Abstract
We describe the one-pot synthesis of twenty polyheterocyclic pyrrolo[3,4-b]pyridin-5-ones via a cascade process (Ugi-3CR/aza Diels-Alder/N-acylation/aromatization) in 20 to 95% overall yields, as well as four pharmacologically promising analogues via an improved cascade process (Ugi-3CR/aza Diels-Alder/N-acylation/aromatization/SN2): two piperazine-linked pyrrolo[3,4-b]pyridin-5-ones in 33 and 34%, and a couple of Falipamil aza-analogues in 30 and 35% overall yields. It is worth highlighting the good substrate scope found, because final products are furnished with alkyl, aryl, and heterocyclic substituents. The use of chain-ring tautomerizable isocyanides (as key reagents for the Ugi-type three component reaction) allowed for a rapid and efficient assembly of the polysubstituted oxindoles, which were used in situ toward the complex products, conferring features like robustness, sustainability, and the one-pot approach to this synthetic methodology.
1. Introduction
Falipamil (1) is a calcium-channel-blocker of high interest in the medicinal chemistry due to its bradycardic, vagolytic, and anti-ischemic properties [1,2,3] (Figure 1). From a structural point of view, the compound 1 is an isoindolin-1-one, a benzo[d]fused heterocycle found in many natural and synthetic products exhibiting biological activity [4]. Moreover, the pyrrolo[3,4-b]pyridin-5-one is a pyrrolo[b]fused polyheterocyclic system that can be considered an aza-analogue of the isoindolin-1-one core, because the fused benzene is replaced by a pyridine. Similarly, various bioactive products contain the pyrrolo[3,4-b]pyridin-5-one system within their structures. Besides, piperazine is a common structural motif in bioactive products, and thus it is considered a privileged linker in medicinal chemistry [5]. For example, Kung et al. described that various compounds containing the isoindolin-1-one system that are piperazine-linked to other heterocycles exhibit strong binding affinity to the 5-hydroxytryptamine 1A receptor [6]. In the same way, Couture et al. synthesized various piperazine-linked isoindolin-1-ones like the compound 2 (Figure 1), bearing in mind its possible use for further SAR studies [7]. It is worthy to note that in the same work, Falipamil (1) and various related analogues, such as the product 3 (Figure 1), were synthesized successfully via a stepwise (multistep) strategy. Indeed, almost all reports describing the synthesis of isoindolin-1-ones and pyrrolo[3,4-b]pyridin-5-ones, including those piperazine-linked to other heterocyclic systems, have been synthesized using stepwise methodologies [8].
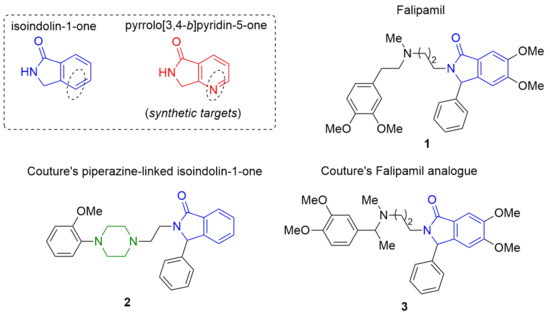
Figure 1.
Isoindolin-1-one and one of its aza-analogues (pyrrolo[3,4-b]pyridin-5-one).
We have investigated new one-pot synthetic strategies, mainly based on multicomponent reactions (MCR) to construct novel polyheterocyclic compounds with potential applications in different fields of knowledge such as agrochemistry, materials and polymers science, optics, and medicinal chemistry [9]. In this context, we have reported some one-pot syntheses on a series of novel, fused, polyheterocyclic pyrrolo[3,4-b]pyridin-5-one-based compounds via an Ugi-3CR/aza Diels-Alder/N-acylation/aromatization cascade sequence combined with further cyclization processes like free-radical mediated [10], Pummerer [11], Pictet-Spengler [12], and Pomeranz-Fritsch [13]. In the same way, we developed an oxidative [14] and a repetitive version [15] of this robust one-pot cascade process (Ugi-3CR/aza Diels-Alder/N-acylation/aromatization). Additionally, by using this methodology (one-pot approach), diverse aza-analogues of natural products, such as (±)-Nuevamine, (±)-Lennoxamine, and Magallanesine, were synthesized successfully [13]. On the other hand, to the best of our knowledge, the synthesis of Falipamil aza-analogues (or its piperazine-linked analogues) has not been previously reported either via stepwise, or one-pot cascade, or via Multicomponent Reactions (MCR)-based strategies.
Thus, it can be found in the literature of our own reports [10,11,12,13,14,15], which are previous works to the present one, and in the above-mentioned methodology from the Couture’s group [7], which is a close work to the present one, in which Falipamil (1) and some of its analogues (2–3) are synthesized efficiently, but via a stepwise strategy, resulting in larger times and generally using harsh conditions. Moreover, pioneering works from Bienaymé and Zhu [16,17] allowed the synthesis of pyrrolo[3,4-b]pyridin-5-ones via a cascade process (Ugi-3CR/aza Diels-Alder/N-acylation/aromatization) in good yields, short reaction times, and under relatively milder conditions with respect to stepwise methods. Thus, the main hypothesis behind the present work is that novel polyheterocyclic pyrrolo[3,4-b]pyridin-5-ones, including some piperazine-linked and Falipamil aza-analogues, can be synthesized efficiently via a cascade process (Ugi-3CR/aza Diels-Alder/N-acylation/aromatization) followed by a SN2 reaction under a new and robust MW-assisted one-pot process. Hence, this synthetic approach is in line with the idea extracted from the remarkable work by Danishefsky et al. “the development of novel synthetic strategies toward analogues of natural products with potential application in medicinal chemistry will always be worthy to be investigated” [18].
2. Results and Discussion
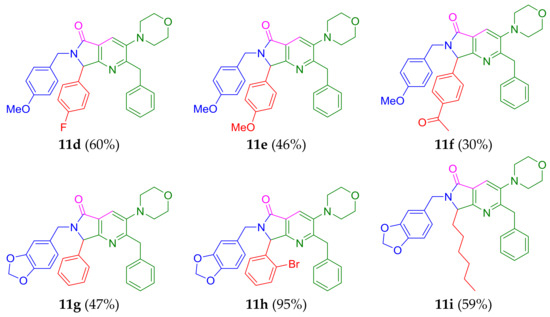
The synthesis of the desired pyrrolo[3,4-b]pyridin-5-ones 11a–i was performed using our previously optimized conditions [10]: scandium(III) triflate as the Lewis-acid catalyst (3% mol) [19], microwaves (MW) as heat source to reduce reaction times [20], and benzene as the solvent. Thus, 1.0 equiv. of the corresponding benzylamines 4a–c (R1 = a H, b 4-OMe, c 3,4-[-O-CH2-O-]) were combined with 1.0 equiv. of the corresponding aldehydes 5a–f (R2 = a Ph, b 2-BrPh, c 4-FPh, d 4-OMePh, e 4-AcPh, f n-Hex) in benzene [0.5 M] at 65 °C under MW heating conditions (55 W) to give the Schiff bases 6a–i, which were activated in situ by scandium (III) triflate as Lewis-acid catalyst to produce the iminium-like intermediates 7a–i. Then, 1.2 equiv. of the isocyanide 8a (prepared in three steps from the racemic phenylalanine [21]) were added sequentially to provide the corresponding 5-aminooxazoles 9a–i in quantitative yields. These intermediates used as in situ were prepared to access to the polyheterocycles 11a–i in one-pot manner. Consequently, when intermediates 9a–i were detected by TLC (by typical features such as Rf and spot nature [22]), 1.4 equiv. of the maleic anhydride (10) [23] was added to give the desired products 11a-i via a cascade triple process: N-acylation/aza Diels-Alder cycloaddition/aromatization (decarboxylation-dehydration) (Scheme 1).
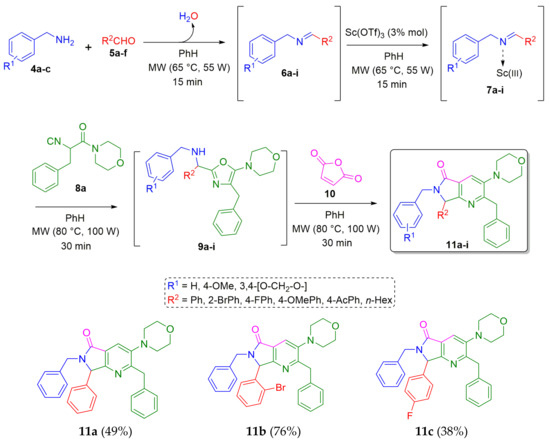
Scheme 1.
Synthesis of pyrrolo[3,4-b]pyridin-5-ones via a cascade process (Ugi-3CR/aza Diels-Alder/N-acylation/aromatization).
Although some yields of products 11 were moderate, these results can be considered satisfactory due to the molecular complexity of final products and the fact that they were synthesized in one-pot manner. This methodology was also demonstrated to have highly atomic economy, since various new C-C and C-N bonds were created, and only two molecules of water and one of carbon dioxide were lost in all the process. In addition, compounds 11c and 11d contain a fluorine atom in their structures. It is well known that the incorporation of fluorine atoms into the structure of potentially bioactive molecules often enhances their pharmacokinetic properties such as lipophilicity, oral bioavailability, and metabolic resistance [24]. Interestingly, the product 11h was synthesized in 95% yield (the highest among all the products), probably due to the high nucleophilicity of the piperonyl amine (doubly activated by the di-oxamethylene group in its 3,4-positions) combined with the highly activated 2-bromobenzaldehyde.
Continuing with our efforts to gain substrate scope, we synthesized the series of morpholine-containing analogues 11j–o. Thus, the 3-morpholinopropan-1-amine (4d) was combined sequentially with the aldehydes 5a,c–d,f,g (R1 = a Ph, c 4-FPh, d 4-OMePh, f n-Hex, g 4-ClPh), isocyanides 8a–b (X = a O, b CH2) and maleic anhydride 10 via an Ugi-3CR/aza Diels-Alder/N-acylation/aromatization cascade process (Scheme 2).
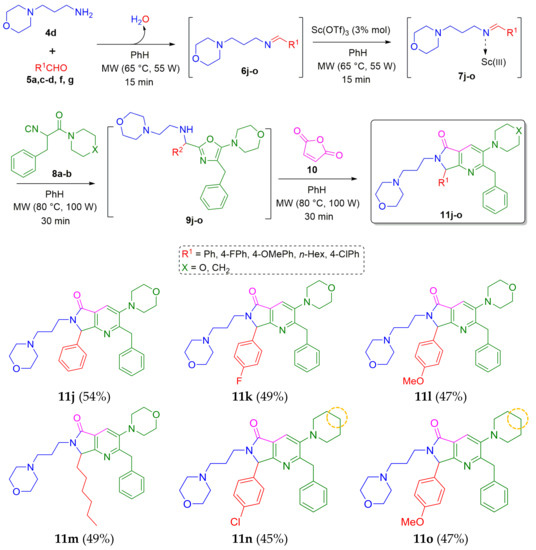
Scheme 2.
Synthesis of morpholine-based polyheterocyclic pyrrolo[3,4-b]pyridin-5-ones via a cascade process (Ugi-3CR/aza Diels-Alder/N-acylation/aromatization).
It is worth highlighting the structural versatility of the products, because they are provided with alkyl, aryl, and heterocyclic substituents. The use of the piperidine-containing isocyanide 8b to synthesize the analogues 11n (45%) and 11o (47%) appears not to alter the efficiency of the reactions, since their yields remained close to those of the other analogues 11j–m (47–54%).
A further example (in which the amino component of the MCR is modified) was performed by utilizing the tryptamine (4e) to prepare the polyheterocyclic analogue 11p in 65% yield. Thus, the tryptamine (4e) was combined sequentially with 2-bromobenzaldehyde (5b), isocyanide 8a, and maleic anhydride (10) via an Ugi-3CR/aza Diels-Alder/N-acylation/aromatization cascade process (Scheme 3).
