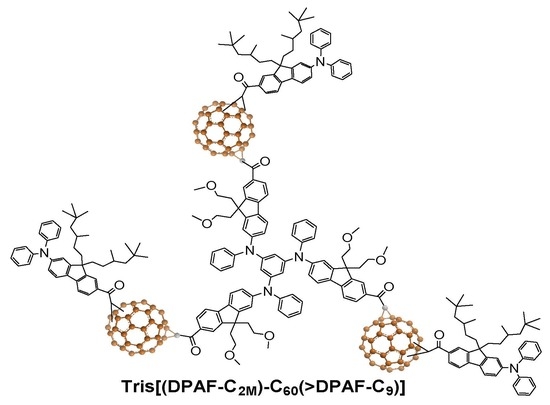
Synthesis and Intramolecular Energy- and Electron-Transfer of 3D-Conformeric Tris(fluorenyl-[60]fullerenylfluorene) Derivatives
Abstract
1. Introduction
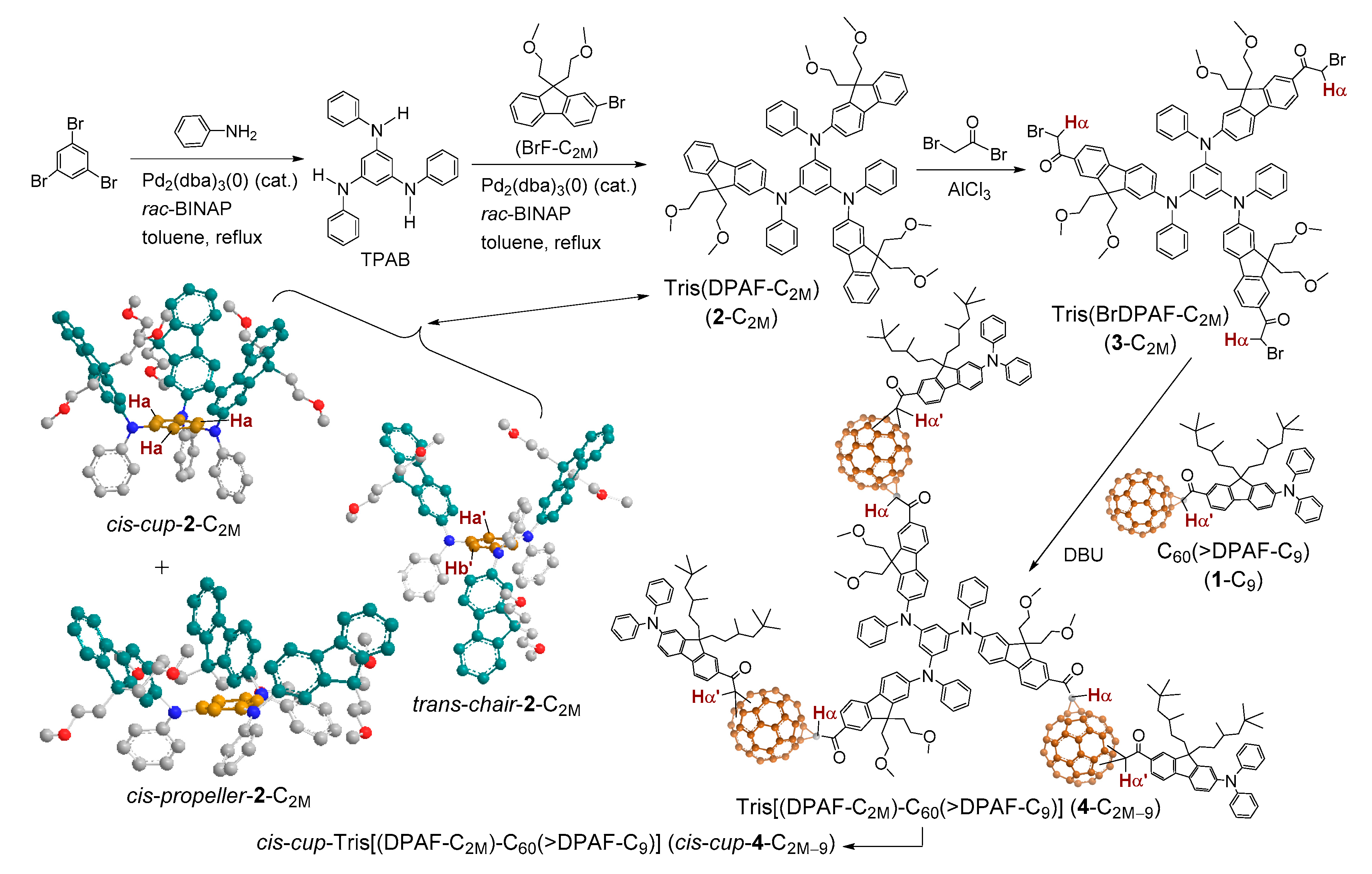
2. Results and Discussion
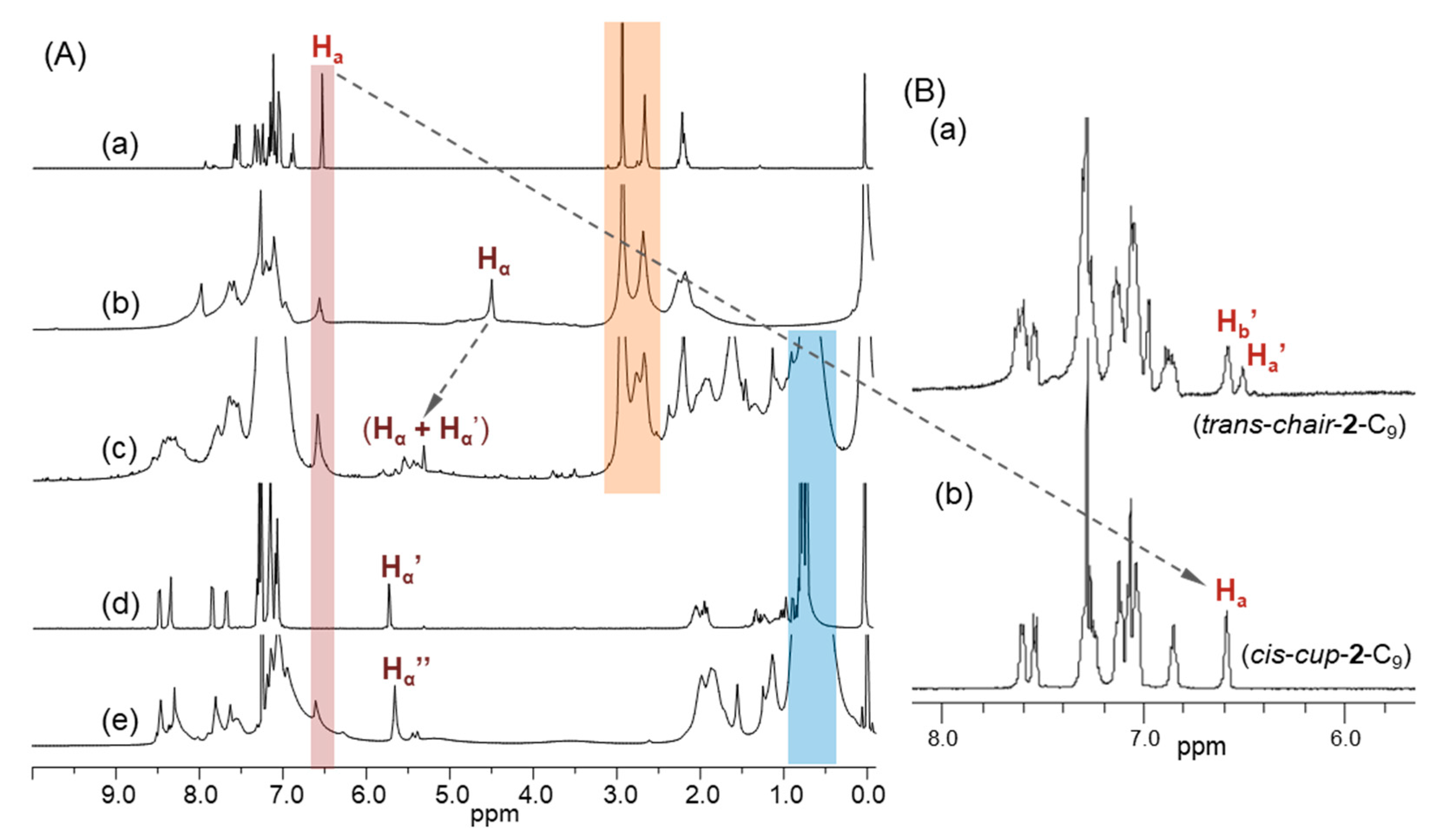
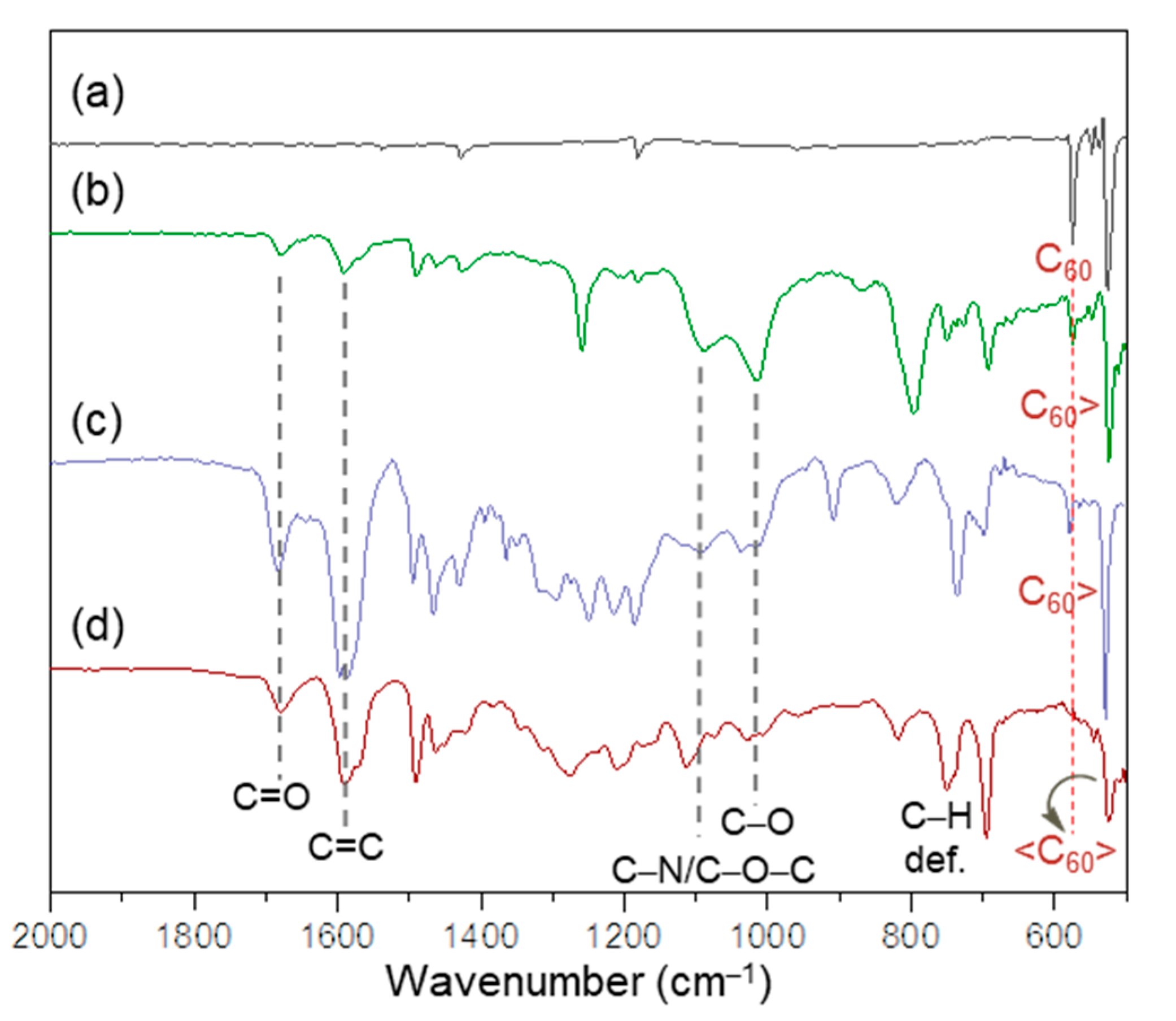
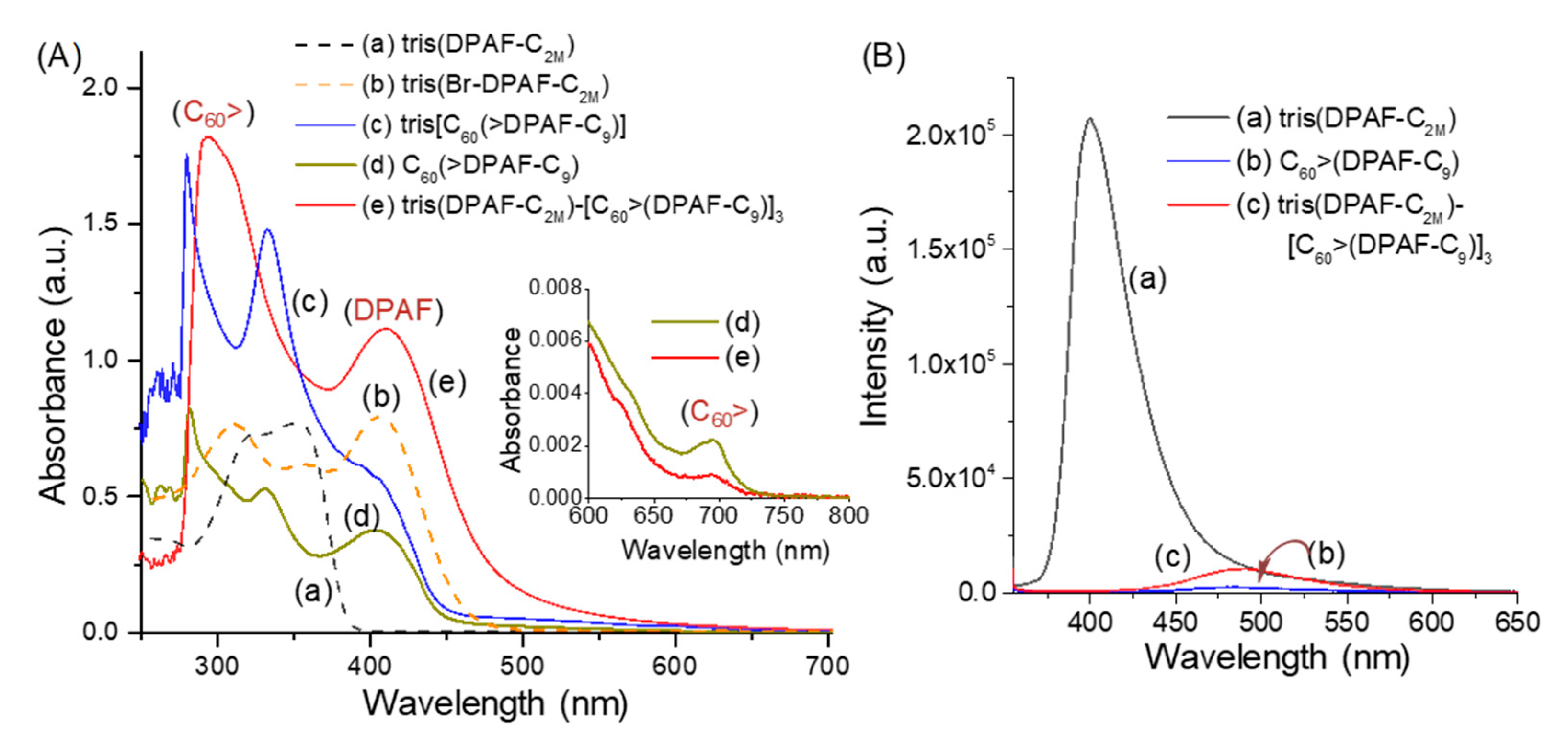
2.1. Spectroscopic Characterization of Synthetic 3D Configurated Fullerenyl Nanomaterials
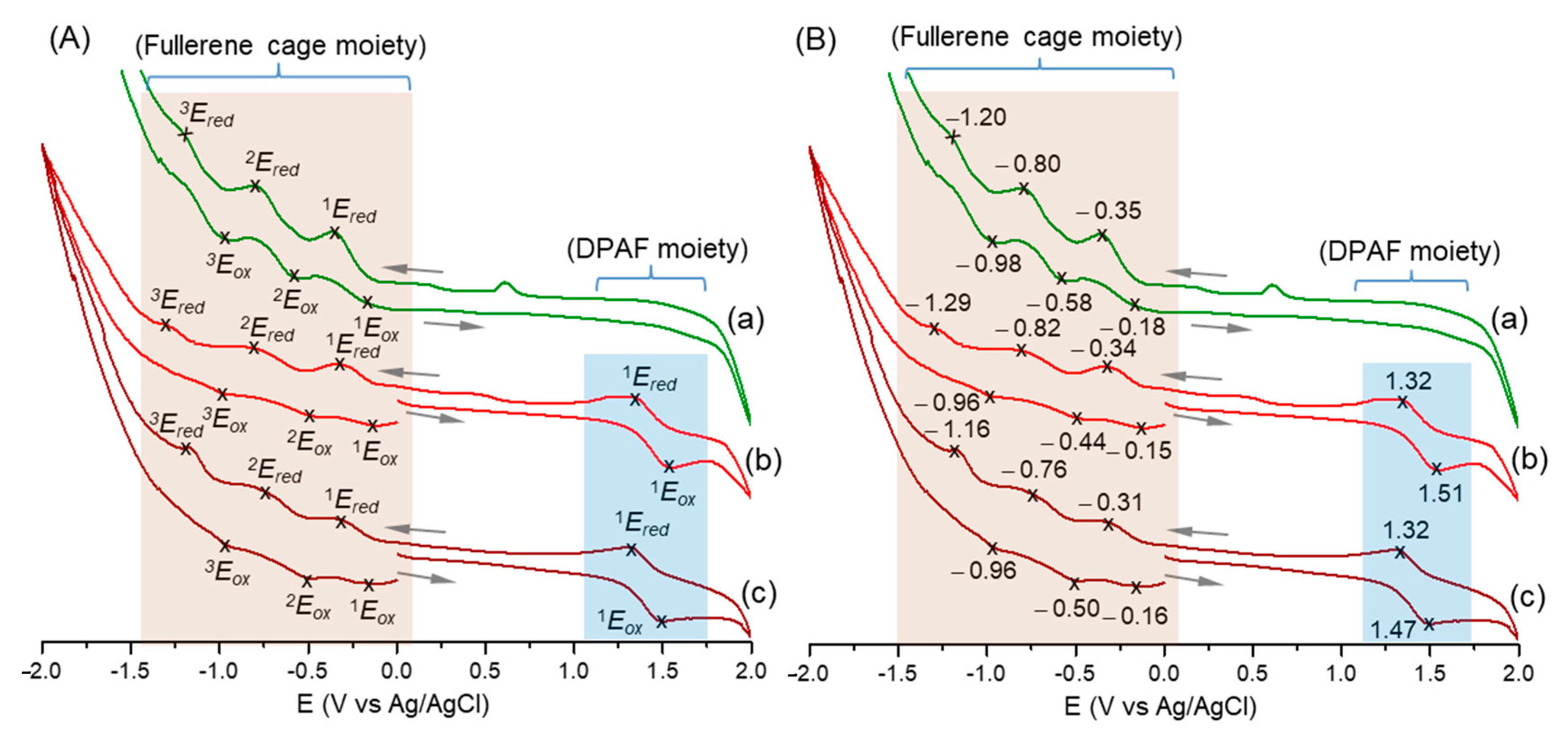
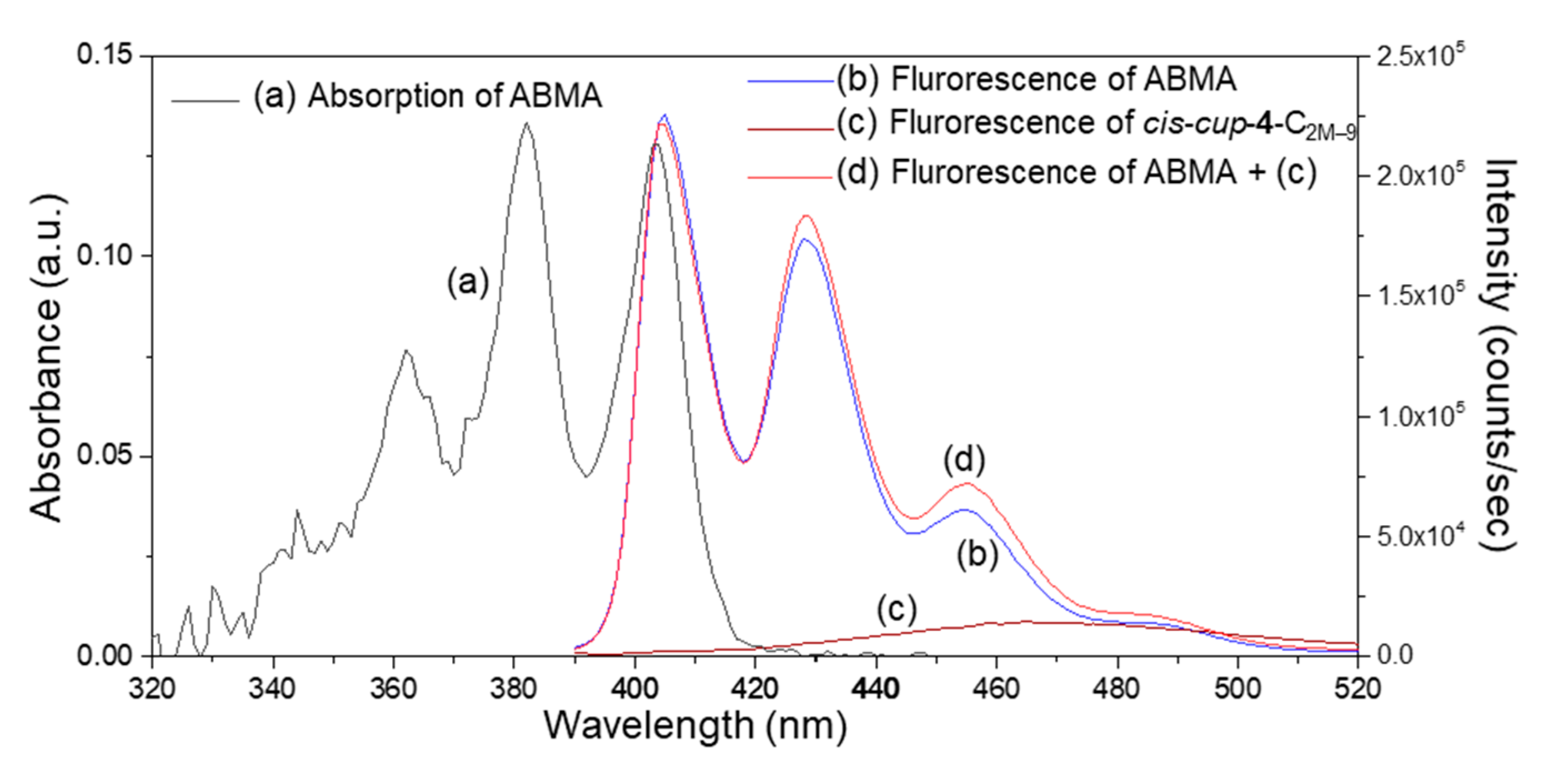
2.2. Photophysical and Physical Properties of 3D Conformeric Fullerenyl Nanomaterials
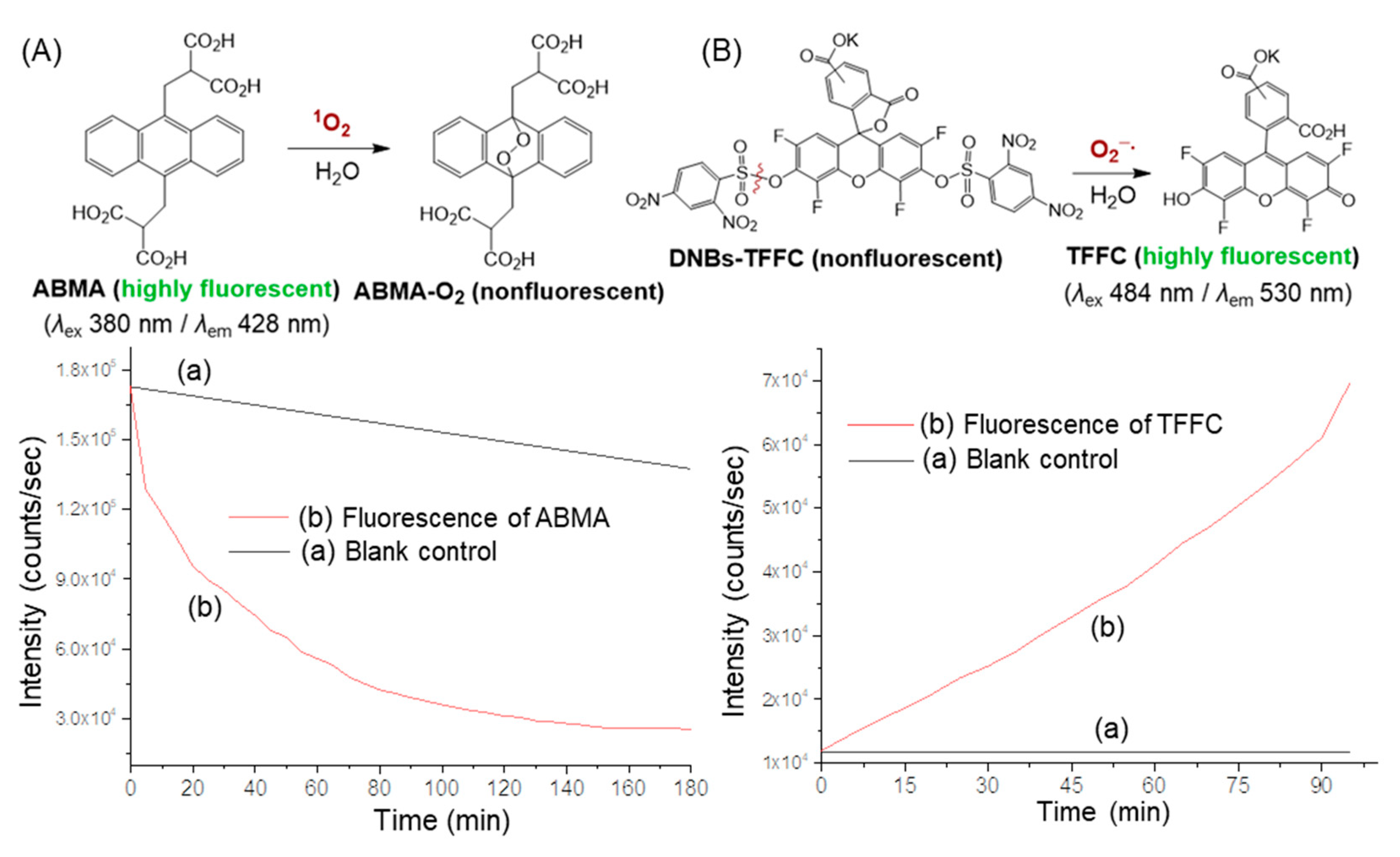
2.3. Evidence of Intramolecular Energy- and Electron-Transfer Events within cis-cup-4-C2M–9 by Detection of Corresponding Reactive Oxygen Species (ROS)
3. Experimental Section
3.1. Chemicals and Reagents
3.2. Instruments for Spectroscopic Measurements
3.3. Synthesis of N1,N3,N5-Tris(9,9-di(methoxyethyl)fluoren-2-yl)-1″,3″,5″-tris(phenylamino)-benzene as Tris(DPAF-C2M) (2-C2M)
3.4. Synthesis of N1,N3,N5-Tris(7-α-bromoacetyl-9,9-di(methoxyethyl)fluoren-2-yl)-1″,3″,5″-tris-(phenylamino)benzene as Tris(BrDPAF-C2M) (3-C2M)
3.5. Synthesis of N1,N3,N5-Tris(7-(1,2-dihydro-1,2-methanofullerene[60]-61-carbonyl)-9,9-di(methoxyethyl)fluoren-2-yl)-1″,3″,5″-tris(phenylamino)benzene) as Tris[(DPAF-C2M)-C60(>DPAF-C9)] (4-C2M–9)
3.6. ROS Measurements Using singlet oxygen (1O2)-Sensitive Fluorescent Probe
3.7. ROS Measurements Using Superoxide Radical (O2−·)-Sensitive Fluorescent Probe
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- El-Khouly, M.E.; Ito, O. Intermolecular and supramolecular photoinduced electron transfer processes of fullerene–porphyrin/phthalocyanine systems. J. Photochem. Photobiol. C Photochem. Rev. 2004, 5, 79–104. [Google Scholar]
