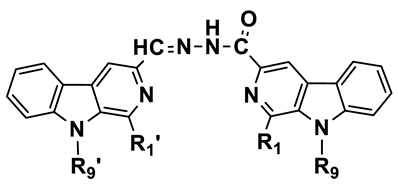
Abstract
Utilizing a pharmacophore hybridization approach, we have designed and synthesized a novel series of 28 new heterobivalent β-carbolines. The in vitro cytotoxic potential of each compound was evaluated against the five cancer cell lines (LLC, BGC-823, CT-26, Bel-7402, and MCF-7) of different origin—murine and human, with the aim of determining the potency and selectivity of the compounds. Compound 8z showed antitumor activities with half-maximal inhibitory concentration (IC50) values of 9.9 ± 0.9, 8.6 ± 1.4, 6.2 ± 2.5, 9.9 ± 0.5, and 5.7 ± 1.2 µM against the tested five cancer cell lines. Moreover, the effect of compound 8z on the angiogenesis process was investigated using a chicken chorioallantoic membrane (CAM) in vivo model. At a concentration of 5 μM, compound 8z showed a positive effect on angiogenesis. The results of this study contribute to the further elucidation of the biological regulatory role of heterobivalent β-carbolines and provide helpful information on the development of vascular targeting antitumor drugs.
1. Introduction
Cancer remains a leading cause of death in developed and developing countries, although much significant progress has been achieved recently [1]. Cancer resistance to therapy is becoming a common phenomenon that threatens the current strategies against this disease. For that reason, we need to discover new anticancer agents. One of the successful and effective methods for the discovery of new anticancer drugs from natural products is synthesis of novel compounds through chemical structural modifications on the basis of leading compounds.
β-Carbolines are a large group of heterocyclic compounds with a 9H-pyrido[3,4-b]indole structural unit. They compose a class of alkaloids that are widely distributed in nature, including plants, foodstuffs, marine creatures, insects, mammals, human tissues, and body fluids [2]. In the last few decades, there have been intense research efforts in the design and development of β-carbolines as a new class of antitumor agents. A large number of β-carboline derivatives have been prepared in search of more potent antitumor agents. The structure–activity relationships (SARs) of these β-carbolines have been extensively investigated [3,4,5,6,7,8,9,10]. Research has indicated that this class of compounds exert their antitumor effects through multiple mechanisms of action, including intercalating into DNA [11,12,13] and inhibiting topoisomerases I and II [14,15], cyclin-dependent kinase (CDK) [16,17], polo-like kinase 1 (PLK1) [18], kinesin-like protein Eg5 [19], and IκB kinases [20].
Among frequently studied novel bioactive chemical entities, the acylhydrazone scaffold (−CONHN=) has attracted considerable attention for decades due to its broad applications ranging from medicinal agents to agrochemicals to functional materials. Many compounds containing this moiety have been reported, and many reports demonstrate that the introduction of this pharmacophore may have high potential for antitumor activity [21,22,23,24,25,26]. The acylhydrazone moiety is able to act as pharmacophore or auxophore subunit in different pharmaceutic classes, with a variety of action profiles, depending on the other functionalities present in the molecular structure [27]. For example, MylotargTM (gemtuzumab ozogamicin; Pfizer) [28] (Figure 1) is a humanized anti-CD33 monoclonal antibody linked covalently to the cytotoxic agent N-acetyl gamma calicheamicin. Peterson reported PAC-1 (Figure 1), another N-acylhydrazone small-molecule, induces apoptotic death in cancer cells via the chelation of inhibitory zinc from procaspase-3, which leads to autocatalytic activation and subsequent generation of caspase-3 [29]. Carbazochrome (Figure 1), a semicarbazone-related compound, has been used as a hemostatic agent and is specifically indicated for capillary and parenchymal hemorrhage [30].
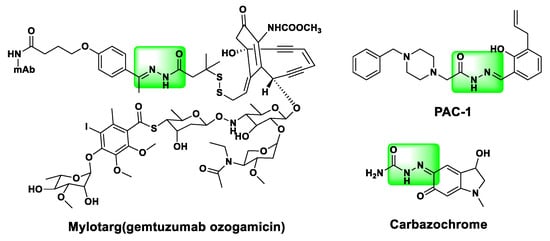
Figure 1.
Structures of Mylotarg, PAC-1, and carbazochrome.
Our research group [31,32,33,34] has focused on incorporating substituents into positions 1, 2, 3, 7, and 9 of the β-carboline nucleus as antitumor agents. Structure–activity relationship (SAR) analysis indicated that (1) the β-carboline moiety was associated with their potential antitumor activities, and (2) the introduction of appropriate substituents into positions 1, 3, and 9 of the β-carboline nucleus enhanced their antitumor potencies. Previous research has shown that some antitumor agents when dimerized via an appropriate linker can lead to significantly improved antitumor effects, giving 100- to 500-fold improvement over the corresponding monomers [35,36,37,38].
So our group reported the synthesis, in vitro evaluation, in vivo efficacies, and SARs of the new homobivalent β-carbolines and heterobivalent β-carbolines with alkyl or alkylamino spacers in positions 1, 3, 7, and 9 of the β-carboline nucleus (Figure 2) [39,40,41,42]. In these homobivalent β-carbolines, 1-Methyl-9-[4-(1-methyl-β-carboline-9-yl)butyl]-β-carboline (B-9-3) [43,44] exhibited potent antitumor activity. The pharmacological mechanisms showed that B-9-3 selectively induces apoptosis of endothelial cells, in part through disruption of VEGF-A/VEGFR2 signaling [45], and also acts on the TGF-β signaling pathway [46]. Compounds B-3 [39] and B-4 [40] exhibited significant angiogenesis inhibitory effects in chicken chorioallantoic membrane (CAM) assay, and the anti-angiogenetic potency was comparable or more potent with the drug Endostar.
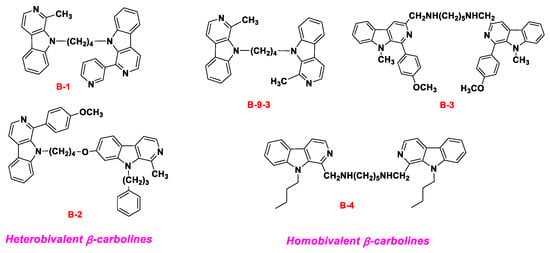
Figure 2.
The chemical structure of the representative reported diremic β-carbolines.
Continuing our studies to develop effective cytotoxic agents, the objective of this study was to synthesize potential anticancer compounds that are hybrids of β-carboline and acylhydrazone fragments (Figure 3). We have evaluated their cytotoxic activities for the first time, and the study also includes an investigation of the mechanism of action of these compounds for angiogenesis inhibition. These findings as well as our study of the SARs of the new compounds are discussed.
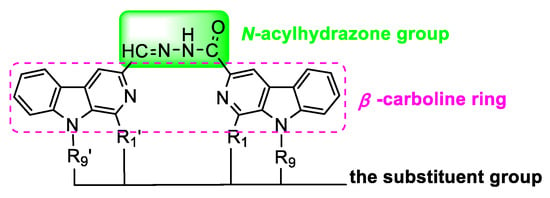
Figure 3.
Hybrids of β-carboline and acylhydrazone fragments giving the target heterobivalent β-carbolines.
2. Results and Discussion
2.1. Chemistry
The syntheses of compounds 8a–ab are depicted in Scheme 1, Scheme 2 and Scheme 3. Monovalent β-carbolines 6a–l and 7a–l were synthesized according to previously published methods [39,47,48]. Using L-tryptophan as starting material, the tetrahydro-β-carboline skeleton (2a–g) was constructed via Pictet–Spengler cyclization. Then, the obtained carboxylic acid 2 reacted with thionyl chloride and ethanol to form ethyl ester 3, which subsequently reacted with sulfur in xylene to afford compounds 4a–g. Then compounds 4a–g were reduced to their corresponding alcohols by lithium borohydride (LiBH4) in dry THF to provide compounds 5a–g, and further oxidized by MnO2 in CH3CN to afford the key intermediates, the 3-carboxaldehyde derivatives 6a–g [39]. Alternatively, refluxing of compounds 4a–g with 80% hydrazine hydrate in ethanol gave the other key intermediates, hydrazides 7a–g [39] (see Scheme 1).
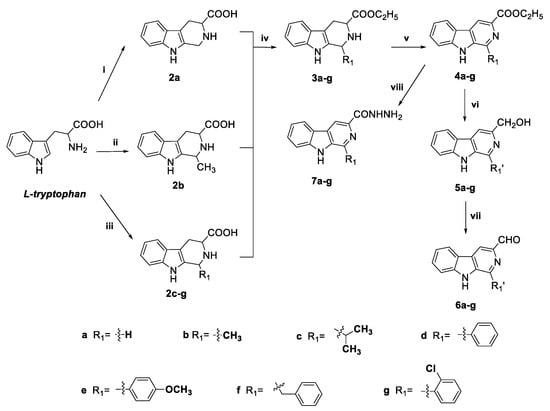
Scheme 1.
Synthesis of the key intermediates 6a–g, 7a–g. Reagents and conditions: (i) NaOH, H2O, formaldehyde, reflux, 3 h; (ii) H2SO4, H2O, acetaldehyde, room temperature, 3 h; (iii) acetic acid, R1CHO, reflux, 3 h; (iv) ethanol, SOCl2, reflux, 4 h; (v) xylene, S8, reflux, 8 h; (vi) THF, LiBH4, stirred at RT; (vii) CH3CN, MnO2, reflux, 2 h. (viii) hydrazine hydrate, ethanol, reflux, 4 h.
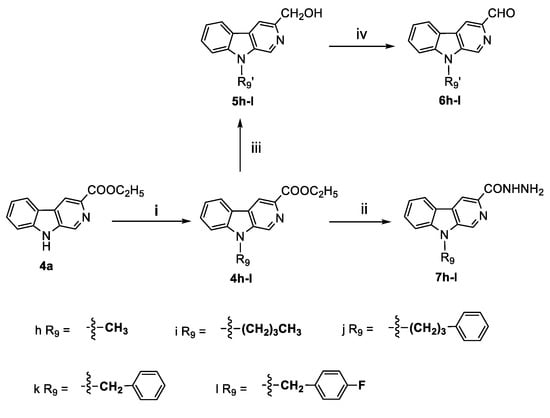
Scheme 2.
Synthesis of the key intermediates 6h–l, 7h–l. Reagents and conditions: (i) DMF, NaH, alkyl halogenide, stirred at RT; (ii) hydrazine hydrate, ethanol, reflux, 4 h. (iii) THF, LiBH4, stirred at RT; (iv) CH3CN, MnO2, reflux, 2 h.
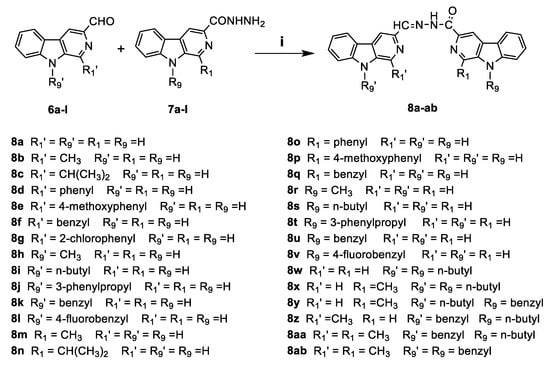
Scheme 3.