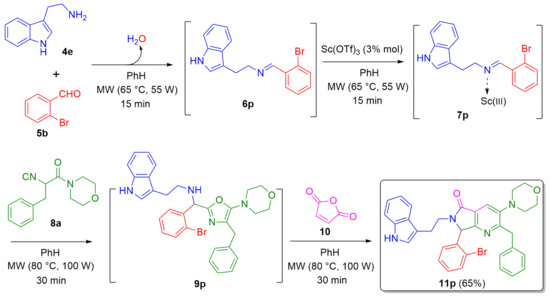
Scheme 3.
Synthesis of a tryptamine-based polyheterocyclic pyrrolo[3,4-b]pyridin-5-one via a cascade process (Ugi-3CR/aza Diels-Alder/N-acylation/aromatization).
Having in mind the idea of functionalizing the amine moiety of the pyrrolo[3,4-b]pyridin-5-ones to construct more complex polyheterocycles, the alcohol-containing analogues 11q–r were synthesized in 43 and 20 % yields, respectively. Thus, 2-aminoethan-1-ol (4f) was combined sequentially with the corresponding aldehydes 5f,h (R1 = f n-Hex, h 3-OMePh), the isocyanide 8a and maleic anhydride (10), using the previously detailed methodology to afford the expected products 11q–r (Scheme 4).
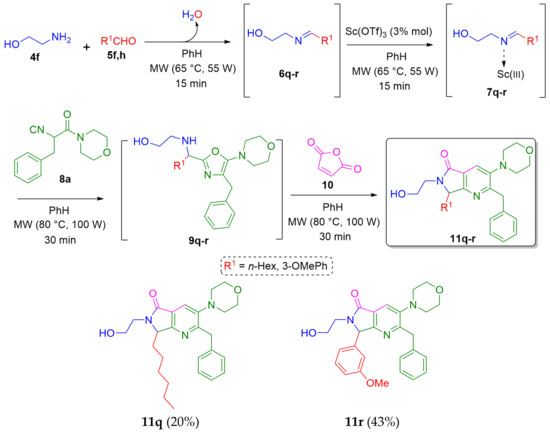
Scheme 4.
Synthesis of N-alcohol-functionalized pyrrolo[3,4-b]pyridin-5-ones via a cascade process (Ugi-3CR/aza Diels-Alder/N-acylation/aromatization).
Then, with the aim of evaluating the introduction of an additional step in the one-pot process, a further SN2 reaction was adapted to our cascade process. The first objective was to prepare the bromine-containing analogue 11s as a common precursor to introduce a variety of amines through a sequential SN2 reaction. Thus, 2-bromoethan-1-amine 4g was used as starting material, which reacted sequentially with the benzaldehyde (5a), isocyanide 8a, and maleic anhydride (10) to furnish the product 11s in 31% yield (Scheme 5).
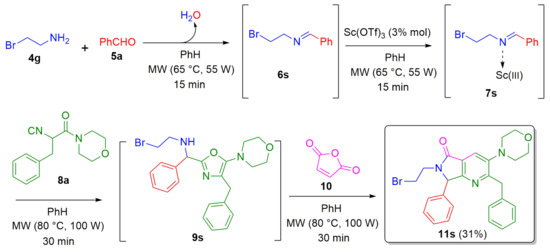
Scheme 5.
Synthesis of the bromide-functionalized pyrrolo[3,4-b]pyridin-5-one via a cascade process (Ugi-3CR/aza Diels-Alder/N-acylation/aromatization).
Subsequently, the availability of the SN2 reaction was tested by treatment of 11s with a mixture of morpholine (12a) and triethylamine in acetonitrile as the solvent using MW as heat source (80 °C) for 30 minutes to synthesize the morpholine-containing polyheterocyclic pyrrolo[3,4-b]pyridin-5-one 11t in 35% yield (Scheme 6). Since the chain length between the two heterocyclic systems was shortened by one methylene, the product 11t is a shorter analogue of the morpholine-containing polyheterocyclic pyrrolo[3,4-b]pyridin-5-ones 11j–o (Scheme 2).
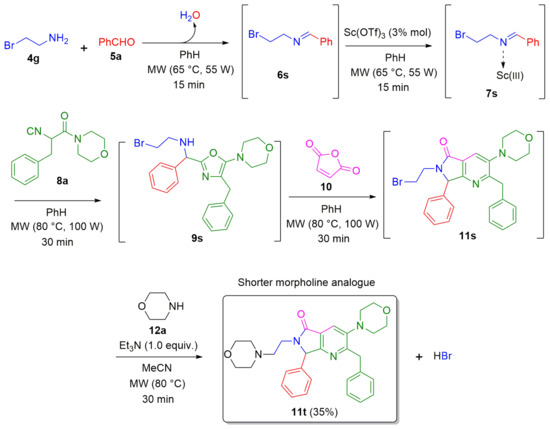
Scheme 6.
Synthesis of a morpholine-containing pyrrolo[3,4-b]pyridin-5-one via an improved one-pot process (Ugi-3CR/aza Diels-Alder/N-acylation/aromatization/SN2).
Following the proposed cascade process, the more complex polyheterocyclic pyrrolo[3,4-b]pyridin-5-ones 11u–x were synthesized from 11s or from other of its in situ prepared bromine-containing analogues 11s’. Then, strongly inspired by the work from Couture et al. [7], we synthesized the pair of piperazine-linked analogues 11u–v in 34 and 33% yields, respectively, through the same one-pot procedure. It is noteworthy that the secondary amine used for the SN2 reaction (last step) was 1-(2-methoxyphenyl)piperazine (12b), which contains one of the most valued linkers in medicinal chemistry (piperazine). Finally, we synthesized the Falipamil aza-analogues 11w–x in 30 and 35% yields, respectively, by following the procedure of preparation of the analogues 11u–v. The secondary amine used for both derivatives was N-methyl-3,4,-dimethoxyphenethylamine (12c) (Scheme 7). It is worthy to note that the products 11w–x present morpholine and benzyl groups attached to the pyrrolo[3,4-b]pyridin-5-one scaffold, which may be interpreted as a little loss of structural analogy to falipamil. However, both morpholine [25,26] and benzyl [27] are substituents that are relatively easy to remove from aromatic rings just to recover certain analogy to falipamil.
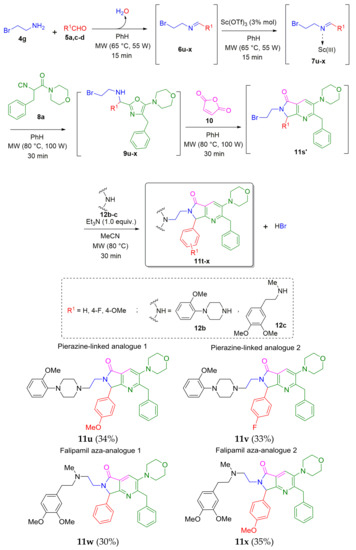
Scheme 7.
Synthesis of polyheterocyclic pyrrolo[3,4-b]pyridin-5-ones via an improved one-pot process (Ugi-3CR/aza Diels-Alder/N-acylation/aromatization/SN2).
All the products herein reported 11a–x were fully characterized using IR, NMR, and HRMS or EA techniques (See the Supplementary Materials for further details). Thus, the compound 11a was selected to discuss briefly the 1H and 13C-NMR spectra. The pyridine proton (position 4) appears at 7.97 ppm due to the mesomeric effect coming from the N-sp2. Besides, the CH in which the three components of the MCR converge has a peak value of 5.30 ppm. As seen, this proton is alkylic. However, it belongs to a pyrrolidinone system. With respect to 13C, there are two key peaks worth highlighting. The first one is the peak at 167.1 ppm, which belongs to a γ-lactam-carbonyl carbon. The second one is just the CH in which all the reagents converge. That peak appears at 64.5 ppm, a non-typic shift for an alkylic carbon atom. It is important to mention that despite several attempts to obtain adequate crystals for X-ray analysis for at least one product were conducted; these were unsuccessful.
A plausible reaction mechanism is depicted in Scheme 8, which is supported on computational calculations previously reported [28]. Thus, a condensation between amines 4 and the corresponding aldehydes 5 occurs to give the Schiff bases 6, which are nucleophilically attacked by the respective isocyanides 8 to afford the nitrilium ions 13. The later undergo a chain-ring tautomerization to give the 5-aminooxazoles 9 as products of the Ugi-3CR, which react with maleic anhydride (10) in two possible pathways: (a) intermolecular N-acylation/intramolecular aza Diels-Alder cycloaddition (through 14) or (b) intermolecular aza Diels-Alder cycloaddition/intramolecular N-acylation (through 15) to provide the O-bridged intermediate 16. The latter undergoes a decarboxylation to give 17, followed by a dehydration to provide the pyrrolo[3,4-b]pyridin-5-ones 11 (Scheme 6). It is worth noting that a close DFT-based study was reported recently by Gámez-Montaño et al. to support a plausible reaction mechanism for the construction of the isoinsolin-1-one core via an Ugi-type reaction [29]. Besides, as was discussed, the pyrrolo[3,4-b]pyridin-5-one is the ‘aza-version’ of the isoinsolin-1-one core.
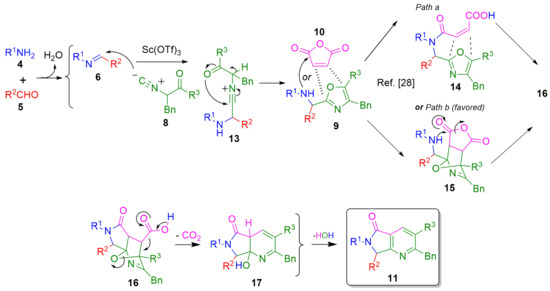
Scheme 8.
Plausible reaction mechanism (adapted from Ref. [9] with permission from the Royal Society of Chemistry).
3. Experimental Section
3.1. General Information, Instrumentation, Software, and Chemicals
1H and 13C Nuclear Magnetic Resonance (NMR) spectra were acquired on a Bruker Advance III spectrometer (500 MHz, Fällande, Uster, Switzerland). The solvent used for NMR samples was deuterated chloroform (CDCl3). Chemical shifts are reported in parts per million (δ/ppm). Coupling constants are reported in Hertz (J/Hz). Internal reference for NMR spectra was tetramethylsilane (TMS) at 0.00 ppm. Multiplicities of the signals are reported using the standard abbreviations: singlet (s), doublet (d), triplet (t), quartet (q), and multiplet (m). NMR spectra were analyzed using the MestreNova software (Ver. 10.0.1–14719). Infrared (IR) spectra were acquired on a Perkin Elmer GX spectrometer (Norwalk, CT, USA) using the Attenuated Total Reflectance (ATR) method. The absorbance peaks are reported in reciprocal centimeters (υmax/cm−1). IR spectra were analyzed using the Report Builder software (Ver. 2.01). Elemental analyses (CHNS) were determined in a Perkin Elmer 2400 Series II Analyzer (Norwalk, CT, USA). High Resolution Mass Spectroscopy (HRMS) spectra were acquired on a Jeol JMS–GC Mate II spectrometer (Akishima, Tokyo, Japan). HRMS samples were injected directly (direct probe) and analyzed by the Electron Impact (EI) method. HRMS spectra were analyzed using the Jeol-data analysis software. Melting points were determined on a Fisher-Johns apparatus (Suwanee, GA, USA) and are uncorrected. Microwave assisted reactions were performed in closed vessel mode on a CEM Discover MW-reactor (Matthews, NC, USA). Reaction progress was monitored by Thin Layer Chromatography (TLC) on precoated plates with Kieselgel 60 (F254), and the spots were visualized under Ultraviolet (UV) light (254 or 365 nm). Flash columns packed with silica-gel Merck 60 (230–400 nm) and glass preparative plates (20 × 20 cm) coated with Kieselgel 60 (F254) doped with UV indicator were used to purify the products. Mixtures in different proportions (v/v) of hexanes (Hex) with ethyl acetate (EtOAc) or ethyl acetate (EtOAc) with ethanol (EtOH) were used to run TLC, silica-gel columns, preparative plates, and to measure the Retention Factors (Rf) (using the same mobile phase for all these experiments per product). All starting materials and solvents were used as received without further purification, distillation, or dehydration. Chemical structures were drawn using the ChemBioDraw software (Ver. 13.0.2.3020). The purity for all synthesized products (up to 98%) was assessed by NMR.
