Abstract
Euphorbia species were widely used in traditional medicines for the treatment of several diseases. From the aerial parts of Egyptian endemic plant, Euphorbia sanctae-catharinae, three new premyrsinane diterpenoids, namely, euphosantianane E–G (1–3), alongside four known triterpenes, 9,19-cyclolanostane-3β,24S-diol (4), 25-methoxycycloartane-3β,24S-diol (5), 25-methylenecycloartan-3β,24R-diol (6), and 25-methylenecycloartan-3β,24S-diol (7), were isolated and identified. The chemical structures were proven depending upon spectroscopic analysis, including FTIR, HRFABMS, and 1D/2D-NMR. The chemotaxonomic significance of the isolated compounds, especially diterpenes from E. sanctae-catharinae compared to those documented from different Euphorbia species was also studied via agglomerative hierarchical clustering (AHC). The Egyptian endemic Euphorbia sanctae-catharina was grouped with E. bupleuroides, E. fidjiana, E. fischeriana, E. pithyusa subsp. cupanii, E. prolifera, and E. seguieriana, where myrsinol diterpenoids were the characteristic compounds.
1. Introduction
From the times of ancient Egyptian civilization, medicinal plants have been used as resources of several medicinal treatments. Egypt has unique biodiversity, including different ecological zones such as the Nile-Delta areas, Mediterranean coasts, Red Sea coast, and deserts. Because of these variations in ecological and natural factors between these zones, it is characterized by a diversity of wild and/or endemic medicinal plants. It also includes 13 pharmacopeia ones, 60 endemic ones, and 529 species used for medical purposes [1,2]. The Sinai Peninsula and especially Saint Katherine is one of the most interesting and promising resources of medicinal plants. The medicinal plants located in Sinai have shown unique phytochemicals with potent biological activities which have piqued the interest of many scientists, especially biologists and phytochemists, for further investigations [1,3,4,5].
It is well known that Euphorbia species all over the world have different chemical and biological diversity. Several Euphorbia plants have been used in folk medicines against several remedies, such as skin diseases, warts, gonorrhea, migraine, and intestinal parasites. Phytochemical characterization of these plants affords highly bioactive isoprenoids [6,7,8]. Diterpenes represent the main constituents from the plants of this genus, including jatrophanes, premyrsinanes, Abietanes, tiglianes, ingenanes, lathyranes, and myrsinols [6,7,9].
Several bioactivities were described for the different extracts and isolated metabolites of these plants, such as anti-inflammatory [10], antifungal [11], antiviral [12], antispasmodic [11], cytotoxic [9,13], antimutagenic [14], antibacterial [15,16], and hepatoprotective [6,7].
Among the 60 identified endemic species to Egypt, three Euphorbia species (E. bivonae Steud., E. punctata Delile, and E. sanctae-catharinae Fayed (synonym of E. obovata Decne.)) were recorded. Recently, our group successfully isolated and identified nine premyrsinanes diterpenoids as well as three flavonoids from E. sanctae-catharinae and evaluated their cytotoxic activities [9]. Continuing our work on the investigation of new metabolites from medicinal plants, we performed here the isolation and identification of seven terpenoids, including three new premyrsinanes (1–3, Euphosantianane E–G), alongside four known triterpenes (4–7) (Figure 1).
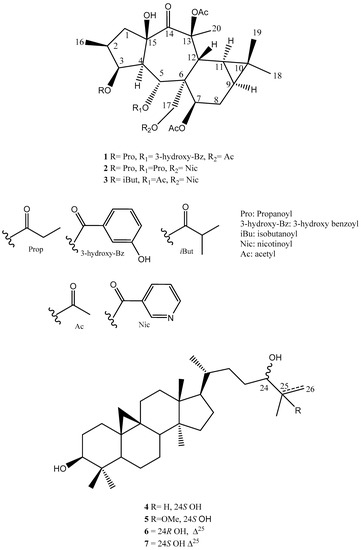
Figure 1.
Isolated compounds from Euphorbia sanctae-catharinae.
2. Results and Discussion
2.1. Structure Elucidation of the Isolated Compounds
The chemical characterization of the CH2Cl2–MeOH extract of the air-dried powder of the Egyptian endemic plant, E. sanctae-catharinae, using different chromatographic tools, afforded three new premyrsinane types diterpenes (1–3) alongside four known triterpenes (4–7), presented in Figure 1. The chemical structures of these seven isolated compounds were established depending upon the modern spectroscopic analysis.
Euphosantianane E (1) was isolated as a colorless oil and exhibited positive optical rotation ( + 18.0 in MeOH). The chemical formula was assigned as C36H46O13 (cal. 686.2938) depending upon the HRFABMS that exhibit molecular ion peak at m/z 709.2930 (M + Na)+ and displayed 14 degrees of unsaturation. FTIR absorption bands characteristic for OH and carbonyl esters were observed, respectively, at 3380 and 1732 cm−1, alongside with the aromatic ring absorption bands at 1442 and 723 cm−1. 1H-NMR spectrum (Table 1) of 1 exhibited proton signals for three oxygenated methines at δH 5.35 br t (J = 3.48 Hz), 6.42 d (J = 11.46 Hz) and 4.77 d (J = 6.72 Hz); one methylene at δH 4.35 d (J = 11.70 Hz) and 4.67 d (J = 11.70 Hz); three characteristic methyles singlets of acetates at 2.11 s (6H) and 2.12 s. Additionally, four singlet methyl signals at δH 0.94 s, 1.04 s (6H), and 1.57 s, as well as one triplet methyl signal at δH 0.98 t (J = 7.5 Hz). Additionally, two methine protons characteristic to cyclopropane moiety were identified at δH 0.73 m (2H). 13C-NMR (Table 1) spectrum displayed 36 carbon signals that were characterized by DEPT-135 and HMQC experiments to 12 quaternary carbons (including five carbonyl esters at δC 173.5, 170.8 (2 × C), and 170.0, 168.9; one ketone at δC 204.1 and two oxygenated carbons at δC 84.2 and 85.7); 12 methines (including three oxygenated at δC 78.2, 70.6 and 70.5); four methylene carbons (including one oxygenated at δC 62.8); and eight methyl groups (including three methyles of acetates at δC 20.8, and 21.4 (2 × C) and one methyl of propanoyl group at δC 8.8). 1D and 2D-NMR spectra of 1 were closely related to previously reported premyrsinanes with clear differences in type of substitution in C-5. A complete assignment of 1 was compatible with previously reported euphosantianane A [9].

