Discovery of 2-(1-(3-(4-Chloroxyphenyl)-3-oxo- propyl)pyrrolidine-3-yl)-1H-benzo[d]imidazole-4-carboxamide: A Potent Poly(ADP-ribose) Polymerase (PARP) Inhibitor for Treatment of Cancer
Abstract
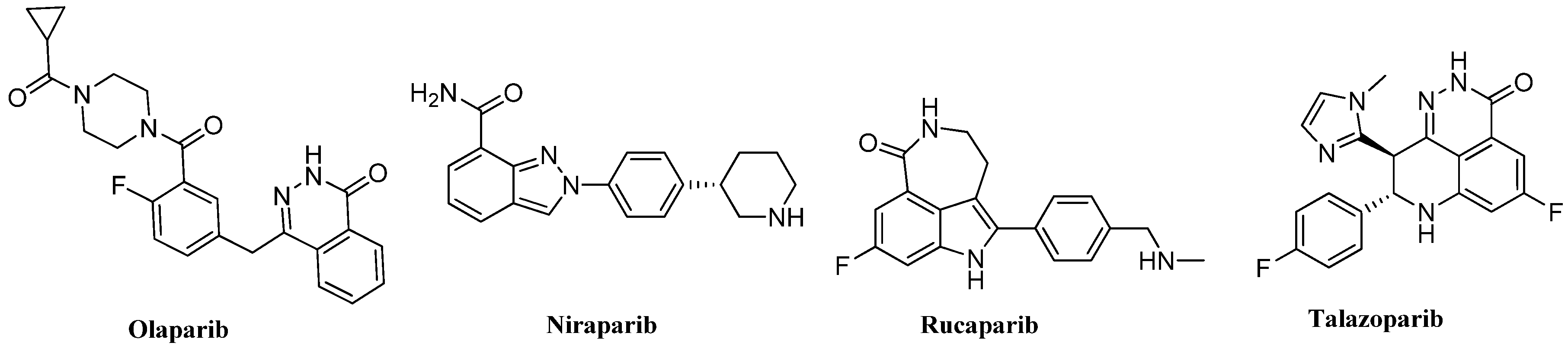
1. Introduction
2. Results and Discussion
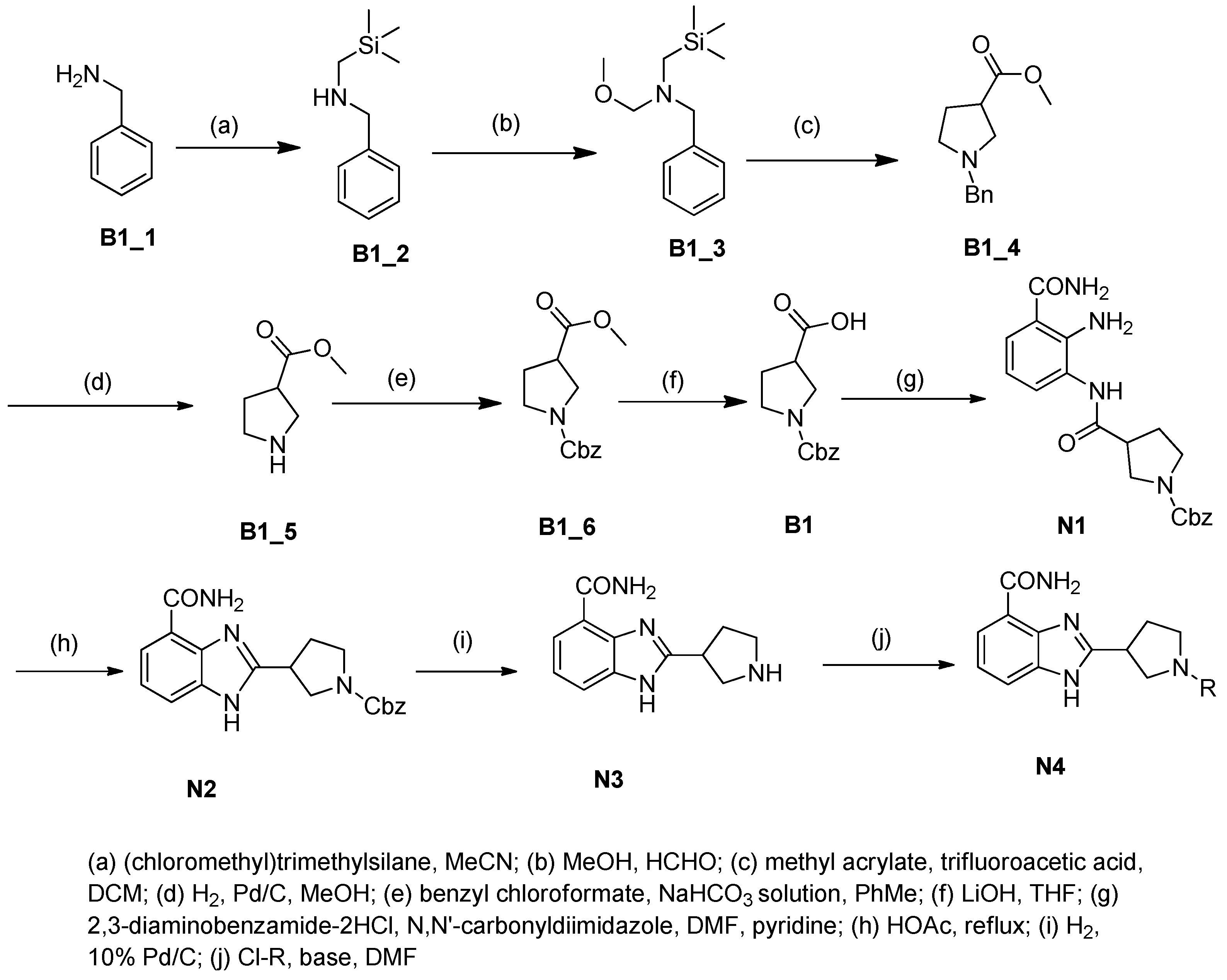
2.1. Chemistry
2.2. PARP Inhibition Assay
2.3. Cell Proliferation Assay
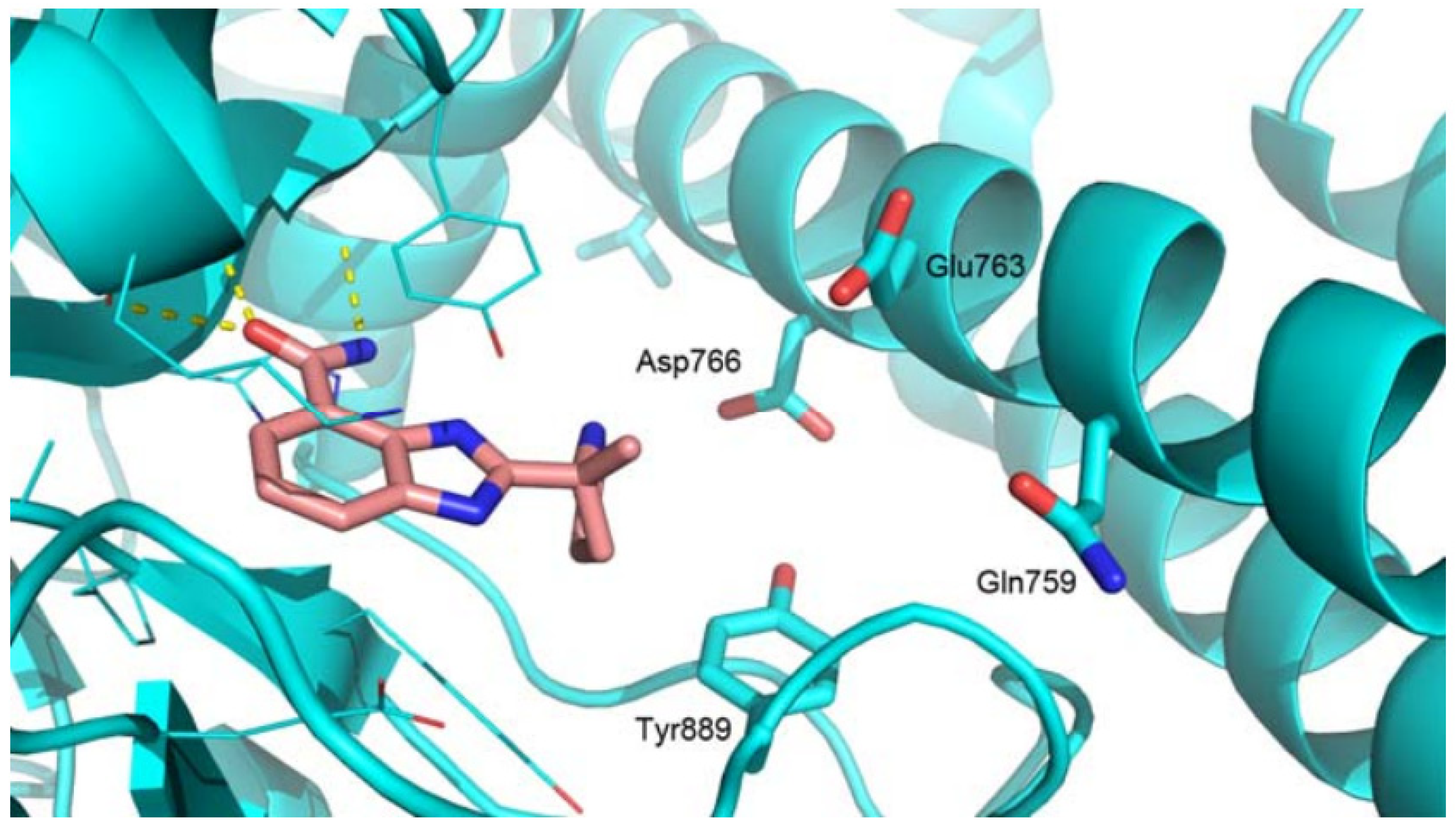
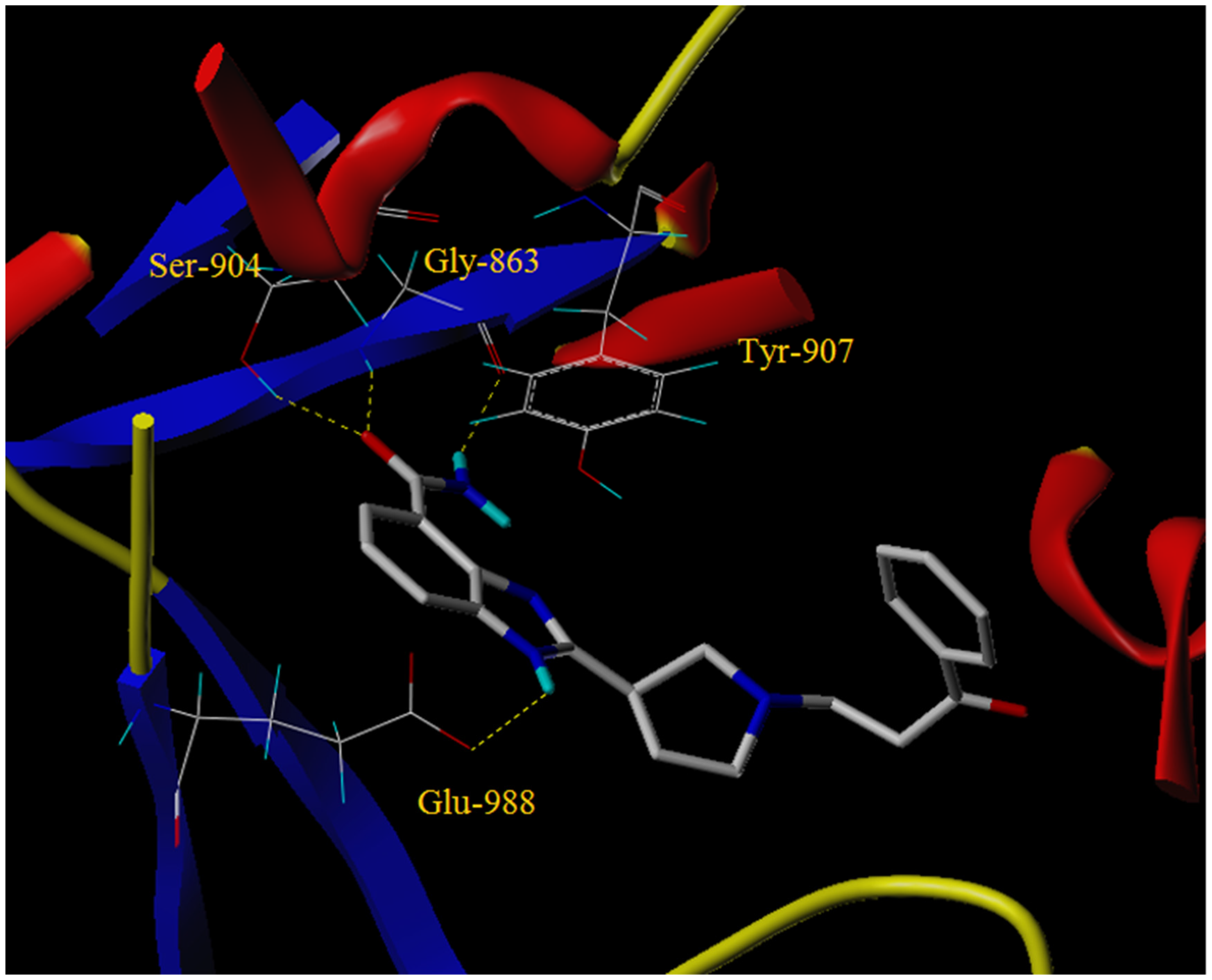
2.4. Molecular Docking
3. Materials and Methods
3.1. Genereral Informations
3.2. Chemistry
3.2.1. Procedure A: Synthesis of 2-(Pyrrolidin-3-yl)-1H-benzo[d]imidazole-4-carboxamide (N3)
3.2.2. Procedure B: Synthesis of 5ca, 5cb, 5cc, 5cd, 5ce, 5ch, 5ci, 5cj, 5ck and 5cp
3.2.3. Procedure C: Synthesis of 5cf and 5cg
3.2.4. Procedure D: Synthesis of 5cl and 5cm
3.2.5. Procedure E: Synthesis of 5cn and 5co
3.3. PARP Inhibition Assay
3.4. Cell Proliferation Assay
3.5. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Virág, L.; Szabó, C. The therapeutic potential of poly(ADP-ribose)polymerase inhibitors. Pharmacol. Rev. 2002, 54, 375–429. [Google Scholar] [CrossRef] [PubMed]
- Jagtap, P.; Szabó, C. Poly(ADP-Ribose)polymerase and the therapeutic effects of its inhibitors. Nat. Rev. Drug Discov. 2005, 4, 421–440. [Google Scholar] [CrossRef]