3.2. Synthesis and Characterization of the Polysubstituted Pyrrolo[3,4-b]pyridin-5-ones 11a–s
General procedure 1 (GP-1): The corresponding amines (0.1 mmol, 1.0 equiv.) and the corresponding aldehydes (1.0 equiv.) were placed in a 10 mL sealed CEM Discover microwave reaction tube and diluted in benzene [0.5 M]. The mixture was stirred and irradiated (MW, 65 °C, 55 W) for 15 min, and then Sc(OTf)3 (0.03 equiv.) was added. The mixture was stirred and irradiated (MW, 65 °C, 55 W) for 15 min, and then the corresponding isocyanides (1.2 equiv.) were added. The mixture was stirred and irradiated (MW, 80 °C, 100 W) for 30 min, and then maleic anhydride (1.4 equiv.) was added. Finally, the new reaction mixture was stirred and irradiated (MW, 80 °C, 100 W) for 30 min. Then, the solvent was removed to dryness under vacuum. The crude was diluted in dichloromethane (5.0 mL), washed with a concentrated aqueous solution of NaHCO3 (3 × 25 mL), and then washed with brine (3 × 25 mL). The organic layer was dried using anhydrous Na2SO4 and then filtered over a celite pad. The solvent was removed to dryness under vacuum. The residue was purified immediately using a silica-gel column chromatography followed by preparative TLC using mixtures of Hex–EtOAc or EtOAc-EtOH (v/v) in different proportions as mobile phase to afford the corresponding polyheterocyclic pyrrolo[3,4-b]pyridin-5-ones 11a–s.
3.2.1. 2,6-Dibenzyl-3-morpholino-7-phenyl-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-one (11a)
According to GP-1, benzylamine (10.7 mg), benzaldehyde (10.2 µL), scandium (III) triflate (1.5 mg), 2-isocyano-1-morpholino-3-phenylpropan-1-one (29.3 mg), and maleic anhydride (13.7 mg) were reacted together in PhH (0.2 mL) to afford the product 11a (23.3 mg, 49%) as a yellow solid; mp = 128–130 °C; Rf = 0.54 (Hex–AcOEt = 1/1, v/v); FT–IR (ATR) υmax/cm−1 921, 1114, 1263, 1442, 1695; 1H-NMR (500 MHz, CDCl3, 25 °C): δ 2.81–2.88 (m, 4H), 3.79–3.84 (m, 5H), 4.19 (d, J = 13.9 Hz, 1H), 4.31 (d, J = 13.9 Hz, 1H), 5.30 (s, 1H), 5.45 (d, J = 14.8 Hz, 1H), 7.13–7.18 (m, 7H), 7.22–7.23 (m, 2H), 7.29–7.34 (m, 3H), 7.38–7.41 (m, 3H), 7.97 (s, 1H); 13C-NMR (126 MHz, CDCl3, 25 °C): δ 40.0 (CH2), 43.9 (CH2), 53.0 (CH2), 64.5 (CH), 67.1 (CH2), 123.9 (Cqar), 124.1 (CHar), 126.1 (CHar), 127.7 (CHar), 128.1 (CHar), 128.2 (CHar), 128.5 (CHar), 128.7 (CHar), 128.8 (2 CHar), 129.0 (CHar), 135.3 (Cqar), 136.8 (Cqar), 139.2 (Cqar), 147.8 (Cqar), 160.5 (Cqar), 162.1 (Cqar), 167.1 (Cq); HRMS (EI): calcd. for C31H30N3O2+ 476.2338, found 476.2341.
3.2.2. 2,6-Dibenzyl-7-(2-bromophenyl)-3-morpholino-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-one 11b
According to GP-1, benzylamine (10.7 mg), 2-bromobenzaldehyde (11.7 µL), scandium (III) triflate (1.5 mg), 2-isocyano-1-morpholino-3-phenylpropan-1-one (29.3 mg), and maleic anhydride (13.7 mg) were reacted together in PhH (0.2 mL) to afford the product 11b (42.0 mg, 76%) as a white solid; mp = 109–111 °C; Rf = 0.53 (Hex–AcOEt = 1/1, v/v); FT–IR (ATR) υmax/cm−1 940, 1015, 1112, 1441, 1694; 1H-NMR (500 MHz, CDCl3, 25 °C): δ 2.81–2.84 (m, 4H), 3.00–3.82 (m, 4H), 3.86 (d, J = 14.8 Hz, 1H), 4.12 (d, J = 13.7 Hz, 1H), 4.27 (d, J = 13.7 Hz, 1H), 5.32 (d, J = 14.7 Hz, 1H), 5.99 (s, 1H), 6.74–6.76 (m, 1H), 7.12–7.28 (m, 12H), 7.65–7.67 (m, 1H), 7.89 (s, 1H); 13C-NMR (126 MHz, CDCl3, 25 °C): δ 39.8 (CH2), 44.3 (CH2), 53.0 (CH2), 63.2 (CH), 67.1 (CH2), 123.6 (Cqar), 123.8 (CHar), 125.6 (Cqar), 126.1 (CHar), 127.7 (CHar), 127.9 (2 CHar), 128.1 (CHar), 128.6 (CHar), 128.7 (CHar), 128.9 (CHar), 129.9 (CHar), 133.5 (CHar), 134.8 (Cqar), 136.5 (Cqar), 139.1 (Cqar), 147.7 (Cqar), 160.3 (Cqar), 162.2 (Cqar), 167.4 (Cq); Elemental analysis: calcd. for C31H28BrN3O2 C 67.15, H 5.09, N 7.58%, found C 66.65, H 5.29, N 7.34%.
3.2.3. 2,6-Dibenzyl-7-(4-fluorophenyl)-3-morpholino-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-one 11c
According to GP-1, benzylamine (10.7 mg), 4-fluorobenzaldehyde (10.7 µL), scandium (III) triflate (1.5 mg), 2-isocyano-1-morpholino-3-phenylpropan-1-one (29.3 mg), and maleic anhydride (13.7 mg) were reacted together in PhH (0.2 mL) to afford the product 11c (18.8 mg, 38%) as a yellow solid; mp = 152–154 °C; Rf = 0.54 (Hex–AcOEt = 1/1, v/v); FT–IR (ATR) υmax/cm−1 920, 1114, 1220, 1441, 1696; 1H-NMR (500 MHz, CDCl3, 25 °C): δ 2.79–2.85 (m, 4H), 3.76–3.81 (m, 5H), 4.17 (d, J = 13.9 Hz, 1H), 4.27 (d, J = 13.9 Hz, 1H), 5.24 (s, 1H), 5.41 (d, J = 14.9 Hz, 1H), 7.05–7.17 (m, 10H), 7.24–7.31 (m, 4H), 7.92 (s,1H); 13C-NMR (126 MHz, CDCl3, 25 °C): δ 40.0 (CH2), 43.8 (CH2), 53.0 (CH2), 63.7 (CH), 67.1 (CH2), 116.0 (d, oJCF = 21.0 Hz, CF), 123.7 (Cqar), 123.9 (CHar), 126.2 (CHar), 127.8 (CHar), 128.1 (CHar), 128.4 (CHar), 128.7 (CHar), 128.8 (CHar), 129.8 (d, mJCF = 8.3 Hz, CF), 131.1 (d, pJCF = 2.6 Hz, CF), 136.6 (Cqar), 139.1 (Cqar), 147.9 (Cqar), 160.3 (Cqar), 162.1 (Cqar), 162.8 (d, iJCF = 247.5 Hz, CF), 166.9 (Cq); Elemental analysis: calcd. for C31H28FN3O2 C 75.44, H 5.72, N 8.51%, found C 75.10, H, 5.75, N 8.42%.
3.2.4. 2-Benzyl-7-(4-fluorophenyl)-6-(4-methoxybenzyl)-3-morpholino-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-one 11d
According to GP-1, (4-methoxyphenyl)methanamine (13.1 µL), 4-fluorobenzaldehyde (10.7 µL), scandium (III) triflate (1.5 mg), 2-isocyano-1-morpholino-3-phenylpropan-1-one (29.3 mg), and maleic anhydride (13.7 mg) were reacted together in dry PhH (0.2 mL) to afford the product 11d (31.4 mg, 60%) as a white solid; mp = 145–147 °C; Rf = 0.52 (Hex–AcOEt = 1/1, v/v); FT–IR (ATR) υmax/cm−1 917, 1114, 1221, 1246, 1441, 1509, 1694, 2852; 1H-NMR (500 MHz, CDCl3, 25 °C): δ 2.78–2.85 (m, 4H), 3.72 (d, J = 14.7 Hz, 1H), 3.75 (s, 3H), 3.79 (t, J = 4.6 Hz, 4H), 4.16 (d, J = 13.9 Hz, 1H), 4.27 (d, J = 13.9 Hz, 1H), 5.24 (s, 1H), 5.35 (d, J = 14.7 Hz, 1H), 6.81–6.83 (m, 2H), 7.05–7.14 (m, 11H), 7.91 (s, 1H); 13C-NMR (126 MHz, CDCl3, 25 °C): δ 39.9 (CH2), 43.2 (CH2), 53.0 (CH2), 55.2 (CH3), 63.6 (CH), 67.1 (CH2), 114.1 (CHar), 115.9 (d, oJCF = 21.7 Hz, CF), 123.8 (CHar), 123.9 (CHar), 126.1 (CHar), 128.1 (CHar), 128.7 (CHar), 129.8 (CHar), 131.2 (d, pJCF = 2.5 Hz, CF), 139.2 (Cqar), 147.8 (Cqar), 159.2 (Cqar), 160.3 (Cqar), 162.0 (Cqar), 162.8 (d, iJCF = 247.4 Hz, CF), 166.8 (Cq); Elemental analysis: calcd. for C32H30FN3O3 C 73.40, H 5.78, N 8.03%, found C 73.45, H 6.13, N 8.15%.
3.2.5. 2-Benzyl-6-(4-methoxybenzyl)-7-(4-methoxyphenyl)-3-morpholino-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-one 11e
According to GP-1, (4-methoxyphenyl)methanamine (13.1 µL), 4-methoxybenzaldehyde (12.2 µL), scandium (III) triflate (1.5 mg), 2-isocyano-1-morpholino-3-phenylpropan-1-one (29.3 mg), and maleic anhydride (13.7 mg) were reacted together in PhH (0.2 mL) to afford the product 11e (24.6 mg, 46%) as a yellow solid; mp = 166–168 °C; Rf = 0.42 (Hex–AcOEt = 1/1, v/v); FT–IR (ATR) υmax/cm−1 701, 940, 1015, 1112, 1441, 1694, 2842; 1H-NMR (500 MHz, CDCl3, 25 °C): δ 2.76–2.84 (m, 4H), 3.70 (d, J = 14.7 Hz, 1H), 3.80–3.92 (m, 10H), 4.15 (d, J = 13.8 Hz, 1H), 4.29 (d, J = 13.8 Hz, 1H), 5.21 (s, 1H), 5.34 (d, J = 14.7 Hz, 1H), 6.82–6.83 (m, 2H), 6.88–6.90 (m, 2H), 7.02–7.04 (m, 2H), 7.10–7.15 (m, 7H), 7.91 (s, 1H); 13C-NMR (126 MHz, CDCl3, 25 °C): δ 40.0 (CH2), 43.0 (CH2), 53.0 (CH2), 55.2 (CH3), 55.3 (CH3), 63.9 (CH), 67.1 (CH2), 114.1 (CHar), 114.4 (CHar), 123.9 (CHar), 124.0 (Cqar), 126.1 (CHar), 127.1 (Cqar), 128.1 (CHar), 128.7 (CHar), 129.0 (Cqar), 129.3 (CHar), 129.8 (CHar), 139.3 (Cqar), 147.7 (Cqar), 159.1 (Cqar), 159.8 (Cqar), 160.8 (Cqar), 161.9 (Cqar), 166.7 (Cq); HRMS (EI): calcd. for C33H34N3O4+ 536.2549, found 536.2611.