Synthesis of the asymmetric dimeric β-carboline derivatives 8a–ab. Reagents and conditions: (i) methanol, reflux, 4–6 h.
The N9-alkylated derivative of compound 4a was prepared by the action of sodium hydride (NaH) in anhydrous N,N-dimethylformamide (DMF) followed by the addition of alkyl halide to afford compounds 4h–l, and following this, the intermediates 6h–l and 7h–l were prepared according to the same method for compounds 6a–g and 7a–g (see Scheme 2). Finally, the synthesis of compounds 8a–ac (see Scheme 3) containing the acylhydrazone fragment was accomplished by the condensation of compounds 7a–l with the corresponding aldehydes 6a–l. We obtained the products easily in moderate to good yields when the aldehyde was 1 equiv and when the reactions were carried out in ethanol under reflux conditions. The structures of all compounds were confirmed by 1H-NMR, 13C-NMR (see Supplementary Materials), and high-resolution mass spectra (HRMS).
2.2. MTT Assay of Compounds 8a–8ab
From the synthetic route mentioned above, we obtained a series of novel heterobivalent β-carbolines. All of the target compounds were assayed for anticancer activity in various cancer cell lines including LLC (Lewis lung carcinoma), BGC-823 (gastric carcinoma), CT-26 (murine colon carcinoma), Bel-7402 (liver carcinoma), and MCF-7 (breast carcinoma), using the MTT method. The half-maximal inhibitory concentration (IC50) values for each compound with respect to the five cancer cell lines were calculated, and the results are summarized in Table 1. These values represent the concentrations at which a 50% decrease in cell growth is observed after 72 h of incubation in the presence of the drug compared with control cells treated with DMSO or positive control Cisplatin (DDP) under similar conditions.

Table 1.
Cytotoxic activity of acylhydrazone linked heterobivalent β-carbolines 8a–ab in vitroa (IC50, µMb).
For the first experiment, we examined the influence of the substituents in position 1 of the β-carboline core on cytotoxic activities. In order to enhance the range of substituents, we designed 11 novel compounds with methyl and isopropyl substitutions and different patterns of aryl rings substituted by electron withdrawing (Cl) and donating (OCH3) groups in the C-1 position of β-carboline. Of these 11 compounds, most of them showed medium or marginal cytotoxic activities in all cell lines. Interestingly, compounds 8b, 8c, 8d, and 8o were selectively active against BGC823 cells with IC50 values lower than 10 µM. In particular, compound 8b was more potent against BGC823 cells than against the four other cell lines, with potencies in the double-digit µM range. Compounds 8m (R1 = CH3) and 8q (R1 = benzyl) were exceptional; they showed no distinct difference between each other, and their IC50 values were in the ranges of 13.8–24.7 μM and 13.3–24.5 μM, respectively. Next, we examined the influence of the substituents in position 9 of the β-carboline ring on antiproliferative effects. Compound 8s, with an n-butyl group, was found to be the most potent agent among the heterobivalent β-carbolines, with IC50 values of 2.4 ± 0.6 µM (for BGC823) and 3.1 ± 1.2 µM (for CT26). Introduction of benzyl to the R9 position on β-carboline yielded compound 8u, and it demonstrated higher cytotoxic activity than other compounds against all tested tumor cell lines, except for the BGC823 cell line.
Among all these novel molecules, the cytotoxic potencies of most heterobivalent β-carbolines (8a–ab) showed no distinct differences, and the IC50 values of this class of compounds ranged from 10 to 100 µM. 8a and 8p had poor inhibitory activities with IC50 values above 50 µM. Compound 8z exhibited the most potent anticancer activity against the LLC, BGC-823, CT-26, Bel-7402, and MCF-7 cell lines, with IC50 values of 9.9 ± 0.9, 8.6 ± 1.4, 6.2 ± 2.5, 9.9 ± 0.5, and 5.7 ± 1.2 µM, respectively.
2.3. Inhibition of Angiogenesis in the Chicken Chorioallantoic Membrane (CAM) Assay
The CAM assay was deployed to assess the inhibitory effect of compound 8z on neovascularization. In this experiment, we used Combretastatin A4 disodium phosphate (CA4P) as a positive control. The inhibitory effects of compound 8z on angiogenesis in CAM are shown in Figure 4A. At the dose 0.5 μM, the reference anti-angiogenic drug CA4P elicited 21% inhibition of angiogenesis, and compound 8z did not show a significant anti-angiogenic activity at this concentration (13% inhibition). The anti-angiogenetic activity of compound 8z was comparable with CA4P in an in vivo CAM assay at the 5 μM level. In this assay, 8z inhibited blood vessel formation by 45%, compared to 47% inhibition induced by CA4P (p < 0.05). At 50 μM, CA4P significantly inhibited blood vessel formation, eliciting 78% inhibition (Figure 4B).
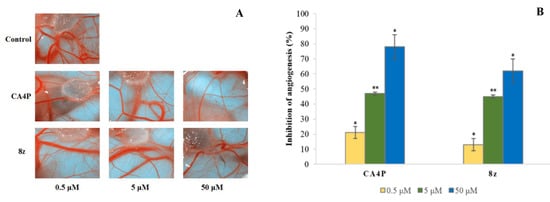
Figure 4.
In vivo anti-angiogenic effect of compounds 8z in CAM assay. (A) The representative photos of the experiments. (B) The anti-angiogenetic activity of compound 8z was semi-quantitatively analyzed using Graph Pad Prism 5.0. The data represent the mean values ± SD of at least three independent determinations. (**, p < 0.05; *, p < 0.5).
3. Materials and Methods
3.1. Reagents and General Methods
MTT was obtained from Sigma-Aldrich (Darmstadt, Germany) and Cisplatin from Qilu pharmaceutical (Jinan, China). Other commercially available starting materials and solvents were reagent grade and were purchased from Adamas-beta and used without further purification. Reactions and products were routinely monitored by thin-layer chromatography (TLC) on silica gel F254 plates (Qingdao Haiyang Inc., Qingdao, China). 1H-NMR and 13C-NMR spectra were recorded at room temperature on a Bruker Avance III HD 400 instrument (Bruker Company, Bremen, Gemany) using tetramethylsilane as the internal reference. Chemical shifts (δ) were reported in ppm relative to the residual solvent peak, and the multiplicity of each signal was designated by the following abbreviations—s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet. Coupling constants (J) were quoted in Hz. HRMS were recorded on a Bruker ultrafleXtreme MALDI-TOF/TOF-MS and Thermo Scientific LTQ Orbitrap XL (Thermo Fisher Scientific Inc, Waltham, USA). Column chromatography was performed with silica gel (200–300 mesh, Qingdao Haiyang Inc., Qingdao, China).
3.2. General Procedure for the Preparation of 6a–lc
A mixture of compound 5a (1.98 g, 10 mmol) and activated MnO2 (30 mmol) in CH3CN (60 mL) was stirred under reflux for 2 h. After completion of the reaction (monitored by TLC), the products were cooled to room temperature and filtered through Celite. The filtrate was passed through silica gel and washed with dichloromethane, and the solvent was removed under reduced pressure. The residue was crystallized from acetone or acetone–petroleum ether to give the corresponding compound 6a. Products 6b–l were prepared according to the same method as 6a.
1-benzyl-β-carboline-3-carbaldehyde (6f): The compound was obtained as a white solid with 81% yield. 1H-NMR (400 MHz, CDCl3) δ 10.24 (s, 1H, CHO), 8.68 (s, 1H, ArH), 8.64 (s, 1H, ArH), 8.14 (d, J = 8.0 Hz, 1H, ArH), 7.51 (d, J = 8.0 Hz, 1H, ArH), 7.43 (d, J = 8.4 Hz, 1H, ArH), 7.35–7.30 (m, 4H, ArH), 7.25–7.23 (m, 2H, ArH), 4.61 (s, 2H, ArCH2). 13C-NMR (100 MHz, CDCl3) δ 193.41, 144.21, 143.97, 137.68, 129.04, 129.00, 128.94, 128.81, 128.77, 127.08, 127.01, 121.92, 121.80, 121.20, 114.09, 112.11, 41.56.
1-(2-chlorophenyl)-β-carboline-3-carbaldehyde (6g): The compound was obtained as a light yellow solid with 72% yield. 1H-NMR (400 MHz, CDCl3) δ 10.28 (s, 1H, CHO), 8.79 (s, 1H, ArH), 8.39 (s, 1H, ArH), 8.25 (d, J = 8.0 Hz, 1H, ArH), 7.69 – 7.66 (m, 1H, ArH), 7.64–7.59 (m, 2H, ArH), 7.56–7.49 (m, 3H, ArH), 7.40 (t, J = 7.6 Hz, 1H, ArH). 13C-NMR (100 MHz, CDCl3) δ 193.33, 144.43, 141.54, 140.61, 136.39, 136.02, 132.89, 132.03, 130.79, 130.32, 129.56, 129.42, 127.71, 122.22, 122.16, 121.48, 114.17, 111.97.
3.3. General Procedure for the Preparation of Compounds 7a–l
We added 85% hydrazine hydrate (10 mL) to a solution of compound 4a (2.40 g, 10 mmol) in ethanol (100 mL), and then the mixture was refluxed for 8 h. Following the completion of reaction (as demonstrated by TLC), the resulting mixture was cooled to 5 °C and the precipitate was collected by filtration. The crude product was further purified first by washing with ethanol and then by recrystallization in ethanol to obtain compound 7a with a yield of 85%. Product 7b–l was prepared according to the same method as 7a.
1-isopropyl-β-carboline-3-carbohydrazide (7c): The compound was obtained as a white solid with 91% yield. 1H-NMR (400 MHz, DMSO-d6) δ 9.47 (s, 1H, NH), 8.65 (s, 1H, ArH), 8.35 (d, J = 7.6 Hz, 1H, ArH), 7.65 (d, J = 8.0 Hz, 1H, ArH), 7.60–7.55 (m, 1H, ArH), 7.30–7.26 (m, 1H, ArH), 4.58 (s, 2H, NH2), 3.75–3.64 (m, 1H, CH), 1.43 (d, J = 6.8 Hz, 6H, CH3). 13C-NMR (100 MHz, DMSO-d6) δ 164.65, 149.72, 141.16, 138.99, 134.81, 128.67, 128.22, 122.44, 121.92, 120.27, 112.62, 112.19, 31.21, 21.81.
1-benzyl-β-carboline-3-carbohydrazide (7f): The compound was obtained as a white solid with 94% yield. 1H-NMR (400 MHz, DMSO-d6) δ 9.54 (s, 1H, NH), 8.69 (s, 1H, ArH), 8.37 (d, J = 8.0 Hz, 1H, ArH), 7.67 (d, J = 8.4 Hz, 1H, ArH), 7.63–7.57 (m, 1H, ArH), 7.51 (d, J = 7.2 Hz, 2H, ArH), 7.34–7.25 (m, 4H, ArH), 7.20–7.15 (m, 1H, ArH), 4.60 (s, 2H, NH2), 4.51 (s, 2H, ArCH2). 13C-NMR (100 MHz, DMSO-d6) δ 164.43, 143.63, 141.29, 139.36, 139.33, 135.69, 129.36, 129.05, 128.92, 128.82, 128.76, 126.65, 122.60, 121.88, 120.44, 112.68, 112.59, 61.00.
