Abstract
The work reports the facile synthesis of novel α-aminophosphonate derivatives coupled with indole-2,3-dione moieties, namely the diethyl(substituted phenyl/heteroaryl)(2-(2-oxoindolin-3-ylidene)hydrazinyl)methylphosphonates derivatives 4(a–n). One-pot three component Kabachnik-Fields reactions were used to synthesize these derivatives. The reaction was carried out at room temperature by stirring in presence of ceric ammonium nitrate (CAN) as a green catalyst. The structures of the synthesized compounds were established by spectral studies. The synthesized derivatives 4(a–n) were evaluated for their in vitro anticancer activity against six human cancer cell lines by the SRB assay method. The cancer cell lines used in this research work are SK-MEL-2 (melanoma), MCF-7 (breast cancer), IMR-32 (neuroblastoma) MG-63 (human osteosarcoma), HT-29 (human colon cancer) and Hep-G2 (human hepatoma). All the synthesized derivatives inhibited the cell proliferation. Importantly, all the target compounds showed no cytotoxicity towards normal tissue cells (GI50 > 250 µM). A docking study was performed to predict the mode of action. Docking results indicate that the compounds have good binding with the enzyme tyrosine kinase as well as with microtubules, which makes them dual inhibitors. The result of in-silico bioavailability studies suggests that the compounds from the present series have good oral drug-like properties and are non-toxic in nature. In vivo acute oral toxicity study results indicate that the compounds can be considered safe, and therefore could be developed in the future as good anticancer agents or as leads for the design and synthesis of novel anticancer agents.
1. Introduction
The number of patients dying across the globe because of cancer and the non-availability of effective, non-toxic anticancer drugs in the present drug market continues to increase. Consequently, preventing this fatal disease is more challenging and hence the invention of novel anticancer agents is of paramount significance.
During carcinogenesis, an angiogenic switch occurs and several angiogenic growth factors stimulate their receptor tyrosine kinases (RTKs) to initiate multiple pro-angiogenic events [1]. A therapeutic strategy to inhibit these key angiogenic proteins or their RTKs was envisioned [2,3,4]. Multiple inhibitors targeting the different types of RTKs receptors have been studied by scientists all over the world to synthesize target-oriented drug molecules. Epidermal growth factor receptor (EGFR), vascular endothelial growth factor (VEGF) and/or platelet-derived growth factor receptor (PDGFR-2) are now used clinically. These RTKs are noted to have multi-kinase effects [5], and this appears to be imperative for improved anticancer activity.
The most successful anticancer drugs in clinical use acting by inhibiting tubulin polymerization are vincristine, vinblastine, vindesine, etc. [6]. The destabilizing agents bind to tubulin at different binding sites, including the vinca domain and the colchicines site [6].
Combination cancer chemotherapy is not a new idea. Recent studies indicate that the combination of antiangiogenic agents with cytotoxic agents is more effective in cancer treatment [7]. Examples of RTK inhibitors as the anti-angiogenic component along with cytotoxic chemotherapeutic agents [8,9] are lapatinib with carboplatin, paclitaxel and trastuzumab used in metastatic breast cancer [10,11] and docetaxel, gemcitabine and pazopanib as a treatment for soft tissue sarcoma [12], etc. The advantage of combination chemotherapy, particularly with RTK inhibitors, is reduced redundancy [9]. It is also beneficial when RTK inhibitors are combined with conventional cancer therapeutics [8,9]. The RTK inhibitors are cytostatic in nature and the tubulin inhibitors are cytotoxic in nature. If the scientists were to design the drug that contains a pharmacophore which hascytotoxic plus cytostatic pharmacological properties, then such a drug could be most promising in treating cancer patients. In keeping with the principles of combination chemotherapy [8,9], such single entities would act at a time at two or more distinct targets. Combination chemotherapy can prevent or delay the emergence of resistance, avoid drug–drug interactions, and circumvent pharmacokinetic problems and overlapping toxicities. Therefore, we thought to combine RTK inhibitory and cytotoxic activities in a single molecule to afford combination chemotherapeutics via a single agent [13,14].
The indole scaffold can be a keystone to discover drug-like kinase inhibitor molecules. Variation of substituents on the indole scaffold may have a great impact on the pharmacokinetic and pharmacodynamic behavior of the resultant molecule [15]. Tyrosine kinases are enzymes responsible for the activation of many proteins by signal transduction cascades. A pharmaceutical drug that inhibits tyrosine kinases is tyrosine kinase inhibitor (TKI) [16].
α-Aminophosphonates are among the most studied bioactive organophosphorus derivatives. They are used as enzyme inhibitors [17], inhibitors of serine hydrolase [18], peptide mimics [19], antiviral [20], antibacterial [21], antifungal [22], anticancer [23], anti-HIV [24], antibiotics [25], herbicidal [26] agents, etc. Indoles possess various medicinal properties like antibacterial, antifungal, anti-malarial, anticonvulsant and anti-inflammatory effects, etc. [27].
Isatin, chemically known as 1-H-indole-2,3-dione, and its derivatives possess a broad range of pharmacological properties. They are extensively utilized as a starting material for the synthesis of a broad range of heterocyclic compounds and as substrates for drug synthesis. In terms of its mode of action, isatin itself is proposed to inhibit cancer cell proliferation via interaction with extracellular signal-related protein kinases (ERKs), thereby promoting apoptosis. These compounds inhibit cancer cell proliferation and tumor growth via interaction with a variety of intracellular targets such as DNA, telomerase, tubulin, P glycoprotein, protein kinases and phosphatases [28]. Isatin-based hydrazones have been identified as inhibitors of the protein tyrosine phosphatase Shp2 [29]. The protein tyrosine phosphatase Shp2 plays a key role in cell signaling, cell proliferation, differentiation and migration [30].
The design protocol for the target molecules is revealed in Figure 1. Marketed anticancer drugs such as sunitinib [31] and oratinib contain a 2-oxoindolin-3-ylidene moiety. Ilmofosin and Edelfosin contain a phosphonatemoiety. A recently marketed anticancer drug, toceranib phosphate [32] contains a 2-oxoindol-3-ylidene as well as a phosphonate moiety. The biological importance of the 2-oxoindolin-3-ylidene scaffold and α-aminophosphonates and the ongoing interest of our research group [16,33,34,35,36] in the synthesis of anticancer agents encouraged us to synthesize coupled derivatives containing isatin-based hydrazones and α-aminophosphonates with the hope of obtaining novel hybrid derivatives with better anticancer activity and minimal toxicity.
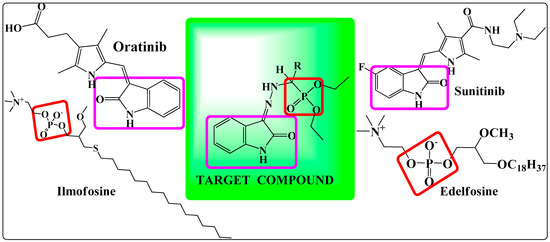
Figure 1.
Designing protocol for target compounds 4(a–n).
The one pot three-component reaction of aromatic/heterocyclic aldehydes, amines and triethylphosphite, also known as the Kabachnik–Fields reaction [37] was performed using various catalysts like Cu(OTf)2[38], VB1 [39], Al(OTf)3 [40], ZrOCl2·8H2O [41], YbCl3 [42], lanthanide triflates [43], Mg(ClO4)2 [44], LiClO4[45] etc. The Kabachnik–Fields reaction was also promoted in the presence of ceric (IV) ammonium nitrate (CAN) as a green catalyst. CAN catalyst has advantages like the very small amount of this catalyst is needed to complete reactions in most of the cases, lower costs, ecofriendly nature, high reactivity, non-toxicity, and ease of handling [46].
Fourteen new diethyl (substituted phenyl/heteroaryl)(2-(2-oxoindolin-3ylidene)hydrazinyl)methyl phosphonates derivatives 4(a–n) were synthesized at room temperature. The synthesized compounds were screened for their in-vitro anticancer activity against six human cancer cell lines by the SRB assay method. The cancer cell lines used in this research work are SK-MEL-2 (melanoma), MCF-7 (breast cancer), IMR-32 (neuroblastoma), MG-63 (human osteosarcoma), HT-29 (human colon cancer) and Hep-G2 (human hepatoma). All the synthesized derivatives 4(a–n) were also tested for their cytotoxic effects on normal cell lines i.e., NIH/3T3 (murine embryonic fibroblast) by the SRB assay method. The results showed that the synthesized compounds inhibited the proliferation of these selected cancer cell lines at moderate to high rates. In order to explore the anticancer activity of the designed derivatives they were subjected to a computational molecular docking study. The synthesized compounds that demonstrated potential in vitro anticancer activities were further screened for their in vivo acute oral toxicity study and gross behavioral studies using Swiss albino mice.
2. Results
2.1. Chemistry
The diethyl (substituted phenyl/heteroaryl)(2-(2-oxoindolin-3ylidene)hydrazinyl)methyl phosphonate derivatives 4(a–n) were synthesized as summarized in Scheme 1. 3-Hydrazonoindolin-2-one (1) was synthesized by reacting indole-2,3-dione (isatin) with hydrazine hydrate in methanol in the presence of glacial acetic acid as a catalyst by the conventional method using molecular sieves. 3-Hydrazonoindolin-2-one (1) was also synthesized by an ultrasonication-assisted green method by replacement of methanol with ethanol.
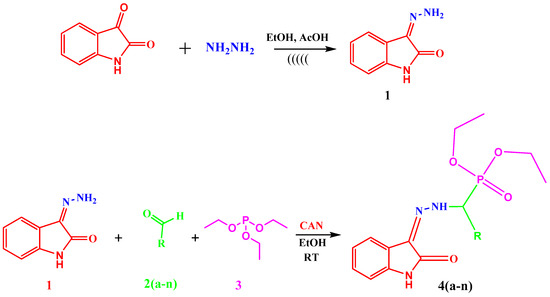
Scheme 1.