Table 1.
1H (600 Hz) and 13C (150 Hz) NMR (CDCl3) of 1–3a,b.
1H–1H COSY correlations of H-4 (δH 2.37, dd, J = 3.78, 11.5 Hz)/H-3 (δH 5.35 brt, J = 3.48 Hz), H-4/H-5 (δH 6.42 d, J = 11.46 Hz), H-9 (δH 0.73 m)/H-8 (δH 3.50 brd, J = 6.54 Hz), and H-8/H-7 (δH 4.77, d, 6.72 Hz) confirmed the oxygenation of C-3 (δC 78.2), C-5 (δC 70.5), and C-7 (δC 70.6). Additionally, H3-16 (δH 1.04, s), H-12 (δH 3.48, s), and H-5 showed correlation with C-3, C-5, and C-7 in HMBC analyses, respectively (Figure 2). The HMBC spectra showed the following correlation: H-17 at δH 4.67 (d, J = 11.70 Hz)/C-5 (J3); H-3/C-15 (84.2; J3); H-4/C-15 (J2); H-4/C-14 (204.1; J3); H3-20 at δH 1.57(s)/C-14 (J3); H-12/C-13 (δC 85.7; J2) confirmed the hydroxylation of C-15, oxygenation of C-13 and C-17, and localization of ketone group in C-14 (Figure 2). As described in several reports, the premyrsinanes isolated from Euphorbia plants are usually characterized by variation of functionality at C-3, C-5, C-7, and/or C-17 [9,17,18]. The 1D and 2D-NMR as well as mass spectrum exhibited the replacement of 3-methylbutyryl moiety (euphosantianane A, Hegazy et al., 2018) with 3-hydroxy benzoyl moiety. This substitution was deduced depending upon the HMBC correlations of H-5/(O=C)-1`` (δC 168.9; J3), H-3`` at δH 7.39 d (J = 1.56 MHz)/C-1``, and H-7`` at δH 7.58 dd (J = 1.68, 7.98 MHz). Further, the hydroxylation of C-4`` was confirmed with the aromatic quaternary carbon at δC 161.9 (C-4``) alongside the HMBC correlations of H-6`` at δH 6.80 t (J = 9.18 MHz)/C-4``.
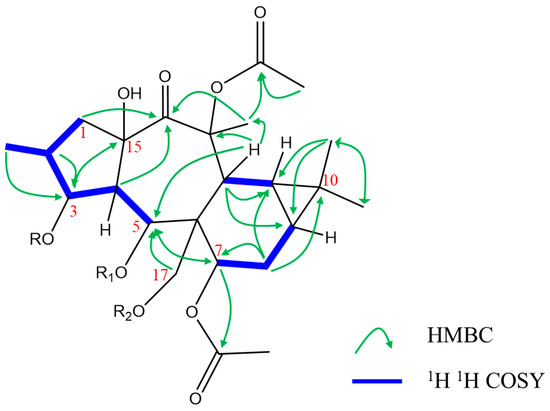
Figure 2.
Selected significant 1H 1H COSY, and Key HMBC correlations of 1–3.
The relative configuration of 1 was determined depending upon NOESY correlations (Figure 3). The trans and α orientation H-4 and H2-17, respectively, was established depending upon the biosynthetic pathways of these types of myrsinols [9,17,18]. Starting from this reference point, NOESY correlations of H-4α/H-3 and H-3/H-2 deduced the α configuration of H-3 and H-2. NOESY correlations of H-12β/H-5, H-9α/H-11α, and H-11α/H-20 elucidated the β orientation of H-5 and Me-20. From all these described data, 1 was assigned as premyrsinol-3β-propanoyl-5α-(3-hydroxy)-benzoyl-7β,13β,17α-triacetate (Euphosantianane E).
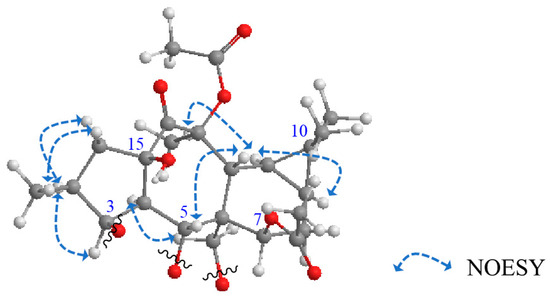
Figure 3.
Significant NOESY correlations of 1–3.
Euphosantianane F (2, Figure 1) was isolated as a colorless oil and exhibited positive optical rotation ( + 24.4 in MeOH). Based on the HRFABMS ion peak at m/z 685.3089 (M), the molecular formula of 2 was determined as C36H47NO12 (cal. 685.3098), exhibiting 14 degrees of unsaturation. FTIR bands for OH and carbonyl esters were characterized at 3441 and 1736 cm−1, respectively, in addition to the aromatic ring absorption bands at 1434 and 741 cm−1. 1D-NMR data (Table 1) and 2D (1H–1H COSY, HMQC, and HMBC, Figure 2) exhibited that 2 is very close to euphosantianane A (Hegazy et al., 2018), except the substitution at C-5 and C-17 was replaced by propanoyl and nicotinoyl, respectively. This substitution was deduced by the downfield shift of C-5 by 1.9 ppm to be at δC 70.7 and the upfield shift of C-17 by 1.0 ppm to be at δC 62.6, in addition to the HMBC and 1H–1H COSY related to propanoyl and nicotinoyl substituents, respectively. Depending on NOESY, compound 2 showed the same stereochemistry of 1. Thus, 2 was identified as premyrsinol-3β,5α-dipropanoyl-7β,13β-diacetyl-17α-nicotinoate (euphosantianane F).