- Ame´, J.-C.; Spenlehauer, C.; de Murcia, G. The PARP superfamily. BioEssays 2004, 26, 882–893. [Google Scholar] [CrossRef]
- Burkle, A. Physiology and pathophysiology of poly(ADP-ribosyl)ation. BioEssays 2001, 23, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.J.; Ashworth, A. PARP inhibitors: Synthetic lethality in the clinic. Science 2017, 355, 1152–1158. [Google Scholar] [CrossRef] [PubMed]
- Almahli, H.; Hadchity, E.; Jaballah, M.Y.; Daher, R.; Ghabbour, H.A.; Kabil, M.M.; Al-shakliah, N.S.; Eldehna, W.M. Development of novel synthesized phthalazinone-based PARP-1 inhibitors with apoptosis inducing mechanism in lung cancer. Bioorg. Chem. 2018, 77, 443–456. [Google Scholar] [CrossRef]
- Malyuchenko, N.V.; Kotova, E.Y.; Kulaeva, O.I.; Kirpichnikov, M.P.; Studitskiy, V.M. PARP1 inhibitors: Antitumor drug design. Acta Nat. 2015, 7, 27–37. [Google Scholar]
- Menear, K.A.; Adcock, C.; Boulter, R.; Cockcroft, X.L.; Copsey, L.; Cranston, A.; Dillon, K.J.; Drzewiecki, J.; Garman, S.; Gomez, S.; et al. 4-[3-(4-cyclopropanecarbonylpiperazine-1-carbonyl)-4-fluorobenzyl]-2H-phtha-lazin-1-one: A novel bioavailable inhibitor of poly(ADP-ribose) polymerase-1. J. Med. Chem. 2008, 51, 6581–6591. [Google Scholar] [CrossRef] [PubMed]