One pot three component synthesis of diethyl (substituted phenyl/heteroaryl)(2-(2-oxoindolin-3ylidene)hydrazinyl)methyl phosphonates derivatives 4(a–n).
α-Aminophosphonate derivatives 4(a–n) were synthesized by the Kabachnik-Fields method by reacting 3-hydrazonoindolin-2-one (1), substituted aldehydes 2(a–n) and triethylphosphite (3) via a one pot synthetic step in the presence of CAN as a green catalyst. CAN activates the imine formation due to which addition of phosphite to furnish a phosphonium intermediate is facilitated. This phosphonium intermediate reacts with water to give the title compounds 4(a–n). The mechanism of synthesis is as shown in Figure S1 in the Supplementary File.
The diethyl (substituted phenyl/heteroaryl)(2-(2-oxoindolin-3ylidene)hydrazinyl)methyl phosphonates derivatives 4(a–n) were thus synthesized using the multicomponent reactions (MCRs). The α-Aminophosphonate derivatives 4(a–n) can be synthesized using the multicomponent reaction (MCR) concept or by using multistep reactions. In the multistep reactions first 3-hydrazonoindolin-2-one (1) and substituted aldehydes 2(a–n) are allowed to react to give a Schiff base intermediate which is extracted and used for next step. In the next step the Schiff base intermediate is allowed to react with triethylphosphite (3) to give the final title compounds 4(a–n). In our research work we have carried out one pot synthesis in presence of CAN as green catalyst which makes our synthetic route green.
All the synthesized compounds were characterized and confirmed by spectral analysis like; FTIR, 1H-NMR, 13C-NMR, 31P-NMR, MS and elemental analysis. The purity of the synthesized compounds was determined by thin layer chromatography (TLC). The melting points were determined in open capillary tubes and are uncorrected. Physical constants data and the time required for completion of reactions for the diethyl (substituted phenyl/hetery)(2-(2-oxoindolin-3-ylidene)hydrazinyl)methylphosphonate 4(a–n) are summarized in Table 1.

Table 1.
Physical constant data for diethyl (substitutedphenyl/hetery)(2-(2-oxoindolin-3-ylidene) hydrazinyl)methylphosphonates 4(a–n).
2.2. In Vitro Anticancer Screening
The in vitro anticancer activity of novel series of α-aminophosphonate derivatives 4(a–n), was evaluated by SRB assay against six human cancer cell lines. The human cancer cell lines used are MCF-7, IMR-32, SK-MEL-2, MG-63, HT-29 and Hep-G2. Adriamycin was used as the positive control. The results obtained are summarized in Table 2. All the synthesized derivatives 4(a–n) were also tested for their cytotoxic effects on normal cell lines i.e., NIH/3T3 (murine embryonic fibroblast) by the SRB assay method.

Table 2.
In vitro Anticancer activity data of the novel synthesized compounds 4(a–n).
From the in vitro anticancer screening data the substitution of a 4-hydroxy-3-methoxyphenyl moiety in compound 4g resulted in good inhibitory activity against SK-MEL-2 with a GI50 value of 24.0 µM. The compounds 4b, 4f, 4h and 4e show moderate in vitro anticancer activity against SK-MEL-2 with GI50 values of 41.4 µM, 51.6 µM, 51.0 µM and 55.2 µM, respectively, while the others show less activity. The 4-chlorophenyl moiety in compound 4b exhibited good inhibitory activity against MCF-7, with a GI50 value of 67.2 µM. The 4-hydroxy-3-ethoxyphenyl moiety in compound 4h exhibited good inhibitory activity against MCF-7 with a GI50 value of 70.7 µM. All the novel synthesized compounds were found to have moderate in vitro anticancer activity for IMR-32 when compared to the standard drug Adriamycin.
All the synthesized derivatives 4(a–n) were found to be potent anticancer agents against the MG-63 (human osteosarcoma) cell line, with GI50 values similar to that of Adriamycin i.e., the standard drug. The compounds 4d, 4e, 4f, 4i, 4j, 4l, 4m and 4n have shown equipotent anticancer activity to that of adriamycin employed as the standard drug against the HT-29 (human colon cancer) cell line. The compounds 4i and 4l showed equipotent anticancer activity to that of standard drug Adriamycin against the Hep-G2 (human hepatoma) cell line.
The induction of apoptosis by chemotherapeutic agents has always been a favorite choice in developing anti-cancer therapeutics. To find out whether the treatment with the novel synthesized compounds could lead to loss of cell viability and induction of apoptosis, the MCF-7, IMR-32 SK-MEL-2, MG-63, HT-29 and Hep-G2 cancer cell lines were treated with the GI50 concentrations of the novel synthesized compounds 4(a–n). Cell morphology was observed at the GI50 concentration of the synthesized compounds 4(a–n) and photographs were taken under a Nikon Eclipse Ti-S Inverted Research Microscope and the images were processed using the NIS-Elements software. The images of the in vitro anticancer activity of the active compounds from the synthesized compounds 4(a–n) on the MCF-7, IMR-32, SK-MEL-2, MG-63, HT-29 and Hep-G2 cancer cell lines are shown in Figures S2–S7, respectively, in the Supplementary File. At the GI50 concentration of the potent novel compound 4f there distinguishing morphological changes were observed in IMR-32 cancer cells such as cell detachment, cell wall deformation, cell shrinkage and reduced number of viable cells in contrast to control cells, which can be clearly observed in Figure S3. It can be concluded from Figure S4 that at the GI50 concentration of the most active compound 4g there were distinctive morphological changes such as cell detachment, cell wall deformation, cell shrinkage and reduced number of viable cells in SK-MEL-2 cancer cell lines in comparison to control cells.
The synthesized compounds 4(a–n) showed no cytotoxicity towards normal tissue cells. It’s very vital for cancer treatment that the anticancer drugs have properties of high efficiency and low toxicity. The synthesized compounds 4(a–n) were found to be selective towards cancer cells since they did not exhibit cytotoxicity even at GI50 > 250 μM on normal tissue cells.
2.3. Docking Study
A molecular docking study was carried out in order to find the anticancer activity potential of the synthesized compounds. The synthesized compounds have an indole nucleus in their structure which is similar to that present in anticancer drugs such as vincristine, vinblastine and sunitinib. Sunitinib acts by inhibition of tyrosine kinase therefore, a docking study was carried out using tyrosine kinase. Vincristine and vinblastine bind to the microtubular proteins of the mitotic spindle, leading to crystallization of the microtubule and mitotic arrest or cell death. Therefore, a docking study was carried out using microtubules. In the present study the synthesized compounds are also evaluated for their in-vitro anticancer activity against SK-20 MEL-2 (melanoma), MCF-7 (breast cancer), IMR-32 (neuroblastoma) MG-63 (human osteosarcoma), HT-29 (human colon cancer) and Hep-G2 (human hepatoma) cell lines and based on this assumption synthesized compounds were docked against microtubules and tyrosine kinases (TRKs) to determine their possible mode of inhibition.
In order to identify and analyse binding affinity, binding mode and molecular interactions of the synthesized compounds in active site of receptor molecular docking study was carried out against microtubules and tyrosine kinases (TRKs). Tyrosine kinases (TRKs) are indispensable for numerous processes in the cell. These enzymes catalyze phosphorylation of different cellular substrates. Phosphorylation in turn regulates various cellular functions. Normally, their activity is stringently regulated. However, under pathological conditions, TRKs can be deregulated, leading to alterations in the phosphorylation and resulting in uncontrolled cell division, inhibition of apoptosis, and other abnormalities and consequently to diseases. Inhibition of TRKs has been shown to be a promising therapeutic strategy. Tubulin α and β heterodimer polypeptide chains are found to be an important drug target in breast cancer treatment [47]. Tubulin heterodimer polypeptide chains of α and β tubulin (50 kDa each in size) are the basic structural components of microtubules which are hollow tubes of approximately measure about 25 nm in diameter. Microtubules is important component of cell which is cytoskeletal polymers involved in various cellular functions like as mitosis, organization of intracellular structure and intracellular transport, as well as ciliary and flagellar motility. The α and β heterodimers are very significant for cellular process. In Homosapiens, there are six α-tubulin and seven β-tubulin isotypes identified and in the molecular expression of each isotype studied it is found that the molecular expression varies in different tissues and cells [48,49,50]. It is found that tubulin-binding drugs have different affinities for different isotypes of α and β, which affects the overall efficacy in different cancers. There are chemically diverse classes of compounds that bind to the tubulin–microtubule hetrodimer system. Tubulin-binding agents are potent mitotic poisons [51,52]. The literature reports of types of compounds that are synthesized in this project and the result of anticancer activity obtained prompted us to take up of docking study against two targets i.e., TRKs and tubulin inhibitors.
Depending upon the binding affinity, docking score −Log (ki), molecular interaction values given in Table 3. The methyl phosphonate derivatives such as 4f, 4g, 4d and 4e that show docking scores in between 6.031 to 5.333 are found among the most active ones. The compounds 4a, 4b, 4h, 4c and 4j are moderately active, with docking scores of 5.111 to 4.511. The compound 4i is the least active amongst the entire series of synthesized compounds, with a docking score 3.599.

Table 3.
Molecular docking study data of the compounds 4(a–n) in TRK.
The most active compounds 4f against IMR-32 showed overall a very efficient binding mode and penetration of the active site cavity by forming various interactions with active site residues such as ILE758, VAL654, THR870, LYS870, CYS678, and ASP818. The active site residue ILE758 formed hydrogen bond interactions with hydrazine groups with a distance of 1.65 Å. The amino acid CYS678 forms the hydrogen bond interactions with the hydroxyl group of the aryl ring with a distance of 3.15 Å. The amino acids LYS870 and VAL654 form the H bond interactions with the oxoindolinene ring and phosphonate group with distances of 2.25 and 2.75 Å as shown in Figure 2 and Figure 3.