Euphosantianane G (3, Figure 1) was obtained as a colourless oil and exhibited positive optical rotation ( 53.2 in MeOH). The HRFABMS ion peak assigned at m/z 708.3090 (M + Na)+ indicated the molecular formula as C36H47NO12 (cal. 685.3098) with 14 degrees of unsaturation. Hydroxyls and carbonyl ester FTIR bands at 3430 and 1728 cm−1, respectively, as well as the aromatic ring absorption bands at 1458 and 716 cm−1 were identified. 1D-NMR data (Table 1), 1H–1H COSY and HMBC (Figure 2) of 3 are very close to euphosantianane D [9] (Hegazy et al., 2018), with the usual exception of different substitutions at C-3 and C-5, in which isobutanoyl and acetyl groups were inserted, respectively. The downfield shift of C-3 by 1.3 ppm found at δC 78.6 deduced the substitution of this carbon with the acetyl group instead of 3-dimethylbutanoyl in euphosantianane D [9]. One methylene group was detected in 1H and 13C-NMR at δH 2.15 m and δC 27.6, respectively, along with at two methyle groups at δH 1.05 d (J = 7.02 Hz; 6H) and at δC 8.9 that were characterized by DEPT-135 and HMQC. Strong 1H–1H COSY and HMBC correlations of H3-3`/H-2`, H3-3`/C-2` (J2), H3-3`/(C=O)-1` (δC 175.1, J3), and H-3 at δH 5.20 s/(C=O)-1` were observed that deduced the localization of isobutanoyl group at C-3 (δC 78.6). The NOESY experiment confirmed that 3 has the same absolute configuration of 1 and 2. Thus, 3 was identified as premyrsinol-3β-isobutyroyl-5α,7β,13β-triacetoyl-17α-nicotinoate (euphosantianane G, 3).
In addition to these three new premyrsinane diterpenes, euphosantianane E–G (1–3), four known triterpenes, 9,19-cyclolanostane-3β,24S-diol (4) [19], 25-methoxycycloartane-3β,24S-diol (5) [20], 25-methylenecycloartan-3β,24R-diol (6), and 25-methylenecycloartan-3β,24S-diol (7) [21,22], were characterized (Figure 1).
2.2. Study of Chemosystematic Significance
Euphorbia is one of the most diverse and very large genera among flowering plants. It has a worldwide distribution, and it is found as herbs, shrubs or trees. It is characterized by the presence of milky latex. More than 2000 species are distributed at cosmopolitan, but especially tropical, subtropical, and warm-temperate regions [23]. Euphorbia is represented by 36 species in the flora of Egypt. Three of these Euphorbia are endemic to Egypt, namely, E. bivonae Steud., E. punctata Delile, and E. sanctae-catharinae Fayed (synonym of Euphorbia obovata Decne).
In our previous work on the chemical composition of the endemic E. sanctae-catharinae of Egypt, nine premyrsinanes diterpenes and three flavonoids were isolated and identified from E. sanctae-catharina. Moreover, in the present study, four cycloartane triterpenes and three new myrsinol compounds were identified for the first time from the endemic E. sanctae-catharinae.
In order to correlate the chemical composition of this endemic species to Egypt with other Euphorbia species, agglomerative hierarchical clustering (AHC) analysis was performed. Based on diterpenoid chemical composition from 32 Euphorbia species in addition to E. sanctae-catharinae, the AHC analysis revealed these plants categorized into nine groups (Figure 4). The largest group comprised ten Euphorbia species (E. amygdaloides, E. bungei, E. characias, E. dendroides, E. esula, E. formosana, E. helioscopia, E. peplus, E. sororia, and E. tuckeyana). These species showed a close correlation to each other due to the presence of jatrophane diterpenoid compounds. These species mainly contained jatrophane terpenoids, where amygdaloidins A–L were reported in E. amygdaloides [24]. Helioscopinolides A, B, and C were reported in E. formosana [25], while euphocharacins A–L were isolated from E. characias [26]. The jatrophane diterpenoids euphodendrophanes A–P, abeodendroidin, and epiabeodendroidin F were isolated from E. dendroides [27,28,29], while esulatin A–M, esulone A, and esulone B were isolated from E. esula [30].
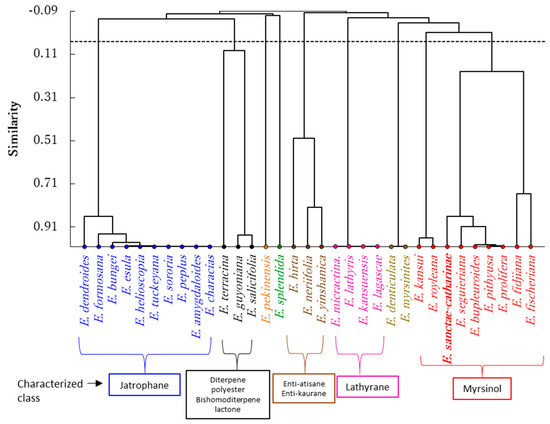
Figure 4.
Agglomerative hierarchical clustering (AHC) of 32 Euphorbia species and the present studied endemic Euphorbia (E. sanctae-catharinae) based on the terpenoid compounds.