- Escudero, D. Revising intramolecular photoinduced electron transfer (PET) from first-principles. Acc. Chem. Res. 2016, 49, 1816–1824. [Google Scholar] [CrossRef] [PubMed]
- Ito, O.; D’Souza, F. Recent advances in photoinduced electron transfer processes of fullerene-based molecular assemblies and nanocomposites. Molecules 2012, 17, 5816–5835. [Google Scholar] [PubMed]
- Wu, W.; Zhao, J.; Sun, J.; Guo, S. Light-harvesting fullerene dyads as organic triplet photosensitizers for triplet–triplet annihilation upconversions. J. Org. Chem. 2012, 77, 5305–5312. [Google Scholar] [CrossRef] [PubMed]
- Ziessel, R.; Allen, B.D.; Rewinska, D.B.; Harriman, A. Selective triplet-state formation during charge recombination in a fullerene/bodipy molecular dyad (bodipy=borondipyrromethene). Chem. Eur. J. 2009, 15, 7382–7393. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wu, W.; Sun, J.; Guo, S. Triplet photosensitizers: From molecular design to applications. Chem. Soc. Rev. 2013, 42, 5323–5351. [Google Scholar] [CrossRef]
- Chea, Y.; Yuan, X.; Cai, F.; Zhaoa, J.; Zhaoa, X.; Xub, H.; Liu, L. Bodipy−corrole dyad with truxene bridge: Photophysical properties and application in triplet−triplet annihilation upconversion. Dyes Pigments 2019, 171, 107756. [Google Scholar] [CrossRef]
- Kamkaew, A.; Lim, S.H.; Lee, H.B.; Kiew, L.V.; Chung, L.Y.; Burgess, K. BODIPY dyes in photodynamic therapy. Chem. Soc. Rev. 2013, 42, 77–88. [Google Scholar] [CrossRef]
- Natali, M.; Campagna, S.; Scandola, F. Photoinduced electron transfer across molecular bridges: Electron- and hole-transfer superexchange pathways. Chem. Soc. Rev. 2014, 43, 4005–4018. [Google Scholar] [CrossRef]