- Loh, V.M.; Cockcroft, X.L.; Dillon, K.J.; Dixon, L.; Drzewiecki, J.; Eversley, P.J.; Gomez, S.; Hoare, J.; Kerrigan, F.; Matthews, I.T.W.; et al. Phthalazinones. Part 1: The design and synthesis of a novel series of potent inhibitors of poly(ADP ribose) polymerase. Bioorg. Med. Chem. Lett. 2005, 15, 2235–2238. [Google Scholar] [CrossRef]
- Scott, L.J. Niraparib: First global approval. Drugs 2017, 77, 1029–1034. [Google Scholar] [CrossRef]
- Kanjanapan, Y.; Lheureux, S.; Oza, A.M. Niraparib for the treatment of ovarian cancer. Expert Opin. Pharmaco. 2017, 18, 631–640. [Google Scholar] [CrossRef]
- Jones, P.; Altamura, S.; Boueres, J.; Ferrigno, F.; Fonsi, M.; Giomini, C.; Lamartina, S.; Monteagudo, E.; Ontoria, J.M.; Orsale, M.V.; Palumbi, M.C.; et al. Discovery of 2-{4-[(3S)-piperidin-3-yl]phenyl}-2H-indazole-7-carboxamide (MK-4827): A novel oral poly (ADP-ribose)polymerase (PARP) inhibitor efficacious in BRCA-1 and -2 mutant tumors. J. Med. Chem. 2009, 52, 7170–7185. [Google Scholar] [CrossRef] [PubMed]
- Murai, J.; Huang, S.Y.; Das, B.B.; Renaud, A.; Zhang, Y.; Doroshow, J.H.; Ji, J.; Takeda, S.; Pommier, Y. Trapping of PARP-1 and PARP-2 by clinical PARP inhibitors. Cancer Res. 2012, 72, 5588–5599. [Google Scholar] [CrossRef]
- Canan Koch, S.S.; Thoresen, L.H.; Tikhe, J.G.; Maegley, K.A.; Almassy, R.J.; Li, J.; Yu, X.-H.; Zook, S.E.; Kumpf, R.A.; et al. Novel tricyclic poly(ADP-ribose) polymerase-1 inhibitors with potent anticancer chemopotentiating activity: Design, synthesis, and X-ray cocrystal structure. J. Med. Chem. 2002, 45, 4961–4974. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.; Kaye, S.; Yap, T. PARP inhibitors: The race is on. Brit. J. Cancer 2016, 114, 713–715. [Google Scholar] [CrossRef] [PubMed]
- Penning, T.D.; Zhu, G.-D.; Gandhi, V.B.; Gong, J.; Liu, X.; Shi, Y.; Klinghofer, V.; Johnson, E.F.; Donawho, C.K.; Frost, D.J.; et al. Discovery of the Poly(ADP-ribose) Polymerase (PARP) Inhibitor 2-[(R)-2-methylpyrrolidin-2-yl]-1H-benzimidazole-4-carboxamide (ABT-888) for the Treatment of Cancer. J. Med. Chem. 2009, 52, 514–523. [Google Scholar] [CrossRef]
- Penning, T.D.; Zhu, G.-D.; Gandhi, V.B.; Gong, J.; Thomas, S.; Lubisch, W.; Grandel, R.; Wernet, W.; Park, C.H.; Fry, E.H.; et al. Discovery and SAR of 2-(1-propylpiperidin-4-yl)-1H-benzimidazole-4-carboxamide: A potent inhibitor of poly (ADP-ribose) polymerase (PARP) for the treatment of cancer. Bioorg. Med. Chem. 2008, 16, 6965–6975. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, D.; Ficco, R.P.; Dain, D.; Ginski, M.; Lautar, S.; Lee Wisdom, K.; Linag, S.; Lin, Q.; Lu, M.X.-C.; Morgan, L.; et al. Design and synthesis of poly(ADP-ribose)polymerase-1 (PARP-1) inhibitors. Part 4: Biological evaluation of imidazobenzodiazepines as potent PARP-1 inhibitors for treatment of ischemic injuries. Bioorg. Med. Chem. 2003, 11, 3695–3707. [Google Scholar] [CrossRef]