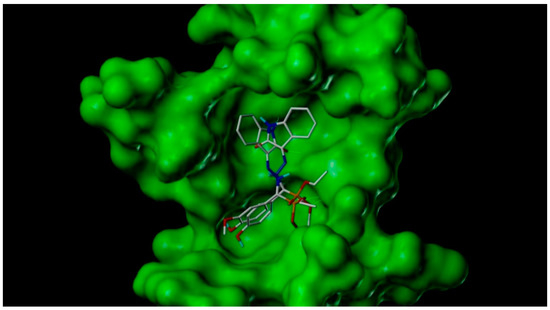
Figure 2.
3D representation of binding modes of most active forms of methylphosphonate derivatives 4f and 4g in the putative active site of TRKs enzyme.
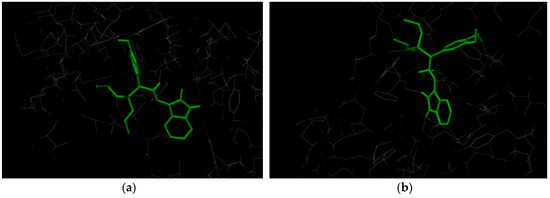
Figure 3.
3D representation of binding modes and molecular interactions of methylphosphonate derivatives (a): 4f and (b): 4g.
The molecular docking study was also carried out into the active site of tubulin (PDB ID: 1SA0). The molecular docking data against tubulin are shown in Table 4.

Table 4.
Molecular docking of the synthesized compounds 4(a–n) in tubulin receptor.
Among all the synthesized phosphonate derivatives 4l, 4f, 4m and 4n have highest potential of inhibitory activity compared the other synthesized derivatives and the standard ADR against the cancer cell lines MG-63, HT-29 and HEP-G2. The other synthesized derivatives also have shown very good inhibitory and binding interactions, indicated in the form of the GI50 value, low total docking score, polar score and high crash score data shown in Table 4.
The most active phosphonate derivatives 4l (6.1242) and 4f (5.2831) have shown efficient binding mode and penetrating active site cavity in tubulin (1AS0) by forming hydrogen bond interactions with active site residues such as LEU252, ASN258, ALA316, ALA317, LYS352, ALA354, MET259, LEU248, ALA254 and LEU255, etc., as shown in Figure 4a,b.The most active derivative 4l interacts with the active site ASN258 residue forming a hydrogen bond with phenol ring, and hydrogen atom interactions with a distance of 2.00 Å. The (N-H) hydrogen atom of the indole ring interacts with amino acid residues LYS352 and ALA317 forming hydrogen bond interactions with a distance of 2.80 and 1.86 Å respectively. The hydrophobic amino acids ALA316, ALA317, VAL 318, LEU248, LEU255 and MET259 interact with the aryl ring π-electrons and alkyl groups to form π-alkyl and π-σ interactions as shown in Figure 4a.
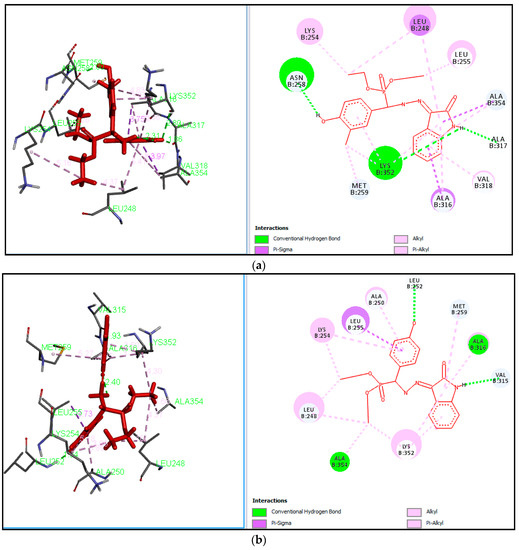
Figure 4.
(a) Binding pose and molecular interactions of 4l into the active site of tubulin (PDB ID: 1SA0); (b) Binding pose and molecular interactions of 4f into the active site of tubulin (PDB ID: 1SA0).
The second most active phosphonate derivative 4f (5.2831) formed hydrogen bond interactions between the hydroxyl group oxygens of the phenyl ring and the hydrophobic amino acid LEU252 with a distance of 1.92 Å, whereas amino acid VAL315 interacts with the indole ring (-N-H) at a distance of 1.93 Å. Hydrophobic active site amino acid residues like LEU248, MET259, ALA250, LEU295 and the charged amino acid active site residues LYS254 and LYS352 interact with the aryl ring π- electrons and alkyl groups to form π-alkyl and π-σ interactions as shown in Figure 4b.
2.4. In Silico ADMET Predictions
At the beginning of the drug discovery and development process, prediction of drug-like properties of lead compounds is an imperative task as it is key step towards the success of the lead compounds. It has been observed that most active agents that show good biological activity but fail in clinical trials it is because of their poor drug like properties. The drug-like properties have been estimated by analyzing the absorption and distribution properties. We calculated and analyzed a range of physical descriptors and pharmaceutical relevant properties for ADMET prediction by using FAF Drugs 2 [53], and the data are as shown in Table 5. All the novel synthesized compounds exhibited noteworthy values for the various parameters analyzed and exhibited good drug-like characteristics based on Lipinski’s rule of five and its variants that characterized that these agents are likely to be orally active. The data obtained for all the synthesized methyl phosphonate derivatives 4(a–n) are within the range of accepted values. None of the synthesized compounds violated the Lipinski’s rule [54,55] of five. The value of polar surface area (PSA), Log P and H/C ratio of synthesized compounds 4(a–n) indicated good oral bioavailability. The parameters, like number of rotatable bonds and number of rigid bonds are linked with the intestinal absorption. All the synthesized compounds 4(a–n) have shown good intestinal absorption. All the novel synthesized compounds 4(a–n) were established to be non-toxic in nature. In silico assessment of all the novel synthetic compounds has shown that they have very good pharmacokinetic properties which is reflected in their physicochemical values and which is ultimately contributing for pharmacological properties of these molecules. By using a FAF Drugs 2 it was predicted that the compounds exhibited good % absorption (ABS) ranging from 66.82 to 76.98%, as shown in Table 5.

Table 5.
Pharmacokinetic parameters of the synthesized compounds 4(a–n) for good oral bioavailability.
2.5. In Vivo Acute Oral Toxicity Study and Gross Behavioral Studies
Swiss albino mice treated with the newly synthesized compounds diethyl(4-chlorophenyl)(2-(2-oxoindolin-3-ylidene)hydrazinyl)methylphosphonate (4b) and (Z)-diethyl(3-ethoxy-4-hydroxy-phenyl)(2-(2-oxoindolin-3-ylidene)hydrazinyl)methylphosphonate (4h) were found to be free of any toxicity as per the acceptable range given by the OECD Guideline no. 425. No mortality was observed in mice when treated with the newly synthesized compounds 4b and 4h up to 2000 mg/kg. The results of the in vivo studies indicates that the lethal dose of the compounds 4b and 4h is above 2000 mg/kg body weight in mice, which points out that the compounds 4b and 4h can be considered to be safe and could be further developed in the coming years as potential anticancer agents. The data of the in vivo acute oral toxicity study and gross behavioral studies of the newly synthesized compounds 4b and 4h are summarized in Table 6.

Table 6.
In vivo acute oral toxicity study and gross behavioral studies of the compounds 4b and 4h.
3. Materials and Methods
3.1. General
All the chemicals used for synthesis were obtained from Merck (Darmstadt, Germany), Sigma (Mumbai, Maharashtra, India), Qualigens Fine chemicals (Mumbai, Maharashtra, India) and Himedia (Mumbai, Maharashtra, India).
3.2. Instrumentation
The FTIR spectra were obtained by means of a FTIR-4000 instrument (JASCO, Tokyo, Japan) and peaks were given in terms of wave number (cm−1). The 1H-NMR and 13C-NMR spectra of the synthesized compounds were recorded on an Avance II 400 NMR spectrometer (Bruker, Biospin AG Industriestrasse 26, CH-8117, Fallanden, Switzerland)at 400/100 MHz frequency in CDCl3 and using TMS as internal standard (chemical shift δ in ppm). The 31P-NMR spectra of compounds were recorded in CDCl3 using phosphoric acid (H3PO4) as external standard (chemical shift δ in ppm). The mass spectra were executed on a Micromass Q-Tof system (Waters, UK). Elemental analyses were done with a FLASHEA 112 analyzer (Shimadzu, Mumbai, Maharashtra, India) and all analyses were consistent (within 0.4%) with theoretical values. A Vibra Cell VCX-500 ultrasound synthesizer (Sonics, Newtown, CT, USA) equipped with a solid probe was employed for the synthesis of intermediate 1. In vitro anti-cancer activity screening of the synthesized compounds was accomplished at the Anti-Cancer Drug screening facility (ACDSF) at ACTREC (Tata Memorial Centre, Navi Mumbai, India).
3.3. Synthesis
3.3.1. Synthesis of 3-Hydrazonoindolin-2-one (1)
(A) Conventional method [56]
A mixture of indole-2,3-dione (isatin, 0.01 mol) and hydrazine hydrate (0.01 mol) in methanol (15 mL) was refluxed for 3–4 h in the presence of molecular sieves. The separated crystals were filtered, washed with a little amount of ethanol, dried and recrystallized from chloroform. The melting point of the synthesized compound 1 was found to be 284 °C, Yield 82%.
(B) Ultrasonication Method
Equimolar quantities of indole-2,3-dione (isatin, 0.01 mmol) and hydrazine hydrate (0.01 mmol) in the presence of glacial acetic acid (0.02 mmol) in absolute ethanol (5 mL) was sonicated by keeping the reaction mixture in an acoustic box containing an ultrasonic solid probe at 25–40 °C and at 25 amplitude for 15–20 min. The reaction mixture was concentrated and cooled. The obtained solid was filtered and dried. Recrystalization of the synthesized compounds was done using ethanol. Yield: 95%; melting point: 279–284 °C (uncorrected). Ultrasound method is thus seen to be better than the conventional method because it gives a better yield in 15–20 min against 3–4 h required for the conventional method. Also the amount of solvent required is less than that required for conventional method.