- Imahori, H.; Sakata, Y. Donor-linked fullerenes: Photoinduced electron transfer and its potential application. Adv. Mater. 1997, 9, 537–546. [Google Scholar]
- D’Souza, F.; Ito, O. Photosensitized electron transfer processes of nanocarbons applicable to solar cells. Chem. Soc. Rev. 2012, 41, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Bottari, G.; Torre, G.; Guldi, D.M.; Torres, T. Covalent and noncovalent phthalocyanine-carbon nanostructure systems: Synthesis, photoinduced electron transfer, and application to molecular photovoltaics. Chem. Rev. 2010, 110, 6768–6816. [Google Scholar] [CrossRef] [PubMed]
- Daly, B.; Ling, J.; Prasanna de Silva, A. Current developments in fluorescent PET (photoinduced electron transfer) sensors and switches. Chem. Soc. Rev. 2015, 44, 4203–4211. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Wang, M.; Huang, Y.-Y.; Chiang, L.Y.; Hamblin, M.R. Photodynamic therapy with decacationic [60]fullerene monoadducts: Effect of a light absorbing e−-donor antenna and micellar formulation. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 795–808. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yin, R.; Wang, M.; Huang, Y.-Y.; Landi, G.; Vecchio, D.; Chiang, L.Y.; Hamblin, M.R. Antimicrobial photodynamic inactivation with decacationic functionalized fullerenes: Oxygen independent photokilling in presence of azide and new mechanistic insights. Free Radic. Biol. Med. 2014, 79, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Segura, J.L.; Martin, N. [60]Fullerene dimer. Chem. Soc. Rev. 2000, 29, 13–25. [Google Scholar] [CrossRef]