3.2.6. 7-(4-Acetylphenyl)-2-benzyl-6-(4-methoxybenzyl)-3-morpholino-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-one 11f
According to GP-1, (4-methoxyphenyl)methanamine (13.1 µL), 4-acetylbenzaldehyde (14.8 mg), scandium (III) triflate (1.5 mg), 2-isocyano-1-morpholino-3-phenylpropan-1-one (29.3 mg), and maleic anhydride (13.7 mg) were reacted together in PhH (0.2 mL) to afford the product 11f (16.4 mg, 30%) as a yellow solid; mp = 149–151 °C; Rf = 0.34 (Hex–AcOEt = 1/1, v/v); FT–IR (ATR) υmax/cm−1 917, 1035, 1114, 1247, 1441, 1687, 2852; 1H-NMR (500 MHz, CDCl3, 25 °C): δ 2.62 (s, 3H), 2.79–2.86 (m, 4H), 3.72 (d, J = 14.8 Hz, 1H), 3.78 (s, 3H), 3.79–3.82 (m, 4H), 4.12 (d, J = 14.0 Hz, 1H), 4.27 (d, J = 13.9 Hz, 1H), 5.29 (s, 1H), 5.39 (d, J = 14.7 Hz, 1H), 6.81–6.83 (m, 2H), 7.07–7.13 (m, 7H), 7.24–7.26 (m, 2H), 7.91 (s, 1H), 7.96–7.97 (m, 2H); 13C-NMR (126 MHz, CDCl3, 25 °C): δ 126.7 (CH3), 40.0 (CH2), 43.5 (CH2), 53.0 (CH2), 55.3 (CH3), 63.8 (CH), 67.1 (CH2), 114.2 (CHar), 123.8 (Cqar), 124.0 (CHar), 126.2 (CHar), 128.2 (2 CHar), 128.5 (Cqar), 128.7 (Cqar), 129.0 (CHar),129.8 (CHar), 137.3 (Cqar),139.1 (Cqar), 140.9 (Cqar),148.0 (Cqar), 159.2 (Cqar), 159.8 (Cqar), 162.1 (Cqar), 167.0 (Cq), 197.5 (Cq); Elemental analysis: calcd. for C34H33N3O4 C 74.57, H 6.07, N 7.67%, found C 74.55, H 6.26, N 7.49%.
3.2.7. 6-(benzo[d][1,3]dioxol-5-ylmethyl)-2-benzyl-3-morpholino-7-phenyl-6,7-dihidro-5H-pyrrolo[3,4-b]pyridin-5-one 11g
According to GP-1, piperonylamine (12.5 µL), benzaldehyde (10.2 µL), scandium (III) triflate (1.5 mg), 2-isocyano-1-morpholino-3-phenylpropan-1-one (29.3 mg), and maleic anhydride (13.7 mg) were reacted together in PhH (0.2 mL) to afford the product 11g (24.4 mg, 47%) as a yellow solid; mp = 126–128 °C; Rf = 0.51 (Hex–AcOEt = 1/1, v/v); FT–IR (ATR) υmax/cm−1 927, 1036, 1114, 1243, 1442, 1694; 1H-NMR (500 MHz, CDCl3, 25 °C): δ 2.77–2.84 (m, 4H), 3.68 (d, J = 14.8 Hz, 1H), 3.78–3.80 (m, 4H), 4.15 (d, J = 13.9 Hz, 1H), 4.28 (d, J = 13.9 Hz, 1H), 5.27 (s, 1H), 5.32 (d, J = 14.7 Hz, 1H), 5.90 (d, J = 1.5 Hz, 1H), 5.92 (d, J = 1.5 Hz, 1H), 6.62 (dd, J = 1.5, 7.8 Hz, 1H), 6.71–6.72 (m, 2H), 7.12–7.15 (m, 7H), 7.35–7.38 (m, 3H), 7.90 (s,1H); 13C-NMR (126 MHz, CDCl3, 25 °C): δ 40.0 (CH2), 43.6 (CH2), 53.0 (CH2), 64.3 (CH), 67.1 (CH2), 101.1 (CH2), 108.3 (CHar), 108.9 (CHar), 121.9 (CHar), 123.8 (Cqar), 123.9 (CHar), 126.1 (CHar), 128.1 (2 CHar), 128.6 (CHar), 128.8 (CHar), 129.0 (CHar), 130.7 (Cqar), 135.3 (Cqar), 139.2 (Cqar), 147.1 (Cqar), 147.8 (Cqar), 148.0 (Cqar), 160.5 (Cqar), 162.0 (Cqar), 166.9 (Cq); HRMS (EI): calcd. for C32H30N3O4+ 520.2236, found 520.2208.
3.2.8. 6-(Benzo[d][1,3]dioxol-5-ylmethyl)-2-benzyl-7-(2-bromophenyl)-3-morpholino-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-one 11h
According to GP-1, piperonylamine (12.5 µL), 2-bromobenzaldehyde (11.7 µL), scandium (III) triflate (1.5 mg), 2-isocyano-1-morpholino-3-phenylpropan-1-one (29.3 mg), and maleic anhydride (13.7 mg) were reacted together in PhH (0.2 mL) to afford the product 11h (56.7 mg, 95%) as a yellow solid; mp = 109–111 °C; Rf = 0.51 (Hex–AcOEt = 1/1, v/v); FT–IR (ATR) υmax/cm−1 701, 940, 1015, 1112, 1441, 1694; 1H-NMR (500 MHz, CDCl3, 25 °C): δ 2.83–2.85 (m, 4H), 3.79 (d, J = 14.7 Hz, 1H), 3.83–3.85 (m, 4H), 4.12–4.16 (m, 1H), 4.31 (d, J = 13.7 Hz, 1H), 5.24 (d, J = 14.7 Hz, 1H), 5.93 (dd, J = 1.5, 6.1 Hz, 2H), 6.01 (s, 1H), 6.65 (dd, J = 1.7, 7.9 Hz, 1H), 6.71–6.77 (m, 4H), 7.13–7.24 (m, 6H), 7.69–7.71 (m, 1H), 7.90 (s, 1H); 13C-NMR (126 MHz, CDCl3, 25 °C): δ 39.9 (CH2), 44.1 (CH2), 53.0 (CH2), 63.1 (CH), 67.1 (CH2), 101.0 (CH2), 108.3 (CHar), 109.1 (CHar), 122.2 (CHar), 123.6 (Cqar), 123.8 (CHar), 125.6 (Cqar), 126.1 (CHar), 127.9 (CHar), 128.0 (CHar), 128.1 (CHar), 129.0 (CHar), 129.9 (CHar), 130.3 (Cqar), 133.5 (CHar), 134.9 (Cqar), 139.1 (Cqar), 147.1 (Cqar), 147.7 (Cqar), 147.9 (Cqar), 160.3 (Cqar), 162.2 (Cqar), 167.3 (Cq); HRMS (EI): calcd. for C32H29BrN3O4+ 598.1341, found 598.1360.
3.2.9. 6-(Benzo[d][1,3]dioxol-5-ylmethyl)-2-benzyl-7-hexyl-3-morpholino-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-one 11i
According to GP-1, piperonylamine (12.5 µL), heptanal (14.0 µL), scandium (III) triflate (1.5 mg), 2-isocyano-1-morpholino-3-phenylpropan-1-one (29.3 mg), and maleic anhydride (13.7 mg) were reacted together in PhH (0.2 mL) to afford the product 11i (31.3 mg, 59%) as a yellow viscous liquid; Rf = 0.53 (Hex–AcOEt = 1/1, v/v); FT–IR (ATR) υmax/cm−1 701, 928, 1038,1115, 1243, 1441, 1691; 1H-NMR (500 MHz, CDCl3, 25 °C): δ 0.72–0.78 (m, 1H), 0.85 (t, J = 7.16 Hz, 3H), 1.10–1.22 (m, 7H), 1.84–1.93 (m, 1H), 2.01–2.15 (m, 1H), 2.83–2.89 (m, 4H), 3.83–3.86 (m, 4H), 4.11 (d, J = 14.9 Hz, 1H), 4.29 (d, J = 14.0 Hz, 1H), 4.39–4.42 (m, 2H), 5.26 (d, J = 14.9 Hz, 1H), 5.94 (dd, J = 1.4, 3.7 Hz, 2H), 6.76 (d, J = 7.8 Hz, 2H), 6.78–6.82 (m, 2H), 7.16–7.19 (m, 1H), 7.23–7.28 (m, 5H), 7.88 (s, 1H); 13C-NMR (126 MHz, CDCl3, 25 °C): δ 14.0 (CH3), 22.3 (CH2), 22.5 (CH2), 29.1 (2 CH2), 31.6 (CH2), 40.0 (CH2), 43.7 (CH2), 53.1 (CH2), 59.8 (CH), 67.2 (CH2), 101.1 (CH2), 108.3 (CHar), 108.7 (CHar), 121.5 (Cqar), 123.6 (Cqar), 124.7 (Cqar), 126.2 (CHar), 128.2 (CHar), 128.8 (CHar), 130.9 (Cqar), 139.5 (Cqar), 147.1 (Cqar), 147.5 (Cqar), 148.0 (Cqar), 160.4 (Cqar), 161.3 (Cqar), 167.1 (Cq); Elemental analysis: calcd. for C32H37N3O4 C 72.84, H 7.07, N 7.96%, found C 72.76, H 7.31, N 7.76%.
3.2.10. 2-Benzyl-3-morpholino-6-(3-morpholinopropyl)-7-phenyl-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-one 11j
According to GP-1, 3-morpholinopropan-1-amine (14.6 µL), benzaldehyde (10.2 µL), scandium (III) triflate (1.5 mg), 2-isocyano-1-morpholino-3-phenylpropan-1-one (29.3 mg), and maleic anhydride (13.7 mg) were reacted together in PhH (0.2 mL) to afford the product 11j (27.7 mg, 54%) as a yellow viscous liquid; Rf = 0.20 (Hex–AcOEt = 1/1, v/v); FT–IR (ATR) υmax/cm−1 698, 1031, 1115, 1252, 1443, 1512, 1689; 1H-NMR (500 MHz, CDCl3, 25 °C): δ 1.67–1.73 (m, 1H), 1.77–1.82 (m, 1H), 2.29–2.37 (m, 6H), 2.76–2.83 (m, 4H), 2.99–3.05 (m, 1H), 3.58–3.66 (m, 4H), 3.76–3.79 (m, 4H), 3.94–3.99 (m, 1H), 4.20 (d, J = 13.9 Hz, 1H), 4.28 (d, J = 13.9 Hz, 1H), 5.49 (s, 1H), 7.08–7.11 (m, 1H), 7.14–7.15 (m, 4H), 7.16–7.18 (m, 2H), 7.32–7.35 (m, 3H), 7.87 (s, 1H); 13C-NMR (126 MHz, CDCl3, 25 °C): δ 25.0 (CH2), 38.7 (CH2), 40.0 (CH2), 53.0 (CH2), 53.6 (CH2), 56.0 (CH2), 65.6 (CH), 66.8 (CH2), 67.1 (CH2), 123.7 (CHar), 124.2 (Cqar), 126.1 (CHar), 128.0 (CHar), 128.1 (CHar), 128.6 (CHar), 128.7 (CHar), 128.9 (CHar), 135.7 (Cqar), 139.3 (Cqar), 147.8 (Cqar), 160.4 (Cqar), 161.8 (Cqar), 167.2 (Cq); HRMS (EI): calcd. for C31H36N4O3 512.2787, found 512.2795.