- Costatino, G.; Macchiarulo, A.; Camaioni, E.; Pellicciari, R. Modeling of poly(ADP- ribose)polymerase (PARP) inhibitors. Docking of ligands and quantitative structure- activity relationship analysis. J. Med. Chem. 2001, 44, 3786–3794. [Google Scholar] [CrossRef]
- Murai, J.; Huang, S.-Y.N.; Renaud, A.; Zhang, Y.; Ji, J.; Takeda, S.; Morris, J.; Teicher, B.; Doroshow, J.H.; Promier, Y. Stereospecific PARP trapping by BMN-673 and comparison with Olaparib and Rucaparib. Mol. Cancer Ther. 2014, 13, 433–443. [Google Scholar] [CrossRef]
- Pommier, Y.; O’ Connor, M.J.; de Bono, J. Laying a trap to kill cancer cells: PARP inhibitors and their mechanisms of action. Sci. Transl. Med. 2016, 8, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Barkalow, J.H.; Breting, J.; Gaede, B.J.; Haight, A.R.; Henry, R.; Kotecki, B.; Mei, J.; Pearl, K.B.; Tedrow, J.S.; Viswanath, S.K. Process development for ABT-472, a benzimidazole PARP inhibitor. Org. Process Res. Dev. 2007, 11, 693–698. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 5ca–5cp are available from the authors. |


| Compound | R | PARP-1 Inhibition % (10 nM) | PARP-2 Inhibition % (10 nM) | PARP-1 IC50 (nM) | PARP-2 IC50 (nM) |
|---|---|---|---|---|---|
| 5ca |  | 17.6 | 34.5 | / | / |
| 5cb | 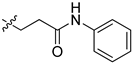 | 19.9 | 41.2 | / | / |