- Shirai, Y.; Osgood, A.J.; Zhao, Y.; Kelly, K.F.; Tour, J.M. Directional control in thermally driven single-molecule nanocars. Nano Lett. 2005, 5, 2330–2334. [Google Scholar] [CrossRef]
- Akimov, A.V.; Nemukhin, A.V.; Moskovsky, A.A.; Kolomeisky, A.B.; Tour, J.M. Molecular dynamics of surface-moving thermally driven nanocars. J. Chem. Theory Comput. 2008, 4, 652–656. [Google Scholar] [CrossRef]
- Sasaki, T.; Osgood, A.J.; Kiappes, J.L.; Kelly, K.F.; Tour, J.M. Synthesis of a porphyrin-fullerene pinwheel. Org. Lett. 2008, 10, 1377–1380. [Google Scholar] [CrossRef]
- Zhang, J.; Porfyrakis, K.; Morton, J.J.L.; Sambrook, M.R.; Harmer, J.; Xiao, L.; Ardavan, A.; Briggs, G.A.D.; Briggs, G. Photoisomerization of a fullerene dimer. J. Phys. Chem. 2008, C 112, 2802–2904. [Google Scholar] [CrossRef]
- Wang, J.L.; Duan, X.F.; Jiang, B.; Gan, L.B.; Pei, J.; He, C.; Li, Y.F. Nanosized rigid π-conjugated molecular heterojunctions with multi[6 0]fullerenes: Facile synthesis and photophysical properties. J. Org. Chem. 2006, 71, 4400–4410. [Google Scholar] [CrossRef] [PubMed]
- Lόpez-Andarias, J.; Bauza, A.; Sakai, N.; Frontera, A.; Matile, S. Remote control of anion-π catalysis on fullerene-centered catalytic triads. Angew. Chem. 2018, 130, 11049–11053. [Google Scholar] [CrossRef]
- Sabirov, D.S. Polarizability of C60 fullerene dimer and oligomers: The unexpected enhancement and its use for rational design of fullerene-based nanostructures with adjustable properties. RSC Adv. 2013, 3, 19430. [Google Scholar] [CrossRef]
- Pankratyev, E.Y.; Tukhbatullina, A.A.; Sabirov, D.S. Dipole polarizability, structure, and stability of [2+2]-linked fullerene nanostructures (C60)n (n ≤ 7). Phys. E Low-Dimens. Syst. Nanostruct. 2017, 86, 237–242. [Google Scholar] [CrossRef]