9-n-butyl-β-carboline-3-carbohydrazide (7i): The compound was obtained as a light yellow solid with 80% yield. 1H-NMR (400 MHz, DMSO-d6) δ 9.70 (s, 1H, NH), 9.05 (d, J = 1.2 Hz, 1H, ArH), 8.84 (d, J = 0.8 Hz, 1H, ArH), 8.44 (d, J = 8.0 Hz, 1H, ArH), 7.77 (d, J = 8.4 Hz, 1H, ArH), 7.68–7.63 (m, 1H, ArH), 7.36–7.31 (m, 1H, ArH), 4.59–4.54 (m, 4H, NH2, –CH2CH2CH2CH3), 1.85–1.76 (m, 2H, –CH2CH2CH2CH3), 1.35–1.24 (m, 2H, –CH2CH2CH2CH3), 0.88 (t, J = 7.2 Hz, 3H, –CH2CH2CH2CH3). 13C-NMR (100 MHz, DMSO-d6) δ 164.37, 141.62, 140.00, 137.58, 131.70, 129.16, 128.14, 122.87, 121.16, 120.55, 114.14, 110.97, 43.05, 31.38, 20.20, 14.14.
9-(3-phenylpropyl)-β-carboline-3-carbohydrazide (7j): The compound was obtained as a gray powder with 86% yield. 1H-NMR (400 MHz, DMSO-d6) δ 9.69 (s, 1H, NH), 9.00 (d, J = 1.2 Hz, 1H, ArH), 8.83 (d, J = 0.8 Hz, 1H, ArH), 8.44 (d, J = 8.0 Hz, 1H, ArH), 7.72 (d, J = 8.4 Hz, 1H, ArH), 7.68–7.62 (m, 1H, ArH), 7.37–7.31 (m, 1H, ArH), 7.29–7.22 (m, 2H, ArH), 7.21–7.13 (m, 3H, ArH), 4.60 (t, J = 7.2 Hz, 2H, ArCH2CH2CH2), 4.56 (s, 2H, NH2), 2.67 (t, J = 7.6 Hz, 2H, ArCH2CH2CH2), 2.19–2.10 (m, 2H, ArCH2CH2CH2). 13C-NMR (101 MHz, DMSO-d6) δ 164.34, 141.59, 141.55, 140.08, 137.52, 131.62, 129.20, 128.80, 128.58, 128.24, 126.37, 122.92, 121.22, 120.62, 114.16, 110.89, 43.03, 32.85, 30.84.
9-(4-fluorobenzyl)-β-carboline-3-carbohydrazide (7l): The compound was obtained as a white solid with 96% yield. 1H-NMR (400 MHz, DMSO-d6) δ 9.76 (s, 1H, NH), 9.11 (d, J = 1.2 Hz, 1H, ArH), 8.89 (d, J = 1.2 Hz, 1H, ArH), 8.48 (d, J = 7.6 Hz, 1H, ArH), 7.83 (d, J = 8.4 Hz, 1H, ArH), 7.68–7.63 (m, 1H, ArH), 7.39–7.34 (m, 1H, ArH), 7.33–7.29 (m, 2H, ArH), 7.17–7.11 (m, 2H, ArH), 5.85 (s, 2H, ArCH2), 4.61 (s, 2H, NH2). 13C-NMR (100 MHz, DMSO-d6) δ 164.30, 162.52 (d, J = 242.1 Hz), 141.65, 140.50, 137.58, 133.86 (d, J = 3.2 Hz), 131.90, 129.51, 129.43, 129.39, 128.59, 122.98, 121.17 (d, J = 46.3 Hz), 115.99 (d, J = 21.4 Hz), 114.25, 111.18, 45.88.
3.4. General Procedure for the Preparation of Heterobivalent β-Carbolines 8a–ab
We added β-carboline-3-carbaldehyde 6a–l (1 mmol) to a solution of β-carboline-3-carbohydrazide 7a–l (1 mmol) in EtOH (50 mL), and then the reaction mixture was refluxed for 5 h. The solution was allowed to cool to room temperature. Then, the precipitates formed and were filtered, and the crude product was recrystallized with ethanol to afford compounds 8a–ab.
N′-((9H-pyrido[3,4-b]indol-3-yl)methylene)-9H-pyrido[3,4-b]indole-3-carbohydrazide (8a): The compound was obtained as a yellow solid with 93% yield. 1H-NMR (400 MHz, DMSO-d6) δ 12.13 (s, 1H, NH), 12.11 (s, 1H, NH), 9.28 (s, 1H, ArH), 9.16 (d, J = 0.8 Hz, 1H, ArH), 9.02 (s, 1H, ArH), 8.63 (s, 1H, ArH), 8.46 (d, J = 8.0 Hz, 1H, CH), 8.34 (d, J = 8.0 Hz, 1H, ArH), 7.80 (s, 1H, ArH), 7.73 (d, J = 8.0 Hz, 1H, ArH), 7.70 (d, J = 8.0 Hz, 1H, ArH), 7.68–7.61 (m, 2H, ArH), 7.39–7.32 (m, 2H, ArH). 13C-NMR (100 MHz, DMSO-d6) δ 162.68, 142.36, 141.65, 141.50, 140.77, 139.78, 137.85, 135.54, 133.55, 133.31, 129.49, 129.16, 128.80, 128.66, 122.81, 122.53, 121.47, 121.24, 120.74, 120.59, 118.99, 115.46, 112.99, 112.78. HRMS m/z calculated for C24H17N6O+ (M + H)+: 405.1458; found 405.1459.
N′-((1-methyl-9H-pyrido[3,4-b]indol-3-yl)methylene)-9H-pyrido[3,4-b]indole-3-carbohydrazide (8b): The compound was obtained as a yellow solid with 84% yield. 1H-NMR (400 MHz, DMSO-d6) δ 12.15 (s, 1H, NH), 12.02 (s, 1H, NH), 9.08 (d, J = 0.8 Hz, 1H, ArH), 9.03 (s, 1H, ArH), 8.46 (d, J = 8.0 Hz, 1H, CH), 8.44 (s, 1H, ArH), 8.29 (d, J = 8.0 Hz, 1H, ArH), 7.74 (s, 1H, ArH), 7.73–7.69 (m, 2H, ArH), 7.66–7.60 (m, 2H, ArH), 7.34 (t, J = 7.6 Hz, 2H, ArH), 3.20 (s, 3H, CH3). 13C-NMR (100 MHz, DMSO-d6) δ 162.54, 141.96, 141.86, 141.48, 141.36, 140.92, 139.96, 137.89, 134.27, 133.02, 129.17, 129.13, 128.75, 128.02, 122.80, 122.45, 121.71, 121.48, 120.66, 120.60, 117.15, 115.48, 112.88, 112.81, 20.96. HRMS m/z calculated for C25H19N6O+ (M + H)+: 419.1615; found 419.1620.
N′-((1-isopropyl-9H-pyrido[3,4-b]indol-3-yl)methylene)-9H-pyrido[3,4-b]indole-3-carbohydrazide (8c): The compound was obtained as a yellow solid with 89% yield. 1H-NMR (400 MHz, DMSO-d6) δ 15.64 (s, 1H, CONH), 12.10 (s, 1H, NH), 12.05 (s, 1H, NH), 9.07 (s, 1H, ArH), 9.02 (s, 1H, ArH), 8.49 (d, J = 8.0 Hz, 1H, CH), 8.46 (s, 1H, ArH), 8.30 (d, J = 8.0 Hz, 1H, ArH), 7.78 (s, 1H, ArH), 7.75–7.69 (m, 2H, ArH), 7.67–7.62 (m, 2H, ArH), 7.38–7.33 (m, 2H, ArH), 4.02–3.91 (m, 1H, CH(CH3)2), 1.76 (d, J = 7.2 Hz, 6H, CH(CH3)2). 13C-NMR (100 MHz, DMSO-d6) δ 162.76, 151.03, 142.16, 141.51, 141.38, 141.17, 139.54, 138.00, 132.90, 132.63, 129.25, 129.20, 128.88, 128.60, 122.87, 122.35, 121.72, 121.45, 120.66, 120.62, 117.59, 115.71, 112.87, 112.82, 31.57, 21.63. HRMS m/z calculated for C27H23N6O+ (M + H)+: 447.1928; found 447.1932.
N′-((1-phenyl-9H-pyrido[3,4-b]indol-3-yl)methylene)-9H-pyrido[3,4-b]indole-3-carbohydrazide (8d): The compound was obtained as a yellow solid with 96% yield. 1H-NMR (400 MHz, DMSO-d6) δ 15.58 (s, 1H, CONH), 12.06 (s, 1H, NH), 11.95 (s, 1H, NH), 8.99 (s, 1H, ArH), 8.65 (s, 1H, ArH), 8.44 (d, J = 8.4 Hz, 2H, CH, ArH), 8.35 (d, J = 8.0 Hz, 1H, ArH), 8.26–8.23 (m, 2H, ArH), 7.85 (s, 1H, ArH), 7.82–7.73 (m, 4H, ArH), 7.69–7.59 (m, 3H, ArH), 7.41–7.35 (m, 1H, ArH), 7.35–7.29 (m, 1H, ArH). 13C-NMR (100 MHz, DMSO-d6) δ 162.83, 142.56, 142.51, 142.15, 141.43, 140.85, 139.47, 137.75, 137.64, 133.10, 132.78, 130.10, 129.75, 129.47, 129.44, 129.32, 129.16, 128.59, 122.81, 122.36, 121.46, 121.41, 120.91, 120.57, 118.56, 115.52, 113.36, 112.74. HRMS m/z calculated for C30H21N6O+ (M + H)+: 481.1771; found 481.1777.
N′-((1-(4-methoxyphenyl)-9H-pyrido[3,4-b]indol-3-yl)methylene)-9H-pyrido[3,4-b]indole-3-carbohydrazide (8e): The compound was obtained as a yellow solid with 91% yield. 1H-NMR (400 MHz, DMSO-d6) δ 15.53 (s, 1H, CONH), 12.12 (s, 1H, NH), 11.90 (s, 1H, NH), 9.01 (s, 1H, ArH), 8.59–8.57 (m, 2H, ArH), 8.45 (d, J = 7.6 Hz, 1H, CH), 8.33 (d, J = 7.6 Hz, 1H, ArH), 8.23 (d, J = 8.8 Hz, 2H, ArH), 7.83 (s, 1H, ArH), 7.74 (d, J = 8.4 Hz, 1H, ArH), 7.70 (d, J = 8.4 Hz, 1H, ArH), 7.68–7.60 (m, 2H, ArH), 7.39–7.31 (m, 4H, ArH), 4.02 (s, 3H, OCH3). 13C-NMR (100 MHz, DMSO-d6) δ 162.86, 160.53, 142.53, 142.45, 142.07, 141.48, 140.97, 139.51, 137.76, 132.92, 132.80, 131.10, 130.00, 129.88, 129.30, 129.18, 128.65, 122.82, 122.29, 121.50, 121.42, 120.84, 120.58, 118.19, 115.63, 114.72, 113.36, 112.75, 56.05. HRMS m/z calculated for C31H23N6O2+ (M + H)+: 511.1877; found 511.1878.
