.6 (c 0.27, MeOH); UV (MeOH) λmax (log ε): 212 (3.94), 272 (2.79) nm; IR (KBr) νmax 3406, 2925, 2873, 1742, 1629, 1460, 1383, 1291, 1260, 1050, 984, and 938 cm−1; HREIMS m/z 212.1043 [M]+ (C11H16O4); 1H- and 13C-NMR: see Table 2.
3.4. Preparation of Butenolide Derivatives 4a–4d
Bracteanolide A (20 mg) was dissolved in the corresponding alcohol (10 mL). Concentrated hydrochloric acid (3 drops) was added and the solution was refluxed and stirred for 12 h. The solvent was evaporated under vacuum to produce a yellow residue that was diluted with distilled water and fractionated twice with dichloromethane. The organic extracts were combined and dried over magnesium sulfate to give butenolide derivatives 4a–4d, in approximately 70% yield.
5-O-Ethyl bracteanolide A (4a): Yield 73%; IR (KBr) νmax 3368, 3098, 2974, 1728, 1605, 1512, 1373, 1342, 1300, 1281, 1200, 1177, 1115, 1015, 972, and 945 cm−1; 1H-NMR (acetone-d6, 400 MHz): δH 7.28 (d, 1H, J = 2.1 Hz), 7.21 (dd, 1H, J = 8.3, 2.1 Hz), 6.92 (d, 1H, J = 8.3 Hz), 6.38 (s, 1H), 6.36 (s, 1H), 3.84 (m, 2H), 1.24 (t, 3H, J = 7.1 Hz); 13C-NMR: δC 170.5, 161.7, 148.8, 145.4, 121.7, 120.9, 115.5, 114.7, 112.2, 102.1, 64.2, 14.5.
5-O-n-Propylbracteanolide A (4b): Yield 70%; IR (KBr) νmax 3472, 3167, 2970, 2878, 1713, 1605, 1512, 1408, 1342, 1300, 1285, 1200, 1177, 1123, 1030, 964, and 949 cm−1; 1H-NMR (acetone-d6, 400 MHz): δH 7.28 (d, 1H, J = 1.9 Hz), 7.21 (dd, 1H, J = 8.3, 1.9 Hz), 6.93 (d, 1H, J = 8.3 Hz), 6.38 (s, 1H), 6.37 (s, 1H), 3.77 (t, 2H, J = 7.1 Hz), 1.63 (tq, 2H, J = 7.0 Hz), 0.92 (t, 3H, J = 7.0 Hz); 13C NMR: δC 170.5, 161.7, 148.7, 145.4, 121.7, 121.0, 115.5, 114.8, 112.2, 102.3, 70.1, 22.6, 9.9.
5-O-i-Propylbracteanolide A (4c): Yield 67%; IR (KBr) νmax 3472, 3159, 2978, 2920, 2808, 1713, 1605, 1512, 1408, 1381, 1323, 1342, 1300, 1281, 1196, 1150, 1126, 1030, 961, and 922 cm−1; 1H-NMR (acetone-d6, 400 MHz): δH 7.26 (d, 1H, J = 2.1 Hz), 7.19 (dd, 1H, J = 8.3, 2.1 Hz), 6.92 (d, 1H, J = 8.3 Hz), 6.44 (s, 1H), 6.33 (s, 1H), 4.22 (m, 2H), 1.33 (t, 3H, J = 6.1 Hz), 1.23 (t, 3H, J = 6.1 Hz); 13C NMR: δC 170.6, 162.1, 148.7, 145.3, 121.8, 120.9, 115.5, 114.8, 112.1, 101.4, 72.8, 22.8, 21.6.
5-O-n-Butylbracteanolide A (4d): Yield 69%; IR (KBr) νmax 3476, 3171, 2963, 2936, 2874, 1732, 1605, 1512, 1412, 1373, 1342, 1300, 1200, 1126, 1030, 972, 941, and 929 cm−1; 1H-NMR (acetone-d6, 400 MHz): δH 7.27 (d, 1H, J = 1.9 Hz), 7.21 (dd, 1H, J = 8.3, 1.9 Hz), 6.92 (d, 1H, J = 8.3 Hz), 6.38 (s, 1H), 6.36 (s, 1H), 3.78 (m, 2H), 1.60 (dq, 2H, J = 6.6 Hz), 1.37 (tq, 2H, J = 6.2 Hz), 0.89 (t, 3H, J = 7.4 Hz); 13C NMR: δC 170.5, 161.7, 148.8, 145.4, 121.6, 120.9, 115.5, 114.7, 112.2, 102.3, 68.1, 31.4, 18.9, 13.1.
