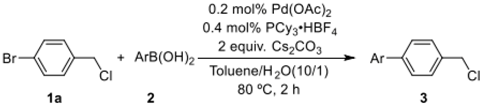
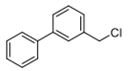
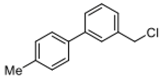
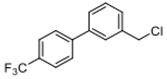
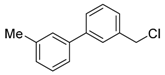
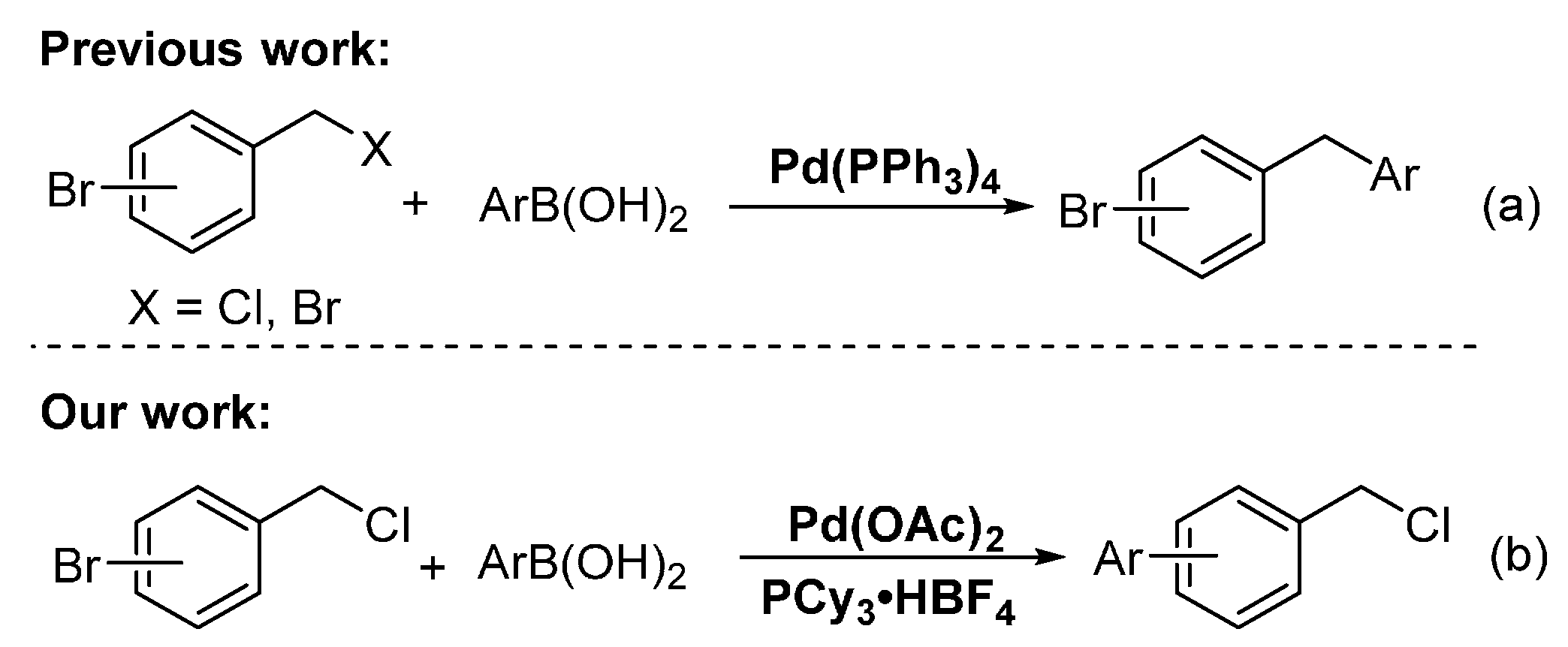Abstract
Highly selective C(sp2)–C(sp2) cross-coupling of dihalogenated hydrocarbons comprising C(sp2)–Br and C(sp3)–Cl bonds with arylboronic acids is reported. This highly selective coupling reaction of the C(sp2)–Br bond is successfully achieved using Pd(OAc)2 and PCy3·HBF4 as the palladium source and ligand, respectively. A series of chloromethyl-1,1′-biphenyl compounds are obtained in moderate-to-excellent yields. Moreover, this protocol can be extended to the one-pot dual arylation of 1-bromo-4-(chloromethyl)benzene with two arylboronic acids, leading to diverse unsymmetrical 4-benzyl-1,1′-biphenyl derivatives.
1. Introduction
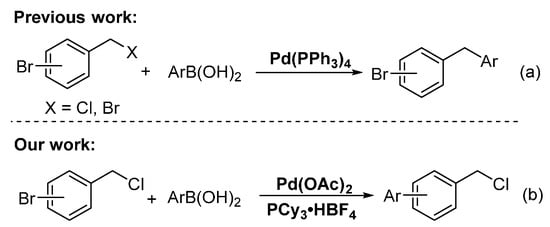
Transition-metal-catalyzed cross-coupling reactions between electrophiles and arylboronic acids are an important method in the C–C bond formation [1,2,3]. Over the past decades, aryl and benzyl halides have been used as electrophiles for constructing C(sp2)–C(sp2) and C(sp3)–C(sp2) bonds, respectively [4,5,6,7,8,9,10]. To the best of our knowledge, the selective reaction of dihalogenated hydrocarbons containing the C(sp2)–X and C(sp3)–X bonds with arylboronic acids in the presence of palladium catalyst has been rarely reported [11,12,13,14,15,16]. Duchêne and Thibonnet reported the Pd(PPh3)4-catalyzed selective coupling reaction of C(sp3)–Br at the benzylic position of the starting bromobenzyl bromides with arylboronic acids, affording C(sp3)–C(sp2) as the coupling products (Scheme 1a) [11,12]. Gueiffier also developed a Pd(PPh3)4-catalyzed one-pot two-step reaction of bromobenzyl chloride with arylboronic acids by first C(sp3)–C(sp2) coupling and subsequent C(sp2)–C(sp2) coupling, affording numerous new unsymmetrical methylene-linked biaryl systems (Scheme 1a)[13]. Çetinkaya et al. achieved the palladium-catalyzed C(sp2)–C(sp2) coupling reactions with 1-bromo-4-(bromomethyl)benzene and arylboronic acids by using saturated N-heterocarbene ligands [17]. In 2010, Maseras conducted an experimental and theoretical study on the role of phosphine ligands in palladium-catalyzed Suzuki cross-coupling of competitive and selective C(sp3)–Br versus C(sp2)–Br bond activation [18]. Their results indicated that as a less-hindered phosphine, PPh3 is associated with a bisligated form of the catalyst, which favors the activation of the C(sp3)–Br bond of the α-bromosulfoxide side. As the more hindered phosphine, P(1-napthyl)3 is related to the monoligated form of the catalyst, which promotes the activation of the C(sp2)–Br bond of the bromoaryl moiety.
Scheme 1.
Selective palladium-catalyzed Suzuki-Miyaura coupling reaction. (a) C(sp3)–C(sp2) coupling; (b) C(sp2)–C(sp2) coupling.
Inspired by this study and based on our previous studies [19,20,21,22,23,24,25], we reported Pd-catalyzed, highly selective C(sp2)-Br bond coupling reactions of o-(or m-, or p-)chloromethyl bromobenzene with arylboronic acids in the presence of the ligand PCy3·HBF4, which does not afford C(sp3)–C(sp2) coupling products; instead, the reaction provided highly selective of C(sp2)–C(sp2) coupling products (Scheme 1b).