3.3.2. General Procedure for the Synthesis of Diethyl(substituted phenyl/heteroaryl)(2-(2-oxoindolin-3-ylidene)hydrazinyl)methylphosphonates
Equimolar quantities of 3-hydrazonoindolin-2-one (1, 1 mmol), a substituted aromatic aldehyde/heteroaldehyde 2(a–n) (1 mmol) and triethylphosphite (3, 1 mmol) were stirred at room temperature in absolute ethanol, in the presence of ceric ammonium nitrate (CAN, 0.003 mmol) as a catalyst. The TLC method was used to verify the completion of the reaction. After completion of the reaction, the reaction mixture was cooled and poured into water, filtered and the solid obtained was dried and recrystallized using ethanol. The time required for completion of reaction varies from 70 min to 89 min. The details are shown in Table 1. Our work represents a one pot Kabachnik-Fields synthesis of diethyl (substitutedphenyl/heteroaryl)(2-(2-oxoindolin-3-ylidene)hydrazinyl)-methylphosphonate derivatives from 3-hydrazonoindolin-2-one and substituted aromatic aldehyde/heteroaldehydes using CAN as a green catalyst at room temperature with better yield 84–95%.
Diethyl(phenyl)(2-(2-oxoindolin-3-ylidene)hydrazinyl)methylphosphonate (4a): Yield: 90%; M.P. 195–196 °C; 1H-NMR (CDCl3) δ: 1.2 (t, J = 7.11 Hz, 6H, 2×OCH2CH3),3.17 (d, J = 8.51 Hz, 1H, -CH), 4.70 (q, J = 7.11 Hz, 4H, 2×OCH2CH3), 7.10 (m, 9H, -CH), 8.61 (s, 1H, -NH), 10.90 (s, 1H, -NH of indole); 13C-NMR (CDCl3) δ: 16.31, 60.11, 63.32, 110.32, 119.25, 124.32, 126.25, 126.52, 128.12, 128.32, 129.22, 130.32, 162.11; 31P-NMR (CDCl3) δ: 19.90; ESI-MS: m/z calculated for C19H22N3O4P (M + H+): 388.84; found: 389.88 (M+1); IR (KBr) cm−1: 3340.31 (N-H stretching), 2960.41 (CH stretching of aromatic), 2837.21 (CH stretching of alkyl), 2300.23 (N-H stretching), 1620.33 (C=O stretching of amide), 1466.55 (CH Bending of CH2); Elemental analysis calculated for C19H22N3O4P: C, 58.91; H, 5.72; N, 10.85; P, 7.58; found; C, 58.88; H, 5.75; N, 10.87; P, 7.60.
Diethyl(4-chlorophenyl)(2-(2-oxoindolin-3-ylidene)hydrazinyl)methylphosphonate (4b): Yield 92%; M.P. 150–152 °C; 1H-NMR (CDCl3) δ: 1.20 (t, J = 7.11 Hz, 6H, 2×OCH2CH3), 2.53 (d, J = 8.11 Hz, 1H, -CH), 4.10 (q, J = 7.11 Hz, 4H, 2×OCH2CH3), 7.40 (m, 8H, -CH), 8.58 (s, 1H, -NH), 11.55 (s, 1H, -NH of indole); 13C-NMR (CDCl3) δ: 16.15, 40.17, 60.25, 78.07, 110.26, 117.96,127.79, 128.21, 128.83, 129.54, 130.19, 158.78, 164.52; 31P-NMR (CDCl3) δ: 18.84; ESI-MS: m/z calculated for C19H21ClN3O4P (M+1): 421.09; found: 422.33 (M+1); IR (KBr) cm−1: 3350.41 (N-H stretching), 2970.06 (CH stretching of aromatic), 2800.22 (CH stretching of alkyl), 2350.36 (N-H stretching), 1710.01 (C=O stretching of amide), 1454.75 (CH Bending of CH2); Elemental analysis calculated for C19H21ClN3O4P: C, 54.10; H, 5.02; N, 9.96; P, 7.34; found; C, 54.12; H, 5.04; N, 9.99; P, 7.39
Diethyl(4-flurophenyl)(2-(2-oxoindolin-3-ylidene)hydrazinyl)methylphosphonate (4c): Yield 95%; M.P. 176–180 °C; 1H-NMR (CDCl3) δ: 1.31 (t, J = 7.11 Hz, 6H, 2×OCH2CH3), 3.64 (d, J=8.45 Hz, 1H, -CH), 4.41 (q, J = 7.11 Hz, 4H, 2×OCH2CH3), 8.79 (m, 8H, -CH), 8.84 (s, 1H, -NH), 10.14 (s, 1H, -NH of indole); 13C-NMR (CDCl3) δ: 18.12, 65.21, 68.21, 123.32, 114.21, 117.14, 120.85, 127.55, 128.85, 131.36, 144.74, 146.96, 161.11, 164.85; 31P-NMR (CDCl3) δ: 18.54; ESI-MS: m/z calculated for C19H21FN3O4P (M + H+): 406.13; found: 407.20 (M + H+); IR (KBr) cm−1:3340.11 (N-H stretching), 2910.16 (CH stretching of aromatic), 2800.48 (CH stretching of alkyl), 2200.50 (N-H stretching), 1620.17 (C=O stretching of amide), 1464.47 (CH Bending of CH2); Elemental analysis calculated for C19H21FN3O4P: C, 56.30; H, 5.22; N, 10.37; P, 7.64 found; C, 56.33; H, 5.23; N, 10.40; P, 7.67
(Z)-Diethyl(4-methoxyphenyl)(2-(2-oxoindolin-3-ylidene)hydrazinyl)methylphosphonate (4d): Yield 89%; M.P. 178–179 °C; 1H-NMR (CDCl3) δ: 1.25 (t, J = 7.11 Hz, 6H, 2×OCH2CH3), 2.54 (s, 3H, O CH3), 3.42 (d, J = 8.41 Hz, 1H, -CH), 4.11 (q, J = 7.11 Hz, 4H, 2×OCH2CH3), 7.00 (m, 8H, -CH), 8.60 (s, 1H, -NH), 10.94 (s, 1H, -NH of indole); 13C-NMR (CDCl3) δ: 14.23, 55.13, 78.34, 79.88, 99.49, 110.93, 113.77, 119.25, 122.98, 126.47, 128.23, 129.85, 133.99, 144.85, 160.22, 168.98;31P-NMR (CDCl3) δ: 19.84; ESI-MS: m/z calculated for C20H24N3O5P (M + H+): 417.15; found: 418.42 (M + H+); IR (KBr) cm−1: 3350.11 (N-H stretching), 3070.76 (CH stretching of aromatic), 2800.96 (CH stretching of alkyl), 2300.11 (N-H stretching), 1610.47 (C=O stretching of amide), 1025.74 (-O- stretching); Elemental analysis calculated for C20H24N3O5P: C, 57.55; H, 5.80; N, 10.07; P, 7.42 found; C, 57.58; H, 5.82; N, 10.10; P, 7.45.
Diethyl(3,4-dimethoxyphenyl)(2-(2-oxoindolin-3-ylidene)hydrazinyl)methylphosphonate (4e): Yield 90%; M.P. 189–190 °C; 1H-NMR (CDCl3) δ: 1.21 (t, J = 7.11 Hz, 6H, 2×OCH2CH3), 2.55 (s, 6H, OCH3), 3.67 (d, J = 8.45 Hz, 1H, -CH), 4.21 (q, J = 7.11 Hz, 4H, 2×OCH2CH3), 7.37 (m, 7H, -CH), 8.42 (s, 1H, -NH), 10.03 (s, 1H, -NH of indole); 13C-NMR: (CDCl3) δ: 20.22, 58.69, 60.21, 61.12, 66.32, 111.78, 120.78, 121.36, 131.85, 132.11, 133.25, 141.74, 148.23, 150.41, 151.12, 167.47; 31P-NMR (CDCl3) δ: 18.94; ESI-MS: m/z calculated for C21H26N3O6P (M + H+): 448.16; found: 449.44 (M + H+); IR (KBr)cm−1: 3250.01 (N-H stretching), 2890.76 (CH stretching of aromatic), 2800.57 (CH stretching of alkyl), 2350.78 (N-H stretching), 1650.23 (C=O stretching of amide), 1002.44 (-O- stretching); Elemental analysis calculated for C21H26N3O6P: C, 56.37; H, 5.86; N, 9.39; P, 6.92 found; C, 56.40; H, 5.89; N, 9.41; P, 6.94.
Diethyl(4-hydroxyphenyl)(2-(2-oxoindolin-3-ylidene)hydrazinyl)methylphosphonate (4f): Yield 88%; M.P. 140–142 °C; 1H-NMR: (CDCl3) δ: 1.31 (t, J = 7.11 Hz, 6H, 2×OCH2CH3), 3.71 (d, J = 8.41 Hz, 1H, -CH), 4.54 (q, J = 7.11 Hz, 4H, 2×OCH2CH3), 5.61 (s, 1H, OH), 7.20 (m, 8H, -CH), 8.53 (s, 1H, -NH), 10.44 (s, 1H, -NH of indole); 13C-NMR (, CDCl3) δ: 17.26, 61.25, 69.74, 115.47,116.23, 117.63, 123.52, 129.85, 130.47, 134.52, 136.12, 145.32, 156.54, 164.41; 31P-NMR (CDCl3) δ:19.64; ESI-MS: m/z calculated for C19H22N3O5P (M + H+): 404.13; found: 405.37 (M + H+); IR (KBr) cm−1: 3600.10 (aromatic OH), 3440.44 (N-H stretching), 2980.88 (CH stretching of aromatic), 2800.01 (CH stretching of alkyl), 2280.21 (N-H stretching), 1710.22 (C=O stretching of amide); Elemental analysis calculated for C19H22N3O5P: C, 56.57; H, 5.50; N, 10.42; P, 7.68 found; C, 56.60; H, 5.54; N, 10.44; P, 7.70.