- Tukhbatullina, A.; Shepelevich, I.; Sabirov, D.S. Exaltation of polarizability as a common property of fullerene dimers with divers intercage bridges. Fuller. Nanotub. Carbon Nanostruct. 2018, 26, 661–666. [Google Scholar] [CrossRef]
- Swart, M.; van Duijnen, P.T. Rapid determination of polarizability exaltation in fullerene-based nanostructures. J. Mater. Chem. 2015, C3, 23–25. [Google Scholar] [CrossRef]
- Wang, M.; Su, C.; Yu, T.; Tan, L.-S.; Hu, B.; Urbas, A.; Chiang, L.Y. Novel photoswitchable dielectric properties on nanomaterials of electronic core-shell γ-FeOx@Au@fullerosomes for GHz frequency applications. Nanoscale 2016, 8, 6589–6599. [Google Scholar] [CrossRef]
- Wang, M.; Yu, T.; Tan, L.-S.; Urbas, A.; Chiang, L.Y. Tunability of rf-responses by plasmonic dielectric amplification using branched e−-polarizable C60-adducts on magnetic nanoparticles. J. Phys. Chem. C 2016, 120, 17711–17721. [Google Scholar] [CrossRef]
- Wang, M.; Yu, T.; Tan, L.-S.; Urbas, A.; Chiang, L.Y. Enhancement of photoswitchable dielectric property by conducting electron donors on plasmonic core-shell gold-fluorenyl C60 nanoparticles. J. Phys. Chem. C 2018, 122, 12512–12523. [Google Scholar] [CrossRef]
- Padmawar, P.A.; Rogers, J.O.; He, G.S.; Chiang, L.Y.; Canteenwala, T.; Tan, L.-S. Large cross-section enhancement and intramolecular energy transfer upon multiphoton absorption of hindered diphenylaminofluorene-C60 dyads and triads. Chem. Mater. 2006, 18, 4065–4074. [Google Scholar] [CrossRef]
- Padmawar, P.A.; Canteenwala, T.; Tan, L.-S.; Chiang, L.Y. Synthesis and characterization of photoresponsive diphenylaminofluorene chromophore adducts of [60]fullerene. J. Mater. Chem. 2006, 16, 1366–1378. [Google Scholar] [CrossRef]