Corea et al. [31] identified several types of jatrophane compounds in E. peplus, such as pepluanin A, B, and C, euphopeplin A, 2α,5α,7β,8α,9α, 14β-hexaacetoxy-3β-benzoyloxy-15-hydroxyjatropha-6(17), 11E-diene, and 5α,8α,9β,10β, 14α-pentaacetoxy-3β-benzoy-loxy-15-hydroxypepluane. However, E. helioscopia was reported as a rich plant with jatrophane diterpenes, where it contained euphohelin A–E, euphoheliosnoids A–C, and helioscopianoids A–Q [32,33,34]. Sororianolide A–C were identified from E. sororia [35], while tuckeyanol A and B and euphotuckeyanol were isolated from E. tuckeyana [36].
Therefore, the predominance of jatrophane compounds in theses Euphorbia species reflected the similarity of the chemistry of these species and led to a clustering of them together in one group of the AHC (Figure 4). Nevertheless, different diterpenoid classes such as lathyrane, enti-atisane, ingole, and bishomoditerpene lactone were also isolated from some of these species [24,34].
On the other hand, E. kansuensis, E. lagascae, E. lathyris, and E. micractina were closely related to each other and grouped together (Figure 4). This group was characterized by lathyrane-type diterpenoids, where E. lagascae has been reported to possess latilagascene A–F and jolkinol B [37]. While euphorbia factor L8, euphorbia factor L7a and 7b, euphorbia factor L3, jolkinol B, isolathyrol, 7-hydroxylathyrol, and lathyrol were isolated from E. lathyris [38,39].
Another group comprised E. hirta, E. neriifolia, and E. yinshanica and was characterized by the presence of enti-atisane and enti-kaurane diterpenoids [40,41,42]. Euphorbia guyoniana, E. salicifolia, and E. terracina represented another group, and these plants are characterized by the presence of diterpene polyester and bishomoditerpene lactone. Terracinolide A and B were identefied in E. terracina [43], guyonianin C–F were isolated from E. guyoniana [44,45], and euphosalicin 1–3 as well as salicinolide were idenfied in E. salicifolia [46].
Our studied endemic species, E. sanctae-catharinae, was grouped with E. bupleuroides, E. fidjiana, E. fischeriana, E. pithyusa subsp. cupanii, E. prolifera, and E. seguieriana. However, the Pearson correlation coefficient analysis revealed that E. sanctae-catharinae showed a close correlation to E. bupleuroides (0.888), followed by E. prolifera (0.880), then E. pithyusa subsp. cupanii (0.870) based on the composition of the terpenoid composition (Figure 4). Myrsinol diterpenoids were the main constituents of these Euphorbia species [9,47,48,49], while other diterpenoid compounds such as abietane, ent-kaurane, ent-atisane, and lathyrane types as well as cycloartane triterpene were also identified in these plants [6,50]. Hegazy et al. [9] identified several myrsinol compounds form E. sanctae-catharinae, such as euphosantianane A–D. In addition, the present study revealed the presence of an additional three new myrsinol compounds (euphosantianane E–G). However, E. prolifera was richer in the myrsinol diterpenoids where it comprises numerous compounds including for examples, proliferin A–D, euphorprolitherin B and D, euphorbiaproliferin A–I, and euphorbialoid [17,47,48,49,51,52].
Euphorbia pithyusa subsp. cupanii was reported to have myrsinol compounds [18], while Jeske et al. [50] stated that E. seguieriana contained 12 myrsinol compounds. Therefore, the results exhibited that our studied endemic species (E. sanctae-catharinae) was grouped with theses Euphorbia species due to the predominance of myrsinol diterpenes. Nevertheless, the present phytochemical study showed the presence of four cycloartane triterpenes (25-methylenecycloartan-3β,24R-diol, 9,19-cyclolanostane-3β,24S-diol, and 25-methylenecycloartan-3β,24S-diol,) which was already reported from some Euphorbia species like, E myrsinites [53], E. denticulate [54], and E. spinidens [55].
On the other hand, cycloartane triterpenes were identified in both E. denticulate and E. myrsinites, and thus grouped together. However, E. kansui and E. royleana were similar to each other, chacterized by ingole compounds [56,57,58], while either E. pekinensis or E. splendida showed dissimilarity with all tested Euphorbia species.
From previously reported flavonoids from Euphorbia species, flavonoid and their glycosides were isolated and identified from some of these plants, such as E. condylocarpa, E. virgata, E. chamaesyce, E. hirta, and E. magalanta [59,60,61,62], in addition to E. sanctae-catharinae [9]. For example, quercetin-3-O-α-rhamnopyranoside and kaempferol-3-O-rhamnoside were isolated from E. condylocarpa collected from Iraq [60], and this is in total agreement with the isolated compound from E. sanctae-catharinae.
3. Conclusions
From the Egyptian endemic plant, E. sanctae-catharinae, three new premyrsinanes, euphosantianane E–G (1–3), alongside the known triterpenes (4–7), were characterized using modern spectroscopic tools. For first time, the chemotaxonomic significance of isolated compounds from E. sanctae-catharinae especially diterpenes compared to those documented from different Euphorbia ecospecies was also studied, and it was found to be closely correlated to E. bupleuroides, E. prolifera, and E. pithyusa subsp. cupanii based on the composition of the terpenoid compound classes.
4. Materials and Methods
4.1. General Experimental Procedures
As described in our previously reported protocol by Hegazy et al. [9].
4.2. Plant Material
The aerial parts of E. sanctae-catharinae were collected from Wadi Jibaal, Protectorate of Saint Katherine, South Sinai, Egypt during the flowering stage in April 2016 under the permission of the Protectorate for scientific purposes. A voucher specimen (#16-212) has been deposited in the herbarium of the National Research Centre. The authentication of the plant was kindly performed by Prof. Mona Marzouk, Professor of Taxonomy, NRC, Cairo, Egypt.