2. Results and Discussion
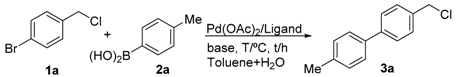
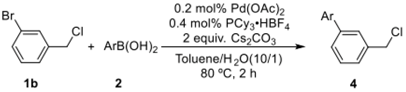
Initially, 1-bromo-4-(chloromethyl)benzene and p-tolylboronic acid were selected as model substrates to optimize the reaction conditions. Table 1 summarizes the results obtained. The screened bases were examined by using PCy3·HBF4 and Pd(OAc)2 as the ligand and palladium source, respectively, in toluene/water (1/0.1) at 80 °C for 2 h; Cs2CO3 was the most effective base, affording the desired product in 99% yield (entry 5). On the other hand, other bases such as K2CO3, K3PO4·3H2O, NaOH, and NEt3 afforded the desired products in 16–84% yields (entries 1–4). Remarkably, the ligand was found to play an important role in this reaction, and PPh3 was not effective for this selective C(sp2)–C(sp2) coupling reaction (entry 6). Moreover, with the decrease in the reaction temperature to 60 °C, the product was obtained in only 74% yield (entry 7). With the decrease in the catalyst amount from 1 mol % to 0.2 mol %, the desired product was still obtained in a gas chromatography-mass spectrometer (GC–MS) yield of 99% (entries 8–10). However, with the decrease in the catalyst loading to 0.1 mol %, the yield was significantly reduced (entry 11). Finally, the combination of Pd(OAc)2 (0.2 mol %)/PCy3·HBF4 (0.4 mol %) and Cs2CO3 (2 equiv.) at 80 °C for 2 h in toluene/water (1 mL/0.1 mL) was found to constitute the optimum reaction conditions.

Table 1.
Optimized reaction conditions a.
With the optimized reaction conditions in hand, the substrate scope of this selective Suzuki–Miyaura reaction was examined. First, the coupling reactions of 1-bromo-4-(chloromethyl)benzene with arylboronic acid were explored under the optimized reaction conditions. 4-Substituted arylboronic acids bearing electron-donating or electron-withdrawing groups selectively underwent the coupling reaction, affording corresponding products 3b–3g in 75–93% yields (Table 2). Furthermore, the selective coupling reaction of 1-bromo-4-(chloromethyl)benzene, with m-tolylboronic acid, and (3-chlorophenyl)boronic acid afforded the desired products 3h and 3i in 98% and 73% yields, respectively. Sterically demanding ortho substituents, such as o-tolylboronic acid, did not impair the coupling reaction, affording the desired product 3j in 90% yield. However, (2,3-difluorophenyl)boronic acid and (2,6-dimethylphenyl)boronic acid as substrates afforded coupling products 3k and 3l, respectively, in low yields. In addition, thiophen-3-ylboronic acid, naphthalen-2-ylboronic acid, and 4-vinylphenylboronic acid were tolerated, affording desired products 3n–3p in 57–86% yields.

Table 2.
Selective coupling reaction of 1-bromo-4-(chloromethyl)benzene with arylboronic acid a.
Next, the selective coupling reactions of 1-bromo-3-(chloromethyl)benzene with various arylboronic acids were investigated (Table 3). The results indicated that neither the electronic property nor the steric hindrance of the substrates clearly affects the coupling reaction: The desired products 4a–4h were obtained in 73–95% yields.

Table 3.
Selective coupling reaction of arylboronic acid with 1-bromo-3-(chloromethyl)benzene a.
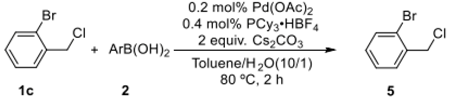
To further ascertain the application scope of the catalytic system, the reaction of 1-bromo-2-(chloromethyl)benzene with arylboronic acids was examined. The present catalytic method can be applied for the selective coupling of 1-bromo-2-(chloromethyl)benzene with arylboronic acids, affording the desired products 5a–5h in yields of 80–95% (Table 4).

Table 4.
Selective coupling reaction of arylboronic acid with 1-bromo-2-(chloromethyl)benzene a.
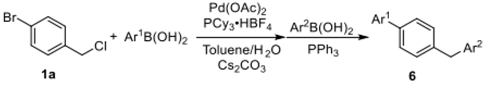
A one-pot dual Suzuki coupling reaction for successively substituting 4-bromobenzyl chloride with distinct aryl groups was contemplated, which could provide a straightforward route for obtaining diverse 4-benzyl-1,1′-biphenyl derivatives (Table 5). First, 4-bromobenzyl chloride was treated with 1.1 equivalent of p-tolylboronic acid in the presence of 2 mol % of Pd(OAc)2, 0.4 mol % of PCy3·HBF4, and 5.0 equiv. of Cs2CO3 in a mixture of toluene and water (10:1). After heating for 2 h at 80 °C, 1.0 equivalent of arylboronic acid and 4.0 mol % of PPh3 were added to the reaction system. The reaction mixture was stirred for 5 h at 80 °C, affording the desired products 6a–6d in 57–96% yields.

Table 5.
One-pot dual arylations of 1-bromo-4-(chloromethyl)benzene a.
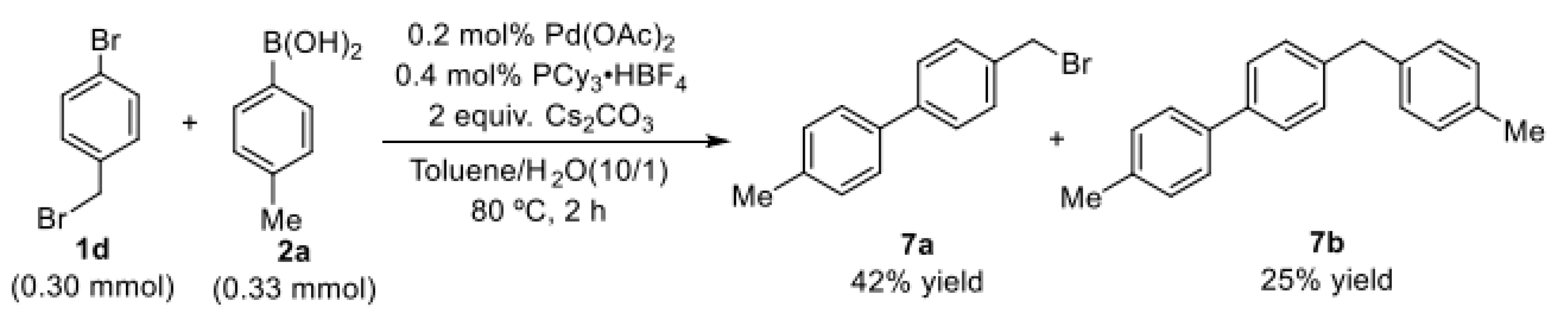
Next, the coupling reactions of 1-bromo-4-(bromomethyl)benzene (1d) with p-tolylboronic acid (2a) were investigated under the optimized conditions. We found that the selective reaction of C(sp2)–Br bond with arylboronic acids could proceed smoothly to give 42% of the desired product 7a, also accompanied by 25% yield of bis-coupling product 7b (Scheme 2). This experimental result shows that the selectivity of the reaction depends not only on the regulatory effect of the phosphine ligand but also on the structure of the C(sp3)–X bond.
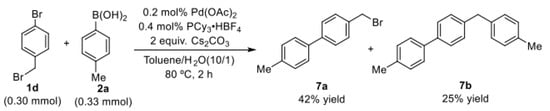
Scheme 2.
Selective coupling reaction of 1-bromo-4-(bromo methyl)benzene with p-tolylboronic acid.
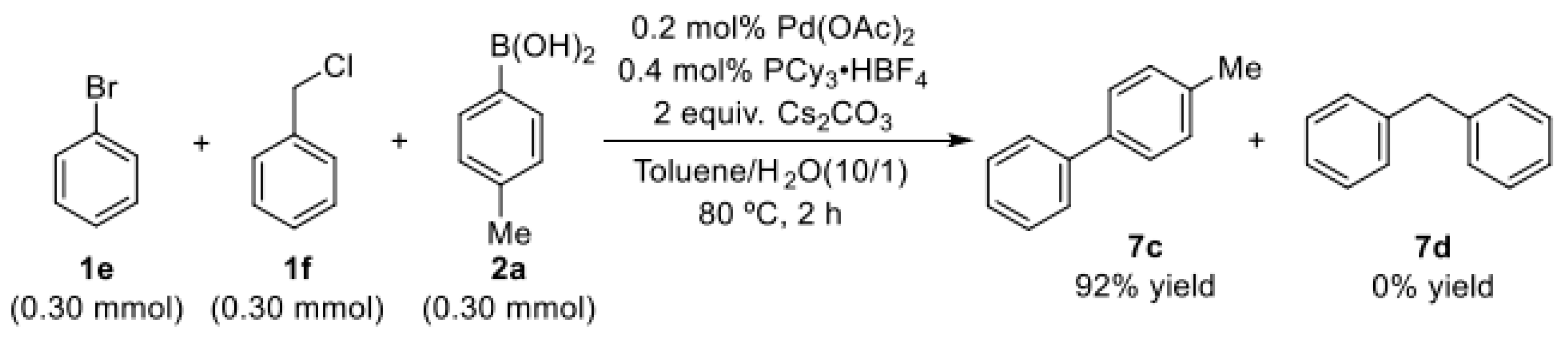
The selectivity depended exclusively on the palladium ligands.