N′-((1-benzyl-9H-pyrido[3,4-b]indol-3-yl)methylene)-9H-pyrido[3,4-b]indole-3-carbohydrazide (8f): The compound was obtained as a yellow solid with 82% yield. 1H-NMR (400 MHz, DMSO-d6) δ 12.19 (s, 1H, NH), 11.95 (s, 1H, NH), 9.06 (s, 1H, ArH), 8.88 (s, 1H, ArH), 8.50 (s, 1H, ArH), 8.47 (d, J = 7.6 Hz, 1H, CH), 8.29 (d, J = 7.6 Hz, 1H, ArH), 7.78–7.72 (m, 4H, ArH), 7.69–7.59 (m, 4H, ArH), 7.37–7.32 (m, 2H, ArH), 7.23 (t, J = 7.6 Hz, 2H, ArH), 7.13 (t, J = 7.2 Hz, 1H, ArH), 4.90 (s, 2H, ArCH2). 13C-NMR (100 MHz, DMSO-d6) δ 162.61, 144.08, 142.15, 141.48, 141.44, 140.83, 139.92, 139.26, 137.88, 133.58, 132.82, 131.50129.39, 129.23, 129.11, 128.95, 128.82, 126.84, 122.85, 122.44, 121.68, 121.45, 120.80, 120.64, 117.69, 115.64, 112.96, 112.78. HRMS m/z calculated for C31H23N6O+ (M + H)+: 495.1928; found 495.1932.
N′-((1-(2-chlorophenyl)-9H-pyrido[3,4-b]indol-3-yl)methylene)-9H-pyrido[3,4-b]indole-3-carbohydrazide (8g): The compound was obtained as a yellow solid with 81% yield. 1H-NMR (400 MHz, DMSO-d6) δ 12.04 (s, 1H, NH), 11.85 (s, 1H, NH), 8.92 (s, 1H, ArH), 8.70 (s, 1H, ArH), 8.41 (d, J = 8.0 Hz, 1H, CH), 8.37 (d, J = 8.0 Hz, 1H, ArH), 7.96 (d, J = 1.2 Hz, 1H, ArH), 7.90–7.85 (m, 3H, ArH), 7.84–7.79 (m, 1H, ArH), 7.77–7.72 (m, 1H, ArH), 7.70–7.55 (m, 5H, ArH), 7.40–7.35 (m, 1H, ArH), 7.31 (t, J = 7.2 Hz, 1H, ArH). 13C-NMR (100 MHz, DMSO-d6) δ 162.71, 142.01, 141.37, 140.58, 140.53, 139.50, 137.61, 136.59, 133.76, 133.32, 132.75, 132.47, 131.09, 130.30, 129.58, 129.55, 129.09, 128.46, 127.95, 122.75, 122.50, 121.40, 121.38, 120.86, 120.52, 118.77, 115.25, 113.09, 112.70. HRMS m/z calculated for C30H20ClN6O+ (M + H)+: 515.1382; found 515.1386.
N′-((9-methyl-9H-pyrido[3,4-b]indol-3-yl)methylene)-9H-pyrido[3,4-b]indole-3-carbohydrazide (8h): The compound was obtained as an ivory solid with 84% yield. 1H-NMR (400 MHz, DMSO-d6) δ 12.14 (s, 1H, NH), 9.45 (s, 1H, ArH), 9.15 (s, 1H, ArH), 9.01 (s, 1H, ArH), 8.64 (s, 1H, ArH), 8.46 (d, J = 8.0 Hz, 1H, CH), 8.36 (d, J = 8.0 Hz, 1H, ArH), 7.83 (d, J = 8.4 Hz, 1H, ArH), 7.80 (s, 1H, ArH), 7.74 (d, J = 7.6 Hz, 1H, ArH), 7.70 (d, J = 8.4 Hz, 1H, ArH), 7.63 (t, J = 7.6 Hz, 1H, ArH), 7.41 (t, J = 7.6 Hz, 1H, ArH), 7.34 (t, J = 7.4 Hz, 1H, ArH), 4.15 (s, 3H, CH3). 13C-NMR (100 MHz, DMSO-d6) δ 162.68, 142.51, 142.47, 141.50, 140.63, 139.79, 137.86, 136.16, 133.45, 132.20, 129.61, 129.18, 128.68, 128.32, 122.82, 122.60, 121.47, 120.92, 120.60, 118.70, 115.47, 112.77, 111.11, 30.24. HRMS calculated for C25H19N6O [M + H]+: 419.1615; found 419.1615.
N′-((9-n-butyl-9H-pyrido[3,4-b]indol-3-yl)methylene)-9H-pyrido[3,4-b]indole-3-carbohydrazide (8i): The compound was obtained as an ivory solid with 79% yield. 1H-NMR (400 MHz, DMSO-d6) δ 12.12 (s, 1H, NH), 9.46 (s, 1H, ArH), 9.17 (s, 1H, ArH), 9.02 (s, 1H, ArH), 8.64 (s, 1H, ArH), 8.47 (d, J = 7.6 Hz, 1H, CH), 8.36 (d, J = 8.0 Hz, 1H, ArH), 7.84 (d, J = 8.4 Hz, 1H, ArH), 7.80 (s, 1H, ArH), 7.75–7.68 (m, 2H, ArH), 7.63 (t, J = 7.6 Hz, 1H, ArH), 7.40 (t, J = 7.6 Hz, 1H, ArH), 7.34 (t, J = 7.6 Hz, 1H, ArH), 4.67 (t, J = 7.2 Hz, 2H, –CH2CH2CH2CH3), 1.92–1.84 (m, 2H, –CH2CH2CH2CH3), 1.42–1.31 (m, 2H, –CH2CH2CH2CH3), 0.92 (t, J = 7.6 Hz, 3H, –CH2CH2CH2CH3). 13C-NMR (100 MHz, DMSO-d6) δ 162.70, 142.48, 141.84, 141.51, 140.60, 139.77, 137.85, 135.67, 133.51, 132.16, 129.61, 129.19, 128.69, 128.42, 122.82, 122.69, 121.48, 121.00, 120.90, 120.60, 118.83, 115.47, 112.78, 111.30, 43.26, 31.50, 20.27, 14.19. HRMS calculated for C28H25N6O [M + H]+: 461.2084; found 461.2088.
N′-((9-(3-phenylpropyl)-9H-pyrido[3,4-b]indol-3-yl)methylene)-9H-pyrido[3,4-b]indole-3-carbohydrazide (8j): The compound was obtained as a yellow solid with 84% yield. 1H-NMR (400 MHz, DMSO-d6) δ 12.15 (s, 1H, NH), 9.35 (s, 1H, ArH), 9.15 (d, J = 1.2 Hz, 1H, ArH), 9.03 (s, 1H, ArH), 8.64 (s, 1H, ArH), 8.47 (d, J = 7.6 Hz, 1H, CH), 8.36 (d, J = 8.0 Hz, 1H, ArH), 7.81–7.78 (m, 2H, ArH), 7.73–7.68 (m, 2H, ArH), 7.66–7.63 (m, 1H, ArH), 7.42–7.38 (m, 1H, ArH), 7.37–7.34 (m, 1H, ArH), 7.32–7.27 (m, 3H, ArH), 7.25–7.20 (m, 3H, ArH), 4.69 (t, J = 7.2 Hz, 2H, ArCH2CH2CH2), 2.74 (t, J = 7.2 Hz, 2H, ArCH2CH2CH2), 2.27–2.18 (m, 2H, ArCH2CH2CH2). 13C-NMR (100 MHz, DMSO-d6) δ 162.70, 142.55, 141.79, 141.57, 141.51, 140.59, 139.77, 137.85, 135.59, 133.44, 131.99, 129.63, 129.20, 128.84, 128.82, 128.69, 128.62, 126.43, 122.83, 122.72, 121.48, 121.06, 120.96, 120.61, 118.85, 115.50, 112.78, 111.19, 43.12, 32.84, 30.87. HRMS calculated for C33H27N6O [M + H]+: 523.2241; found 523.2242.
N′-((9-benzyl-9H-pyrido[3,4-b]indol-3-yl)methylene)-9H-pyrido[3,4-b]indole-3-carbohydrazide (8k): The compound was obtained as a yellow solid with 86% yield. 1H-NMR (400 MHz, DMSO-d6) δ 12.32 (s, 1H, NH), 12.06 (s, 1H, NH), 9.13 (d, J = 0.8 Hz, 1H, ArH), 9.04 (s, 1H, ArH), 9.01 (d, J = 1.2 Hz, 1H, ArH), 8.92 (s, 1H, ArH), 8.83 (d, J = 0.8 Hz, 1H, ArH), 8.48 (t, J = 8.0 Hz, 2H, ArH, CH), 7.79 (d, J = 8.4 Hz, 1H, ArH), 7.71 (d, J = 8.4 Hz, 1H, ArH), 7.67–7.63 (m, 1H, ArH), 7.38–7.23 (m, 8H, ArH), 5.83 (s, 2H, ArCH2). 13C-NMR (100 MHz, DMSO-d6) δ 161.86, 150.06, 144.04, 141.98, 141.53, 139.51, 137.86, 137.69, 137.05, 132.81, 129.36, 129.26, 129.19, 128.79, 128.76, 128.00, 127.35, 127.31, 122.96, 122.90, 121.43, 121.30, 120.74, 120.64, 115.62, 112.80, 111.71, 111.15, 46.53. HRMS calculated for C31H23N6O [M + H]+: 495.1928; found 495.1922.
N′-((9-(4-fluorobenzyl)-9H-pyrido[3,4-b]indol-3-yl)methylene)-9H-pyrido[3,4-b]indole-3-carbohydrazide (8l): The compound was obtained as a light gray solid with 92% yield. 1H-NMR (400 MHz, DMSO-d6) δ 12.31 (s, 1H, NH), 12.04 (s, 1H, NH), 9.15 (d, J = 0.8 Hz, 1H, ArH), 9.03 (s, 1H, ArH), 9.00 (d, J = 1.2 Hz, 1H, ArH), 8.91 (s, 1H, ArH), 8.82 (d, J = 1.2 Hz, 1H, ArH), 8.48 (dd, J = 8.0, 4.8 Hz, 2H, CH, ArH), 7.81 (d, J = 8.4 Hz, 1H, ArH), 7.71 (d, J = 8.4 Hz, 1H, ArH), 7.68–7.61 (m, 2H, ArH), 7.38–7.30 (m, 4H, ArH), 7.17–7.11 (m, 2H, ArH), 5.82 (s, 2H, ArCH2). 13C-NMR (100 MHz, DMSO-d6) δ 161.96 (d, J = 241.8 Hz), 161.84, 150.05, 144.12, 141.86, 141.53, 139.51, 137.85, 136.94, 133.92 (d, J = 3.1 Hz), 132.80, 129.53, 129.45, 129.40, 129.24, 128.81, 128.79, 123.00 (d, J = 9.6 Hz), 121.43, 121.34, 120.80, 120.64, 116.11 (d, J = 21.3 Hz), 115.62, 112.79, 111.70, 111.12, 45.79. HRMS calculated for C31H22FN6O [M + H]+: 513.1834; found 513.1832.