| 5cc | 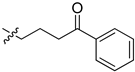 | 42.8 | 62.3 | 12.2 | 5.8 |
| 5cd |  | −1.1 | 7.2 | / | / |
| 5ce | 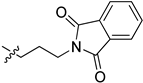 | 22.0 | 74.5 | / | / |
| 5cf |  | 5.0 | 9.0 | / | / |
| 5cg | 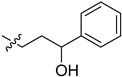 | −2.1 | 6.5 | / | / |
| 5ch | 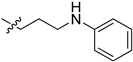 | 47.5 | 66.2 | 7.1 | 3.3 |
| 5ci |  | 59.2 | 62.5 | 5.9 | 4.5 |
| 5cj |  | 65.7 | 65.6 | 3.9 | 4.2 |
| 5ck |  | 6.0 | 37.1 | / | / |
| 5cl |  | 26.8 | 46.4 | / | / |
| 5cm |  | 24.8 | 32.8 | / | / |
| 5cn |  | 19.4 | 39.1 | / | / |
| 5co |  | 38.4 | 66.2 | 11.1 | 5.7 |
| 5cp |  | 68.0 | 76.7 | 3.6 | 3.2 |
| pc1 | Veliparib | 63.7 | 78.3 | 5.3 | 1.6 |

| Compound ID | R | MDA-MB-436 IC50 (μM) | CAPAN-1 IC50 (μM) |
|---|---|---|---|
| 5ca |  | 22.9 | >100 |
| 5cb |  | 90.4 | >100 |
| 5cc |  | 31.9 | 20.7 |
| 5cd |  | 55.0 | 82.5 |
| 5ce |  | >100 | >100 |
| 5cf |  | 61.0 | >100 |
| 5cg |  | 74.1 | >100 |
| 5ch |  | >100 | >100 |
| 5ci |  | 38.6 | 48.1 |
| 5cj |  | 17.4 | 11.4 |
| 5ck |  | >100 | >100 |
| 5cl |  | >100 | >100 |
| 5cm |  | >100 | >100 |
| 5cn |  | >100 | >100 |
| 5co |  | >100 | >100 |
| 5cp | 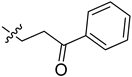 | 19.8 | 15.5 |
| pc1 | Veliparib | >100 | >100 |