Diethyl(4-hydroxy-3-methoxyphenyl)(2-(2-oxoindolin-3-ylidene)hydrazinyl)methylphosphonates (4g): Yield: 94%; M.P. 112–114 °C; 1H-NMR (CDCl3) δ: 1.37 (t, J = 7.11 Hz, 6H, 2×OCH2CH3), 2.58 (s, 3H, OCH3), 3.36 (d, J = 8.41 Hz, 1H, CH), 4.14 (q, J = 7.11 Hz, 4H, 2×OCH2CH3), 6.84 (s, 1H, OH), 7.73 (m, 7H, CH), 9.77 (s, 1H, -NH), 10.55 (s, 1H, -NH of indole); 13C-NMR (CDCl3) δ: 16.05, 40.17, 55.40, 62.57, 110.36, 116.74, 120.48, 125.29, 132.66,134.25, 136.24, 138.59, 138.90, 144.46, 150.94, 190.22; 31P-NMR (CDCl3) δ: 19.94; ESI-MS: m/z calculated for C20H24N3O6P (M + H+): 434.14; found: 435.39 (M + H+); IR (KBr) cm−1:3610.45 (aromatic OH), 3450.64 (N-H stretching), 2996.74 (CH stretching of aromatic), 2830.74 (CH stretching of alkyl), 2310.21 (N-H stretching), 1680.12 (C=O stretching of amide), 1030.14 (-O-stretching); Elemental analysis calculated for C20H24N3O6P: C, 55.43; H, 5.51; N, 9.70; found; C, 55.49; H, 5.60; N, 9.75.
(Z)-Diethyl(3-ethoxy-4-hydroxyphenyl)(2-(2-oxoindolin-3-ylidene)hydrazinyl)methylphosphonate (4h): Yield 92%; M.P. 160–162 °C; 1H-NMR (CDCl3) δ: 1.40 (t, J = 7.11 Hz, 9H, 3×OCH2CH3), 3.90 (d, J = 8.41 Hz, 1H, CH), 4.51 (q, J = 7.11 Hz, 6H, 3×OCH2CH3), 5.42 (s, 1H, OH), 7.71 (m, 7H, CH), 8.8 (s, 1H, -NH),10.72 (s, 1H, -NH of indole); 13C-NMR (CDCl3) δ: 16.21, 16.52,18.21, 61.52, 64.85, 68.74, 117.12, 116.41, 119.32, 121.92, 122.74, 130.96, 133.12, 136.24, 141.23, 142.74, 167.74; 31P-NMR (CDCl3) δ: 18.65; ESI-MS: m/z calculated for C21H26N3O6P (M + H+): 448.16; found: 449.40 (M + H+); IR (KBr) cm−1: 3550.50 (-OH),3420.32 (N-H stretching), 2999.45 (CH stretching of aromatic), 2813.87 (CH stretching of alkyl), 2350.52 (N-H stretching), 1710.72 (C=O stretching of amide), 1020.42 (-O- stretching); Elemental analysis calculated for C21H26N3O6P: C, 56.37; H, 5.86; N, 9.39; P, 6.92 found; C, 56.40; H, 5.88; N, 9.41; P, 6.94.
(Z)-Diethyl(2-(2-oxoindolin-3-ylidene)hydrazinyl)(thiophen-2-yl)methylphosphonate (4i): Yield 87%; M.P. 179–182 °C; 1H-NMR (CDCl3) δ: 1.01 (t, J = 7.11 Hz, 6H, 2×OCH2CH3), 3.64 (d, J = 8.44 Hz, 1H, CH), 3.82 (s, 1H, CH), 4.51 (q, J = 7.11 Hz, 4H, 2×OCH2CH3), 7.81 (m, 6H, CH), 8.57 (s, 1H, -NH), 10.77 (s, 1H, -NH of indole); 13C-NMR: (CDCl3) δ: 17.12, 63.52, 69.96, 119.12, 120.35, 124.18, 128.47, 129.96, 130.18, 150.52, 152.74, 155.54, 165.65, 170.65,174.96; 31P-NMR (CDCl3) δ: 18.45; ESI-MS: m/z calculated for C17H20N3O4PS (M + H+): 394.09; found: 395.48 (M + H+);IR (KBr) cm−1: 3520.72 (N-H stretching), 2912.88 (CH stretching of aromatic), 2800.32 (CH stretching of alkyl), 2340.54 (N-H stretching), 1710.72 (C=O stretching of amide); Elemental analysis calculated for C17H20N3O4PS: C, 51.90; H, 5.12; N, 10.68; P, 7.87; S, 8.15 found; C, 51.91; H, 5.14; N, 10.70; P, 7.89; S, 8.17.
(Z)-Diethylfuran-2-yl(2-(2-oxoindolin-3-ylidene)hydrazinyl)methylphosphonate (4j): Yield 84%; M.P. 176–178 °C; 1H-NMR (CDCl3) δ: 1.20 (t, J=7.11 Hz, 6H, 2×OCH2CH3), 3.81 (s, 1H, CH), 3.42 (d, J = 8.44 Hz, 1H, CH), 4.62 (q, J = 7.11 Hz, 4H, 2×OCH2CH3), 7.98 (m, 6H, CH), 8.44 (s, 1H, -NH), 10.45 (s, 1H, -NH of indole); 13C-NMR (CDCl3) δ: 22.21, 50.11, 65.52, 112.31, 119.95, 121.36, 128.63, 130.36, 131.21, 133.65, 147.33, 151.25, 153.23, 155.39, 164.21; 31P-NMR (CDCl3) δ: 18.56; ESI-MS: m/z calculated for C17H20N3O5P (M + H+): 378.12; found: 379.33 (M + H+);IR (KBr)cm−1: 3280.32 (NH stretching), 2945.46 (CH aromatic), 2850.63 (CH stretching of alkyl), 2440.85 (C=N stretching), 1710.33 (C=O stretching), 1070.10 (-O- stretching); Elemental analysis calculated for C17H20N3O5P: C, 54.11; H, 5.34; N, 11.14; P, 8.21 found; C, 54.14; H, 5.36; N, 11.16; P, 8.24.
(Z)-Diethyl (2-hydroxyphenyl)(2-(2-oxoindolin-3-ylidene)hydrazinyl)methylphosphonate (4k): Yield 88%; M.P.152–154 °C; 1H-NMR (DMSO-d6) δ: 1.29 (t, J = 7.11 Hz, 6H, 2×OCH2CH3), 4.07 (q, J = 7.12 Hz, 4H, 2×OCH2CH3),5. 35 (s, 1H, OH), 6.80–8.08 (m, 9H, aromatic), 8.0 (s, 1H, -NH), 10.46 (s, 1H, -NH of indole); 13C-NMR: (DMSO-d6) δ ppm: 16.31, 61.33,62.24, 115.41, 117.45, 119.05, 121.33, 121.56, 124.42, 128.46, 128.73, 129.11, 131.91, 133.22, 134.59, 141.44, 155.81, 168.44; 31P-NMR (CDCl3) δ: 19.56; ESI-MS: m/z calculated for C19H22N3O5P (M + H+): 403.13; found: 404.37 (M + H+); IR (KBr) cm−1: NH 3313.45 (N-H stretching), 3033.42 (C-H stretching of aromatic), 2653.64 (C-H stretching of alkyl), 2133.63 (C=N Stretching), 1723.05 (C-O stretching), 1690.80 (C=O stretching), 1554.65 (C-N Stretching), 1034.65 (O- stretching),Elemental Analysis calculated for C19H22N3O5P: C, 56.57; H, 5.50; N, 10.42; P, 7.68 found; C, 56.59; H, 5.52; N, 10.44; P, 7.69.
(Z)-Diethyl (4-hydroxy-3-methylphenyl)(2-(2-oxoindolin-3-ylidene)hydrazinyl)methylphosphonate (4l): Yield 75%; M.P.190–192 °C; 1H-NMR (DMSO-d6) δ: 1.29 (t, J = 7.11 Hz, 6H, 2×OCH2CH3), 2.15 (s, 1H, CH3), 4.07.(q, J = 7.12 Hz, 4H, 2×OCH2CH3), 5. 35 (s, 1H, OH), 6.80–8.08 (m, 9H, aromatic), 8.0 (s, 1H, -NH), 10.45 (s, 1H, -NH of indole); 13C-NMR: (DMSO-d6) δ ppm: 15.32, 16.33, 62.22, 67.72, 115.4, 118.44, 119.93, 124.49, 124.88, 125.94, 128.32, 129.81, 130.59, 131.72, 133.49, 141.45, 152.21, 168.41;31P-NMR (CDCl3) δ: 19.56; ESI-MS: m/z calculated for C20H24N3O5P (M + H+): 417.15; found: 417.40; IR (KBr) cm−1: NH 3303.34 (N-H stretching), 3013.32 (C-H stretching of aromatic), 2653.64 (C-H stretching of alkyl), 2133.63 (C=N Stretching), 1723.05 (C-O stretching), 1690.80 (C=O stretching), 1554.65 (C-N Stretching), 1034.65 (O- stretching),Elemental Analysis calculated for C20H24N3O5P: C, 57.55; H, 5.80; N, 10.07; P, 7.42 found; C, 57.59; H, 5.82; N, 10.09; P, 7.43.