N′-((9H-pyrido[3,4-b]indol-3-yl)methylene)-1-methyl-9H-pyrido[3,4-b]indole-3-carbohydrazide (8m): The compound was obtained as a yellow solid with 85% yield. 1H-NMR (400 MHz, DMSO-d6) δ 12.05 (s, 1H, NH), 12.02 (s, 1H, NH), 11.83 (s, 1H, NH), 8.95 (d, J = 0.8 Hz, 1H, ArH), 8.90 (s, 1H, ArH), 8.85 (s, 1H, ArH), 8.78 (s, 1H, ArH), 8.42 (t, J = 8.4 Hz, 2H, CH, ArH), 7.69–7.57 (m, 4H, ArH), 7.35–7.28 (m, 2H, ArH), 2.94 (s, 3H, CH3). 13C-NMR (100 MHz, DMSO-d6) δ 161.84, 150.09, 143.33, 141.66, 141.61, 141.28, 138.85, 136.87, 136.60, 133.94, 129.05, 128.88, 128.76, 127.95, 122.77, 122.70, 121.90, 121.33, 120.55, 120.26, 113.68, 112.71, 112.63, 111.84, 20.87. HRMS calculated for C25H19N6O [M + H]+: 419.1615; found 419.1617.
N′-((9H-pyrido[3,4-b]indol-3-yl)methylene)-1-isopropyl-9H-pyrido[3,4-b]indole-3-carbohydrazide (8n): The compound was obtained as a yellow solid with 91% yield. 1H-NMR (400 MHz, DMSO-d6) δ 15.65 (s, 1H, CONH), 12.17 (s, 1H, NH), 12.06 (s, 1H, NH), 9.22 (s, 1H, ArH), 8.88 (s, 1H, ArH), 8.65 (s, 1H, ArH), 8.42 (d, J = 7.6 Hz, 1H, CH), 8.34 (d, J = 7.6 Hz, 1H, ArH), 7.80 (s, 1H, ArH), 7.74–7.60 (m, 4H, ArH), 7.40–7.30 (m, 2H, ArH), 3.92–3.82 (m, 1H, CH(CH3)2), 1.68 (d, J = 6.8 Hz, 6H, CH(CH3)2). 13C-NMR (100 MHz, DMSO-d6) δ 162.96, 149.74, 142.52, 141.59, 141.29, 140.56, 139.03, 135.63, 135.24, 132.99, 129.52, 128.88, 128.79, 128.53, 122.59, 121.98, 121.19, 120.75, 120.50, 119.37, 113.80, 112.96, 112.71, 31.12, 22.22. HRMS calculated for C27H23N6O [M + H]+: 447.1928; found 447.1926.
N′-((9H-pyrido[3,4-b]indol-3-yl)methylene)-1-phenyl-9H-pyrido[3,4-b]indole-3-carbohydrazide (8o): The compound was obtained as a yellow solid with 91% yield. 1H-NMR (400 MHz, DMSO-d6) δ 15.97 (s, 1H, CONH), 12.27 (s, 1H, NH), 12.03 (s, 1H, NH), 9.05 (d, J = 5.2 Hz, 2H, ArH), 8.63 (s, 1H, ArH), 8.50 (d, J = 8.0 Hz, 1H, CH), 8.39 (d, J = 6.8 Hz, 2H, ArH), 8.33 (d, J = 7.6 Hz, 1H, ArH), 7.91 (t, J = 7.6 Hz, 2H, ArH), 7.81 (s, 1H, ArH), 7.79–7.72 (m, 3H, ArH), 7.68–7.62 (m, 2H, ArH), 7.39–7.34 (m, 2H, ArH). 13C-NMR (100 MHz, DMSO-d6) δ 162.62, 142.40, 142.07, 141.63, 141.12, 140.82, 139.66, 138.23, 135.57, 134.93, 132.94, 130.58, 129.75, 129.51, 129.20, 128.74, 122.61, 122.56, 121.71, 121.18, 120.84, 120.72, 119.28, 114.68, 113.22, 112.93. HRMS calculated for C30H21N6O [M + H]+: 481.1771; found 481.1772.
N′-((9H-pyrido[3,4-b]indol-3-yl)methylene)-1-(4-methoxyphenyl)-9H-pyrido[3,4-b]indole-3-carbohydrazide (8p): The compound was obtained as a yellow solid with 87% yield. 1H-NMR (400 MHz, DMSO-d6) δ 15.89 (s, 1H, CONH), 12.27 (s, 1H, NH), 11.96 (s, 1H, NH), 9.07 (s, 1H, ArH), 9.00 (s, 1H, ArH), 8.64 (s, 1H, ArH), 8.47 (d, J = 7.6 Hz, 1H, CH), 8.37–8.32 (m, 2H, ArH), 7.81 (s, 1H, ArH), 7.75 (dd, J = 8.0, 4.4 Hz, 2H, ArH), 7.69–7.61 (m, 2H, ArH), 7.44 (d, J = 8.8 Hz, 2H, ArH), 7.39–7.32 (m, 2H, ArH), 4.04 (s, 3H, OCH3). 13C-NMR (100 MHz, DMSO-d6) δ 162.69, 160.72, 142.41, 142.01, 141.63, 141.10, 140.77, 139.53, 135.60, 134.70, 133.00, 130.57, 130.49, 130.34, 129.52, 129.08, 128.77, 122.58, 122.54, 121.75, 121.20, 120.78, 120.73, 119.33, 114.92, 114.17, 113.22, 112.94, 56.01. HRMS calculated for C31H23N6O2 [M + H]+: 511.1877; found 511.1874.
N′-((9H-pyrido[3,4-b]indol-3-yl)methylene)-1-benzyl-9H-pyrido[3,4-b]indole-3-carbohydrazide (8q): The compound was obtained as an ivory solid with 83% yield. 1H-NMR (400 MHz, DMSO-d6) δ 12.14 (s, 1H, CONH), 11.99 (s, 1H, NH), 11.86 (s, 1H, NH), 8.98 (s, 1H, ArH), 8.91 (d, J = 2.4 Hz, 2H, ArH), 8.81 (s, 1H, ArH), 8.45 (d, J = 8.0 Hz, 1H, CH), 8.40 (d, J = 7.6 Hz, 1H, ArH), 7.72–7.66 (m, 2H, ArH), 7.65–7.55 (m, 4H, ArH), 7.35–7.28 (m, 4H, ArH), 7.21 (t, J = 7.2 Hz, 1H, ArH), 4.65 (s, 2H, ArCH2). 13C-NMR (100 MHz, DMSO-d6) δ 161.65, 150.29, 143.62, 143.18, 141.67, 141.39, 139.26, 138.89, 136.90, 136.13, 133.99, 129.39, 129.08, 129.04, 128.96, 128.83, 128.78, 126.71, 122.77, 122.70, 121.89, 121.32, 120.66, 120.27, 113.96, 112.77, 112.64, 111.94. HRMS calculated for C31H23N6O [M + H]+: 495.1928; found 495.1929.
N′-((9H-pyrido[3,4-b]indol-3-yl)methylene)-9-methyl-9H-pyrido[3,4-b]indole-3-carbohydrazide (8r): The compound was obtained as a light yellow solid with 81% yield. 1H-NMR (400 MHz, DMSO-d6) δ 12.31 (s, 1H, CONH), 11.82 (s, 1H, NH), 9.14 (s, 1H, ArH), 9.03 (s, 1H, ArH), 8.94 (d, J = 0.8 Hz, 1H, ArH), 8.92 (s, 1H, ArH), 8.78 (s, 1H, ArH), 8.52 (d, J = 7.6 Hz, 1H, CH), 8.41 (d, J = 8.0 Hz, 1H, ArH), 7.79 (d, J = 8.4 Hz, 1H, ArH), 7.73–7.68 (m, 1H, ArH), 7.64 (d, J = 8.4 Hz, 1H, ArH), 7.62–7.57 (m, 1H, ArH), 7.41 – 7.36 (m, 1H, ArH), 7.32–7.28 (m, 1H, ArH), 4.10 (s, 3H, CH3). 13C-NMR (100 MHz, DMSO-d6) δ 161.77, 150.35, 143.37, 142.39, 141.65, 139.73, 138.38, 136.86, 133.88, 131.51, 129.35, 129.04, 128.75, 128.36, 122.96, 122.70, 121.34, 121.09, 120.81, 120.25, 115.37, 112.62, 111.79, 110.90, 30.18. HRMS calculated for C25H19N6O [M + H]+: 419.1615; found 419.1618.
N′-((9H-pyrido[3,4-b]indol-3-yl)methylene)-9-n-butyl-9H-pyrido[3,4-b]indole-3-carbohydrazide (8s): The compound was obtained as a light yellow solid with 92% yield. 1H-NMR (400 MHz, DMSO-d6) δ 12.28 (s, 1H, CONH), 11.82 (s, 1H, NH), 9.15 (d, J = 0.8 Hz, 1H, ArH), 9.04 (d, J = 0.8 Hz, 1H, ArH), 8.95 (d, J = 0.8 Hz, 1H, ArH), 8.92 (s, 1H, ArH), 8.79 (s, 1H, ArH), 8.52 (d, J = 7.6 Hz, 1H, CH), 8.41 (d, J = 8.0 Hz, 1H, ArH), 7.81 (d, J = 8.4 Hz, 1H, ArH), 7.71–7.69 (m, 1H, ArH), 7.65 (d, J = 8.0 Hz, 1H, ArH), 7.62–7.57 (m, 1H, ArH), 7.40–7.35 (m, 1H, ArH), 7.32–7.28 (m, 1H, ArH), 4.63 (t, J = 6.8 Hz, 2H, –CH2CH2CH2CH3), 1.88–1.80 (m, 2H, –CH2CH2CH2CH3), 1.36–1.26 (m, 2H, –CH2CH2CH2CH3), 0.89 (t, J = 7.2 Hz, 3H, –CH2CH2CH2CH3). 13C-NMR (100 MHz, DMSO-d6) δ 161.76, 150.35, 143.36, 141.75, 141.65, 139.72, 137.94, 136.87, 133.89, 131.55, 129.35, 129.04, 128.75, 128.44, 123.07, 122.69, 121.34, 121.19, 120.80, 120.25, 115.45, 112.62, 111.80, 111.11, 49.07, 31.41, 20.21, 14.17. HRMS calculated for C28H25N6O [M + H]+: 461.2084; found 461.2085.
N′-((9H-pyrido[3,4-b]indol-3-yl)methylene)-9-(3-phenylpropyl)-9H-pyrido[3,4-b]indole-3-carbohydrazide (8t): The compound was obtained as a yellow solid with 88% yield. 1H-NMR (400 MHz, DMSO-d6) δ 12.32 (s, 1H, CONH), 11.85 (s, 1H, NH), 9.10 (s, 1H, ArH), 9.06 (s, 1H, ArH), 8.97 (s, 1H, ArH), 8.94 (s, 1H, ArH), 8.80 (s, 1H, ArH), 8.52 (d, J = 7.6 Hz, 1H, CH), 8.39 (d, J = 8.0 Hz, 1H, ArH), 7.76 (d, J = 8.0 Hz, 1H, ArH), 7.71–7.64 (m, 2H, ArH), 7.62–7.58 (m, 1H, ArH), 7.38 (t, J = 7.6 Hz, 1H, ArH), 7.32–7.25 (m, 3H, ArH), 7.19–7.16 (m, 3H, ArH), 4.65 (t, J = 7.2 Hz, 2H, ArCH2CH2CH2), 2.69 (t, J = 7.6 Hz, 2H, ArCH2CH2CH2), 2.22–2.14 (m, 2H, ArCH2CH2CH2). 13C-NMR (100 MHz, DMSO-d6) δ 161.81, 150.40, 143.37, 141.70, 141.64, 141.50, 139.79, 137.86, 136.88, 133.94, 131.43, 129.37, 129.03, 128.81, 128.73, 128.58, 128.55, 126.39, 123.09, 122.67, 121.34, 121.25, 120.85, 120.24, 115.49, 112.63, 111.84, 110.99, 43.06, 32.85, 30.82. HRMS calculated for C33H27N6O [M + H]+: 523.2241; found 523.2242.