4.3. Extraction and Isolation
Air dried aerial parts (one kg) were ground, and extracted with CH2Cl2:MeOH (1:1) at room temperature, filtered and then concentrated under vacuum afforded black gum (63 g). The extract was then subjected to silica gel flash column chromatography (5 × 60 cm) and eluted with n-hexane/EtOAc step gradient. Eight main fractions (ES-1:ES-8) were obtained after thin layer chromatography (TLC, Kieselgel 60 F254, 0.25 mm, Merck, Darmstadt, Germany) examinations of similar ones. Fraction ES-4 (724 mg), subjected to further fractionation via reversed phas ODS column (3×60 cm) with MeOH: H2O 4:1, afforded 3 main subfractions (ES-4A–C) after TLC examinations. Subfraction ES-4B, eluted by MeOH:H2O (4:1) by a reversed phase HPLC (20 × 250 cm), afforded compounds 1 (2.8 mg), 3 (3.2 mg), 6 (14.6 mg), and 7 (12.9 mg). Moreover, subfraction ES-5 (564 mg) was further fractionated over ODS column (3 × 60 cm) with MeOH: H2O 7:3 that afforded 2 main subfractions (ES-5A,B). Subfraction ES-5B was eluted by MeOH:H2O (85:15) over a reversed phase HPLC (20 × 250 cm) and afforded compounds 2 (3.4 mg), 4 (15.1 mg), and 5 (7.8 mg).
4.4. Spectroscopic Data of Euphosantianane E–G (1–3)
Euphosantianane E (premyrsinol-3β-propanoyl-5α-(3-hydroxy)-benzoyl-7β,13β,17α-triacetate, 1): colorless oil; +18.0 (c 0.01, MeOH); FT-IR (KBr): 3380, 1732, 1462 and 728 cm−1; 1H and 13C-NMR spectral data, see Table 1 and Supplementary Materials (S1–S9); HRFABMS: m/z 709.2930 (M + Na)+; C36H46O13 (cal. 686.2938).
Euphosantianane F (premyrsinol-3β,5α-dipropanoyl-7β,13β-diacetyl-17α-nicotinoate, 2): colorless oil; + 24.4 (c 0.01, MeOH); FT-IR (KBr): 3441, 1736, 1434 and 741 cm−1; 1H and 13C-NMR spectral data, see Table 1 and Supplementary Materials (S10–S17); HRFABMS: m/z 685.3089 (M), C36H47NO12 (cal. 685.3098).
Euphosantianane G (premyrsinol-3β-isobutyroyl-5α,7β,13β-triacetoyl-17α-nicotinoate, 3): colorless oil; + 53.2 (c 0.01, MeOH); FT-IR (KBr): 3430, 1728, 1458 and 716 cm−1 1H and 13C-NMR spectral data, see Table 1 and Supplementary Materials (S18–S25); HRFABMS: m/z 708.3090 (M + Na)+, C36H47NO12 (cal. 685.3098).
4.5. Statistical Analysis
A data matrix of 15 terpene classes from 33 Euphorbia species was subjected to agglomerative hierarchical clustering (AHC). This matrix was designed based on the numbers of the identified terpenoid compounds from 32 Euphorbia species (collected from the literature review) as well as that identified in the present study from the endemic species to Egypt (E. sanctae-catharinae). This analysis was performed by XLSTAT statistical computer software package, version 2018 (Addinsoft, New York, NY, USA).
Supplementary Materials
The Supplementary Materials are available online.
Author Contributions
A.I.E., T.A.M., and M.-E.F.H. contributed to the extraction, isolation, purification, identification, and manuscript preparation. A.M.A.-E. contributed to the evaluation of chemotaxonomic significance. A.M.A., A.A.S., S.L.A.-R., and B.A.D. contributed to guiding experiments, and manuscript preparations. M.-E.F.H. was the group leader, organizing and guiding the experiments, structure elucidation, and manuscript writing. All authors discussed the results, commented on the paper, and approved the final manuscript.
Funding
This research was funded by King Saud University through research group No (RG-1440-113; A.M.A.) and the Alexander von Humboldt Foundation (Georg Foster Research Fellowship for Experienced Researcher, for M.-E.F.H.), Germany.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group No (RG-1440-113). Further, the authors like to sincerely thanks the National Research Centre, and Department of Botany, Faculty of Science, Mansoura University, Egypt. Prof. Mohamed Hegazy gratefully acknowledges the financial support from Alexander von Humboldt Foundation “Georg Foster Research Fellowship for Experienced Researcher”.
Conflicts of Interest
The authors declare that there is no conflict of interest.