- Luo, H.; Fujitsuka, M.; Araki, Y.; Ito, O.; Padmawar, P.; Chiang, L.Y. Inter- and intramolecular photoinduced electron-transfer processes between C60 and diphenylaminofluorene in solutions. J. Phys. Chem. B 2003, 107, 9312–9318. [Google Scholar] [CrossRef]
- Hu, R.; Lager, E.; Aguilar-Aguilar, A.; Liu, J.; Lam, J.W.Y.; Sung, H.H.Y.; Williams, I.D.; Zhong, Y.; Wong, K.S.; Pena-Cabrera, E.; et al. Twisted intramolecular charge transfer and aggregation-induced emission of BODIPY derivatives. J. Phys. Chem. C 2009, 113, 15845–15853. [Google Scholar] [CrossRef]
- Kang, N.-G.; Kokubo, K.; Jeon, S.; Wang, M.; Lee, C.-L.; Canteenwala, T.; Tan, L.-S.; Chiang, L. Synthesis and photoluminescent properties of geometrically hindered cis-tris(diphenyl-aminofluorene) as precursors to light-emitting devices. Molecules 2015, 20, 4635–4654. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-T.; Wang, M.; Kokubo, K.; Kang, N.-G.; Wolf, L.; Tan, L.-S.; Chen, C.-T.; Chiang, L. New 3D-stereoconfigurated cis-tris(fluorenylphenylamino)-benzene with large steric hindrance to minimize π–π stacking in thin-film devices. Dyes Pigments 2018, 149, 377–386. [Google Scholar] [CrossRef]
- Chiang, L.Y.; Padmawar, P.A.; Canteenwala, T.; Tan, L.-S.; He, G.S.; Kannan, R.; Vaia, R.; Lin, T.-C.; Zheng, Q.; Prasad, P.N. Synthesis of C60-diphenylaminofluorene dyad with large 2PA cross-sections and efficient intramolecular two-photon energy transfer. Chem. Commun. 2002, 1854–1855. [Google Scholar] [CrossRef]
- Jeon, S.; Wang, M.; Ji, W.; Tan, L.-S.; Cooper, T.; Chiang, L.Y. Broadband two-photon absorption characteristics of highly photostable fluorenyl-dicyanoethylenylated [60]fullerene dyads. Molecules 2016, 21, 647. [Google Scholar] [CrossRef]