N′-((9H-pyrido[3,4-b]indol-3-yl)methylene)-9-benzyl-9H-pyrido[3,4-b]indole-3-carbohydrazide (8u): The compound was obtained as a light yellow solid with 80% yield. 1H-NMR (400 MHz, DMSO-d6) δ 12.28 (s, 1H, CONH), 11.82 (s, 1H, NH), 9.19 (s, 1H, ArH), 9.07 (s, 1H, ArH), 8.95 (s, 1H, ArH), 8.90 (s, 1H, ArH), 8.78 (s, 1H, ArH), 8.55 (d, J = 8.0 Hz, 1H, CH), 8.41 (d, J = 8.0 Hz, 1H, ArH), 7.84 (d, J = 8.0 Hz, 1H, ArH), 7.70–7.63 (m, 2H, ArH, NH), 7.62–7.57 (m, 1H, ArH), 7.39 (t, J = 7.5 Hz, 1H, ArH), 7.34–7.24 (m, 6H, ArH), 5.91 (s, 2H, ArCH2). 13C-NMR (100 MHz, DMSO-d6) δ 161.70, 150.39, 143.33, 141.89, 141.64, 140.14, 138.04, 137.60, 136.87, 133.89, 131.84, 129.54, 129.23, 129.04, 128.84, 128.75, 128.09, 127.41, 123.17, 122.70, 121.40, 121.34, 121.11, 120.25, 115.52, 112.63, 111.80, 111.35, 46.73. HRMS calculated for C31H23N6O [M + H]+: 495.1928; found 495.1927.
N′-((9H-pyrido[3,4-b]indol-3-yl)methylene)-9-(4-fluorobenzyl)-9H-pyrido[3,4-b]indole-3-carbohydrazide (8v): The compound was obtained as a light yellow solid with 90% yield. 1H-NMR (400 MHz, DMSO-d6) δ 12.27 (s, 1H, CONH), 11.82 (s, 1H, NH), 9.20 (d, J = 1.2 Hz, 1H, ArH), 9.06 (d, J = 1.2 Hz, 1H, ArH), 8.94 (d, J = 1.2 Hz, 1H, ArH), 8.90 (s, 1H, ArH), 8.78 (s, 1H, ArH), 8.55 (d, J = 7.6 Hz, 1H, CH), 8.41 (d, J = 8.0 Hz, 1H, ArH), 7.86 (d, J = 8.4 Hz, 1H, ArH), 7.71–7.57 (m, 3H, ArH), 7.39 (t, J = 7.6 Hz, 1H, ArH), 7.35–7.28 (m, 3H, ArH), 7.19–7.12 (m, 2H, ArH), 5.90 (s, 2H, ArCH2). 13C-NMR (100 MHz, DMSO-d6) δ 162.00 (d, J = 242.1 Hz), 161.68, 150.43, 143.34, 141.77, 141.64, 140.21, 137.94, 136.88, 133.90, 133.82 (d, J = 3 Hz), 131.81, 129.59, 129.51, 129.04, 128.89, 128.74, 123.20, 122.70, 121.39 (d, J = 10.2 Hz), 121.17, 120.25, 116.05 (d, J = 21.3 Hz), 115.52, 112.62, 111.80, 111.32, 49.07. HRMS calculated for C31H22FN6O [M + H]+: 513.1834; found 513.1835.
9-n-butyl-N′-((9-n-butyl-9H-pyrido[3,4-b]indol-3-yl)methylene)-9H-pyrido[3,4-b]indole-3-carbohydrazide (8w): The compound was obtained as a yellow solid with 91% yield. 1H-NMR (400 MHz, DMSO-d6) δ 12.28 (s, 1H, CONH), 9.15 (s, 1H, ArH), 9.10 (s, 1H, ArH), 9.03 (s, 1H, ArH), 8.91 (s, 1H, ArH), 8.80 (s, 1H, ArH), 8.52 (d, J = 8.0 Hz, 1H, CH), 8.45 (d, J = 8.0 Hz, 1H, ArH), 7.82 (d, J = 8.4 Hz, 1H, ArH), 7.76 (d, J = 8.4 Hz, 1H, ArH), 7.71–7.64 (m, 2H, ArH), 7.40–7.32 (m, 2H, ArH), 4.63 (t, J = 6.8 Hz, 2H, –CH2CH2CH2CH3), 4.55 (t, J = 6.8 Hz, 2H, –CH2CH2CH2CH3), 1.88–1.79 (m, 4H, –CH2CH2CH2CH3), 1.37–1.27 (m, 4H, –CH2CH2CH2CH3), 0.92–0.88 (m, 6H, –CH2CH2CH2CH3). 13C-NMR (100 MHz, DMSO-d6) δ 161.75, 150.25, 143.59, 141.85, 141.75, 139.70, 137.95, 136.96, 132.61, 131.57, 129.36, 129.17, 128.44, 128.40, 123.08, 122.88, 121.19, 121.09, 120.80, 120.40, 115.45, 111.64, 111.12, 110.90, 43.12, 42.99, 31.42, 31.34, 20.24, 20.22, 14.18. HRMS calculated for C32H33N6O [M + H]+: 517.2710; found 517.2706.
9-n-butyl-N′-((9-n-butyl-9H-pyrido[3,4-b]indol-3-yl)methylene)-1-methyl-9H-pyrido[3,4-b]indole-3-carbohydrazide (8x): The compound was obtained as a yellow solid with 88% yield. 1H-NMR (400 MHz, CDCl3) δ 11.32 (s, 1H, CONH), 9.04 (s, 1H, ArH), 8.92 (s, 1H, ArH), 8.84 (s, 1H, ArH), 8.59 (s, 1H, CH), 8.25 (d, J = 8.0 Hz, 1H, ArH), 8.22 (d, J = 8.0 Hz, 1H, ArH), 7.64–7.59 (m, 2H, ArH), 7.47 (dd, J = 8.4, 2.8 Hz, 2H, ArH), 7.35 (t, J = 7.6 Hz, 2H, ArH), 4.54 (t, J = 7.6 Hz, 2H, –CH2CH2CH2CH3), 4.36 (t, J = 7.2 Hz, 2H, –CH2CH2CH2CH3), 3.09 (s, 3H, CH3), 1.94–1.81 (m, 4H, –CH2CH2CH2CH3), 1.53–1.36 (m, 4H, –CH2CH2CH2CH3), 1.00 (t, J = 7.6 Hz, 3H, –CH2CH2CH2CH3), 0.97 (t, J = 7.2 Hz, 3H, –CH2CH2CH2CH3). 13C-NMR (100 MHz, CDCl3) δ 161.29, 148.92, 142.53, 141.84, 141.81, 139.67, 137.77, 137.00, 136.59, 130.81, 129.58, 129.19, 128.72, 128.53, 122.59, 121.98, 121.69, 121.48, 120.56, 120.27, 113.75, 113.00, 109.97, 109.60, 44.84, 43.34, 33.02, 31.27, 23.71, 20.52, 20.21, 13.87, 13.81. HRMS calculated for C33H35N6O [M + H]+: 531.2867; found 531.2873.
9-benzyl-N′-((9-n-butyl-9H-pyrido[3,4-b]indol-3-yl)methylene)-1-methyl-9H-pyrido[3,4-b]indole-3-carbohydrazide (8y): The compound was obtained as a yellow solid with 84% yield. 1H-NMR (400 MHz, CDCl3) δ 11.27 (s, 1H, CONH), 9.03 (s, 1H, ArH), 8.99 (s, 1H, ArH), 8.84 (s, 1H, ArH), 8.55 (s, 1H, CH), 8.26 (dd, J = 10.4, 8.0 Hz, 2H, ArH), 7.64–7.55 (m, 2H, ArH), 7.47 (d, J = 8.4 Hz, 1H, ArH), 7.41–7.34 (m, 3H, ArH), 7.33–7.23 (m, 3H, ArH), 7.01–6.95 (m, 2H, ArH), 5.83 (s, 2H, ArCH2), 4.37 (t, J = 7.2 Hz, 2H, –CH2CH2CH2CH3), 2.93 (s, 3H, CH3), 1.94–1.87 (m, 2H, –CH2CH2CH2CH3), 1.47–1.37 (m, 2H, –CH2CH2CH2CH3), 0.96 (t, J = 7.2 Hz, 3H, –CH2CH2CH2CH3). 13C-NMR (100 MHz, CDCl3) δ 161.19, 149.29, 142.63, 142.26, 141.78, 140.15, 138.33, 137.49, 137.06, 131.05, 129.80, 129.13, 129.07, 128.91, 128.64, 127.72, 125.32, 122.56, 122.05, 121.75, 121.51, 121.03, 120.23, 113.81, 113.00, 110.11, 109.59, 48.30, 43.32, 31.27, 23.32, 20.52, 13.82. HRMS calculated for C36H33N6O [M + H]+: 565.2710; found 565.2715.
N′-((9-benzyl-1-methyl-9H-pyrido[3,4-b]indol-3-yl)methylene)-9-n-butyl-9H-pyrido[3,4-b]indole-3-carbohydrazide (8z): The compound was obtained as a yellow solid with 85% yield. 1H-NMR (400 MHz, CDCl3) δ 11.22 (s, 1H, CONH), 9.06 (s, 1H, ArH), 8.92 (s, 1H, ArH), 8.78 (s, 1H, ArH), 8.50 (s, 1H, CH), 8.25 (t, J = 7.2 Hz, 2H, ArH), 7.65–7.61 (m, 1H, ArH), 7.56–7.48 (m, 2H, ArH), 7.39–7.32 (m, 3H, ArH), 7.31 – 7.23 (m, 3H, ArH), 7.00 (d, J = 6.4 Hz, 2H, ArH), 5.76 (s, 2H, ArCH2), 4.39 (t, J = 7.2 Hz, 2H, –CH2CH2CH2CH3), 2.88 (s, 3H, CH3), 1.94–1.87 (m, 2H, –CH2CH2CH2CH3), 1.46–1.36 (m, 2H, –CH2CH2CH2CH3), 0.97 (t, J = 7.2 Hz, 3H, –CH2CH2CH2CH3). 13C-NMR (100 MHz, CDCl3) δ 161.36, 149.11, 142.47, 142.18, 141.57, 140.82, 138.81, 137.87, 137.70, 136.08, 130.00, 129.88, 129.05, 128.95, 128.81, 128.75, 127.59, 125.40, 122.41, 122.16, 121.76, 121.48, 120.69, 120.57, 115.31, 111.46, 109.91, 109.81, 48.26, 43.48, 31.32, 23.00, 20.52, 13.81. HRMS calculated for C36H33N6O [M + H]+: 565.2710; found 565.2703.