To demonstrate the importance of phosphine ligand for palladium-catalyzed selective coupling reactions of C(sp2)–Br bond or C(sp3)–Cl bond with arylboronic acid, the external competition experiment was performed. To this end, we designed an experiment in which mixtures of bromobenzene and (chloromethyl)benzene were allowed to react with p-tolylboronic acid (Scheme 3). As expected, the formation of the Csp2–Csp2 cross-coupling product (7c) was achieved in the competitive experiment when PCy3∙HBF4 was used as the phosphine ligand in the palladium catalyst.
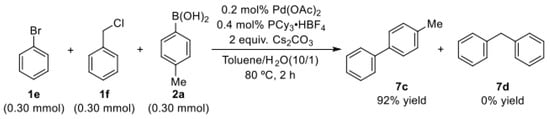
Scheme 3.
Selective coupling reaction of bromobenzene or (chloromethyl)benzene with p-tolylboronic acid.
3. Materials and Methods
Chemicals were obtained commercially and used as received. Nuclear magnetic resonance (NMR) spectra were recorded on a Bruker DPX–400 spectrometer (Bruker Co., Billerica, MA, USA) using tetramethylsilane (TMS) as the internal standard. Electric impact ionization (EI)–Mass spectrum was measured on a gas chromatography time of flight high resolution mass spectrometry (GCTOF-HRMS) (Waters Co, Milford, MA, USA). or GC-MS (Agilent 7890A/5975C, Santa Clara, CA, USA) instrument. Electrospray ionization (ESI)–Mass spectrum was measured on a matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF MS) (Bruker Co., Bremen, Germany). To all copies of 1H NMR, 13C NMR and HRMS spectra, please see Figures S2–S45 in Supplementary Materials. All products were isolated by short chromatography on a silica gel (200–300 mesh) column using petroleum ether (60–90 °C), unless otherwise noted. Arylboronic acids and o-(or m-, or p-)chloromethyl bromobenzene were of analytical grade quality, purchased from Adamas-beta Pharmaceuticals, Inc. (Shanghai, China).
3.1. General Procedure for the Selective Coupling Reaction of o-(or m-, or p-)chloromethyl Bromobenzene with Arylboronic Acid
A Schlenk tube (20 mL) was charged with o-(or m-, or p-)chloromethyl bromobenzene (0.3 mmol), arylboronic acid (0.33 mmol), Pd(OAc)2 (0.2 mol %), PCy3·HBF4 (0.4 mol %), and Cs2CO3 (2 equiv.). The tube was degassed for 30 s and then was filled with argon. This operation was repeated three times. After toluene (1.0 mL) and H2O (0.1 mL) were added under argon atmosphere, the resulting reaction mixture was stirred at 80 °C for 2 h under argon. After the completion of the reaction, the reaction mixture was allowed to cool to room temperature. The solution was quenched with water (10 mL) and extracted with EtOAc (3 × 10 mL). The combined EtOAc extracts were dried over anhydrous Na2SO4 and filtered, followed by solvent removal under reduced pressure. The residue was purified by flash column chromatography on silica gel using petroleum ether/EtOAc as the eluent.
3.2. General Procedure for One-Pot Dual Arylations of 1-Bromo-4-(chloromethyl)benzene
A Schlenk tube (20 mL) was charged with 1-bromo-4-(chloromethyl)benzene (0.3 mmol), arylboronic acid (0.33 mmol), 2 mol % Pd(OAc)2, 0.4 mol % PCy3·HBF4, and 5 equiv. Cs2CO3. The tube was degassed for 30 s and then was filled with argon. This operation was repeated for three times. After toluene (1.0 mL) and H2O (0.1 mL) were added under argon atmosphere, the resulting reaction mixture was stirred at 80 °C for 2 h under argon. After the completion of the reaction, the solution was allowed to cool to room temperature. Then, another arylboronic acid (0.33 mmol) and 4 mol % PPh3 were introduced under argon. The reaction mixture was heated at 80 °C for 5 h. The solution was quenched with water (10 mL) and extracted with EtOAc (3 × 10 mL). The combined EtOAc extracts were dried over anhydrous Na2SO4, filtrated, and then the solvent was removed under reduced pressure. The residue was purified by flash column chromatography on silica gel with PE/EtOAc as the eluent.
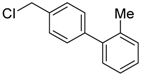
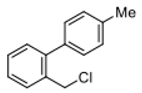
4-(chloromethyl)-4′-methyl-1,1′-biphenyl (3a) [26]: Colorless oil (64.3 mg, 99%). 1H-NMR (400 MHz, CDCl3) δ 7.57 (d, J = 8.4 Hz, 2H), 7.48 (d, J = 8.4 Hz, 2H), 7.44 (d, J = 8.0 Hz, 2H), 7.25 (d, J = 8.0 Hz, 2H), 4.63 (s, 2H), 2.40 (s, 3H). 13C-NMR (100 MHz, CDCl3) δ 141.48, 137.75, 137.53, 136.28, 129.69, 129.18, 127.43, 127.10, 46.27, 21.27.
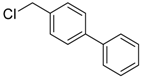
4-(chloromethyl)-1,1′-biphenyl (3b) [27]: Yellow oil (47.9 mg, 79%). 1H-NMR (400 MHz, CDCl3) δ 7.58 (d, J = 7.2 Hz, 4H), 7.51–7.40 (m, 4H), 7.39–7.32 (m, 1H), 4.63 (s, 2H). 13C-NMR (100 MHz, CDCl3) δ 141.53, 140.63, 136.58, 129.19, 128.96, 127.67, 127.63, 127.26, 46.19.
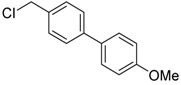
4-(chloromethyl)-4′-methoxy-1,1′-biphenyl (3c) [28]: Colorless oil (60.7 mg, 87%). 1H-NMR (400 MHz, CDCl3) δ 7.54 (d, J = 8.8 Hz, 2H), 7.52 (d, J = 8.8 Hz, 2H), 7.44 (d, J = 8.4 Hz, 2H), 6.98 (d, J = 8.8 Hz, 2H), 4.63 (s, 2H), 3.85 (s, 3H). 13C-NMR (100 MHz, CDCl3) δ 159.50, 141.16, 135.94, 133.15, 129.19, 128.29, 127.17, 114.41, 55.51, 46.30.
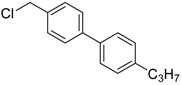
4-(chloromethyl)-4′-propyl-1,1′-biphenyl (3d): Colorless oil (60.9 mg, 83%). 1H-NMR (400 MHz, CDCl3) δ 7.57 (d, J = 8.4 Hz, 2H), 7.50 (d, J = 8.4 Hz, 2H), 7.44 (d, J = 8.4 Hz, 2H), 7.26 (s, 1H), 7.24 (d, J = 2.8 Hz, 1H), 4.63 (s, 2H), 2.65–2.60 (m, 2H), 1.68 (dq, J = 14.8, 7.2 Hz, 2H), 0.97 (t, J = 7.3 Hz, 3H). 13C-NMR (100 MHz, CDCl3) δ 142.33, 141.51, 137.97, 136.24, 129.16, 129.09, 127.44, 127.07, 46.27, 37.84, 24.69, 14.03. HRMS (ESI) m/z calcd for C16H17ClNa+ (M + Na)+ 267.09110, found 267.09195.
