The authors wish to make the following corrections to their paper [1]. The authors regret that in the above-mentioned paper, Figure 2 and the associated text were adapted without attribution from “Molecular Interactions (Noncovalent Interactions) and the Behaviors of Biological Macromolecules” by Loren Dean Williams [2]. The authors sincerely thank Prof. Loren Dean Williams for bringing this to our attention.
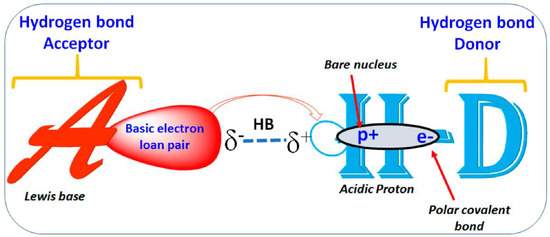
Figure 2.
The pictorial illustration of hydrogen bond interaction, where HB acceptor/donor can be an F, O, N, or S atom in the molecule. Electron polarization and exposure of a positive proton on either side are shown schematically.
References
- Mishra, S.K.; Suryaprakash, N. Intramolecular Hydrogen Bonding Involving Organic Fluorine: NMR Investigations Corroborated by DFT-Based Theoretical Calculations. Molecules 2017, 22, 423. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.D. Molecular Interactions (Noncovalent Interactions) and the Behaviors of Biological Macromolecules. Available online: http://ww2.chemistry.gatech.edu/~lw26/structure/molecular_interactions/mol_int.html (accessed on 26 February 2019).
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).