N′-((9-benzyl-1-methyl-9H-pyrido[3,4-b]indol-3-yl)methylene)-9-n-butyl-1-methyl-9H-pyrido[3,4-b]indole-3-carbohydrazide (8aa): The compound was obtained as a yellow solid with 83% yield. 1H-NMR (400 MHz, CDCl3) δ 11.30 (s, 1H, CONH), 8.90 (d, J = 4.4 Hz, 2H, ArH), 8.51 (s, 1H, CH, ArH), 8.26 (d, J = 7.6 Hz, 1H, ArH), 8.22 (d, J = 7.6 Hz, 1H, ArH), 7.63–7.59 (m, 1H, ArH), 7.56–7.52 (m, 1H, ArH), 7.46 (d, J = 8.4 Hz, 1H, ArH), 7.38–7.29 (m, 4H, ArH), 7.29–7.22 (m, 2H, ArH), 7.01–6.98 (m, 2H, ArH), 5.74 (s, 2H, ArCH2), 4.51 (t, J = 7.6 Hz, 2H, –CH2CH2CH2CH3), 3.06 (s, 3H, CH3), 2.88 (s, 3H, CH3), 1.88–1.80 (m, 2H, –CH2CH2CH2CH3), 1.51–1.42 (m, 2H, –CH2CH2CH2CH3), 1.00 (t, J = 7.6 Hz, 3H, –CH2CH2CH2CH3). 13C-NMR (100 MHz, CDCl3) δ 161.26, 142.48, 142.10, 141.79, 140.81, 139.64, 137.73, 137.68, 136.55, 136.02, 129.90, 129.54, 129.06, 128.78, 128.52, 127.60, 125.40, 125.32, 122.17, 121.97, 121.73, 121.68, 120.71, 120.55, 113.67, 111.45, 109.97, 109.91, 48.24, 44.82, 33.01, 23.71, 22.93, 20.21, 13.86. HRMS calculated for C37H35N6O [M + H]+: 579.2867; found 579.2861.
9-benzyl-N′-((9-benzyl-1-methyl-9H-pyrido[3,4-b]indol-3-yl)methylene)-1-methyl-9H-pyrido[3,4-b]indole-3-carbohydrazide (8ab): The compound was obtained as a yellow solid with 85% yield. 1H-NMR (400 MHz, CDCl3) δ 11.25 (s, 1H, CONH), 8.96 (s, 1H, ArH), 8.90 (s, 1H, ArH), 8.48 (s, 1H, ArH), 8.28–8.25 (m, 2H, ArH, CH), 7.58–7.52 (m, 2H, ArH), 7.39–7.32 (m, 4H, ArH), 7.30–7.26 (m, 3H, ArH), 7.25–7.21 (m, 2H, ArH), 7.00–6.95 (m, 5H, ArH), 5.78 (s, 2H, ArCH2), 5.74 (s, 2H, ArCH2), 2.88 (s, 3H, CH3), 2.86 (s, 3H, CH3). 13C-NMR (100 MHz, CDCl3) δ 161.08, 142.44, 142.22, 142.14, 140.82, 140.09, 138.28, 137.70, 137.49, 136.98, 136.05, 129.83, 129.73, 129.11, 129.04, 128.88, 128.72, 127.70, 127.58, 125.39, 125.30, 122.14, 122.01, 121.75, 121.72, 120.99, 120.68, 113.67, 111.34, 110.09, 109.90, 48.24, 23.31. HRMS calculated for C40H33N6O [M + H]+: 613.2710; found 613.2719.
3.5. MTT Assay
Target compounds were assayed by the MTT method for determining cytotoxic activity as described previously [41]. The panel of cell lines included the human umbilical vein cell line EA.HY926, Lewis lung carcinoma (LLC), gastric carcinoma (BGC-823), murine colon carcinoma (CT-26), liver carcinoma (Bel-7402), and breast carcinoma (MCF-7). Cell lines were obtained from Shanghai Cell Institute, Chinese Academy of Science. Growth inhibition rates were calculated with the following equitation: Inhibition ratio (%) = 100%. The half-maximal inhibitory concentration (IC50) of each compound was calculated using GraphPad Prism software (version 6.0).
3.6. CAM Assay in Vivo
To determine in vivo anti-angiogenic activity of heterobivalent β-carbolines, a CAM assay was performed as previously described [39]. In brief, five-day-old fertilized chicken eggs were purchased from a local hatchery. All the eggs were incubated at 37 °C in an incubator. We injected 0.5 mL of saline, and the eggs were incubated horizontally to allow the CAM to detach from the shell to make a bogus chamber. Compound 8z was prepared in gelatin sponge discs at concentrations of 0.5, 5.0, and 50 µM/disc. CA4P was used as a positive control drug. Discs containing the vehicle only (DMSO) were used as negative controls. A small window opening was made in the shell, and the discs were directly applied onto the CAM. The square opening was covered with sterilized surgical tape, and the embryos were incubated for 48 h at 38.5 °C. The CAMs were photographed under a dissecting microscope, and blood vessels in each CAM were counted. The results are presented as a mean percentage of inhibition compared to the control ± SD, n = 3.
4. Conclusions
On the basis of our previous work, this study has focused on the synthesis of a series of heterobivalent β-carbolines bearing an acylhydrazone bond (8a–ab). All of the target compounds were investigated for their in vitro antiproliferative activity using the MTT-based assay against five cancer cell lines (LLC, BGC-823, CT-26, Bel-7402, and MCF-7). Most compounds showed medium antiproliferative activities against the tested cancer cell lines. In particular, compound 8z showed antitumor activities with IC50 values of 9.9, 8.6, 6.2, 9.9, and 5.7 µM against the LLC, BGC-823, CT-26, Bel-7402, and MCF-7 cell lines, respectively. The anti-angiogenic activity of compound 8z was comparable with CA4P in an in vivo CAM assay at the 5 μM level.
Supplementary Materials
The following are available online, 1H and 13C-NMR spectra for the target compounds are available online.
Author Contributions
Conceptualization, J.Z.; methodology, L.G., X.C., Q.M., and W.F.; formal analysis, L.G. and W.C.; investigation, X.C. and L.G.; writing—original draft preparation, X.C. and L.G.; writing—review and editing, J.Z. and L.G.; project administration, J.Z.
Funding
We gratefully thank the Scientific Research Innovation Project in Shihezi University (No. SHYL-YB201804), the Xinjiang Science and Technology Major Project (No. 2016A03005-1), the Program for Changjiang Scholars and Innovative Research Team in University (No. IRT15R46), and Xinjiang Huashidan Pharmaceutical Research Co. Ltd.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Song, M. Progress in discovery of KIF5B-RET kinase inhibitors for the treatment of non-small-cell lung cancer. J. Med. Chem. 2015, 58, 3672–3681. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.H.; Peng, W.L.; Wang, Z.H.; Xu, A.L. β-Carboline alkaloids: Biochemical and pharmacological functions. Curr. Med. Chem. 2007, 14, 479–500. [Google Scholar] [CrossRef] [PubMed]
- Sathish, M.; Kavitha, B.; Nayak, V.L.; Tangella, Y.; Ajitha, A.; Nekkanti, S.; Alarifi, A.; Shankaraiah, N.; Nagesh, N.; Kamal, A. Synthesis of podophyllotoxin linked β-carboline congeners as potential anticancer agents and DNA topoisomerase II inhibitors. Eur. J. Med. Chem. 2018, 144, 557–571. [Google Scholar] [CrossRef] [PubMed]
- Tokala, R.; Thatikonda, S.; Vanteddu, U.S.; Sana, S.; Godugu, C.; Shankaraiah, N. Design and synthesis of DNA-interactive β-carboline-oxindole hybrids as cytotoxic and apoptosis-inducing agents. ChemMedChem 2018, 13, 1909–1922. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Guo, J.; Yang, Q.X.; Zhu, P.; Miao, J.F.; Gao, W.J.; Peng, Y.F.; Yang, J.Y.; Xu, K.; Xiong, B.; et al. Development of novel β-carboline-based hydroxamate derivatives as HDAC inhibitors with antiproliferative and antimetastatic activities in human cancer cells. Eur. J. Med. Chem. 2018, 144, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.A.; Addo, J.K.; Deokar, H.; Sun, S.; Wang, J.; Li, W.; Suttle, D.P.; Wang, W.; Zhang, R.; Buolamwini, J.K. Synthesis, biological evaluation and modeling studies of new pyrido[3,4-b]indole derivatives as broad-spectrum potent anticancer agents. Drug Des. 2017, 6, 143. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Singh, A.; Kumar, K.; Kumar, V. Recent insights into synthetic β-carbolines with anti-cancer activities. Eur. J. Med. Chem. 2017, 142, 48–73. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, M.; Qian, K.; Lee, K.H.; Morris-Natschke, S.; Peng, S.Q. Novel N-(3-carboxyl-9-benzyl-β-carboline-1-yl) ethylamino acids: Synthesis, anti-tumor evaluation, intercalating determination, 3D QSAR analysis and docking investigation. Eur. J. Med. Chem. 2009, 44, 4153–4161. [Google Scholar] [CrossRef]
- Ikeda, R.; Kurosawa, M.; Okabayashi, T.; Takei, A.; Yoshiwara, M.; Kumakura, T.; Sakai, N.; Morita, O.A.; Ikekita, M.; Nakaike, Y.; et al. 3-(3-Phenoxybenzyl) amino-β-carboline: A novel antitumor drug targeting α-tubulin. Bioorg. Med. Chem. Lett. 2011, 21, 4784–4787. [Google Scholar] [CrossRef]
- Yang, M.; Kuo, P.; Hwang, T.; Chiou, W.; Qian, K.; Lai, C.; Lee, K.; Wu, T. Synthesis, in vitro anti-inflammatory and cytotoxic evaluation, and mechanism of action studies of 1-benzoyl-β-carboline and 1-benzoyl-3-carboxy-β-carboline derivatives. Bioorg. Med. Chem. 2011, 19, 1674–1682. [Google Scholar] [CrossRef]
- Shankaraiah, N.; Siraj, K.P.; Nekkanti, S.; Srinivasulu, V.; Sharma, P.; Senwar, K.R.; Sathish, M.; Vishnuvardhan, M.V.P.S.; Ramakrishna, S.; Jadala, C.; et al. DNA-binding affinity and anticancer activity of β-carboline–chalcone conjugates as potential DNA intercalators: Molecular modelling and synthesis. Bioorg. Chem. 2015, 59, 130–139. [Google Scholar] [CrossRef]
- Taira, Z.; Kanzawas, S.; Dohara, C.; Ishida, S.; Matsumoto, M.; Sakiya, Y. Intercalation of six beta-carboline derivatives into DNA. Jpn. J. Toxicol. Environ. Health 1997, 43, 83–91. [Google Scholar] [CrossRef]
- Cao, R.H.; Peng, W.L.; Chen, H.S.; Ma, Y.; Liu, X.D.; Hou, X.R.; Guan, H.J.; Xu, A.L. DNA binding properties of 9-substituted harmine derivatives. Biochem. Biophys. Res. Commun. 2005, 338, 1557–1563. [Google Scholar] [CrossRef]