3.5. Cell Culture
A murine macrophage cell line RAW264.7 (BCRC No. 60001) was obtained from the Bioresources Collection and Research Center of the Food Industry Research and Development Institute (Hsinchu, Taiwan). Cells were maintained in Dulbecco′s Modified Eagle Medium (DMEM, Sigma, St. Louis, MO, USA) supplemented with 10% fetal bovine serum (FBS, Sigma) in an incubator containing 5% CO2 at 37 °C and subcultured every 3 days using 0.05% trypsin-0.02% EDTA in Ca2+-, Mg2+-free phosphate-buffered saline (DPBS).
3.6. Cell Viability
Raw 264.7 cells (5 × 104 cells/well) were seeded into 96-well plates and incubated for 24 h. Then, cells were treated with different concentrations of samples in the presence of 100 ng/mL LPS. After incubation overnight, the cells were washed twice with DPBS and incubated with 100 μL MTT (0.5 mg/mL) for 3 h. The medium was removed, and MTT formazan was dissolved by 100 μL dimethyl sulfoxide (DMSO). Then, absorbance at 570 nm was read using a microplate reader.
3.7. Measurement of Nitric Oxide/Nitrite
NO production was indirectly measured by determining the nitrite levels in the cultured medium using a colorimetric assay based on the Griess reaction. Cells were treated with different concentrations of samples in the presence of LPS (100 ng/mL) and incubated for 24 h. Then, each supernatant (100 μL) was mixed with the same volume of Griess reagent (1% sulfanilamide, 0.1% naphthylethylenediamine dihydrochloride and 5% phosphoric acid) and incubated for 5 min, and the absorbance at 540 nm was measured using a microplate reader.
4. Conclusions
In summary, phytochemical investigation of the whole plant of Tradescantia albiflora Kunth has led to the isolation and characterization of a butanolide, rosmarinosin B (1), that was isolated from natural sources for the first time, a new butenolide, 5-O-acetyl bracteanolide A (2), and a new apocarotenoid 2β-hydroxyisololiolide (11), together with 25 known compounds (compounds 3–10 and 12–28). The isolated compounds 2–8 and four new synthetic butenolide derivatives 4a–4d, which were synthesized from bracteanolide A (4), were evaluated for their preliminary anti-inflammatory activity against LPS-stimulated NO production in RAW 264.7 cells. Among them, the new synthetic butenolide derivative n-butyl bracteanolide A (4d) exhibited better NO inhibitory activity than the original compound bracteanolide A (4).
Supplementary Materials
Supplementary Materials related to this article can be found online.
Author Contributions
Conceptualization, T.-F.K. and Y.-H.K.; methodology, Y.-H.K. and G.-J.H.; investigation, P.-C.T., H.-C.T., and Y.-C.L.; resources, G.-J.H. and Y.-H.K.; writing—original draft preparation, P.-C.T. and Y.-C.L.; writing—review and editing, P.-C.T., Y.-C.L., and Y.-H.K.; supervision, T.-L.L. and Y.-H.K.
Funding
This work was financially supported by Taiwan Ministry of Health and Welfare Clinical Trial Center (MOHW107-TDU-B-212-123004) and “Chinese Medicine Research Center, China Medical University” from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan (CMRC-CHM-4).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, W.L.; Sheu, S.Y.; Huang, W.D.; Chuang, Y.L.; Tseng, H.C.; Hwang, T.S.; Fu, Y.T.; Kuo, Y.H.; Yao, C.H.; Kuo, T.F. Phytochemicals from Tradescantia albiflora Kunth extracts reduce serum uric acid levels in oxonate-induced rats. Pharm. Mag. 2016, 12, S223–S227. [Google Scholar]
- Wang, G.J.; Chen, S.M.; Chen, W.C.; Chang, Y.M.; Lee, T.H. Selective inducible nitric oxide synthase suppression by new bracteanolides from Murdannia bracteata. J. Ethnopharmacol. 2007, 112, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Vo, Q.H.; Nguyen, P.H.; Zhao, B.T.; Ali, M.Y.; Choi, J.S.; Min, B.S.; Nguyen, T.H.; Woo, M.H. Protein tyrosine phosphatase 1B (PTP1B) inhibitory constituents from the aerial parts of Tradescantia spathacea Sw. Fitoterapia 2015, 103, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Wen, H.; Cui, Y.; Fan, M.; Liu, Z.; Mei, L.; Shao, Y.; Wang, Y.; Tao, Y. Phenolics from Lagotis brevituba Maxim. Nat. Prod. Res. 2017, 31, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Ziosi, P.; Paolucci, C.; Santarelli, F.; Tabanelli, T.; Passeri, S.; Cavani, F.; Righi, P. A two-step process for the synthesis of hydroxytyrosol. ChemSusChem. 2018, 11, 2202–2210. [Google Scholar] [CrossRef]