References
- Eissa, T.; Palomino, O.; Carretero, M.; Gómez-Serranillos, M. Ethnopharmacological study of medicinal plants used in the treatment of CNS disorders in Sinai Peninsula, Egypt. J. Ethnopharmacol. 2014, 151, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Boulos, L. Flora of Egypt Checklist; Al Hadara Publishing: Cairo, Egypt, 2009. [Google Scholar]
- Batanouny, K.H.; Aboutabl, E.; Shabana, M.F.S. Wild Medicinal Plants in Egypt. An Inventory to Support Conservation and Sustainable Use; The Palm Press: Cairo, Egypt, 1999. [Google Scholar]
- Boulos, L.; Gibali, M. List of rare, vulnerable, endangered and endemic species of vascular plants in Sinai Peninsula. In Proceedings of the First Egypt-Hungary Conference on Environment, Cairo, Egypt, 5–7 April 1993; pp. 275–282. [Google Scholar]
- Barakat, N.; Abd El-Gawad, A.; Laudadio, V.; Kabiel, H.; Tufarelli, V.; Cazzato, E. A contribution to the ecology and floristic markers of plant associations in different habitats of Sinai Peninsula, Egypt. Rendiconti Lincei Scienze Fisiche e Naturali 2014, 25, 479–490. [Google Scholar] [CrossRef]
- Vasas, A.; Hohmann, J. Euphorbia diterpenes: Isolation, structure, biological activity, and synthesis (2008–2012). Chem. Rev. 2014, 114, 8579–8612. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.-W.; Su, X.-H.; Kiyota, H. Chemical and pharmacological research of the plants in genus Euphorbia. Chem. Rev. 2008, 108, 4295–4327. [Google Scholar] [CrossRef] [PubMed]
- Elshamy, A.I.; Abd-ElGawad, A.M.; El Gendy, A.E.-N.G.; Assaeed, A.M. Chemical characterization of Euphorbia heterophylla L. essential oils and their antioxidant activity and allelopathic potential on Cenchrus echinatus L. Chem. Biodiv. 2019, 16, e1900051. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, M.-E.; Hamed, A.; Ibrahim, M.; Talat, Z.; Reda, E.; Abdel-Azim, N.; Hammouda, F.; Nakamura, S.; Matsuda, H.; Haggag, E. Euphosantianane A–D: Antiproliferative premyrsinane diterpenoids from the endemic Egyptian plant Euphorbia sanctae-catharinae. Molecules 2018, 23, 2221. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; Yu, L.; Tang, Y.; Zhang, L.; Ding, A.; Luo, D.; Duan, J.-a.; Shen, X. Bioassay-guided separation of the proinflammatory constituents from the roots of Euphorbia kansui. J. Nat. Med. 2010, 98, 64–103. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Khan, A.-U.; Chaudhary, B.A.; Janbaz, K.H.; Uzair, M.; Akhtar, M.; Gilani, A.-H. Antifungal and antispasmodic activities of the extracts of Euphorbia granulata. J. Med. Plants Res. 2012, 6, 19–23. [Google Scholar]
- Esposito, M.L.; Nothias, L.-F.L.; Retailleau, P.; Costa, J.; Roussi, F.; Neyts, J.; Leyssen, P.; Touboul, D.; Litaudon, M.; Paolini, J. Isolation of premyrsinane, myrsinane, and tigliane diterpenoids from Euphorbia pithyusa using a Chikungunya virus cell-based assay and analogue annotation by molecular networking. J. Nat. Prod. 2017, 80, 2051–2059. [Google Scholar] [CrossRef]
- Pracheta, S.V.; Paliwal, R.; Sharma, S. Preliminary phytochemical screening and in vitro antioxidant potential of hydro-ethanolic extract of Euphorbia neriifolia Linn. Int. J. PharmTech Res. 2011, 3, 124–132. [Google Scholar]
- Ibraheim, Z.Z.; Ahmed, A.S.; Abdel-Mageed, W.M. Chemical and biological studies of Euphorbia aphylla. J. Nat. Rem. 2013, 13, 35–45. [Google Scholar]
- Wu, L.; Zhou, P.J.; Wang, X.F. The application of antibacterial components of Euphorbia Humifusa Willd on silk fabrics. Adv. Mater. Res. 2012, 441, 315–319. [Google Scholar] [CrossRef]
- Lirio, L.; Hermano, M.; Fontanilla, M. Note antibacterial activity of medicinal plants from the Philippines. Pharm. Biol. 1998, 36, 357–359. [Google Scholar] [CrossRef]
- Xu, J.; Guo, Y.; Xie, C.; Li, Y.; Gao, J.; Zhang, T.; Hou, W.; Fang, L.; Gui, L. Bioactive myrsinol diterpenoids from the roots of Euphorbia prolifera. J. Nat. Prod. 2011, 74, 2224–2230. [Google Scholar] [CrossRef] [PubMed]
- Appendino, G.; Belloro, E.; Tron, G.C.; Jakupovic, J.; Ballero, M. Diterpenoids from Euphorbia pithyusa subsp. cupanii. J. Nat. Prod. 1999, 62, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Katsuya, E.; Mayumi, M.; Atsuko, O.; Miwako, H.; Hiroyuki, Y. A new method for the determination of absolute configuration of isolated hydroxyl groups: CD spectra of acetates and methoxyglyoxalates. Proc. Symp. Chem. Nat. Prod. 1991, 33, 448–455. [Google Scholar]
- Kikuchi, T.; Akihisa, T.; Tokuda, H.; Ukiya, M.; Watanabe, K.; Nishino, H. Cancer chemopreventive effects of cycloartane-type and related triterpenoids in in vitro and in vivo models. J. Nat. Prod. 2007, 70, 918–922. [Google Scholar] [CrossRef] [PubMed]