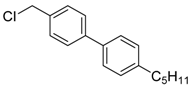
4-(chloromethyl)-4′-pentyl-1,1′-bipheny (3e) [29]: White solid (75.8 mg, 93%). 1H-NMR (400 MHz, CDCl3) δ 7.57 (d, J = 8.4 Hz, 2H), 7.50 (d, J = 8.0 Hz, 2H), 7.44 (d, J = 8.4 Hz, 2H), 7.26 (s, 1H), 7.25 (d, J = 3.2 Hz, 1H), 4.63 (s, 2H), 2.67–2.61 (m, 2H), 1.65 (p, J = 7.4 Hz, 2H), 1.39–1.32 (m, 4H), 0.93–0.88 (m, 3H). 13C-NMR (100 MHz, CDCl3) δ 142.60, 141.51, 137.93, 136.24, 129.16, 129.03, 127.44, 127.09, 46.28, 35.73, 31.70, 31.31, 22.71, 14.19.
4-(chloromethyl)-4′-fluoro-1,1′-biphenyl (3f) [30]: Colorless oil (54.9 mg, 83%). 1H-NMR (400 MHz, CDCl3) δ 7.55 (d, J = 3.2 Hz, 1H), 7.54–7.51 (m, 3H), 7.45 (d, J = 8.0 Hz, 2H), 7.12 (t, J = 8.8 Hz, 2H), 4.63 (s, 2H). 13C-NMR (100 MHz, CDCl3) δ 162.74, 140.53, 136.63, 129.41, 129.25, 128.82, 127.48, 115.85, 46.10.
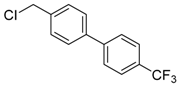
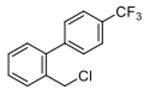
4-(chloromethyl)-4′-(trifluoromethyl)-1,1′-biphenyl (3g) [31]: Colorless oil (60.9 mg, 75%). 1H-NMR (400 MHz, CDCl3) δ 7.69 (d, J = 1.6 Hz, 4H), 7.59 (d, J = 8.4 Hz, 2H), 7.50 (d, J = 8.0 Hz, 2H), 4.64 (s, 2H). 13C-NMR (100 MHz, CDCl3) δ 144.14, 140.03, 137.63, 129.95–129.57 (m), 129.39, 127.79, 127.55, 125.93 (q, J = 4.0 Hz),123.78 (dd, J =420.0, 271.0 Hz) 45.94.
4′-(chloromethyl)-3-methyl-1,1′-biphenyl (3h): Colorless oil (63.7 mg, 98%). 1H-NMR (400 MHz, CDCl3) δ 7.56 (d, J = 8.4 Hz, 2H), 7.42 (d, J = 8.4 Hz, 2H), 7.37 (d, J = 8.8 Hz, 2H), 7.31 (t, J = 7.6 Hz, 1H), 7.16 (d, J = 7.6 Hz, 1H), 4.61 (s, 2H), 2.40 (s, 3H). 13C-NMR (100 MHz, CDCl3) δ 141.61, 140.59, 138.52, 136.45, 129.11, 128.85, 128.40, 128.02, 127.59, 124.35, 46.20, 21.66. HRMS (EI): m/z calcd for C14H13Cl [M]: 216.0723, found [M]: 216.0723.
3-chloro-4′-(chloromethyl)-1,1′-biphenyl (3i): Colorless oil (51.9 mg, 73%). 1H-NMR (400 MHz, CDCl3) δ 7.54 (d, J = 8.4 Hz, 3H), 7.47–7.42 (m, 3H), 7.38–7.30 (m, 2H), 4.62 (s, 2H). 13C-NMR (100 MHz, CDCl3) δ 142.42, 140.02, 137.24, 134.84, 130.18, 129.28, 127.66, 127.56, 127.35, 125.37, 45.99. HRMS (EI): m/z calcd for C13H10Cl2 [M]: 236.0169, found [M]: 236.0160.
4′-(chloromethyl)-2-methyl-1,1′-biphenyl (3j) [32]: Colorless oil (58.5 mg, 90%). 1H-NMR (400 MHz, CDCl3) δ 7.43 (d, J = 8.0 Hz, 2H), 7.32 (d, J = 8.4 Hz, 2H), 7.28–7.25 (m, 2H), 7.25–7.19 (m, 2H), 4.64 (s, 2H), 2.27 (s, 3H). 13C-NMR (100 MHz, CDCl3) δ 142.29, 141.35, 136.06, 135.44, 130.52, 129.86, 129.71, 128.49, 127.60, 125.97, 46.28, 20.59.
4′-(chloromethyl)-2,3-difluoro-1,1′-biphenyl (3k): Yellow oil (33.6 mg, 47%). 1H-NMR (400 MHz, CDCl3) δ 7.54 (d, J = 6.8 Hz, 2H), 7.48 (d, J = 8.4 Hz, 2H), 7.21–7.08 (m, 3H), 4.63 (s, 2H). 13C-NMR (100 MHz, CDCl3) δ 151.25 (dd, J = 247.0, 14.0 Hz), 148.12 (dd, J = 249.0, 14.0 Hz), 137.55, 134.96 (d, J = 4.0 Hz), 130.75 (d, J = 10.0 Hz), 129.45 (d, J = 3.0 Hz), 128.95, 125.46–125.27 (m), 124.29 (dd, J = 7.0, 5.0 Hz), 116.46 (d, J = 20.0 Hz), 45.96. HRMS (EI): m/z calcd for C13H9ClF2 [M]: 238.0369, found [M]: 238.0361.
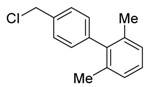
4′-(chloromethyl)-2,6-dimethyl-1,1′-biphenyl (3l): Colorless oil (34.6 mg, 50%). 1H-NMR (400 MHz, CDCl3) δ 7.44 (d, J = 8.0 Hz, 2H), 7.15 (t, J = 2.9 Hz, 2H), 7.13 (s, 1H), 7.10 (d, J = 7.6 Hz, 2H), 4.65 (s, 2H), 2.02 (s, 6H). 13C-NMR (100 MHz, CDCl3) δ 140.29, 139.54, 137.04, 132.09, 129.33, 128.84, 127.43, 122.00, 46.05, 29.85. HRMS (ESI) m/z calcd for C15H16Cl+ (M + H)+ 231.09350, found 231.09262.
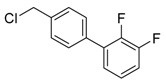
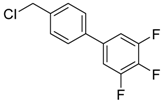
4′-(chloromethyl)-3,4,5-trifluoro-1,1′-biphenyl (3m) [33]: Colorless oil (56.2 mg, 73%). 1H-NMR (400 MHz, CDCl3) δ 7.48 (d, J = 1.6 Hz, 4H), 7.17 (dd, J = 6.4, 6.4 Hz, 2H), 4.62 (s, 2H). 13C-NMR (100 MHz, CDCl3) δ 151.60 (ddd, J = 248.0, 10.0, 4.0 Hz), 141.05–138.04 (m), 138.43, 137.88, 136.74 (td, J = 7.7, 4.6 Hz), 129.46, 127.3, 111.34–111.04 (m), 45.77.
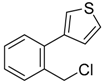
3-(4-(chloromethyl)phenyl)thiophene (3n) [34]: Colorless oil (48.2 mg, 77%). 1H-NMR (400 MHz, CDCl3) δ 7.58 (d, J = 8.4 Hz, 2H), 7.45 (dd, J = 1.6, 1.6 Hz, 1H), 7.40 (d, J = 8.4 Hz, 2H), 7.38 (d, J = 1.2 Hz, 1H), 7.37 (s, 1H), 4.60 (s, 2H). 13C-NMR (100 MHz, CDCl3) δ 141.78, 136.3, 136.13, 129.26, 126.88, 126.52, 126.38, 120.84, 46.21.