| pc2 | Olaparib | 30.2 | >100 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Min, R.; Wu, W.; Wang, M.; Tang, L.; Chen, D.; Zhao, H.; Zhang, C.; Jiang, Y. Discovery of 2-(1-(3-(4-Chloroxyphenyl)-3-oxo- propyl)pyrrolidine-3-yl)-1H-benzo[d]imidazole-4-carboxamide: A Potent Poly(ADP-ribose) Polymerase (PARP) Inhibitor for Treatment of Cancer. Molecules 2019, 24, 1901. https://doi.org/10.3390/molecules24101901
Min R, Wu W, Wang M, Tang L, Chen D, Zhao H, Zhang C, Jiang Y. Discovery of 2-(1-(3-(4-Chloroxyphenyl)-3-oxo- propyl)pyrrolidine-3-yl)-1H-benzo[d]imidazole-4-carboxamide: A Potent Poly(ADP-ribose) Polymerase (PARP) Inhibitor for Treatment of Cancer. Molecules. 2019; 24(10):1901. https://doi.org/10.3390/molecules24101901
Chicago/Turabian StyleMin, Rui, Weibin Wu, Mingzhong Wang, Lin Tang, Dawei Chen, Huan Zhao, Cunlong Zhang, and Yuyang Jiang. 2019. "Discovery of 2-(1-(3-(4-Chloroxyphenyl)-3-oxo- propyl)pyrrolidine-3-yl)-1H-benzo[d]imidazole-4-carboxamide: A Potent Poly(ADP-ribose) Polymerase (PARP) Inhibitor for Treatment of Cancer" Molecules 24, no. 10: 1901. https://doi.org/10.3390/molecules24101901
APA StyleMin, R., Wu, W., Wang, M., Tang, L., Chen, D., Zhao, H., Zhang, C., & Jiang, Y. (2019). Discovery of 2-(1-(3-(4-Chloroxyphenyl)-3-oxo- propyl)pyrrolidine-3-yl)-1H-benzo[d]imidazole-4-carboxamide: A Potent Poly(ADP-ribose) Polymerase (PARP) Inhibitor for Treatment of Cancer. Molecules, 24(10), 1901. https://doi.org/10.3390/molecules24101901