- Scobedo-Martínez, C.; Concepción Lozada, M.; Hernández-Ortega, S.; Villarreal, M.L.; Gnecco, D.; Enríquez, R.G.; Reynolds, W. 1H and 13C-NMR characterization of new cycloartane triterpenes from Mangifera indica. Magn. Reson. Chem. 2012, 50, 52–57. [Google Scholar] [CrossRef]
- Chunhui, M.; Tianfang, H.; Huayi, Q.; Bogang, L.; Guolin, Z. Chemical study of Streptocaulon griffithii. Chin. J. Appl. Environ. Biol. 2005, 11, 265–270. [Google Scholar]
- Boulos, L. Flora of Egypt; Al Hadara Publishing: Cairo, Egypt, 2000; Volume 2. [Google Scholar]
- Corea, G.; Fattorusso, C.; Fattorusso, E.; Lanzotti, V. Amygdaloidins A–L, twelve new 13 α-OH jatrophane diterpenes from Euphorbia amygdaloides L. Tetrahedron 2005, 61, 4485–4494. [Google Scholar] [CrossRef]
- Yu, C.-C.; Hsieh, C.-R.; Hsiao, G.; Chen, P.-Y.; Chang, M.-L.; Yin, H.-W.; Lee, T.-H.; Lee, C.-K. Regulated expressions of mmp-2,-9 by diterpenoids from Euphorbia formosana hayata. Molecules 2012, 17, 2082–2090. [Google Scholar] [CrossRef] [PubMed]
- Helmboldt, H.; Hiersemann, M. Synthetic studies toward jatrophane diterpenes from Euphorbia characias. Enantioselective synthesis of (−)-15-O-Acetyl-3-O-propionyl-17-norcharaciol. J. Org. Chem. 2009, 74, 1698–1708. [Google Scholar] [CrossRef] [PubMed]
- Corea, G.; Fattorusso, E.; Lanzotti, V.; Taglialatela-Scafati, O.; Appendino, G.; Ballero, M.; Simon, P.-N.; Dumontet, C.; Di Pietro, A. Modified jatrophane diterpenes as modulators of multidrug resistance from Euphorbia dendroides L. Bioorg. Med. Chem. 2003, 11, 5221–5227. [Google Scholar] [CrossRef] [PubMed]
- Pešić, M.; Banković, J.; Aljančić, I.S.; Todorović, N.M.; Jadranin, M.; Vajs, V.E.; Tešević, V.V.; Vučković, I.; Momčilović, M.; Marković, I.D. New anti-cancer characteristics of jatrophane diterpenes from Euphorbia dendroides. Food Chem. Toxicol. 2011, 49, 3165–3173. [Google Scholar] [CrossRef]
- Jadranin, M.; Pešić, M.; Aljančić, I.S.; Milosavljević, S.M.; Todorović, N.M.; Podolski-Renić, A.; Banković, J.; Tanić, N.; Marković, I.; Vajs, V.E. Jatrophane diterpenoids from the latex of Euphorbia dendroides and their anti-P-glycoprotein activity in human multi-drug resistant cancer cell lines. Phytochemistry 2013, 86, 208–217. [Google Scholar] [CrossRef]
- Manners, G.D.; Wong, R.Y. The absolute stereochemical characterization of two new jatrophane diterpenes from Euphorbia esula. J. Chem. Soc. Perkin Trans. 1985, 1, 2075–2081. [Google Scholar] [CrossRef]
- Corea, G.; Di Pietro, A.; Dumontet, C.; Fattorusso, E.; Lanzotti, V. Jatrophane diterpenes from Euphorbia spp. as modulators of multidrug resistance in cancer therapy. Phytochem. Rev. 2009, 8, 431–447. [Google Scholar] [CrossRef]
- Kosemura, S.; Shizuri, Y.; Yamamura, S. Isolation and structures of euphohelins, new toxic diterpenes from Euphorbia helioscopia L. Bull. Chem. Soc. Jpn. 1985, 58, 3112–3117. [Google Scholar] [CrossRef]
- Chen, H.; Wang, H.; Yang, B.; Jin, D.-Q.; Yang, S.; Wang, M.; Xu, J.; Ohizumi, Y.; Guo, Y. Diterpenes inhibiting NO production from Euphorbia helioscopia. Fitoterapia 2014, 95, 133–138. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, Y.-W. Three new jatrophone-type diterpenoids from Euphorbia helioscopia. Planta Med. 2005, 71, 283–286. [Google Scholar] [CrossRef]
- Huang, Y.; Aisa, H.A. Three new diterpenoids from Euphorbia sororia L. Helv. Chim. Acta 2010, 93, 1156–1161. [Google Scholar] [CrossRef]
- Duarte, N.; Lage, H.; Ferreira, M.-J.U. Three new jatrophane polyesters and antiproliferative constituents from Euphorbia tuckeyana. Planta Med. 2008, 74, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Duarte, N.; Varga, A.; Cherepnev, G.; Radics, R.; Molnár, J.; Ferreira, M.-J.U. Apoptosis induction and modulation of P-glycoprotein mediated multidrug resistance by new macrocyclic lathyrane-type diterpenoids. Bioorg. Med. Chem. 2007, 15, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Jiao, W.; Dong, W.; Li, Z.; Deng, M.; Lu, R. Lathyrane diterpenes from Euphorbia lathyris as modulators of multidrug resistance and their crystal structures. Bioorg. Med. Chem. 2009, 17, 4786–4792. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-Y.; Liang, Y.-J.; Chen, H.-B.; Zheng, L.-S.; Mi, Y.-J.; Wang, F.; Zhao, X.-Q.; Wang, X.-K.; Zhang, H.; Fu, L.-W. Structure identification of Euphorbia factor L3 and its induction of apoptosis through the mitochondrial pathway. Molecules 2011, 16, 3222–3231. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Ye, D.; Wang, Y.; Zhao, Y.; Pu, J.; Du, X.; Luo, L.; Zhao, Y. Ent-Kaurane diterpenoids from Euphorbia hirta. Rec. Nat. Prod. 2011, 5, 247–251. [Google Scholar]
- Liu, J.-H.; Latif, A.; Ali, M.; Zhang, G.-P.; Xiang, W.-J.; Ma, L.; Arfan, M.; Hu, L.-H. Diterpenoids from Euphorbia neriifolia. Phytochemistry 2012, 75, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.Y.; Wang, H.; Luo, X.D.; Du, Z.Z.; Shen, J.W.; Wu, H.F.; Zhang, X.F. Bisyinshanic acids A and B, two novel diterpene dimers from the roots of Euphorbia yinshanica. Helv. Chim. Acta 2012, 95, 1672–1679. [Google Scholar] [CrossRef]