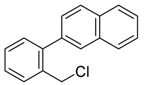
2-(4-(chloromethyl)phenyl)naphthalene (3o) [35]: Colorless oil (65.2 mg, 86%). 1H-NMR (400 MHz, CDCl3) δ 8.00 (s, 1H), 7.90–7.82 (m, 3H), 7.71–7.66 (m, 3H), 7.51–7.44 (m, 4H), 4.62 (s, 2H). 13C-NMR (100 MHz, CDCl3) δ 141.43, 137.93, 136.68, 133.76, 132.86, 129.30, 128.66, 128.36, 127.89, 127.79, 126.53, 126.24, 126.02, 125.53, 46.21.
4-(chloromethyl)-4′-vinyl-1,1′-biphenyl (3p): Colorless oil (39.1 mg, 57%). 1H-NMR (400 MHz, CDCl3) δ 7.59 (d, J = 8.0 Hz, 2H), 7.56 (d, J = 8.0 Hz, 2H), 7.49 (s, 1H), 7.47 (d, J = 2.8 Hz, 2H), 7.45 (s, 1H), 6.76 (dd, J = 10.8, 10.8 Hz, 1H), 5.80 (d, J = 17.6 Hz, 1H), 5.28 (d, J = 10.8 Hz, 1H), 4.64 (s, 2H). 13C-NMR (100 MHz, CDCl3) δ 140.98, 139.91, 137.00, 136.62, 136.44, 129.21, 127.39, 127.31, 126.83, 114.24, 46.17. HRMS (ESI) m/z calcd for C15H13ClNa+ (M + Na)+ 251.05980, found 251.06100.
3-(chloromethyl)-1,1′-biphenyl (4a) [36]: Yellow solid (57.8 mg, 95%). 1H-NMR (400 MHz, CDCl3) δ 7.62–7.59 (m, 2H), 7.58 (d, J = 0.8 Hz, 1H), 7.55 (dd, J = 7.6, 1.2 Hz, 1H), 7.47–7.41 (m, 3H), 7.38 (s, 1H), 7.36 (d, J = 1.6 Hz, 1H), 4.65 (s, 2H). 13C-NMR (100 MHz, CDCl3) δ 141.96, 140.73, 138.10, 129.32, 128.96, 127.69, 127.56, 127.54, 127.36, 127.31, 46.41.
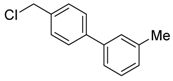
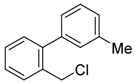
3-(chloromethyl)-4′-methyl-1,1′-biphenyl (4b) [36]: Colorless oil (58.5 mg, 90%). 1H-NMR (400 MHz, CDCl3) δ 7.58 (s, 1H), 7.51 (d, J = 7.6 Hz, 1H), 7.47 (d, J = 8.0 Hz, 2H), 7.40 (t, J = 7.6 Hz, 1H), 7.33 (d, J = 7.6 Hz, 1H), 7.24 (d, J = 7.6 Hz, 2H), 4.62 (s, 2H), 2.38 (s, 3H). 13C-NMR (100 MHz, CDCl3) δ 141.87, 138.04, 137.83, 137.50, 129.67, 129.27, 127.35, 127.26, 127.16, 127.13, 46.46, 21.25.
3-(chloromethyl)-4′-(trifluoromethyl)-1,1′-biphenyl (4c) [37]: Colorless oil (74.7 mg, 92%). 1H-NMR (400 MHz, CDCl3) δ 7.70–7.64 (m, 4H), 7.59 (s, 1H), 7.52 (dt, J = 7.4, 1.6 Hz, 1H), 7.44 (t, J = 7.6 Hz, 1H), 7.41 (d, J = 7.6 Hz, 1H), 4.63 (s, 2H). 13C-NMR (100 MHz, CDCl3) δ 144.23, 140.49, 138.44, 129.78 (d, J = 32.0 Hz), 129.57, 128.45, 127.63, 127.60, 127.46, 125.91 (q, J = 4.0 Hz), 124.38 (q, J = 270.0 Hz), 46.15.
3-(chloromethyl)-3′-methyl-1,1′-biphenyl (4d): Colorless oil (60.4 mg, 93%). 1H-NMR (400 MHz, CDCl3) δ 7.59 (s, 1H), 7.52 (d, J = 7.6 Hz, 1H), 7.40 (q, J = 7.6 Hz, 3H), 7.33 (dd, J = 13.6, 5.6 Hz, 2H), 7.17 (d, J = 7.2 Hz, 1H), 4.63 (s, 2H), 2.41 (s, 3H). 13C-NMR (100 MHz, CDCl3) δ 142.06, 140.70, 138.55, 138.02, 129.24, 128.85, 128.42, 128.08, 127.55, 127.44, 127.36, 124.40, 46.43, 1.66. GC-MS (m/z): 217.
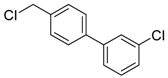
3-chloro-3′-(chloromethyl)-1,1′-biphenyl (4e): Colorless oil (65.4 mg, 92%). 1H-NMR (400 MHz, CDCl3) δ 7.55 (d, J = 4.0 Hz, 2H), 7.49 (d, J = 7.2 Hz, 1H), 7.44 (d, J = 7.2 Hz, 1H), 7.41 (d, J = 7.6 Hz, 1H), 7.39–7.34 (m, 2H), 7.33–7.31 (m, 1H), 4.62 (s, 2H). 13C-NMR (100 MHz, CDCl3) δ 142.56, 140.55, 138.32, 134.87, 130.20, 129.48, 128.14, 127.71, 127.50, 127.45, 127.31, 125.47, 46.23. HRMS (EI): m/z calcd for C13H10Cl2 [M]: 236.0172, found [M]: 236.0160.
3′-(chloromethyl)-2-methyl-1,1′-biphenyl (4f) [32]: Colorless oil (56.6 mg, 87%). 1H-NMR (400 MHz, CDCl3) δ 7.36 (dd, J = 12.4, 7.2 Hz, 3H), 7.27 (d, J = 9.6 Hz, 3H), 7.22 (t, J = 5.4 Hz, 2H), 4.61 (s, 2H), 2.26 (s, 3H). 13C-NMR (100 MHz, CDCl3) δ 142.59, 141.37, 137.43, 135.41, 130.50, 129.84, 129.54, 129.36, 128.60, 127.61, 127.07, 125.95, 46.38, 20.56.
3-(3-(chloromethyl)phenyl)thiophene (4g) [32]: Colorless oil (59.5 mg, 95%). 1H-NMR (400 MHz, CDCl3) δ 7.61 (s, 1H), 7.55 (dt, J = 7.6, 1.6 Hz, 1H), 7.47 (dd, J = 2.4, 2.0 Hz, 2H), 7.43–7.34 (m, 4H), 7.31 (d, J = 8.0 Hz, 1H), 4.62 (s, 2H). 13C-NMR (100 MHz, CDCl3) δ 141.83, 138.13, 136.52, 129.35, 127.38, 126.79, 126.61, 126.52, 126.39, 120.86, 46.35.
2-(3-(chloromethyl)phenyl)naphthalene (4h): Yellow oil (55.3 mg, 73%). 1H-NMR (400 MHz, CDCl3) δ 8.02 (s, 1H), 7.89 (t, J = 7.2 Hz, 2H), 7.85 (d, J = 7.2 Hz, 1H), 7.71 (d, J = 6.4 Hz, 2H), 7.65 (d, J = 7.6 Hz, 1H), 7.52–7.46 (m, 2H), 7.44 (d, J = 7.6 Hz, 1H), 7.38 (d, J = 7.6 Hz, 1H), 4.66 (s, 2H). 13C-NMR (100 MHz, CDCl3) δ 141.86, 138.22, 138.03, 133.76, 132.86, 129.43, 128.66, 128.35, 127.79, 127.64, 126.53, 126.24, 126.07, 125.58, 46.44. HRMS (EI): m/z calcd for C17H10Cl [M]: 252.0723, found [M]: 252.0706.
2-(chloromethyl)-1,1′-biphenyl (5a) [38]: Yellow oil (58.9 mg, 97%). 1H-NMR (400 MHz, CDCl3) δ 7.55–7.51 (m, 1H), 7.44 (d, J = 8.8 Hz, 1H), 7.42–7.33 (m, 6H), 7.27 (d, J = 9.2 Hz, 1H), 4.52 (s, 2H). 13C-NMR (100 MHz, CDCl3) δ 142.21, 140.31, 135.05, 130.64, 130.46, 129.28, 128.64, 128.44, 128.07, 127.59, 44.60.