3.2.11. 2-Benzyl-7-(4-fluorophenyl)-3-morpholino-6-(3-morpholinopropyl)-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-one 11k
According to GP-1, 3-morpholinopropan-1-amine (14.6 µL), 4-fluorobenzaldehyde (10.7 µL), scandium (III) triflate (1.5 mg), 2-isocyano-1-morpholino-3-phenylpropan-1-one (29.3 mg), and maleic anhydride (13.7 mg) were reacted together in PhH (0.2 mL) to afford the product 11k (26.0 mg, 49%) as a yellow viscous liquid; Rf = 0.32 (Hex–AcOEt = 1/1, v/v); FT–IR (ATR) υmax/cm−1 699, 862, 1032, 1115, 1221, 1443, 1508, 1692; 1H-NMR (500 MHz, CDCl3, 25 °C): δ 1.69–1.74 (m, 1H), 1.77–1.83 (m, 1H), 2.32–2.38 (m, 6H), 2.79–2.85 (m, 4H), 2.96–3.02 (m, 1H), 3.61–3.64 (m, 4H), 3.79–3.82 (m, 4H), 4.00–3.95 (m, 1H), 4.22 (d, J = 13.9 Hz, 1H), 4.29 (d, J = 13.9 Hz, 1H), 5.48 (s, 1H), 7.03–7.08 (m, 2H), 7.13–7.18 (m, 7H), 7.87 (s, 1H); 13C-NMR (126 MHz, CDCl3, 25 °C): δ 25.2 (CH2), 38.7 (CH2), 40.1 (CH2), 53.1 (CH2), 53.7 (CH2), 56.1 (CH2), 64.9 (CH), 67.0 (CH2), 67.2 (CH2), 116.1 (d, oJCF = 21.8 Hz, CF), 123.8 (CHar), 124.2 (Cqar), 126.3 (CHar), 128.3 (CHar), 128.8 (CHar), 129.7 (d, mJCF = 8.3 Hz, CF), 131.5 (d, pJCF = 3.2 Hz, CF), 139.3 (Cqar), 148.0 (Cqar), 160.3 (Cqar), 162.0 (Cqar), 162.8 (d, iJCF = 247.6 Hz, CF), 167.2 (CO); HRMS (EI): calcd. for C31H35FN4O3 530.2693, found 530.2694.
3.2.12. 2-Benzyl-7-(4-methoxyphenyl)-3-morpholino-6-(3-morpholinopropyl)-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-one 11l
According to GP-1, 3-morpholinopropan-1-amine (14.6 µL), 4-methoxybenzaldehyde (12.2 µL), scandium (III) triflate (1.5 mg), 2-isocyano-1-morpholino-3-phenylpropan-1-one (29.3 mg), and maleic anhydride (13.7 mg) were reacted together in PhH (0.2 mL) to afford the product 11l (25.5 mg, 47%) as a yellow viscous liquid; Rf = 0.17 (AcOEt–EtOH = 10/1, v/v); FT–IR (ATR) υmax/cm−1 1115, 1252, 1443, 1512, 1689, 2852; 1H-NMR (500 MHz, CDCl3, 25 °C): δ 1.67–1.73 (m, 1H), 1.77–1.83 (m, 1H), 2.30–2.39 (m, 6H), 2.85–2.76 (m, 4H), 2.98–3.03 (m, 1H), 3.62–3.65 (m, 4H), 3.78–380 (m, 7H), 3.91–3.96 (m, 1H), 4.21 (d, J = 13.9 Hz, 1H), 4.30 (d, J = 13.9 Hz, 1H), 5.45 (s, 1H), 6.88–6.89 (m, 2H), 7.07–7.09 (m, 2H), 7.13–7.18 (m, 5H), 7.87 (s, 1H); 13C-NMR (126 MHz, CDCl3, 25 °C): δ 25.1 (CH2), 38.5 (CH2), 40.1 (CH2), 53.1 (CH2), 53.6 (CH2), 55.3 (CH3), 56.1 (CH2), 65.2 (CH), 66.9 (CH2), 67.1 (CH2), 114.4 (CHar), 123.7 (CHar), 124.3 (Cqar), 126.1 (CHar), 127.4 (Cqar), 128.2 (CHar), 128.7 (CHar), 129.3 (CHar), 139.4 (Cqar), 147.8 (Cqar), 159.9 (Cqar), 160.7 (Cqar), 161.8 (Cqar), 167.0 (CO); HRMS (FAB): calcd. for C32H38N4O4 542.2893, found 542.2890.
3.2.13. 2-Benzyl-7-hexyl-3-morpholino-6-(3-morpholinopropyl)-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-one 11m
According to GP-1, 3-morpholinopropan-1-amine (14.6 µL), heptanal (14.0 µL), scandium (III) triflate (1.5 mg), 2-isocyano-1-morpholino-3-phenylpropan-1-one (29.3 mg), and maleic anhydride (13.7 mg) were reacted together in PhH (0.2 mL) to afford the product 11m (25.5 mg, 49%) as a yellow viscous liquid; Rf = 0.40 (Hex–AcOEt = 1/1, v/v); FT–IR (ATR) υmax/cm−1 700, 1031, 1115, 1399, 1446, 1648; 1H-NMR (500 MHz, CDCl3, 25 °C): δ 0.71–0.79 (m, 1H), 0.84 (d, J = 7.1 Hz, 3H), 1.10–1.25 (m, 8H), 1.79–1.91 (m, 3H), 2.23–2.17 (m, 1H), 2.39–2.43 (m, 6H), 2.80–2.87 (m, 4H), 3.23–3.27 (m, 1H), 3.65–3.70 (m, 4H), 3.82 (t, J = 4.6 Hz, 4H), 4.05 (m, 1H), 4.28 (d, J = 14.0 Hz, 1H), 4.43 (d, J = 14.0 Hz, 1H), 4.56 (dd, J = 3.2, 5.7 Hz, 1H), 7.15–7.19 (m, H), 7.22–7.28 (m, 4H), 7.82 (s, 1H); 13C-NMR (126 MHz, CDCl3, 25 °C): δ 14.1 (CH3), 22.5 (2 CH2), 25.4 (CH2), 29.2 (CH2), 29.4 (CH2), 31.6 (CH2), 38.1 (CH2), 40.0 (CH2), 53.1 (CH2), 53.7 (CH2), 56.2 (CH2), 60.5 (CH), 67.0 (CH2), 67.2 (CH2), 123.4 (CHar), 125.0 (Cqar), 126.2 (CHar), 128.3 (CHar), 128.8 (CHar), 139.6 (Cqar), 147.5 (Cqar), 160.3 (Cqar), 161.1 (Cqar), 167.1 (CO); HRMS (EI): calcd. for C31H44N4O3 520.3413, found 520.3414.
3.2.14. 2-Benzyl-7-(4-chlorophenyl)-6-(3-morpholinopropyl)-3-(piperidin-1-yl)-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-one 11n
According to GP-1, 3-morpholinopropan-1-amine (14.6 µL), 4-chlorobenzaldehyde (14.1 mg), scandium (III) triflate (1.5 mg), 2-benzyl-3-oxo-3-(piperidin-1-yl)propanenitrile (29.1 mg), and maleic anhydride (13.7 mg) were reacted together in PhH (0.2 mL) to afford the product 11n (24.5 mg, 45%) as a yellow viscous liquid; Rf = 0.33 (AcOEt–EtOH = 10/1, v/v); FT–IR (ATR) υmax/cm−1 680, 755, 781, 833, 982, 1036, 1168, 1250, 1385, 1515, 1759; 1H-NMR (500 MHz, CDCl3, 25 °C): δ 1.54–1.60 (m, 2H), 1.68–1.73 (m, 5H), 1.76–1.82 (m, 1H), 3.31–3.40 (m, 6H), 2.75–2.81 (m, 4H), 2.94–3.00 (m, 1H), 3.62–3.65 (m, 4H), 3.94–4.00 (m, 1H), 4.16 (d, J = 13.8 Hz, 1H), 4.25 (d, J = 13.9 Hz, 1H), 5.43 (s, 1H), 7.09–7.20 (m, 7H), 7.32–7.33 (m, 2H), 7.80 (s, 1H); 13C-NMR (126 MHz, CDCl3, 25 °C): δ 24.1 (CH2), 25.2 (CH2), 26.5 (CH2), 38.8 (CH2), 39.9 (CH2), 53.7 (CH2), 54.4 (CH2), 56.2 (CH2), 64.9 (CH), 67.0 (CH2), 123.2 (CHar), 123.9 (Cqar), 126.1 (CHar), 128.2 (CHar), 129.0 (CHar), 129.2 (CHar), 129.4 (CHar), 134.6 (2 Cqar), 139.6 (Cqar), 149.7 (Cqar), 159.2 (Cqar), 162.2 (Cqar), 167.6 (CO); HRMS (EI): calcd. for C32H38ClN4O2+ 545.2683, found 545.2639.
3.2.15. 2-Benzyl-7-(4-methoxyphenyl)-6-(3-morpholinopropyl)-3-(piperidin-1-yl)-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-one 11o
According to GP-1, 3-morpholinopropan-1-amine (14.6 µL), 4-methoxybenzaldehyde (12.2 µL), scandium (III) triflate (1.5 mg), 2-benzyl-3-oxo-3-(piperidin-1-yl)propanenitrile (29.1 mg), and maleic anhydride (13.7 mg) were reacted together in PhH (0.2 mL) to afford the product 11o (25.4 mg, 47%) as a yellow viscous liquid; Rf = 0.28 (AcOEt–EtOH = 10/1, v/v); FT–IR (ATR) υmax/cm−1 1115, 1252, 1443, 1512, 1689, 2852; 1H-NMR (500 MHz, CD3OD, 25 °C): δ 1.67–1.74 (m, 1H), 1.77–1.84 (m, 1H), 2.35–2.49 (m, 10H), 2.81–2.88 (m, 4H), 3.06–3.11 (m, 1H), 3.61–3.65 (m, 5H), 3.76–3.78 (m, 2H), 3.79 (s, 3H), 4.24 (d, J = 14.2 Hz, 1H), 4.32 (d, J = 14.1 Hz, 1H), 5.62 (s, 1H), 6.93–6.95 (m, 2H), 7.08–7.16 (m, 7H), 7.98 (s, 1H); 13C-NMR (126 MHz, CD3OD, 25 °C): δ 25.5 (CH2), 39.8 (CH2), 40.7 (CH2), 54.1 (CH2), 54.4 (CH2), 55.8 (CH3), 57.0 (CH2), 66.7 (CH), 67.4 (CH2), 68.1 (CH2), 115.5 (CHar), 125.2 (CHar), 125.6 (Cqar), 127.2 (CHar), 128.3 (Cqar), 129.2 (CHar), 129.7 (CHar), 130.7 (CHar), 140.6 (Cqar), 149.7 (Cqar), 161.7 (Cqar), 162.1 (Cqar), 163.6 (Cqar), 168.9 (CO); HRMS (EI): calcd. for C33H40N4O3 540.3100, found 540.2995.