(Z)-Diethyl (4-nitrophenyl)(2-(2-oxoindolin-3-ylidene)hydrazinyl)methylphosphonate (4m): Yield 90%; M.P.172–174 °C; 1H-NMR (400 MHz, DMSO-d6) δ: 1.29 (t, J = 7.11 Hz, 6H, 2×OCH2CH3), 4.07 (q, J = 7.11 Hz, 4H, 2×OCH2CH3), 5. 35 (s, 1H, OH), 6.80–8.08 (m, 9H, aromatic), 8.0 (s, 1H, -NH), 10.45 (s, 1H, -NH of indole);13C-NMR: (DMSO-d6) δ ppm: 16.37, 62.24, 68.88, 117.76, 119,82, 123.37, 124.43, 127.94, 129.53, 131.22, 134.54, 141.14, 142.39, 145.45, 168.76; 31P-NMR (,CDCl3) δ: 19.56; ESI-MS: m/z calculated for C19H21N4O6P: (M + H+): 432.12, found: 433.37; IR (KBr) cm−1: NH 3303.34 (N-H stretching), 3013.32 (C-H stretching of aromatic), 2653.64 (C-H stretching of alkyl), 2133.63 (C=N Stretching), 1723.05 (C-O stretching), 1690.80 (C=O stretching), 1554.65 (C-N Stretching), 1034.65 (O- stretching); Elemental Analysis calculated for C19H21N4O6P: C, 52.78; H, 4.90; N, 12.96; P, 7.16 found; C, 52.79; H, 4.93; N, 12.98; P, 7.17.
(Z)-Diethyl (4-methylthiazole-5-yl)(2-(2-oxoindolin-3-ylidene)hydrazinyl)methylphosphonate(4n): Yield 70%; M.P. 188–190 °C; 1H-NMR (DMSO-d6) δ: 1.29 (t, J = 7.11 Hz, 6H, 2×OCH2CH3), 2.46 (s, 1H, CH3), 4.07.(q, J = 7.12 Hz, 4H, 2×OCH2CH3), 5. 35 (s, 1H, OH), 6.80–8.08 (m, 9H, aromatic), 8.0 (s, 1H, -NH), 10.45 (s, 1H, -NH of indole); 13C-NMR: (DMSO-d6) δ ppm: 14.73, 16.39, 62.28, 65.77, 117.78, 121.3, 124.48, 129.47, 130.13, 131.1 (C), 133,34, 141.22, 148.77, 151.34, 168.79; 31P-NMR (,CDCl3) δ: 19.54; ESI-MS: m/z calculated for C17H21N4O4PS: (M + H+): 408.10, found: 408.41; IR (KBr) cm−1: NH 3303.34 (N-H stretching), 3013.32 (C-H stretching of aromatic), 2653.64 (C-H stretching of alkyl), 2133.63 (C=N Stretching), 1723.05 (C-O stretching), 1690.80 (C=O stretching), 1554.65 (C-N Stretching), 1034.65 (O-stretching),2723.65 (COOH); Elemental Analysis calculated for C17H21N4O4PS: C, 47.57; H, 5.10; N, 12.33; P, 6.82 found; C, 47.59; H, 5.13; N, 12.38; P, 6.80.
3.4. In Vitro Anticancer Activity
All the newly synthesized compounds were screened for their in vitro anticancer activity against six cancer cell lines: SK-MEL-2, MCF-7, IMR-32, MG-63, HT-29 and Hep-G2 by SRB assay, using adriamycin as a standard drug [57]. All the synthesized derivatives 4(a–n) were also tested for their cytotoxic effect on normal cell lines i.e., NIH/3T3 (murine embryonic fibroblast by the SRB assay method. The cell lines were grown in RPMI 1640 medium containing 10% fetal bovine serum and 2 mM l-glutamine. For the present screening experiments, cells were inoculated into 96 well microtiter plates in 90 µL at 5000 cells per well. After cell inoculation, the microtiter plates were incubated at 37 °C, 5% CO2, 95% air and 100% relative humidity for 24 h prior to addition of experimental drugs. Experimental drugs were solubilized in appropriate solvent to prepare stock of 10−2 concentration. At the time of experiment four 10-fold serial dilutions were made using complete medium. Aliquots of 10 µL of these different drug dilutions were added to the appropriate micro-titer wells already containing 90 µL of medium, resulting in the required final drug concentrations. After compound addition, plates were incubated at standard conditions for 48 h. and assay was terminated by the addition of cold trichloroacetic acid (TCA). Cells were fixed in situ by the gentle addition of 50 µL of cold 30% (w/v) TCA (final concentration, 10% TCA) and incubated for 60 min at 4 °C. The supernatant was discarded; the plates were washed five times with tap water and air dried.
Sulforhodamine B (SRB) solution (50 µL) at 0.4% (w/v) in 1% acetic acid was added to each of the wells, and plates were incubated for 20 min at room temperature. After staining, unbound dye was recovered and the residual dye was removed by washing five times with 1% acetic acid. The plates were air dried. Bound stain was subsequently eluted with 10 mM trizma base, and the absorbance was read on an ELISA plate reader at a wavelength of 540 nm with 690 nm reference wavelength. All the tests were repeated in at least three independent experiments at the concentrations of 10, 20, 40 and 80 µg/mL [57].
3.5. Docking Study
The computational study i.e., molecular docking study, was started by sketching the2D form of the structures of all synthesized compounds using the sketch modules of SYBYL-X 2.1.1. 2D [58] formss of the compounds then subjected to the ligand library preparation module by keeping the preparation protocol as surface for searching where it generates a single lowest strain energy tautomer/stereoisomer and all necessary structural properties were added and finally a 3D prepared conformation of each compounds was stored in SYBYL-Mol2 file format. To perform molecular docking a three dimensional X-ray crystal structure of tubulin (PDB ID: 1SA0 Resolution 3.58 Å) [59] complex with colchicine was used. Many TRK inhibitors (TRKs) have been produced and tested in the clinic by now. The crystal structures of c-kit receptor protein-tyrosine kinase in complex with STI-571 (imatinib or Gleevec) were picked from the Protein Data Bank (PDB) (http://www.rcsb.org/pdb/explore/explore.dostructureId=1T46) (PDB code: 1t46). The synthesized compounds 4(a–n) were subjected to a molecular docking study performed with the Surflex-Dock module of the Sybyl2.1.1 package following standard procedures for understanding the binding interactions with human TRKs1 enzyme (PDB ID: 1t46). All the synthesized compounds showed very good binding interactions in the active site of the selected receptors. The most active compounds from the synthesized series which have exhibited very high docking scores value against selected receptors and had a good binding affinity predicated by non-covalent interactions such as hydrogen bond interaction, VDW interaction, carbon, hydrogen bond interaction, π-Anion interaction, π-π shaped interaction, alkyl interaction, π-σ and π-alkyl interactions. To represent the details of docking score the following terms is used as total score: crash score: as the degree of inappropriate penetration by the ligand into the protein and of interpenetration between ligand atoms that are separated by rotatable bonds of compounds and polar-score which gives an idea about the contribution of the polar non-hydrogen bonding interactions to the total score, as shown in Table 3 and Table 4.
3.6. In-Silico Bioavailability Predictions
The bioavailability properties were predicted and it was seen that the compounds displayed an admirable % ABS (66.82–76.98%, shown in Table 5). Absorption (% ABS) was calculated by: % ABS = 109−(0.345 X TPSA). In the current research study, molecular volume (MV), molecular weight (MW), logarithm of partition coefficient (miLog P), number of hydrogen bond acceptors (n-ON), number of hydrogen bonds donors (n-OHNH), topological polar surface area (TPSA), number of rotatable bonds (n-ROTB), number of rigid bonds (Rig Bond), Rings, ratio H/C and Lipinski’s rule of five were calculated using FAF Drugs 2. None of the synthesized compounds violated the Lipinski’s rule of five or its variants. All the synthesized compounds 4(a–n) were found to be non-toxic, as predicted by using FAF Drugs 2.
3.7. In Vivo Acute Oral Toxicity Study and Gross Behavioral Studies
The in vivo acute oral toxicity study for the newly synthesized scaffolds 4b and 4h was performed according to the OECD Guideline no. 425 [60] using Swiss albino mice (18–22 gm weight) quarantined at the animal house at Y.B. Chavan College of Pharmacy, Aurangabad IAEC approval number CPCSEA/IAEC/P’col-52/2015-16/115. Each group consisted of six mice (overnight fasted) and kept in a colony cage at 25 ± 2 °C with 55% relative humidity and 12 h of light and dark cycle. A specified dose of 100, 250, 500, 750, 1000, 1500 and 2000 mg/kg body weight of mice was administered orally as a single dose. The acute toxic symptoms and the behavioral changes produced by the test compounds were observed continuously for 4 h periods at 8th, 12th and 24th h. Onset of toxic symptoms and the gross behavioral changes were also recorded. These animals were maintained for further 10 days with observations made daily. In case the animal appeared moribund (dying) the animal was sacrificed in a humane way and it is considered to have died because of toxicity.
4. Conclusions
Novel fourteen diethyl (substituted phenyl/heteroaryl)(2-(2-oxoindolin-3ylidene)hydrazinyl)-methylphosphonate derivatives 4(a–n)were synthesized by a one pot reaction using CAN as a green catalyst. The compounds were characterized by TLC, IR, NMR, mass spectrometry and elemental analysis. The in-vitro anticancer activity was evaluated against six human cancer cell lines such as MCF-7, IMR-32, SK-MEL-2, MG-63, HT-29 and Hep-G2by the SRB assay method. Adriamycin was used as a positive control. All the synthesized derivatives have shown excellent in vitro anticancer activity against MG-63 cancer cell lines, equipotent to that of the standard drug adriamycin. The compound 4l with a 4-hydroxy3-methyl group on the phenyl ring was also found to be equipotent to the standard drug adriamycin against the HT-29 and Hep-G2 cancer cell lines. The synthesized compounds 4(a–n) were found to be selective towards cancer cells since they did not exhibit cytotoxicity on normal tissue cells even at GI50 > 250 μM. The treatment of selected cancer cell lines with the synthesized compounds showed apoptotis and morphological changes like cell shrinkage, cell wall deformation and reduced number of viable cells. A computational study i.e., a molecular docking study of the synthesized compounds 4(a–n), was carried out to know the binding interactions of the synthesized derivatives with the tyrosine kinase receptor and microtubules. From the results of the molecular docking study, it was observed that the methyl phosphonate derivatives have dual inhibition potential, inhibiting human TRKS and microtubules.