- Kamal, A.; Sathish, M.; Nayak, V.L.; Srinivasulu, V.; Kavitha, B.; Tangella, Y.; Thummuri, D.; Bagul, C.; Shankaraiah, N.; Nagesh, N. Design and synthesis of dithiocarbamate linked β-carboline derivatives: DNA topoisomerase II inhibition with DNA binding and apoptosis inducing ability. Bioorg. Med. Chem. 2015, 23, 5511–5526. [Google Scholar] [CrossRef]
- Figueiredo, P.O.; Perdomo, R.T.; Garcez, F.R.; Matos, M.F.C.; Carvalho, J.E.; Garcez, W.S. Further constituents of Galianthe thalictroides (Rubiaceae) and inhibition of DNA topoisomerases I and IIa by its cytotoxic β-carboline alkaloids. Bioorg. Med. Chem. Lett. 2014, 24, 1358–1361. [Google Scholar] [CrossRef]
- Song, Y.; Kesuma, D.; Wang, J.; Deng, Y.; Duan, J.; Wang, J.H.; Qi, R.Z. Specific inhibition of cyclin-dependent kinases and cell proliferation by harmine. Biochem. Biophys. Res. Commun. 2004, 317, 128–132. [Google Scholar] [CrossRef]
- Li, Y.; Liang, F.; Jiang, W.; Yu, F.S.; Cao, R.H.; Ma, Q.H.; Dai, X.Y.; Jiang, J.D.; Wang, Y.C.; Si, S.Y. DH334, a β-carboline anticancer drug, inhibits the CDK activity of budding yeast. Cancer Biol. Ther. 2007, 6, 1193–1199. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Guo, L.; Cao, R.H.; Zhao, P.; Jiang, W.; Ma, Q.; Yi, H.; Li, Z.; Jiang, J.D.; et al. DH166, a beta-carboline derivative, inhibits the kinase activity of PLK1. Cancer Biol. Ther. 2009, 8, 2374–2383. [Google Scholar] [CrossRef]
- Barsanti, P.A.; Wang, W.; Ni, Z.; Duhl, D.; Brammeier, N.; Martin, E. The discovery of tetrahydro-β-carbolines as inhibitors of the kinesin Eg5. Bioorg. Med. Chem. Lett. 2010, 20, 157–160. [Google Scholar] [CrossRef]
- Castro, A.C.; Dang, L.C.; Soucy, F.; Grenier, L.; Mazdiyasni, H.; Hottelet, M.; Parent, L.; Pien, C.; Palombella, V.; Adams, J. Novel IKK inhibitors: β-carbolines. Bioorg. Med. Chem. Lett. 2003, 13, 2419–2422. [Google Scholar] [CrossRef]
- Barbosa, V.A.; Barea, P.; Mazia, R.S.; Ueda-Nakamura, T.; Costa, W.F.D.; Foglio, M.A.; Goes Ruiz, A.L.T.; Carvalho, J.E.; Vendramini-Costa, D.B.; Nakamura, C.V.; et al. Synthesis and evaluation of novel hybrids beta-carboline-4-thiazolidinones as potential antitumor and antiviral 766 agents. Eur. J. Med. Chem. 2016, 124, 1093–1104. [Google Scholar] [CrossRef]
- Misra, S.; Ghatak, S.; Patil, N.; Dandawate, P.; Ambike, V.; Adsule, S.; Unni, D.; Venkateswara Swamy, K.; Padhye, S. Novel dual cyclooxygenase and lipoxygenase inhibitors targeting hyaluronan-CD44v6 pathway and inducing cytotoxicity in colon cancer cells. Bioorg. Med. Chem. 2013, 21, 2551–2559. [Google Scholar] [CrossRef][Green Version]
- Cardoso, L.N.F.; Nogueira, T.C.M.; Rodrigues, F.A.R.; Oliveira, A.C.A.; Luciano, M.C.S.; Pessoa, C.; de Souza, M.V.N. N-acylhydrazones containing thiophene nucleus: A new anticancer class. Med. Chem. Res. 2017, 26, 1605–1608. [Google Scholar] [CrossRef]
- Sun, K.; Peng, J.D.; Suo, F.Z.; Zhang, T.; Fu, Y.D.; Zheng, Y.C.; Liu, H.M. Discovery of tranylcypromine analogs with an acylhydrazone substituent as LSD1 inactivators: Design, synthesis and their biological evaluation. Bioorg. Med. Chem. Lett. 2017, 27, 5036–5039. [Google Scholar] [CrossRef]
- Zheng, Y.W.; Ren, J.; Wu, Y.Q.; Meng, X.T.; Zhao, Y.B.; Wu, C.L. Proteolytic unlocking of ultrastable twin-acylhydrazone linkers for lysosomal acid-triggered release of anticancer drugs. Bioconjug. Chem. 2017, 28, 2620–2626. [Google Scholar] [CrossRef]
- Li, F.Y.; Wang, X.; Duan, W.G.; Lin, G.S. Synthesis and in vitro anticancer activity of novel dehydroabietic acid-based acylhydrazones. Molecules 2017, 22, 1087. [Google Scholar] [CrossRef]
- Rodrigues, A.P.; Costa, L.M.; Santos, B.L.; Maia, R.C.; Miranda, A.L.; Barreiro, E.J.; Fraga, C.A. Novel furfurylidene N-acylhydrazones derived from natural safrole: Discovery of LASSBio-1215, a new potent antiplatelet prototype. J. Enzym Inhib. Med. Chem. 2012, 27, 101–109. [Google Scholar] [CrossRef]
- Norsworthy, K.J.; Ko, C.W.; Lee, J.E.; Liu, J.; John, C.S.; Przepiorka, D.; Farrell, A.T.; Pazdur, R. FDA approval summary: Mylotarg for treatment of patients with relapsed or refractory CD33-positive acute myeloid leukemia. Oncologist 2018, 23, 1103–1108. [Google Scholar] [CrossRef]
- Peterson, Q.P.; Goode, D.R.; West, D.C.; Ramsey, K.N.; Lee, J.J.; Hergenrother, P.J. PAC-1 activates procaspase-3 in vitro through relief of zinc-mediated inhibition. J. Mol. Biol. 2009, 388, 144–158. [Google Scholar] [CrossRef]
- Misra, M.C. Drug treatment of haemorrhoids. Drugs 2005, 65, 1481–1491. [Google Scholar] [CrossRef]
- Guo, L.; Cao, R.H.; Fan, W.X.; Ma, Q. Synthesis and biological evaluation of 1,2,7,9-tetrasubstituted harmine derivatives as potential antitumor agents. Chem. J. Chin. Univ. 2014, 35, 518–523. [Google Scholar]
- Guo, L.; Fan, W.X.; Chen, X.M.; Ma, Q.; Cao, R.H. Synthesis and antitumor activities of β-carboline derivatives. Chin. J. Org. Chem. 2013, 33, 332–338. [Google Scholar] [CrossRef]
- Guo, L.; Fan, W.X.; Gan, Z.Y.; Chen, W.; Ma, Q.; Cao, R.H. Design and synthesis of 1-substituted-β-carboline derivatives as potential anticancer agents. J. Chin. Pharm. Sci. 2015, 24, 801–808. [Google Scholar]
- Zhang, G.X.; Cao, R.H.; Guo, L.; Ma, Q.; Fan, W.X.; Chen, X.M.; Li, J.R.; Shao, G.; Qiu, L.Q.; Ren, Z.H. Synthesis and structureeactivity relationships of N2-alkylated quaternary β-carbolines as novel antitumor agents. Eur. J. Med. Chem. 2013, 65, 21–31. [Google Scholar] [CrossRef]
- Posner, G.H.; D’Angelo, J.; O’Neill, P.M.; Mercer, A. Anticancer activity of artemisinin-derived trioxanes. Expert Opin. Ther. Pat. 2006, 16, 1665–1672. [Google Scholar] [CrossRef]
- Alagbala, A.A.; McRiner, A.J.; Borstnik, K.; Labonte, T.; Chang, W.; D’Angelo, J.G.; Posner, G.H.; Foster, B.A. Biological mechanisms of action of novel C-10 non-acetal trioxane dimers in prostate cancer cell lines. J. Med. Chem. 2006, 49, 7836–7842. [Google Scholar] [CrossRef]
- Posner, G.H.; McRiner, A.J.; Paik, I.H.; Sur, S.; Borstnik, K.; Xie, S.; Shapiro, T.A.; Alagbala, A.A.; Foster, B. Anticancer and antimalarial efficacy and safety of artemisininderived trioxane dimers in rodents. J. Med. Chem. 2004, 47, 1299–1301. [Google Scholar] [CrossRef]
- Jung, M.; Lee, S.; Ham, J.; Lee, K.; Kim, H.; Kim, S.K. Antitumor activity of novel deoxoartemisinin monomers, dimers, and trimer. J. Med. Chem. 2003, 46, 987–994. [Google Scholar] [CrossRef]
- Guo, L.; Chen, W.; Fan, W.X.; Ma, Q.; Sun, R.Q.; Shao, G.; Cao, R.H. Synthesis and preliminary evaluation of novel alkyl diamine linked bivalent β-carbolines as angiogenesis inhibitors. Med. Chem. Commun. 2016, 7, 2177–2183. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, G.X.; Guo, L.; Fan, W.X.; Ma, Q.; Zhang, X.D.; Du, R.L.; Cao, R.H. Synthesis and biological evaluation of novel alkyl diamine linked bivalent β-carbolines as angiogenesis inhibitors. Eur. J. Med. Chem. 2016, 124, 249–261. [Google Scholar] [CrossRef]
- Guo, L.; Chen, W.; Cao, R.H.; Fan, W.X.; Ma, Q.; Zhang, J.; Dai, B. Synthesis and structure-activity relationships of asymmetric dimeric β-carboline derivatives as potential antitumor agents. Eur. J. Med. Chem. 2018, 147, 253–265. [Google Scholar] [CrossRef]
- Guo, L.; Ma, Q.; Chen, W.; Fan, W.X.; Zhang, J.; Dai, B. Synthesis and biological evaluation of novel N9-heterobivalent β-carbolines as angiogenesis inhibitors. J. Enzyme Inhib. Med. Chem. 2019, 34, 375–387. [Google Scholar] [CrossRef]
- Shi, B.X.; Cao, R.H.; Fan, W.X.; Guo, L.; Ma, Q.; Chen, X.M.; Zhang, G.X.; Qiu, L.Q.; Song, H.C. Design, synthesis and in vitro and in vivo antitumor activities of novel bivalent β-carbolines. Eur. J. Med. Chem. 2013, 60, 10–22. [Google Scholar] [CrossRef]
- Daoud, A.; Song, J.; Xiao, F.; Shang, J. B-9-3, a novel β-carboline derivative exhibits anti-cancer activity via induction of apoptosis and inhibition of cell migration in vitro. Eur. J. Pharmacol. 2014, 724, 219–230. [Google Scholar] [CrossRef]
- Ma, Q.; Chen, W.; Chen, W. Anti-tumor angiogenesis effect of a new compound: B-9-3 through interference with VEGFR2 signaling. Tumor Biol. 2016, 37, 6107–6116. [Google Scholar] [CrossRef]
- Zhong, H.; Daoud, A.; Han, J.; An, X.; Qiao, C.; Duan, L.; Wang, Y.; Chen, Z.; Zhou, J.; Shang, J. A small β-carboline derivative “B-9-3” modulates TGF-β signaling pathway causing tumor regression in vivo. Front. Pharmacol. 2018, 9, 788. [Google Scholar] [CrossRef]
- Guo, L.; Xie, J.W.; Fan, W.X.; Chen, W.; Dai, B.; Ma, Q. Synthesis and antitumor activities of novel bivalent 1-Heterocyclic-β-carbolines linked by alkylamino spacer. Chin. J. Org. Chem. 2017, 37, 1741–1747. [Google Scholar] [CrossRef]
- Cao, R.H.; Chen, H.S.; Peng, W.L.; Ma, Y.; Hou, X.R.; Guan, H.J.; Liu, X.; Xu, A.L. Design, synthesis and in vitro and in vivo antitumor activities of novel β-carboline derivatives. Eur. J. Med. Chem. 2005, 40, 991–1001. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 8a–ab are available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).