3.2.16. 6-(2-(1H-Indol-3-yl)ethyl)-2-benzyl-7-(2-bromophenyl)-3-morpholino-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-one 11p
According to GP-1, tryptamine (16.0 mg), 2-bromobenzaldehyde (11.7 µL), scandium (III) triflate (1.5 mg), 2-isocyano-1-morpholino-3-phenylpropan-1-one (29.3 mg), and maleic anhydride (13.7 mg) were reacted together in PhH (0.2 mL) to afford the product 11p (39.4 mg, 65%) as a yellow solid; mp = 99–101 °C; Rf = 0.33 (Hex–AcOEt = 1/1, v/v); FT–IR (ATR) υmax/cm−1 420, 695, 738, 1112, 1440, 1674, 3287; 1H-NMR (500 MHz, CDCl3, 25 °C): δ 2.81–2.83 (m, 4H), 3.01–3.07 (m, 1H), 3.17–3.21 (m, 2H), 3.78–3.81 (m, 1H), 3.83–3.84 (m, 4H), 4.21 (d, J = 13.8 Hz, 1H), 4.32 (d, J = 13.9 Hz, 1H), 6.23 (s, 1H), 6.71–6.73 (m, 1H), 7.01 (d, J = 2.1 Hz, 1H), 7.06–7.09 (m, 1H), 7.15–7.23 (m, 8H), 7.28–7.29 (m, 1H), 7.55 (d, J = 7.9 Hz, 1H), 7.69–7.71 (m, 1H), 7.89 (s, 1H), 8.64 (s, 1H); 13C-NMR (126 MHz, CDCl3, 25 °C): δ 29.4 (CH2), 40.0 (CH2), 41.2 (CH2), 53.0 (CH2), 63.9 (CH), 67.1 (CH2), 111.3 (CHar), 112.2 (Cqar), 118.6 (CHar), 119.2 (CHar), 121.9 (CHar), 122.1 (CHar), 123.6 (CHar), 124.3 (Cqar), 125.7 (Cqar), 126.2 (CHar), 127.4 (Cqar), 128.0 (CHar), 128.2 (CHar), 128.9 (CHar), 130.1 (CHar), 133.4 (CHar), 135.1 (Cqar), 136.4 (Cqar), 139.2 (Cqar), 147.9 (Cqar), 160.3 (Cqar), 162.0 (Cqar), 167.4 (CO); HRMS (EI): calcd. for C34H32BrN4O2 607.1708, found 607.1710.
3.2.17. 2-Benzyl-7-hexyl-6-(2-hydroxyethyl)-3-morpholino-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-one 11q
According to GP-1, 2-aminoethan-1-ol (6.0 µL), heptanal (14.0 µL), scandium (III) triflate (1.5 mg), 2-isocyano-1-morpholino-3-phenylpropan-1-one (29.3 mg), and maleic anhydride (13.7 mg) were reacted together in PhH (0.2 mL) to afford the product 11q (8.7 mg, 20%) as a yellow viscous liquid; Rf = 0.22 (AcOEt); FT–IR (ATR) υmax/cm−1 1115, 1399, 1447, 1672; 1H-NMR (500 MHz, CDCl3, 25 °C): δ 0.73–0.78 (m, 1H), 0.84 (t, J = 7.1 Hz, 3H), 1.15–1.21 (m, 7H), 1.83–1.90 (m, 2H), 2.19–2.24 (m, 1H), 2.82–2.85 (m, 4H), 3.44–3.47 (m, 1H), 3.81–3.83 (m, 4H), 3.86–3.89 (m, 2H), 3.96–3.99 (m, 1H), 4.28 (d, J = 14.0 Hz, 1H), 4.42 (d, J = 14.0 Hz, 1H), 4.64–4.66 (m, 1H), 7.16–7.18 (m, 1H), 7.23–7.25 (m, 4H), 7.82 (s, 1H); 13C-NMR (126 MHz, CDCl3, 25 °C): δ 14.4 (CH3), 22.6 (2 CH2), 29.3 (CH2), 29.6 (CH2), 31.7 (CH2), 40.1 (CH2), 44.3 (CH2), 53.2 (CH2), 62.0 (CH2), 62.3 (CH), 67.3 (CH2), 123.6 (CHar), 124.7 (Cqar), 126.3 (CHar), 128.4 (CHar), 128.9 (CHar), 139.6 (Cqar), 147.7 (Cqar), 160.5 (Cqar), 161.6 (Cqar), 168.7 (CO); HRMS (EI): calcd. for C26H36N3O3+ 438.2757, found 438.2777.
3.2.18. 2-Benzyl-6-(2-hydroxyethyl)-7-(3-methoxyphenyl)-3-morpholino-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-one 11r
According to GP-1, 2-aminoethan-1-ol (6.0 µL), 3-methoxybenzaldehyde (12.2 µL), scandium (III) triflate (1.5 mg), 2-isocyano-1-morpholino-3-phenylpropan-1-one (29.3 mg), and maleic anhydride (13.7 mg) were reacted together in PhH (0.2 mL) to afford the product 11r (19.4 mg, 43%) as a yellow viscous liquid; Rf = 0.11 (Hex–AcOEt = 1/1, v/v); FT–IR (ATR) υmax/cm−1 1114, 1261, 1393, 1444, 1680, 2919; 1H-NMR (500 MHz, CDCl3, 25 °C): δ 2.83 (s, 1H), 2.77–2.82 (m, 4H), 3.16–3.19 (m, 1H), 3.40–3.43 (m, 1H), 3.56–3.59 (m, 1H), 3.74 (s, 3H), 3.77–3.79 (m, 4H), 3.95–3.99 (m, 1H), 4.2 (d, J = 14.0 Hz, 1H), 4.29 (d, J = 14.0 Hz, 1H), 5.61 (s, 1H), 6.70–6.71 (m, 1H), 6.77–6.78 (m, 1H), 6.86 (ddd, J = 0.9, 2.6, 8.3 Hz, 1H), 7.13–7.16 (m, 5H), 7.18–7.20 (m, 1H), 7.87 (s, 1H); 13C-NMR (126 MHz, CDCl3, 25 °C): δ 40.0 (CH2), 43.8 (CH2), 53.0 (CH2), 55.3 (CH3), 61.2 (CH2), 66.6 (CH), 67.1 (CH2), 113.7 (CHar), 114.0 (CHar), 120.3 (CHar), 126.2 (CHar), 127.4 (Cqar), 128.2 (CHar), 128.8 (CHar), 129.5 (CHar), 130.0 (CHar), 136.9 (Cqar), 139.2 (Cqar), 147.9 (Cqar), 160.0 (Cqar), 160.4 (Cqar), 162.1 (Cqar), 168.2 (CO); HRMS (EI): calcd. for C27H29N3O4 459.2158, found 459.2158.
3.2.19. 2-Benzyl-6-(2-bromoethyl)-3-morpholino-7-phenyl-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-one 11s
According to GP-1, 22-bromoethan-1-amina (20.5 mg), benzaldehyde (10.2 µL), scandium (III) triflate (1.5 mg), 2-isocyano-1-morpholino-3-phenylpropan-1-one (29.3 mg), and maleic anhydride (13.7 mg) were reacted together in dry PhH (0.2 mL) to afford the product 11s (15.2 mg, 31%) as a yellow viscous liquid; Rf = 0.48 (Hex–AcOEt = 1/1, v/v); FT–IR (ATR) υmax/cm−1 699, 747, 1114, 1391, 1443, 1696; 1H-NMR (500 MHz, CDCl3, 25 °C): δ 2.78–2.82 (m, 4H), 3.32–3.40 (m, 2H), 3.57–3.61 (m, 1H), 3.78–3.80 (m, 4H), 4.21 (d, J = 14.0 Hz, 1H), 4.29 (d, J = 13.9 Hz, 1H), 4.30–4.33 (m, 1H), 5.72 (s, 1H), 7.11–7.18 (m, 8H), 7.36–7.38 (m, 2H), 7.92 (s, 1H); 13C-NMR (126 MHz, CDCl3, 25 °C): δ 29.6 (CH2), 40.1 (CH2), 42.3 (CH2), 53.0 (CH2), 66.3 (CH), 67.1 (CH2), 123.6 (Cqar), 124.0 (CHar), 126.2 (CHar), 128.0 (CHar), 128.2 (CHar), 128.7 (CHar), 128.9 (CHar), 129.1 (CHar), 135.1 (Cqar), 139.1 (Cqar),147.9 (Cqar), 160.4 (Cqar), 162.4 (Cqar), 167.5 (CO); HRMS (EI): calcd. for C26H26BrN3O2 491.1208, found 491.1208.
3.3. One-Pot Synthesis and Characterization of the Polysubstituted Pyrrolo[3,4-b]pyridin-5-ones 11t–x
General procedure 2 (GP-2): The 2-bromoethan-1-amine (0.1 mmol, 1.0 equiv.) and the corresponding aldehydes (1.0 equiv.) were placed in a 10 mL sealed CEM Discover microwave reaction tube and diluted in benzene [0.5 M]. The mixture was stirred and irradiated (MW, 65 °C, 55 W) for 15 min, and then Sc(OTf)3 (0.03 equiv.) was added. The mixture was stirred and irradiated (MW, 65 °C, 55 W) for 15 min, and then the corresponding isocyanides (1.2 equiv.) were added. The mixture was stirred and irradiated (MW, 80 °C, 100 W) for 30 min, and then maleic anhydride (1.4 equiv.) was added. The mixture was stirred and irradiated (MW, 80 °C, 100 W) for 30 min, and then the solvent was removed to dryness under vacuum. Anhydrous acetonitrile [0.5 M] was added. Finally, the corresponding secondary amine (1.0 equiv.) and triethylamine (1.1 equiv.) were sequentially added, and then the new reaction mixture was stirred and irradiated (MW, 80 °C, 100 W) for 30 min. Then, the solvent was removed to dryness under vacuum. The crude was diluted in dichloromethane (5.0 mL), washed with a concentrated aqueous solution of NaHCO3 (3 × 25 mL), and then washed with brine (3 × 25 mL). The organic layer was dried using anhydrous Na2SO4 and then filtered over a celite pad. The solvent was removed to dryness under vacuum. The residue was purified immediately using a silica-gel column chromatography followed by a preparative TLC using mixtures of Hex–EtOAc or EtOAc-EtOH (v/v) in different proportions as mobile phase to afford the corresponding polyheterocyclic pyrrolo[3,4-b]pyridin-5-ones 11t–x.
3.3.1. 2-Benzyl-3-morpholino-6-(2-morpholinoethyl)-7-phenyl-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-one 11t
According to GP-2, 2-bromoethan-1-amine (20.5 mg), benzaldehyde (10.2 µL), scandium (III) triflate (1.5 mg), 2-isocyano-1-morpholino-3-phenylpropan-1-one (29.3 mg), maleic anhydride (13.7 mg), morpholine (8.7 µL), and triethylamine (15.3 µL) were reacted together first in PhH (0.2 mL) and then in MeCN (0.2 mL) to afford the product 11t (17.4 mg, 35%) as a yellow solid; mp = 71–73 °C; Rf = 0.17 (AcOEt); FT–IR (ATR) υmax/cm−1 1015, 1115, 1393, 1443, 1693; 1H-NMR (500 MHz, CDCl3, 25 °C): δ 2.38–2.43 (m, 4H), 2.45–2.50 (m, 1H), 2.56–2.61 (m, 1H), 2.78–2.84 (m, 4H), 3.00–3.06 (m, 1H), 3.66–3.67 (m, 4H), 3.68–3.80 (m, 4H), 4.09–4.14 (m, 1H), 4.21 (d, J = 13.9 Hz, 1H), 4.28 (d, J = 13.9 Hz, 1H), 5.73 (s, 1H), 7.14–7.19 (m, 8H), 7.34–7.36 (m, 2H), 7.88 (s, 1H); 13C-NMR (126 MHz, CDCl3, 25 °C): δ 36.8 (CH2), 40.1 (CH2), 53.1 (CH2), 53.7 (CH2), 57.3 (CH2), 66.2 (CH), 67.0 (CH2), 67.2 (CH2), 123.8 (CHar), 126.2 (CHar), 127.8 (CHar), 128.2 (CHar), 128.6 (CHar), 128.8 (CHar), 129.0 (CHar), 129.6 (Cqar), 135.8 (Cqar), 139.3 (Cqar), 147.7 (Cqar), 160.8 (Cqar), 161.8 (Cqar), 167.2 (Cq); HRMS (EI): calcd. for C30H34N4O3 498.2631, found 498.2631.