The synthesized compounds can act as dual inhibitors, if developed as drug molecules in the future. They can serve as good anticancer agents against cancers for which resistance has developed. The synthesized compounds 4(a–n) showed no cytotoxicity towards normal tissue cells. It’s very vital for cancer treatment that the anticancer drugs have the property of high efficiency and low toxicity. The synthesized compounds 4(a–n) were identified to be selective towards cancer cells in view of the fact that they did not display cytotoxicity even at GI50 > 250 μM on normal tissue cells. In silico bioavailability studies indicated that compounds have a good in silico % absorption (66.82% to 76.98%). All the synthesized compounds 4(a–n) were identified to be non-toxic in nature. All the above information suggests that the novel compounds of the current series can serve as a lead scaffold in the design, development and synthesis of new anticancer agents, especially for bone, liver and melanoma types of cancers.
Supplementary Materials
The following are available online. Figures S1–S7.
Author Contributions
A.P.G.N. research guide; R.I.G. and N.S.S. performed the experiments; S.V.T. performed animal studies and performed the experiments; J.N.S. and M.D.D. performed molecular docking study, J.A.S.V. spectral analysis.
Funding
This research received no external funding.
Acknowledgments
The authors are thankful to Fatma Rafiq Zakaria, Chairman of Maulana Azad Educational Trust and Zahid Zaheer, Principal of Y. B. Chavan College of Pharmacy, for providing the laboratory facility. The authors are grateful to Jyoti Kode for cooperation in performing in vitro testing at Anti-Cancer Drug screening facility (ACDSF) at ACTREC, Tata Memorial Centre, Navi Mumbai.
Conflicts of Interest
The authors declare no conflict of interest.
References and Notes
- Hanahan, D.; Folkman, J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996, 86, 353–364. [Google Scholar] [CrossRef]
- Zhang, X.; Raghavan, S.; Ihnat, M.; Hamel, E.; Zammiello, C.; Bastian, A.; Mooberry, S.L.; Gangjee, A. The design, synthesis and biological evaluation of conformationally restricted 4-substituted-2,6-dimethylfuro[2,3-d]pyrimidines as multi-targeted receptor tyrosine kinase and microtubule inhibitors as potential antitumor agents. Bioorg. Med. Chem. 2015, 23, 2408–2423. [Google Scholar] [CrossRef] [PubMed]
- Kerbel, R.; Folkman, J. Clinical translation of angiogenesis inhibitors. Nat. Rev. Cancer 2002, 2, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K.; Carmeliet, P. SnapShot: Tumor angiogenesis. Cell 2012, 149, 1408–1411. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Waxman, D.J. Combination of antiangiogenesis with chemotherapy for more effective cancer treatment. Mol. Cancer Ther. 2008, 7, 3670–3684. [Google Scholar] [CrossRef] [PubMed]
- Dumontet, C.; Jordan, M.A. Microtubule-binding agents: A dynamic field of cancer therapeutics. Nat. Rev. Drug Discov. 2010, 9, 790–803. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.; Gourley, C.; McNeish, I.; Ledermann, J.; Gore, M.; Jayson, G.; Perren, T.; Rustin, G.; Kaye, S. Targeted anti-vascular therapies for ovarian cancer: Current evidence. Br. J. Cancer 2013, 108, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Holohan, C.; Van Schaeybroeck, S.; Longley, D.B.; Johnston, P.G. Cancer drug resistance: An evolving paradigm. Nat. Rev. Cancer 2013, 13, 714–726. [Google Scholar] [CrossRef] [PubMed]
- Glaxo, S.K.; Combination of Lapatinib with Carboplatin, Paclitaxel and Trastuzumab in Metastatic Breast Cancer. In ClinicalTrials.gov [Internet]. In Bethesda (MD): National Library of Medicine (US). Available online: http://clinicaltrials.gov/show/NCT00367471 NLM Identifier: NCT00367471 (accessed on 6 March 2015).
- Twenty Eight Clinical Trials Were Found on Clinicaltrials.gov (accessed in March 2015) Site in Which RTK Inhibitors Were Being Used in Combination with Antitubulins and Other Chemotherapeutic Agents. Of These, 16 Trials Are Currently in Progress and the Identification Numbers in Clinical Trials.Gov Are Provided Below: NCT01855750; NCT01804530; NCT01606878; NCT01746277; NCT02326285; NCT01620190; NCT01974440; NCT01683994; NCT02378389; NCT01939054; NCT01719302; NCT02191059; NCT02191059; NCT01876082; NCT00567554; NCT00367471NCT02191059; NCT02191059; NCT01876082; NCT00567554; NCT00367471. Available online: https://clinicaltrials.gov/ct2/results?term=receptor+tyrosine+kinase+AND+tubulin&Search=Search (accessed on 3 June 2015).
- Vermont, U.O. Docetaxel, Gemcitabine and Pazopanib as Treatment for Soft Tissue Sarcoma. In ClinicalTrials.gov [Internet]; National Library of Medicine (US): Bethesda, MD, USA, 2015. Available online: http://clinicaltrials.gov/show/NCT01719302 NLM Identifier: NCT01719302 (accessed on 6 March 2015).
- Gangjee, A.; Zaware, N.; Raghavan, S.; Ihnat, M.; Shenoy, S.; Kisliuk, R.L. Single agents with designed combination chemotherapy potential: Synthesis and evaluation of substituted pyrimido[4,5-b]indoles as receptor tyrosine kinase and thymidylate synthase inhibitors and as antitumor agents. J. Med. Chem. 2010, 53, 1563–1578. [Google Scholar] [CrossRef] [PubMed]
- Nikalje, A.G.; Tiwari, S.V.; Sangshetti, J.N.; Damale, M.D. Ultrasound Mediated Synthesis, Biological Evaluation, Docking Study and in vivo acute oral toxicity study of Novel Indolin-2-one Coupled Pyrimidine Derivatives. Res. Chem. Intermed. 2018, 44, 3031–3059. [Google Scholar] [CrossRef]
- Rathi, A.K.; Syed, R.V.; Singh, H.; Shin, H.S.; Patel, R.V. Kinase Inhibitor Indole Derivatives as Anticancer Agents. A Patent Review. Recent Pat. Anticancer Drug Discov. 2016, 12, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Tyrosine-Kinase Inhib. Available online: https://en.wikipedia.org/wiki/Tyrosine-kinase_inhib (accessed on 7 February 2018).
- Li, C.; Song, B.; Yan, K.; Xu, G.; Hu, D.; Yang, S.; Jin, L.; Xue, W.; Lu, P. One Pot Synthesis of α-Aminophosphonates Containing Bromo and 3,4,5-Trimethoxybenzyl Groups under Solvent-free Conditions. Molecules 2007, 12, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Prasad, G.S.; Krishna, J.R.; Manjunath, M.; Reddy, O.V.S.; Krishnaiah, M.; Reddy, C.S.; Puranikd, V.G. Synthesis, NMR, X-ray crystallography and bioactivity of some α-aminophosphonates. Arkivoc 2007, 13, 133–141. [Google Scholar]
- Rao, X.; Song, Z.; He, L. Synthesis and antitumor activity of novel α-aminophosphonates from di-terpenicdehydroabietylamine. Heteroat. Chem. 2008, 19, 512–516. [Google Scholar] [CrossRef]
- Naydenova, E.D.; Todorov, P.T.; Mateeva, P.I.; Zamfirova, R.N.; Pavlov, N.D.; Todorov, S.B. Synthesis and biological activity of novel small peptides with aminophosphonates moiety as NOP receptor ligands. Amino Acids 2010, 39, 1537–1543. [Google Scholar] [CrossRef] [PubMed]
- Tusek-bozic, L.; Juribasic, M.; Traldi, P.; Scarcia, V.; Furlani, A. Synthesis, characterization and antitumor activity of palladium(II) complexes of monoethyl 8-quinolylmethylphosphonate. Polyhedron 2008, 27, 1317–1328. [Google Scholar] [CrossRef]
- Wang, B.; Miao, Z.W.; Wang, J.; Chen, R.Y.; Zhang, X.D. Synthesis and biological evaluation of novel naphthoquinone fused cyclic aminoalkylphosphonates and aminoalkylphosphonic monoester. Amino Acids 2008, 35, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, Z.; Firouzabadi, H.; Iranpoor, N.; Ghaderi, A.; Jafari, M.R.; Jafari, A.A.; Zare, H.R. Design and one-pot synthesis of alpha-aminophosphonates and bis(alpha-aminophosphonates) by iron(III) chloride and cytotoxic activity. See comment in PubMed Commons below. Eur. J. Med. Chem. 2009, 44, 4266–4275. [Google Scholar] [CrossRef] [PubMed]
- Onita, N.; Sisu, I.; Penescu, M.; Purcarea, V.L.; Kurunczi, L. Synthesis, characterization and biological activity of some α-aminophosphonates. Farmacia 2010, 58, 531–545. [Google Scholar]