- Maeda, H.; Yamamoto, K.; Nomura, Y.; Kohno, I.; Hafsi, L.; Ueda, N.; Yoshida, S.; Fukuda, M.; Fukuyasu, Y.; Yamauchi, Y.; et al. A design of fluorescent probes for superoxide based on a nonredox mechanism. J. Am. Chem. Soc. 2005, 127, 68–69. [Google Scholar] [CrossRef]
- Wang, M.; Huang, L.; Sharma, S.K.; Jeon, S.; Thota, S.; Sperandio, F.F.; Nayka, S.; Chang, J.; Hamblin, M.R.; Chiang, L.Y. Synthesis and photodynamic effect of new highly photostable decacationically armed [60]- and [70]fullerene decaiodide monoadducts to target pathogenic bacteria and cancer cells. J. Med. Chem. 2012, 55, 4274–4285. [Google Scholar] [CrossRef][Green Version]
- Wang, M.; Maragani, S.; Huang, L.; Jeon, S.; Canteenwala, T.; Hamblin, M.R.; Chiang, L.Y. Synthesis of decacationic [60]fullerene decaiodides giving photoinduced production of superoxide radicals and effective PDT-mediation on antimicrobial photoinactvation. Eur. J. Med. Chem. 2013, 63, 170–184. [Google Scholar] [CrossRef]
- Sperandio, F.F.; Sharma, S.K.; Wang, M.; Jeon, S.; Huang, Y.-Y.; Dai, T.; Nayka, S.; de Sousa, S.C.O.M.; Chiang, L.Y.; Hamblin, M.R. Photoinduced electron-transfer mechanisms for radical-enhanced photodynamic therapy mediated by water-soluble decacationic C70 and C84O2 fullerene derivatives. Nanomed. Nanotech. Biol. Med. 2013, 9, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wang, M.; Huang, Y.-Y.; El-Hussein, A.; Chiang, L.Y.; Hamblin, M.R. Progressive cationic functionalization of chlorin derivatives for antimicrobial photodynamic inactivation and related vancomycin conjugate. Photochem. Photobiol. Sci. 2018, 17, 638–651. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Wang, M.; Yu, T.; Tan, L.