- Marco, J.A.; Sanz-Cervera, J.F.; Yuste, A.; Jakupovic, J.; Lex, J. Terracinolides A and B, two bishomoditerpene lactones with a novel carbon framework from Euphorbia terracina. J. Org. Chem. 1996, 61, 1707–1709. [Google Scholar] [CrossRef]
- El-Bassuony, A.A. Antibacterial activity of new polyester diterpenes from Euphorbia guyoniana. Asian J. Chem. 2007, 19, 4553–4562. [Google Scholar]
- Hegazy, M.-E.F.; Mohamed, A.E.-H.H.; Aoki, N.; Ikeuchi, T.; Ohta, E.; Ohta, S. Bioactive jatrophane diterpenes from Euphorbia guyoniana. Phytochemistry 2010, 71, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, J.; Evanics, F.; Dombi, G.; Molnár, J.; Szabó, P. Euphosalicin, a new diterpene polyester with multidrug resistance reversing activity from Euphorbia salicifolia. Tetrahedron 2001, 57, 211–215. [Google Scholar] [CrossRef]
- Xu, J.; Jin, D.; Guo, P.; Xie, C.; Fang, L.; Guo, Y. Three new myrsinol diterpenes from Euphorbia prolifera and their neuroprotective activities. Molecules 2012, 17, 9520–9528. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Jin, D.-q.; Guo, Y.; Xie, C.; Ma, Y.; Yamakuni, T.; Ohizumi, Y. New myrsinol diterpenes from Euphorbia prolifera and their inhibitory activities on LPS-induced NO production. Bioorg. Med. Chem. Lett. 2012, 22, 3612–3618. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Jin, D.-q.; Song, H.; Guo, Y.; He, Y. Lathyrane diterpenes from Euphorbia prolifera and their inhibitory activities on LPS-induced NO production. Fitoterapia 2012, 83, 1205–1209. [Google Scholar] [CrossRef] [PubMed]
- Jeske, F.; Jakupovic, J.; Berendsohn, W. Diterpenes from Euphorbia seguieriana. Phytochemistry 1995, 40, 1743–1750. [Google Scholar] [CrossRef]
- Dagang, W.; Sorg, B.; Hecker, E. Oligo-and macrocyclic diterpenes in thymelaeaceae and Euphorbiaceae occurring and utilized in Yunnan (Southwest China). 6. Tigliane type diterpene esters from latex of Euphorbia prolifera. Phytother. Res. 1994, 8, 95–99. [Google Scholar] [CrossRef]
- Li, J.; Xu, L.; Wang, F.P. New cytotoxic myrsinane-type diterpenes from Euphorbia prolifera. Helv. Chim. Acta 2010, 93, 746–752. [Google Scholar] [CrossRef]
- Öksüz, S.; Gürek, F.h.; Gil, R.R.; Pengsuparp, T.; Pezzuto, J.M.; Cordell, G.A. Four diterpene esters from Euphorbia myrsinites. Phytochemistry 1995, 38, 1457–1462. [Google Scholar] [CrossRef]
- Shamsabadipour, S.; Ghanadian, M.; Saeedi, H.; Rahimnejad, M.R.; Mohammadi-Kamalabadi, M.; Ayatollahi, S.M.; Salimzadeh, L. Triterpenes and steroids from Euphorbia denticulata Lam. with anti-Herpes symplex virus activity. Iran. J. Pharm. Res. 2013, 12, 759–767. [Google Scholar]
- Ghannadian, M.; Akhavan, A.; Abdalla, O.; Ayatollahi, A.; Mohammadi-Kamalabadi, M.; Ghazanfari, H. Triterpenes from Euphorbia spinidens with immunomodulatory activity. Res. Pharm. Sci. 2013, 8, 205–210. [Google Scholar] [PubMed]
- Li, X.-L.; Li, Y.; Wang, S.-F.; Zhao, Y.-L.; Liu, K.-C.; Wang, X.-M.; Yang, Y.-P. Ingol and ingenol diterpenes from the aerial parts of Euphorbia royleana and their antiangiogenic activities. J. Nat. Prod. 2009, 72, 1001–1005. [Google Scholar] [CrossRef] [PubMed]
- Khiev, P.; Kim, J.W.; Sung, S.J.; Song, H.-H.; Choung, D.-H.; Chin, Y.-W.; Lee, H.-K.; Oh, S.-R. Ingenane-type diterpenes with a modulatory effect on IFN-γ production from the roots of Euphorbia kansui. Arch. Pharm. Res. 2012, 35, 1553–1558. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.S.; Lee, S.W.; Park, M.H.; Kim, M.S.; Hudson, B.I.; Park, S.-J.; Lee, W.S.; Rho, M.-C. Kansuinine A and Kansuinine B from Euphorbia kansui L. inhibit IL-6-induced Stat3 activation. Planta Med. 2010, 76, 1544–1549. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.S.; Azmi, L.; Mohapatra, P.; Rao, C.V. Flavonoids from whole plant of Euphorbia hirta and their evaluation against experimentally induced gastroesophageal reflux disease in rats. Pharmacogn. Magaz. 2017, 13, 127–134. [Google Scholar]
- Hassan, G.F.; Omer, M.A.; Babadoust, S.; Najat, D.D. Flavonoids from Euphorbia condylocarpa roots. Int. J. Chem. Biochem. Sci. 2014, 6, 56–60. [Google Scholar]
- Kawashty, S.; Abdalla, M.; El-Hadidi, M.; Saleh, N. The chemosystematics of Egyptian Euphorbia species. Biochem. Syst. Ecol. 1990, 18, 487–490. [Google Scholar] [CrossRef]
- Ulubelen, A.; Öksüz, S.; Halfon, B.; Aynehchi, Y.; Mabry, T.J. Flavonoids from Euphorbia larica, E. virgata, E. chamaesyce and E. magalanta. J. Nat. Prod. 1983, 46, 598. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1–7 are available or not from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).