- Zhang, X.; Qu, Y.; Fan, X.; Bores, C.; Feng, D.; Andrei, G.; Snoeck, R.; De Clercq, E.; Loiseau, P.M. Solvent-free synthesis of pyrimidine nucleoside-aminophosphonate hybrids and their biological activity evaluation. Nucleosides Nucleotides Nucleic Acids 2010, 29, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, S.; Li, X.; Fan, H.; Bhadury, P.; Xu, W.; Wu, J.; Wang, Z. Synthesis and antiviral bioactivity of chiral thioureas containing leucine and phosphonate moieties. Molecules 2010, 15, 5112–5123. [Google Scholar] [CrossRef] [PubMed]
- Biswal, S.; Sahoo, U.; Sethy, S.; Kumar, H.K.S.; Banerjee, M. Indole: The Molecule of Diverse Biological Activities. Asian J. Pharm. Clin. Res. 2012, 5, 1–6. [Google Scholar]
- Vine, K.L.; Matesic, L.; Locke, J.M.; Skropeta, D. Recent Highlights in the Development of Isatin-Based Anticancer Agents. In Advances in Anticancer Agents in Medicinal; Bentham Science Publishers: Sharjah, UAE, 2013; Volume 59, pp. 254–312. [Google Scholar]
- Prakash, R.C.; Raja, S. Indolinones as Promising Scaffold as Kinase Inhibitors: A Review. Mini Rev. Med. Chem. 2012, 12, 98–119. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, H.R.; Pireddu, R.; Chen, L.; Luo, Y.; Sung, S.S.; Szymanski, A.M.; Yip, M.L.R.; Guida, W.C.; Sebti, S.M.; Wu, J.; et al. Inhibitors of Src homology-2 domain containing protein tyrosine phosphatase-2 (Shp2) based on oxindole scaffolds. J. Med. Chem. 2008, 51, 4948–4956. [Google Scholar] [CrossRef] [PubMed]
- Hui, K.G.; Bostjan, S.; Knox, J.J. Sunitinib in solid tumors. Expert Opin. Investig. Drugs 2009, 18, 821–834. [Google Scholar]
- Yancey, M.F.; Merritt, D.A.; White, J.A.; Marsh, S.A.; Locuson, C.W. Distribution, metabolism, and excretion of toceranib phosphate (Palladia™, SU11654), a novel tyrosine kinase inhibitor, in dogs. J. Vet. Pharmacol. Ther. 2010, 33, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.V.; Seijas, J.A.; Vazquez-Tato, M.P.; Sarkate, A.P.; Karnik, K.S.; Nikalje, A.P.G. Facile synthesis of novel coumarin derivatives, antimicrobial analysis, enzyme assay, docking study, ADMET prediction and toxicity study. Molecules 2017, 22, 1172. [Google Scholar] [CrossRef] [PubMed]
- Nikalje, A.P.G.; Tiwar, S.V.; Tupe, J.G.; Vyas, K.V.; Qureshi, G. Ultrasound Assisted-synthesis and Biological Evaluation of Piperazinylprop- 1-en-2-yloxy-2H-chromen-2-ones as Cytotoxic Agents. Lett. Drug Des. Discov. 2017, 14, 1195–1205. [Google Scholar] [CrossRef]
- Shailee, V.T.; Siddiqui, S.; Seijas, J.A.; Vazquez-Tato, M.P.; Sarkate, A.P.; Lokwani, D.K.; Nikalje, A.P.G. Microwave-Assisted Facile Synthesis, Anticancer Evaluation and Docking Study of N-((5-(Substituted methylene amino)-1,3,4-thiadiazol-2-yl)methyl) Benzamide Derivatives. Molecules 2017, 22, 995. [Google Scholar] [CrossRef]
- Nimbalkar, U.D.; Seijas, J.A.; Vazquez-Tato, M.P.; Damale, M.G.; Sangshetti, J.N.; Nikalje, A.P.G. Ionic Liquid-Catalyzed Green Protocol for Multi-Component Synthesis of Dihydropyrano[2,3-c]pyrazoles as Potential Anticancer Scaffolds. Molecules 2017, 22, 1628. [Google Scholar] [CrossRef] [PubMed]
- Matveeva, E.D.; Zefirov, N.S. Catalytic Kabachnik-Fields reaction: New horizons for old reaction. Arkivoc 2008, 1, 1–17. [Google Scholar]
- Sayed, I.E.; Kosy, S.M.E.; Magied, M.F.A.; Hamed, M.A.; Gokha, A.A.A.; Sattar, M.A. One-pot Synthesis of Novel α-Aminophosphonate Derivatives Containing a Pyrazole Moiety. J. Am. Sci. 2011, 7, 604–608. [Google Scholar]
- Mandhane, P.G.; Joshi, R.S.; Nagargoje, D.R.; Gill, C.H. Thiamine hydrochloride (VB1): An efficient catalyst for one-pot synthesis of α-aminophosphonates under ultrasonic irradiation. Chin. Chem. Lett. 2011, 22, 563–566. [Google Scholar] [CrossRef]
- Huang, X.C.; Wang, M.; Pan, Y.M.; Tian, X.Y.; Wang, H.S.; Zhang, Y. Synthesis and antitumor activities of novel α-aminophosphonatesdehydroabietic acid derivatives. Bioorg. Med. Chem. Lett. 2013, 23, 5283–5289. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, S.; Chakraborti, A.K. Zirconium(IV) Compounds As Efficient Catalysts for Synthesis of α-Aminophosphonates. J. Org. Chem. 2008, 73, 6029–6032. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Luo, Y.; Wu, J.; Shen, Q.; Chen, H. Facile one-pot synthesis of α-aminophosphonates using lanthanide chloride as catalys. Heteroat. Chem. 2006, 17, 389–392. [Google Scholar] [CrossRef]
- Qian, C.; Huang, T.J. One-Pot Synthesis of α-Amino Phosphonates from Aldehydes Using Lanthanide Triflate as a Catalyst. J. Org. Chem. 1998, 63, 4125–4128. [Google Scholar] [CrossRef]
- Wu, J.; Sun, W.; Xia, H.G.; Sun, X. A facile and highly efficient route to α-amino phosphonates via three-component reactions catalyzed by Mg(ClO4)2 or molecular iodine. Org. Biomol. Chem. 2006, 4, 1663–1666. [Google Scholar] [CrossRef] [PubMed]
- Azizi, N.; Rajabi, F.; Saidi, M.R. A mild and highly efficient protocol for the one-pot synthesis of primary α-amino phosphonates under solvent-free conditions. Tetrahedron Lett. 2004, 45, 9233–9235. [Google Scholar] [CrossRef]
- Sangshetti, J.N.; Kokare, N.D.; Kotharkar, S.A.; Shinde, D.B. Ceric ammonium nitrate catalysed three component one-pot efficient synthesis of 2,4,5-triaryl-1Himidazoles. J. Chem. Sci. 2008, 120, 463–467. [Google Scholar] [CrossRef]
- Lin, C.M.; Ho, H.H.; Pettit, G.R.; Hamel, E. Antimitotic natural products combretastatin A-4 and combretastatin A-2: Studies on the mechanism of their inhibition of the binding of colchicine to tubulin. Biochemistry 1989, 28, 6984–6991. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.A.; Gilmartin, M.E.; Hall, J.L.; Cowan, N.J. Three expressed sequences within the human beta tubulin multigene family each define a distinct isotype. J. Mol. Biol. 1985, 182, 11–20. [Google Scholar] [CrossRef]
- Nicoletti, M.I.; Valoti, G.; Giannakakou, P.; Zhan, Z.; Kim, J.H.; Lucchini, V.; Landoni, F.; Mayo, J.G.; Giavazzi, R.; Fojo, T. Expression of beta-tubulin isotypes in human ovarian carcinoma xenografts and in a sub-panel of human cancer cell lines from the NCI-Anticancer Drug Screen: Correlation with sensitivity to microtubule active agents. Clin. Cancer Res. 2001, 7, 2912–2922. [Google Scholar] [PubMed]
- McKean, P.G.; Vaughan, S.; Gull, K. The extended tubulin super family. J. Cell Sci. 2001, 114, 2723–2733. [Google Scholar] [PubMed]
- Perez, E.A. Microtubule inhibitors: Differentiating tubulin-inhibiting agents based on mechanisms of action, clinical activity, and resistance. Mol. Cancer Ther. 2009, 8, 2086–2095. [Google Scholar] [CrossRef] [PubMed]
- McCarroll, J.A.; Gan, P.P.; Liu, M.; Kavallaris, M. βIII-Tubulin Is a Multifunctional Protein Involved in Drug Sensitivity and Tumorigenesis in Non–Small Cell Lung Cancer. Cancer Rev. 2010, 70, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lagorce, D.; Sperandio, O.; Baell, J.B.; Miteva, M.A.; Villoutreix, B.O. FAF-Drugs3: A web server for compound property calculation and chemical library design. Nucleic Acids Res. 2015, 43, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, L.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 225–226. [Google Scholar] [CrossRef]
- Zhao, Y.; Abraham, M.H.; Lee, J.; Hersey, A.; Luscombe, N.C.; Beck, G.; Sherborne, B.; Cooper, I. Rate-limited steps of human oral absorption and QSAR studies. Pharm. Res. 2002, 19, 1446–1457. [Google Scholar] [CrossRef] [PubMed]
- Ajitha, M.; Rajnarayana, K.; Sarangapani, M. Synthesis of new 2-substituted-[1,3,4]-oxadiazino-[5,6-b]-indoles with H1-antihistaminic, antimuscarinic and antimicrobial activity. Pharmazie 2002, 57, 796–799. [Google Scholar] [CrossRef] [PubMed]
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- SYBYL. Tripos Molecular Modeling Program Package. Available online: http://www.tripos.com/data/SYBYL (accessed on 2 January 2018).
- Berman, H.M.; Battistuz, T.; Bhat, T.N.; Bluhm, W.F.; Bourne, P.E.; Burkhardt, K.; Feng, Z.; Gilliland, G.L.; Iype, L.; Jain, S.; et al. The Protein Data Bank. Nucleic Acids Res. 2002, 58, 899–907. [Google Scholar] [CrossRef]
- OECD. Guideline for Testing of Chemicals. In No. 425: Acute Oral Toxicity Up-and Down-Procedure (UDP); Organisation for Economic Co-Operation and Development: Paris, France, 2008. [Google Scholar]
Sample Availability: Samples of the compounds 4(a–n) are available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).