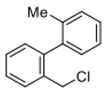
2-(chloromethyl)-4′-methyl-1,1′-biphenyl (5b) [39]: Yellow oil (61.7 mg, 95%). 1H-NMR (400 MHz, CDCl3) δ 7.55–7.50 (m, 1H), 7.38–7.33 (m, 2H), 7.31 (d, J = 8.2 Hz, 2H), 7.28–7.22 (m, 3H), 4.53 (s, 2H), 2.41 (s, 3H). 13C-NMR (100 MHz, CDCl3) δ 142.19, 137.38, 137.29, 135.08, 130.61, 130.50, 129.15, 129.13, 128.61, 127.86, 44.68, 21.33.
2-(chloromethyl)-4′-(trifluoromethyl)-1,1′-biphenyl (5c) [39]: Colorless oil (72.3 mg, 89%). 1H-NMR (400 MHz, CDCl3) δ 7.71 (d, J = 8.4 Hz, 2H), 7.56 (d, J = 7.6 Hz, 3H), 7.46–7.38 (m, 2H), 7.27 (d, J = 10.0 Hz, 1H), 4.48 (s, 2H). 13C-NMR (100 MHz, CDCl3) δ 143.98, 140.80, 135.04, 130.92, 130.28,129.89 (dt, J = 95.0, 30 Hz), 129.68, 128.91, 128.79, 125.43 (q, J = 4.0 Hz), 124.34 (q, J = 270.0 Hz), 44.25.
2-(chloromethyl)-3′-methyl-1,1′-biphenyl (5d) [39]: Yellow oil (59.8 mg, 92%). 1H-NMR (400 MHz, CDCl3) δ 7.56–7.51 (m, 1H), 7.38–7.34 (m, 2H), 7.31 (d, J = 7.2 Hz, 1H), 7.29–7.25 (m, 1H), 7.21 (d, J = 6.8 Hz, 5H), 4.53 (s, 2H), 2.41 (s, 3H). 13C-NMR (100 MHz, CDCl3) δ 142.32, 140.25, 138.06, 135.03, 130.58, 130.40, 130.03, 128.56, 128.31, 128.29, 127.95, 126.33, 44.64, 21.62.
3′-chloro-2-(chloromethyl)-1,1′-biphenyl (5e) [39]: Yellow oil (62.3 mg, 88%). 1H-NMR (400 MHz, CDCl3) δ 7.53 (dd, J = 7.6, 2.0 Hz, 1H), 7.40 (d, J = 1.2 Hz, 1H), 7.38 (dd, J = 4.8, 1.6 Hz, 1H), 7.37–7.32 (m, 3H), 7.32–7.29 (m, 1H), 7.25–7.22 (m, 1H), 4.49 (s, 2H). 13C-NMR (100 MHz, CDCl3) δ 142.04, 140.74, 135.02, 134.33, 130.76, 130.29, 129.67, 129.37, 128.78, 128.55, 127.79, 127.49, 44.31.
2-(chloromethyl)-2′-methyl-1,1′-biphenyl (5f) [40]: Colorless oil (56.5 mg, 87%). 1H-NMR (400 MHz, CDCl3) δ 7.55 (d, J = 6.8 Hz, 1H), 7.42–7.30 (m, 3H), 7.30–7.20 (m, 3H), 7.20–7.11 (m, 2H), 4.43–4.26 (m, 2H), 2.07 (s, 3H). 13C-NMR (100 MHz, CDCl3) δ 141.57, 139.60, 136.16, 135.41, 130.17, 130.09, 129.69, 128.41, 128.01, 127.92, 125.64, 44.26, 20.30.
3-(2-(chloromethyl)phenyl)thiophene (5g) [41]: Colorless oil (59.4 mg, 95%). 1H-NMR (400 MHz, CDCl3) δ 7.51 (dd, J = 7.2 3.6 Hz, 1H), 7.42 (dd, J = 2.8, 1.2 Hz, 1H), 7.39 (dd, J = 5.2, 3.2 Hz, 1H), 7.35 (d, J = 3.2 Hz, 3H), 7.26–7.22 (m, 1H), 4.58 (s, 2H). 13C-NMR (100 MHz, CDCl3) δ 140.44, 136.90, 135.18, 130.92, 130.41, 128.98, 128.80, 128.08, 125.76, 123.44, 44.95.
2-(2-(chloromethyl)phenyl)naphthalene (5h) [42]: Yellow oil (60.6 mg, 80%). 1H-NMR (400 MHz, CDCl3) δ 7.90–7.83 (m, 4H), 7.57–7.51 (m, 2H), 7.51–7.46 (m, 2H), 7.38 (dd, J = 2.8, 2.0 Hz, 1H), 7.37–7.33 (m, 2H), 4.54 (s, 2H). 13C-NMR (100 MHz, CDCl3) δ 142.13, 137.76, 135.26, 133.28, 130.72, 130.66, 128.68, 128.29, 128.16, 128.04, 127.84, 127.49, 126.54
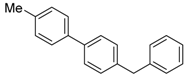
4-benzyl-4′-methyl-1,1′-biphenyl (6a) [43]: Yellow oil (60.4 mg, 78%). 1H-NMR (400 MHz, CDCl3) δ 7.51 (d, J = 2.0 Hz, 1H), 7.48 (dd, J = 3.6, 2.0 Hz, 2H), 7.45 (d, J = 2.0 Hz, 1H), 7.30 (t, J = 6.4 Hz, 2H), 7.26–7.20 (m, 7H), 4.01 (s, 2H), 2.38 (s, 3H). 13C-NMR (100 MHz, CDCl3) δ 141.20, 140.07, 139.11, 138.26, 136.96, 129.59, 129.42, 129.11, 128.65, 127.16, 126.98, 126.26, 41.73, 21.24.
4-(4-methoxybenzyl)-4′-methyl-1,1′-biphenyl (6b) [44]: Yellow oil (83.1 mg, 96%). 1H-NMR (400 MHz, CDCl3) δ 7.49 (s, 1H), 7.47 (d, J = 2.0 Hz, 2H), 7.45 (s, 1H), 7.23 (s, 2H), 7.21 (s, 2H), 7.14 (s, 1H), 7.12 (s, 1H), 6.85 (s, 1H), 6.83 (s, 1H), 3.95 (s, 2H), 3.77 (s, 3H), 2.37 (s, 3H). 13C-NMR (100 MHz, CDCl3) δ 158.14, 140.54, 139.02, 138.29, 136.93, 133.32, 130.03, 129.57, 129.28, 127.13, 126.97, 114.06, 55.41, 40.82, 21.23.
4-(4-fluorobenzyl)-4′-methyl-1,1′-biphenyl (6c): Yellow oil (73.7 mg, 89%).1H-NMR (400 MHz, CDCl3) δ 7.50 (d, J = 8.0 Hz, 2H), 7.46 (d, J = 8.0 Hz, 2H), 7.24–7.20 (m, 4H), 7.17 (dd, J = 8.8, 5.6 Hz, 2H), 6.98 (t, J = 8.8 Hz, 2H), 3.97 (s, 2H), 2.38 (s, 3H). 13C-NMR (100 MHz, CDCl3) δ 161.59 (d, J = 243.9 Hz), 139.86 (d, J = 1.4 Hz), 139.26, 138.16, 137.04, 136.85 (d, J = 3.3 Hz), 130.45 (d, J = 7.8 Hz), 129.60, 129.31, 127.22, 126.98, 115.40 (d, J = 21.2 Hz), 40.86, 21.24. HRMS (ESI) m/z calcd for C20H19FK+ (M + K)+ 315.09459, found 315.09357.