-S.; Chiang, L.Y. 3D-conformer of tris[60]fullerenylated cis-tris(diphenylaminofluorene) as photoswitchable charge-polarizer on GHz-responsive trilayered core-shell dielectric nanoparticles. Molecules 2018, 23, 1873. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Haley, J.; Flikkema, J.; Nalla, V.; Wang, M.; Sfeir, M.; Tan, L.-S.; Cooper, T.; Ji, W.; Hamblin, M.R.; et al. Linear and nonlinear optical properties of light-harvesting hybrid [60]fullerene triads and tetraads with dual NIR two-photon absorption characteristics. J. Phys. Chem. C 2013, 117, 17186–17195. [Google Scholar] [CrossRef] [PubMed][Green Version]
Sample Availability: Sample of tris[(DPAF-C2M)-C60(>DPAF-C9)] is available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, H.; Wang, M.; Tan, L.-S.; Chiang, L.Y. Synthesis and Intramolecular Energy- and Electron-Transfer of 3D-Conformeric Tris(fluorenyl-[60]fullerenylfluorene) Derivatives. Molecules 2019, 24, 3337. https://doi.org/10.3390/molecules24183337
Yin H, Wang M, Tan L-S, Chiang LY. Synthesis and Intramolecular Energy- and Electron-Transfer of 3D-Conformeric Tris(fluorenyl-[60]fullerenylfluorene) Derivatives. Molecules. 2019; 24(18):3337. https://doi.org/10.3390/molecules24183337
Chicago/Turabian StyleYin, He, Min Wang, Loon-Seng Tan, and Long Y. Chiang. 2019. "Synthesis and Intramolecular Energy- and Electron-Transfer of 3D-Conformeric Tris(fluorenyl-[60]fullerenylfluorene) Derivatives" Molecules 24, no. 18: 3337. https://doi.org/10.3390/molecules24183337
APA StyleYin, H., Wang, M., Tan, L.-S., & Chiang, L. Y. (2019). Synthesis and Intramolecular Energy- and Electron-Transfer of 3D-Conformeric Tris(fluorenyl-[60]fullerenylfluorene) Derivatives. Molecules, 24(18), 3337. https://doi.org/10.3390/molecules24183337