3.3.2. 2-Benzyl-7-(4-methoxyphenyl)-6-(2-(4-(2-methoxyphenyl)piperazin-1-yl)ethyl)-3-morpholino-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-one 11u
According to GP-2, 2-bromoethan-1-amine (20.5 mg), 4-methoxybenzaldehyde (12.2 µL), scandium (III) triflate (1.5 mg), 2-isocyano-1-morpholino-3-phenylpropan-1-one (29.3 mg), maleic anhydride (13.7 mg), 1-(2-methoxyphenyl)piperazine (19.2 mg), and triethylamine (15.3 µL) were reacted together first in PhH (0.2 mL) and then in MeCN (0.2 mL) to afford the product 11u (21.5 mg, 34%) as a yellow viscous liquid; Rf = 0.71 (AcOEt–EtOH = 10/1, v/v); FT–IR (ATR) υmax/cm−1 1114, 1241, 1444, 1504, 1689, 2821; 1H-NMR (500 MHz, CDCl3, 25 °C): δ 2.59–2.71 (m, 4H), 2.78–2.82 (m, 6H), 3.05–3.08 (m, 4H), 3.78–3.79 (m, 5H), 3.80 (s, 3H), 3.84 (s, 3H), 4.07–4.13 (m, 1H), 4.22 (d, J = 13.9 Hz, 1H), 4.29 (d, J = 13.9 Hz, 1H), 5.73 (s, 1H), 6.86–6.91 (m, 6H), 7.11–7.16 (m, 7H), 7.89 (s, 1H); 13C-NMR (126 MHz, CDCl3, 25 °C): δ 36.9 (CH2), 40.1 (CH2), 50.5 (CH2), 53.0 (CH2), 53.4 (CH2), 55.3 (CH3), 55.4 (CH3), 56.9 (CH2), 65.8 (CH), 67.1 (CH2), 111.2 (CHar), 114.4 (CHar), 118.2 (CHar), 121.0 (CHar), 123.0 (CHar), 123.9 (CHar), 124.0 (Cqar), 126.1 (CHar), 127.5 (Cqar), 128.2 (CHar), 128.7 (CHar), 129.1 (CHar), 139.3 (Cqar), 141.2 (Cqar), 147.7 (Cqar), 152.2 (Cqar), 159.8 (Cqar), 161.1 (Cqar), 161.8 (Cqar), 167.0 (Cq); HRMS (EI): calcd. for C38H43N5O4 633.3315, found 633.3317.
3.3.3. 2-Benzyl-7-(4-fluorophenyl)-6-(2-(4-(2-methoxyphenyl)piperazin-1-yl)ethyl)-3-morpholino-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-one 11v
According to GP-2, 2-bromoethan-1-amine (20.5 mg), 4-fluorobenzaldehyde (10.7 µL), scandium (III) triflate (1.5 mg), 2-isocyano-1-morpholino-3-phenylpropan-1-one (29.3 mg), maleic anhydride (13.7 mg), 1-(2-methoxyphenyl)piperazine (19.2 mg), and triethylamine (15.3 µL) were reacted together first in PhH (0.2 mL) and then in MeCN (0.2 mL) to afford the product 11v (20.5 mg, 33%) as a yellow viscous liquid; Rf = 0.73 (AcOEt–EtOH = 10/1, v/v); FT–IR (ATR) υmax/cm−1 698, 748, 1027, 1114, 1239, 1444, 1500, 1693, 2819; 1H-NMR (500 MHz, CDCl3, 25 °C): δ 2.52–2.73 (m, 6H), 2.80–2.83 (m, 4H), 3.04–3.08 (m, 4H), 3.69–3.75 (m, 1H), 3.78–3.80 (m, 4H), 3.84 (s, 3H), 4.11–4.16 (m, 1H), 4.23 (q, J =13.9 Hz, 1H), 4.27 (q, J =14.0 Hz, 1H), 5.79 (s, 1H), 6.84–6.86 (m, 1H), 6.91–6.93 (m, 2H), 6.97–7.00 (m, 1H), 7.03–7.07 (m, 3H), 7.11–7.19 (m, 6H), 7.89 (s, 1H); 13C-NMR (126 MHz, CDCl3, 25 °C): δ 37.0 (CH2), 40.1 (CH2), 50.6 (CH2), 53.1 (CH2), 53.4 (CH2), 55.4 (CH3), 57.0 (CH2), 65.6 (CH), 67.1 (CH2), 111.3 (CHar), 116.0 (d, oJCF = 21.7 Hz) (CHar), 118.2 (CHar), 121.0 (CHar), 123.0 (CHar), 123.9 (CHar), 126.2 (CHar), 128.2 (CHar), 128.8 (CHar), 129.5 (d, mJCF = 8.3 Hz) (CHar), 131.6 (d, pJCF = 3.1 Hz) (Cqar), 139.2 (Cqar), 141.2 (Cqar), 147.8 (Cqar), 152.3 (Cqar), 160.6 (Cqar), 161.9 (Cqar), 162.8 (d, iJCF = 247.4 Hz) (Cqar), 167.1 (Cq); HRMS (EI): calcd. for C37H40FN5O3 621.3115, found 621.3113.
3.3.4. 2-Benzyl-6-(2-((3,4-dimethoxybenzyl)(methyl)amino)ethyl)-3-morpholino-7-phenyl-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-one 11w
According to GP-2, 2-bromoethan-1-amine (20.5 mg), benzaldehyde (10.2 µL), scandium (III) triflate (1.5 mg), 2-isocyano-1-morpholino-3-phenylpropan-1-one (29.3 mg), maleic anhydride (13.7 mg), 2-(3,4-dimethoxyphenyl)-N-methylethan-1-amine (23.8 mg), and triethylamine (15.3 µL) were reacted together first in PhH (0.2 mL) and then in MeCN (0.2 mL) to afford the product 11w (18.2 mg, 30%) as a yellow viscous liquid; Rf = 0.35 (AcOEt–EtOH = 10/1, v/v); FT–IR (ATR) υmax/cm−1 700, 1028, 1114, 1236, 1261, 1443, 1515, 1691; 1H-NMR (500 MHz, CDCl3, 25 °C): δ 2.31 (s, 3H), 2.54–2.64 (m, 2H), 2.66–2.74 (m, 2H), 2.80–2.83 (m, 4H), 2.99–3.07 (m, 2H), 3.79–3.81 (m, 4H), 3.82 (s, 3H), 3.84 (s, 3H), 3.85–3.87 (m, 1H), 4.01–4.13 (m, 1H), 4.23 (d, J = 13.9 Hz, 1H), 4.28 (d, J = 14.0 Hz, 1H), 5.66 (s, 1H), 6.67–6.70 (m, 2H), 6.73–6.75 (m, 1H), 7.17–7.18 (m, 7H), 7.36–7.35 (m, 3H), 7.89 (s, 1H); 13C-NMR (126 MHz, CDCl3, 25 °C): δ 33.2 (CH2), 37.8 (CH2), 40.1 (CH2), 42.1 (CH2), 53.1 (CH2), 55.5 (CH2), 55.9 (CH3), 56.0 (CH3), 59.7 (CH2), 66.2 (CH), 67.2 (CH2), 111.4 (CHar), 112.1 (CHar), 120.6 (CHar), 123.8 (CHar), 124.0 (Cqar), 126.2 (CHar), 128.0 (CHar), 128.2 (CHar), 128.7 (Cqar), 128.8 (CHar), 129.0 (CHar), 132.6 (Cqar), 135.8 (Cqar), 139.4 (Cqar), 147.4 (Cqar), 147.8 (Cqar), 148.9 (Cqar), 160.8 (Cqar), 161.8 (Cqar), 167.3 (Cq); HRMS (EI): calcd. for C37H42N4O4 [M] 606.3206, found 606.3208.
3.3.5. 2-Benzyl-6-(2-((3,4-dimethoxybenzyl)(methyl)amino)ethyl)-7-(4-methoxyphenyl)-3-morpholino-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-one 11x
According to GP-2, 2-bromoethan-1-amine (20.5 mg), 4-methoxybenzaldehyde (12.2 µL), scandium (III) triflate (1.5 mg), 2-isocyano-1-morpholino-3-phenylpropan-1-one (29.3 mg), maleic anhydride (13.7 mg), 2-(3,4-dimethoxyphenyl)-N-methylethan-1-amine (23.8 mg), and triethylamine (15.3 µL) were reacted together first in PhH (0.2 mL) and then in MeCN (0.2 mL) to afford the product 11x (22.3 mg, 35%) as a yellow viscous liquid; Rf = 0.33 (Hex–AcOEt = 1/1, v/v); FT–IR (ATR) υmax/cm−1 698, 749, 1027, 1114, 1241, 1444, 1504, 1689, 2849; 1H-NMR (500 MHz, CDCl3, 25 °C): δ 2.29 (s, 3H), 2.52–2.54 (m, 1H), 2.59–2.61 (m, 1H), 2.66–2.67 (m, 2H), 2.79–2.82 (m, 4H), 2.97–3.02 (m, 1H), 3.77–3.80 (m, 9H), 3.81 (s, 3H), 3.82 (s, 3H), 4.05–4.08 (m, 1H), 4.23 (d, J = 13.9, Hz, 1H), 4.29 (d, J = 13.9 Hz, 1H), 5.60 (s, 1H), 6.67–6.69 (m, 2H), 6.72–6.74 (m, 1H), 6.87–6.89 (m, 2H), 7.06–7.08 (m, 2H), 7.10–7.13 (m, 1H), 7.13–7.16 (m, 6H), 7.88 (s, 1H); 13C-NMR (126 MHz, CDCl3, 25 °C): δ 33.3 (CH2), 37.7 (CH2), 40.0 (CH2), 42.1 (CH2), 53.1 (CH2), 55.3 (CH3), 55.6 (CH2), 55.8 (CH3), 55.9 (CH3), 59.8 (CH2), 65.6 (CH), 67.2 (CH2), 111.3 (CHar),112.0 (CHar), 120.5 (CHar), 123.7 (CHar), 124.1 (Cqar), 126.1 (CHar), 127.6 (CHar), 128.1 (CHar), 128.7 (CHar), 129.2 (CHar), 129.5 (Cqar), 132.8 (Cqar), 139.4 (Cqar), 147.3 (Cqar), 147.6 (Cqar), 148.8 (Cqar), 159.8 (Cqar), 161.0 (Cqar), 161.7 (Cqar), 167.0 (Cq); HRMS (EI): calcd. for C38H44N4O5 [M] 636.3312, found 636.3315.
4. Conclusions
The herein described one-pot synthetic strategy, based on multicomponent reactions, allowed for the rapid construction of various new polyheterocyclic compounds (for example, pyrrolo[3,4-b]pyridine-5-ones linked with other heterocycles like morpholines, piperidines, and piperazines). Hence, highly polysubstituted and polyheterocyclic pyrrolo[3,4-b]pyridin-5-ones, including some Falipamil aza-analogues and a pair of piperazine-linked analogues, were synthesized through a robust one-pot process. Likewise, a novel and complex sequence Ugi-3CR/aza Diels-Alder/N-acylation/aromatization (decarboxylation-dehydration) was carried out in a one-pot manner including an additional final SN2 step to provide a wide diversity of polyheterocycles. Despite the molecular complexity of the final products and the high number of formed bonds, modest to good yields were obtained. The high atomic economy (-2H2O and -CO2) provides to this methodology the category of ‘sustainable’. The final products could be considered for further SAR studies, since the pyrrolo[3,4-b]pyridine-5-one scaffold, piperazine-linker, and the drug falipamil are of high interest in medicinal chemistry.