3-((4′-methyl-[1,1′-biphenyl]-4-yl)methyl)thiophene (6d): Colorless oil (45.2 mg, 57%). 1H-NMR (400 MHz, CDCl3) δ 7.50 (d, J = 8.4 Hz, 2H), 7.47 (d, J = 8.0 Hz, 2H), 7.26 (t, J = 2.4 Hz, 2H), 7.23 (d, J = 8.0 Hz, 3H), 6.95 (dd, J = 4.8, 2.8 Hz, 2H), 4.01 (s, 2H), 2.38 (s, 3H). 13C-NMR (100 MHz, CDCl3) δ 139.53, 139.20, 136.99, 129.59, 129.24, 128.61, 127.17, 126.98, 125.81, 121.44, 36.30, 21.24. HRMS (ESI) m/z calcd for C18H17S+ (M + H)+ 265.10455, found 265.10495.
4-(bromomethyl)-4′-methyl-1,1′-biphenyl (7a) [45]: White solid (32.6 mg, 42%). 1H-NMR (400 MHz, CDCl3) δ 7.55 (d, J = 8.4 Hz, 2H), 7.48 (d, J = 8.0 Hz, 2H), 7.45 (d, J = 8.4 Hz, 2H), 7.25 (d, J = 6.4Hz, 2H), 4.55 (s, 2H), 2.40 (s, 3H). 13C-NMR (100 MHz, CDCl3) δ 141.48, 137.69,137.56, 137.58, 129.70, 129.61, 127.48, 127.08, 33.63, 21.27.
4-methyl-4′-(4-methylbenzyl)-1,1′-biphenyl (7b) [46]: White solid, MP: 77–78 °C (20.4 mg, 25%). 1H-NMR (400 MHz, CDCl3) δ 7.51–7.44 (m, 4H), 7.23 (dd, J = 8.0 3.6 Hz, 4H), 7.11 (s, 4H), 3.97 (s, 2H), 2.38 (s, 3H), 2.32 (s, 3H). 13C-NMR (100 MHz, CDCl3) δ 140.38, 139.02, 138.31, 138.16, 136.92, 135.74, 129.57, 129.34, 128.97, 127.13, 126.98, 41.30, 21.23, 21.17.
4-methyl-1,1′-biphenyl (7c) [25]: White solid (46.8 mg, 92%). 1H-NMR (400 MHz, CDCl3) δ 7.57 (dd, J = 8.4, 1.6 Hz, 2H), 7.48 (d, J = 8.4 Hz, 2H), 7.41 (t, J = 7.6 Hz, 2H), 7.31 (t, J = 7.6 Hz, 1H), 7.24 (d, J = 8.0 Hz, 3H), 2.38 (s, 3H).
4. Conclusions
In conclusion, an efficient method for the selective Suzuki–Miyaura coupling of o-(or m-, or p-)chloromethyl bromobenzene with arylboronic acids was described. This Pd-catalyzed highly selective coupling reaction of the C(sp2)–Br bond was achieved by using PCy3·HBF4 as the ligand and Cs2CO3 as the base in a mixture of toluene/water (10:1). A series of chloromethyl-1,1′-biphenyl compounds were obtained in moderate-to-excellent yields. Importantly, the catalytic system exhibited a wide substrate scope and good functional group tolerance. Moreover, this protocol was extended to the one-pot dual arylation of 1-bromo-4-(chloromethyl)benzene, affording numerous unsymmetrical methylene-linked biaryl derivatives.
Supplementary Materials
Supplementary materials are available online. Figures S2–S46: 1H-, 13C-NMR, and HRMS of products.
Acknowledgments
We gratefully acknowledge financial support of this work by the National Natural Science Foundation of China (No. 21563025), the Program for Changjiang Scholars and Innovative Research Team in University (No. IRT_15R46), and the Yangtze River scholar research project of Shihezi University (No. CJXZ201601).
Author Contributions
P.L. and X.-w.M. conceived and designed the experiments; M.-m.P. performed the experiments; B.D. and X.-m.L. contributed reagents/materials/analysis tools; Y.L. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Miyaura, N.; Suzuki, A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Suzuki, A. Recent advances in the cross-coupling reactions of organoboron derivatives with organic electrophiles, 1995–1998. J. Organomet. Chem. 1999, 576, 147–168. [Google Scholar] [CrossRef]
- Suzuki, A. Cross-coupling reactions via organoboranes. J. Organomet. Chem. 2002, 653, 83–90. [Google Scholar] [CrossRef]
- Diedreich, F.; Stang, P.J. Metal-Catalyzed Cross-Coupling Reactions; Wiley-VCH: New York, NY, USA, 1998. [Google Scholar]
- Chowdhury, S.; Georghiou, P. Palladium catalyzed cross-coupling between phenyl- or naphthylboronic acids and benzylic bromides. Tetrahedron Lett. 1999, 40, 7599–7603. [Google Scholar] [CrossRef]
- Oh, C.H.; Lim, Y.M. Double suzuki reactions of organoboronic acids with 1,n-dibromides. Bull. Korean Chem. Soc. 2002, 23, 663–664. [Google Scholar] [CrossRef]
- Lu, T.-Y.; Xue, C.; Luo, F.-T. Palladium-Catalyzed Cross-Coupling Reaction of Aryldioxaborolane with 2-Bromo-N,N-dimethylacetamide. Tetrahedron Lett. 2003, 44, 1587–1590. [Google Scholar] [CrossRef]
- Liu, X.; Deng, M. Remarkable Co-Catalysis by Copper(I) Oxide in the Palladium Catalyzed Cross-Coupling of Arylboronic Acids with Ethyl Bromoacetate. Chem. Commun. 2002, 33, 622–623. [Google Scholar] [CrossRef]
- Gooßen, L. Palladium-Catalyzed Synthesis of Aryl Ketones from Boronic Acids and Carboxylic Acids or Anhydrides. Chem. Commun. 2001, 669–670. [Google Scholar] [CrossRef]
- Netherton, M.R.; Dai, C.; Neuschütz, K.; Fu, G.C. Room-Temperature Alkyl−Alkyl Suzuki Cross-Coupling of Alkyl Bromides that Possess β Hydrogens. J. Am. Chem. Soc. 2001, 123, 10099–10100. [Google Scholar] [CrossRef] [PubMed]
- Langle, S.; Abarbri, M.; Duchêne, A. Selective double Suzuki cross-coupling reactions. Synthesis of unsymmetrical diaryl (or heteroaryl) methanes. Tetrahedron Lett. 2003, 44, 9255–9258. [Google Scholar] [CrossRef]
- Anselmi, E.; Abarbri, M.; Duchêne, A.; Langle-Lamandé, S.; Thibonnet, J. Efficient synthesis of substituted styrenes and biaryls (or heteroaryls) with regioselective reactions of ortho-, meta-, and para-bromobenzyl bromide. Synthesis 2012, 44, 2023–2040. [Google Scholar] [CrossRef]
- Henry, N.; Enguehard-Gueiffier, C.; Thery, I. One-Pot Dual Substitutions of Bromobenzyl Chloride, 2-Chloromethyl-6-halogenoimidazo[1,2-a]pyridine and -[1,2-b]pyridazine by Suzuki–Miyaura Cross-Coupling Reactions. Eur. J. Org. Chem. 2008, 2008, 4824–4827. [Google Scholar] [CrossRef]
- Mahamo, T.; Mogorosi, M.M.; Moss, J.R.; Mapolie, S.F.; Slootweg, J.C.; Lammertsma, K.; Smith, G.S. Neutral palladium (II) complexes with P,N Schiff-base ligands: Synthesis, characterization and application as Suzuki–Miyaura coupling catalysts. J. Organomet. Chem. 2012, 703, 34–42. [Google Scholar] [CrossRef]
- Gurbuz, N.; Özdemir, İ.; Cetinkaya, B.; Seckin, T. Silica-supported 3-4,5-dihydroimidazol-1-yl-propyltriethoxysilanedichloropalladium(II) complex: Heck and Suzuki cross-coupling reactions. Appl. Organomet. Chem. 2003, 17, 776–780. [Google Scholar] [CrossRef]
- Heijnen, D.; Tosi, F.; Vila, C.; Stuart, M.C.; Elsinga, P.H.; Szymanski, W.; Feringa, B.L. Oxygen Activated, Palladium Nanoparticle Catalyzed, Ultrafast Cross-Coupling of Organolithium Reagents. Angew. Chem. Int. Ed. 2017, 129, 3402–3407. [Google Scholar] [CrossRef]