- Govindan, B.; Johnson, A.J.; Viswanathan, G.; Ramaswamy, V.; Koshy, K.C.; Baby, S. Secondary metabolites from the unique bamboo, Melocanna baccifera. Nat. Prod. Res. 2019, 33, 122–125. [Google Scholar] [CrossRef]
- Kimura, J.; Maki, N. New loliolide derivatives from the brown alga Undaria pinnatifida. J. Nat. Prod. 2002, 65, 57–58. [Google Scholar] [CrossRef]
- De Marino, S.; Borbone, N.; Gala, F.; Zollo, F.; Fico, G.; Pagiotti, R.; Iorizzi, M. New constituents of sweet Capsicum annuum L. fruits and evaluation of their biological activity. J. Agric. Food Chem. 2006, 54, 7508–7516. [Google Scholar] [CrossRef]
- Yamano, Y.; Sasaki, H.; Wada, A. Versatile amine-promoted mild methanolysis of 3,5-dinitrobenzoates and its application to the synthesis of colorado potato beetle pheromone. Chem. Pharm. Bull. 2017, 65, 940–944. [Google Scholar] [CrossRef][Green Version]
- Xiong, H.P.; Mi, J.L.; Le, J.M.; Wu, Z.J.; Chen, W.S. Chemical constituents of Ampelopsis japonica. Chem. Nat. Compd. 2017, 53, 791–793. [Google Scholar] [CrossRef]
- Clemente-Tejeda, D.; Bermejo, F.A. Oxidation of alkenes with non-heme iron complexes: Suitability as an organic synthetic method. Tetrahedron 2014, 70, 9381–9386. [Google Scholar] [CrossRef]
- Jia, X.; Yang, D.; Yang, Y.; Xie, H. Carotenoid-derived flavor precursors from Averrhoa carambola fresh fruit. Molecules 2019, 24, 256. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Zhang, W.; Yang, X.; Xiu, F.; Xu, H.; Ying, X.; Stien, D. An isoindole alkaloid from Portulaca oleracea L. Nat. Prod. Res. 2018, 32, 2431–2436. [Google Scholar] [CrossRef] [PubMed]
- Badreddine, A.; Karym el, M.; Zarrouk, A.; Nury, T.; El Kharrassi, Y.; Nasser, B.; Cherkaoui Malki, M.; Lizard, G.; Samadi, M. An expeditious synthesis of spinasterol and schottenol, two phytosterols present in argan oil and in cactus pear seed oil, and evaluation of their biological activities on cells of the central nervous system. Steroids 2015, 99, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Soroka, D.; Sang, S. Oxyphytosterols as active ingredients in wheat bran suppress human colon cancer cell growth: Identification, chemical synthesis, and biological evaluation. J. Agric. Food Chem. 2015, 63, 2264–2276. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, L.; Ren, W.; Zhao, D.; Zhu, Y.; Wu, X. Bioactive metabolites from Chaetomium globosum L18, an endophytic fungus in the medicinal plant Curcuma wenyujin. Phytomedicine 2012, 19, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.H.; Liu, T.; Gu, C.X.; Shao, C.L.; Zhou, J.; Wang, C.Y. Steroids and triterpenoids from the brown alga Kjellmaniella crassifolia. Chem. Nat. Compd. 2012, 48, 158–160. [Google Scholar] [CrossRef]
- Jeong, G.H.; Cho, J.H.; Jo, C.; Lee, S.; Lee, S.S.; Bai, H.W.; Chung, B.Y.; Kim, T.H. Gamma irradiation-assisted degradation of rosmarinic acid and evaluation of structures and anti-adipogenic properties. Food Chem. 2018, 258, 181–188. [Google Scholar] [CrossRef]
- Yang, C.S.; Chen, J.J.; Huang, H.C.; Huang, G.J.; Wang, S.Y.; Sung, P.J.; Cheng, M.J.; Wu, M.D.; Kuo, Y.H. New Benzenoid Derivatives and Other Constituents from Lawsonia inermis with Inhibitory Activity against NO Production. Molecules 2017, 22, 936. [Google Scholar] [CrossRef]
- Korhonen, R.; Lahti, A.; Hamalainen, M.; Kankaanranta, H.; Moilanen, E. Dexamethasone inhibits inducible nitric-oxide synthase expression and nitric oxide production by destabilizing mRNA in lipopolysaccharide-treated macrophages. Mol. Pharm. 2002, 62, 698–704. [Google Scholar] [CrossRef]
- Bordag, N.; Klie, S.; Jurchott, K.; Vierheller, J.; Schiewe, H.; Albrecht, V.; Tonn, J.C.; Schwartz, C.; Schichor, C.; Selbig, J. Glucocorticoid (dexamethasone)-induced metabolome changes in healthy males suggest prediction of response and side effects. Sci. Rep. 2015, 5, 15954. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–10 and 18–28 are available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).