Supplementary Materials
The following are available online: 1H and 13C-NMR spectra of all the products 11a–x Figures S1–S48.
Acknowledgments
A.N.G.-G (25741/CB-2014-236879), G.K.H.-C. (24741/CB-2014-236879), and D.Z.Z. (23890/CB-2014-236879) thank CONACYT for their internships. J.T. acknowledges the financial support provided by SIP-IPN (Grants 20170791 and 20170902) and CONACYT (Grant 178319). J.T. is a fellow of the EDI-IPN and COFAA-IPN programs. I.A.I. acknowledges PAPIIT-UNAM-Mexico (IN101517) and CONACYT (1789) for financial support. A.I.-J. acknowledges QI-DQ-CBI-UAMI for his visiting professor position (40966) and PRODEP-SEP for financial support (12413143). E.G.-Z. acknowledges CONACyT (CB-2014-236879) and PRODEP-SEP (Article Processing Charges and/or Publication Fees) for financial support.
Author Contributions
A.Z.-M. synthesized the polyheterocyclic pyrrolo[3,4-b]pyridin-5-ones herein described. A.B.-M. synthesized the piperazine-containing analogues. D.Z.-Z. synthesized the aza-analogues of falipamil. A.N.G.-G. and G.K.H.-K. integrated the supporting information file. J.T. characterized the products by spectroscopic techniques. I.A.I., A.I.-J., and E.G.-Z. are the responsible researchers who wrote the manuscript and to whom correspondence must be addressed.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Reiffen, M.; Eberlein, W.; Müller, P.; Psiorz, M.; Noll, K.; Heider, J.; Lillie, C.; Kobinger, W.; Luger, P. Specific Bradycardic Agents. 1. Chemistry, Pharmacology, and Structure–Activity Relationships of Substituted Benzazepinones, a New Class of Compounds Exerting Antiischemic Properties. J. Med. Chem. 1990, 33, 1496–1504. [Google Scholar] [CrossRef] [PubMed]
- Rieu, J.P.; Duflos, A.; Tristani, J.C.; Patoiseau, J.F.; Tisne-Versailles, J.; Bessac, A.M.; Bonnafous, R.; Marty, A.; Verscheure, Y.; Bigg, D.C.H. Synthesis and bradycardic activity of a series of substituted 3-aminoalkyl-2,3-dihydro-4H-l,3-benzoxazin-4-ones as potent antiischemics. Eur. J. Med. Chem. 1993, 28, 683–691. [Google Scholar] [CrossRef]
- Boucher, M.; Chassaing, C.; Chapuy, E. Cardiac electrophysiological effects of falipamil in the conscious dog: Comparison with alinidine. Eur. J. Pharmacol. 1996, 306, 93–98. [Google Scholar] [CrossRef]
- Speck, K.; Magauer, T. The chemistry of isoindole natural products. Beilstein J. Org. Chem. 2013, 9, 2048–2078. [Google Scholar] [CrossRef] [PubMed]
- Korch, K.M.; Eidamshaus, C.; Behenna, D.C.; Nam, S.; Horne, D.; Stoltz, B.M. Enantioselective Synthesis of α-Secondary and α-Tertiary Piperazin-2-ones and Piperazines by Catalytic Asymmetric Allylic Alkylation. Angew. Chem. Int. Ed. 2015, 54, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.-P.; Kung, M.-P.; Mu, M.; Kung, H.F. Isoindol-1-one Analogues of 4-(2′-methoxyphenyl)-1 [2′-[N-(2′′-pyridyl)-p-iodobenzamido]ethyl]piperazine (p-MPPI) as 5-HT1A Receptor Ligands. J. Med. Chem. 1998, 41, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Lorion, M.; Couture, A.; Deniau, E.; Grandclaudon, P. Complementary Synthetic Approaches to Constitutionally Diverse N-Aminoalkylated Isoindolinones: Application to the Synthesis of Falipamil and 5-HT1A Receptor Ligand Analogues. Synthesis 2009, 11, 1897–1903. [Google Scholar] [CrossRef]
- Sović, I.; Karminski-Zamola, G. Derivati izoindolina, sinteza i biološka aktivnost. I. Prirodni i sintetski derivati izoindolina. Kem. Ind. 2014, 63, 173–182. [Google Scholar] [CrossRef]
- Ibarra, I.A.; Islas-Jácome, A.; González-Zamora, E. Synthesis of polyheterocycles via multicomponent reactions. Org. Biomol. Chem. 2018, 16, 1402–1418. [Google Scholar] [CrossRef] [PubMed]
- Zamudio-Medina, A.; García-González, M.C.; Padilla, J.; González-Zamora, E. Synthesis of a tetracyclic lactam system of Nuevamine by four-component reaction and free radical cyclization. Tetrahedron Lett. 2010, 51, 4837–4839. [Google Scholar] [CrossRef]
- Islas-Jácome, A.; González-Zamora, E.; Gámez-Montaño, R. A short microwave-assisted synthesis of tetrahydroisoquinolinpyrrolopyridinones by a triple process: Ugi-3CR–aza Diels–Alder/S-oxidation/Pummerer. Tetrahedron Lett. 2011, 52, 5245–5248. [Google Scholar] [CrossRef]
- Islas-Jácome, A.; Cárdenas-Galindo, L.E.; Jerezano, A.V.; Tamariz, J.; González-Zamora, E.; Gámez-Montaño, R. Synthesis of Nuevamine Aza-Analogues by a Sequence: I-MCR–Aza-Diels–Alder–Pictet–Spengler. Synlett 2012, 23, 2951–2956. [Google Scholar] [CrossRef]
- Vázquez-Vera, O.; Sánchez-Badillo, J.S.; Islas-Jácome, A.; Rentería-Gómez, M.A.; Pharande, S.G.; Cortes-García, C.J.; Rincón-Guevara, M.A.; Ibarra, I.A.; Gámez-Montaño, R.; González-Zamora, E. An efficient Ugi-3CR/aza Diels–Alder/Pomeranz–Fritsch protocol towards novel aza-analogues of (±)-nuevamine, (±)-lennoxamine and magallanesine: A diversity oriented synthesis approach. Org. Biomol. Chem. 2017, 15, 2363–2369. [Google Scholar] [CrossRef] [PubMed]
- Islas-Jácome, A.; Gutierrez-Carrillo, A.; García-Garibay, M.A.; González-Zamora, E. One-Pot Synthesis of Nuevamine Aza-Analogues by Combined Use of an Oxidative Ugi Type Reaction and Aza-Diels–Alder Cycloaddition. Synlett 2014, 25, 403–406. [Google Scholar] [CrossRef]
- Zamudio-Medina, A.; García-González, M.C.; Gutierrez-Carrillo, A.; González-Zamora, E. Synthesis of cyclic analogues of hexamethylenebis(3-pyridine)amide (HMBPA) in a one-pot process. Tetrahedron Lett. 2015, 56, 627–629. [Google Scholar] [CrossRef]
- Sun, X.; Janvier, P.; Zhao, G.; Bienaymé, H.; Zhu, J. A Novel Multicomponent Synthesis of Polysubstituted 5-Aminooxazole and Its New Scaffold-Generating Reaction to Pyrrolo[3,4-b]pyridine. Org. Lett. 2001, 3, 877–880. [Google Scholar] [CrossRef] [PubMed]
- Janvier, P.; Sun, X.; Bienaymé, H.; Zhu, J. Ammonium Chloride-Promoted Four-Component Synthesis of Pyrrolo[3,4-b]pyridin-5-one. J. Am. Chem. Soc. 2002, 124, 2560–2567. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.M.; Danishefsky, S.J. On the Reach of Chemical Synthesis: Creation of a MiniPipeline from an Academic Laboratory. Angew. Chem. Int. Ed. 2010, 49, 6032–6056. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Sugiura, M.; Kitagawa, H.; Lam, W.E.-L. Rare-Earth Metal Triflates in Organic Synthesis. Chem. Rev. 2002, 102, 2227–2302. [Google Scholar] [CrossRef] [PubMed]
- Kappe, C.O.; Dallinger, D. Controlled microwave heating in modern organic synthesis: Highlights from the 2004–2008 literature. Mol. Divers. 2009, 13, 71–193. [Google Scholar] [CrossRef] [PubMed]
- Fayol, A.; Housseman, C.; Sun, X.; Janvier, P.; Bienaymé, H.; Zhu, J. Synthesis of α-Isocyano-α-alkyl(aryl)acetamides and their Use in the Multicomponent Synthesis of 5-Aminooxazole, Pyrrolo[3,4-b]pyridin-5-one and 4,5,6,7-Tetrahydrofuro[2,3-c]pyridine. Synthesis 2005, 1, 161–165. [Google Scholar] [CrossRef]
- Rentería-Gómez, M.A.; Morales-Salazar, I.; García-González, N.; Segura-Olvera, D.; Sánchez-Serratos, M.; Ibarra, I.A.; González-Zamora, E.; Gámez-Montaño, R.; Islas-Jácome, A. Ultrasound-assisted synthesis of eight novel and highly functionalized 2-aminonitrile oxazoles via Ugi-3CR. In Proceedings of the 21st International Electronic Conference on Synthetic Organic Chemistry, 1–30 November 2017; Sciforum Electronic Conference Series 2017. Volume 21. [Google Scholar] [CrossRef][Green Version]
- González-López, M.; Shaw, J.T. Cyclic Anhydrides in Formal Cycloadditions and Multicomponent Reactions. Chem. Rev. 2009, 109, 164–189. [Google Scholar] [CrossRef] [PubMed]
- Purser, S.; Moore, P.R.; Sallow, S.; Gouverneur, V. Fluorine in Medicinal Chemistry. Chem. Soc. Rev. 2008, 37, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.-Q.-Q.; Yang, W.-C.; Zhai, D.-D.; Zhang, X.-Y.; Li, S.-Q.; Guan, B.-T. Nickel-catalyzed C–N bond reduction of aromatic and benzylic quaternary ammonium triflates. Chem. Commun. 2016, 52, 10894–10897. [Google Scholar] [CrossRef]
- Tobisu, M.; Nakamura, K.; Chatani, N. Nickel-Catalyzed Reductive and Borylative Cleavage of Aromatic Carbon−Nitrogen Bonds in N-Aryl Amides and Carbamates. J. Am. Chem. Soc. 2014, 136, 5587–5590. [Google Scholar] [CrossRef] [PubMed]
- Chambers, R.R., Jr.; Collins, C.J.; Maxwell, B.E. Reductive Debenzylation of 1-Benzylnaphtalene by a Na-K Alloy. J. Org. Chem. 1985, 50, 4960–4963. [Google Scholar] [CrossRef]
- Islas-Jácome, A.; Rentería-Gómez, A.; Rentería-Gómez, M.A.; González-Zamora, E.; Jiménez-Halla, J.O.C.; Gámez-Montaño, R. Selective reaction route in the construction of the pyrrolo[3,4-b]pyridine-5-one core from a variety of 5-aminooxazoles and maleic anhydride. A DFT study. Tetrahedron Lett. 2016, 57, 3496–3500. [Google Scholar] [CrossRef]
- Rentería-Gómez, A.; Islas-Jácome, A.; Cruz-Jiménez, A.E.; Manzano-Velázquez, J.C.; Rojas-Lima, S.; Jiménez-Halla, J.O.C.; Gámez-Montaño, R. Synthesis of 2-Tetrazolylmethyl-isoindolin-1-ones via a One-Pot UgiAzide/(N-Acylation/exo-Diels−Alder)/Dehydration Process. ACS Omega 2016, 1, 943–951. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 11a–x are available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).