- Gürbüz, N.; Özdemir, I.; Demir, S.; Çetinkaya, B. Improved palladium-catalyzed coupling reactions of aryl halides using saturated N-heterocarbene ligands. J. Mol. Catal. A Chem. 2004, 209, 23–28. [Google Scholar] [CrossRef]
- Mollar, C.; Besora, M.; Maseras, F.; Asensio, G.; Medio-Simón, M. Competitive and Selective Csp3-Br versus Csp2-Br Bond Activation in Palladium-Catalysed Suzuki Cross-Coupling: An Experimental and Theoretical Study of the Role of Phosphine Ligands. Chem. Eur. J. 2010, 16, 13390–13397. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gu, N.; Liu, P.; Ma, X.; Liu, Y.; Xie, J.; Dai, B. Water-soluble salen–Pd complex as an efficient catalyst for Suzuki–Miyaura reaction of sterically hindered substrates in pure water. Tetrahedron 2015, 71, 7985–7989. [Google Scholar] [CrossRef]
- Ma, X.; Liu, Y.; Liu, P.; Xie, J.; Dai, B.; Liu, Z. Palladium-catalyzed direct arylation of polyfluoroarene and facile synthesis of liquid crystal compounds. Appl. Organomet. Chem. 2014, 28, 180–185. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, X.; Xie, J.; Liu, P.; Dai, B.; He, R. Metallomicelles of palladium(II) complexes as efficient catalysts for the Suzuki–Miyaura reaction in neat water. Appl. Organomet. Chem. 2013, 27, 494–498. [Google Scholar] [CrossRef]
- Ma, X.; Liu, Y.; Liu, P.; Xie, J.; Dai, B.; Liu, Z. A rapid and efficient catalysis system for the synthesis of 4-vinylbiphenyl derivatives. Appl. Organomet. Chem. 2013, 27, 707–710. [Google Scholar] [CrossRef]
- Liu, P.; Yan, M.; He, R. Bis(imino) pyridine palladium(II) complexes as efficient catalysts for the Suzuki–Miyaura reaction in water. Appl. Organomet. Chem. 2010, 24, 131–134. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, W.Z.; He, R. Preparation and catalytic properties of bis(imino) pyridine palladium(II) complexes as efficient catalysts for Suzuki cross-coupling reaction in water. Appl. Organomet. Chem. 2009, 23, 135–139. [Google Scholar] [CrossRef]
- Liu, P.; Zhou, L.; Li, X.G.; He, R. Bis(imino) pyridine palladium(II) complexes: Synthesis, structure and catalytic activity. J. Organomet. Chem. 2009, 694, 2290–2294. [Google Scholar] [CrossRef]
- Barclay, L.R.C.; Sonawane, H.R.; Hudson, J.C. Sterically Hindered Aromatic Compounds. IV. Solvolysis of t-Butyl-, Phenyl-, and Trialkyl-benzyl Chlorides in Ethanol–Water. Evidence for Steric Acceleration in 2,4,6-Tri-t-butylbenzyl Chloride. Can. J. Chem. 1972, 50, 2318–2325. [Google Scholar] [CrossRef]
- Han, L.; Xia, J.B.; You, L.; Chen, C. Ketone-catalyzed photochemical C(sp3)–H chlorination. Tetrahedron 2017, 73, 3696–3701. [Google Scholar] [CrossRef] [PubMed]
- Burawoy, A.; Spinner, E. Electronic spectra of organic molecules and their interpretation. Part I. Effect of terminal methyl and substituted methyl groups of conjugated systems on K-bands. J. Chem. Soc. 1955, 2557–2563. [Google Scholar] [CrossRef]
- Kauhanka, M.M.; Kauhanka, U.M. Synthesis and properties of some mesogenic benzyl chlorides. Vestsi Nats. Akad. Navuk Belarusi Ser. Khim. Navuk 2010, 64–67. [Google Scholar]
- Filzen, G.F.; Trivedi, B.K.; Geyer, A.G.; Unangst, P.C.; Bratton, L.D. Compounds that Modulate PPAR Activity and Methods for Their Preparation. WO Patent Application No. 2003084916A2, 24 December 2003. [Google Scholar]
- Bartel, S.; Hahn, M.; Moradi, W.A.; Becker, E.; Roelle, T.; Stasch, J.; Schlemmer, K.; Wunder, F.; Knorr, A. Dicarboxylic Acid Derivatives and Their Use. WO Patent Application No. 2007045433 A1, 26 April 2007. [Google Scholar]
- Li, Z.; Liu, C.X.; Xu, X.; Qiu, Q.; Su, X.; Dai, Y.; Yang, J.; Li, H.; Shi, W.; Liao, C.; et al. Discovery of phenylsulfonyl acetic acid derivatives with improved efficacy and safety as potent free fatty acid receptor 1 agonists for the treatment of type 2 diabetes. Eur. J. Med. Chem. 2017, 138, 458–479. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Hwang, H.S.; Chin, J. Use of the Fetal Reprogramming of a PPAR δ Agonistwo. WO Patent Application No. 2012030165A2, 5 July 2012. [Google Scholar]
- Leivers, M.R.; Roberts, C.D.; Liehr, S.J.R.; Chan, S.A.; Rai, R.; Lauchli, R.; Pham, S.M.; Rajwanshi, V.K.; Ton, T.L. Derivatives of Imidazo [4,5-d] Pyridazine and Their Use as Anti-Viral Compounds. WO Patent Application No. 2009111501 A1, 9 November 2009. [Google Scholar]
- Sabatucci, J.P.; Caufield, C.E.; Greenfield, A.A.; Antane, S. Substituted Naphthylenes for the Treatment of Non-Insulin Dependent Diabetes Mellitus. U.S. Patent Application No. 20130216442 A1, 11 September 2009. [Google Scholar]
- Boettcher, H.; Devant, R.; Greiner, H.; Bartoszyk, G.; Berthelon, J.J.; Noblet, M.; Zeiller, J.J. Preparation of arylamines as central nervous system agents. EP Patent Application No. 707007 A1, 17 April 1996. [Google Scholar]
- Houze, J.; Liu, J.; Ma, Z.H.; Medina, J.C.; Schmitt, M.J.; Sharma, R.; Sun, Y.; Wang, Y.C. Compounds, Pharmaceutical Compositions and Methods for Their Use in Treating Metabolic Disorders. U.S. Patent Application No. 7465804 B2, 16 December 2008. [Google Scholar]
- Standley, E.A.; Jamison, T.F. Simplifying Nickel(0) Catalysis: An Air-Stable Nickel Precatalyst for the Internally Selective Benzylation of Terminal Alkenes. J. Am. Chem. Soc. 2013, 135, 1585–1592. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.J.; Kim, H.J.; Chang, S. Highly Efficient and Versatile Synthesis of Polyarylfluorenes via Pd-Catalyzed C−H Bond Activation. Org. Lett. 2009, 20, 4588–4591. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, L.M.; Leung, W.P.; Raston, C.L.; Twiss, P.; White, A.H. Axially asymmetric metal alkyls. Part 1. Lithium alkyls of 2,2′-dimethylbiphenyl and its trimethylsilylmethylated compounds: crystal structures of [{Li(Me2NCH2CH2NMe2)}2{(2-CHRC6H4)2}] for R = H (polymeric) and R = SiMe3(monomeric). J. Chem. Soc. Dalton Trans. 1984, 321–329. [Google Scholar]
- Suga, A.; Kazuta, K.; Morihira, K.; Matsuda, K.; Kakuta, H.; Moritani, H.; Iizumi, Y. Novel Benzylamine Derivative. WO Patent Application No. 19959518121 A1, 6 July 1995. [Google Scholar]
- Biediger, R.J.; Bui, H.; Henry, K.M.; Thrash, T. Modulators of c3a Receptor and Methods of Use Thereof. WO Patent Application No. 2008079371 A1, 3 July 2008. [Google Scholar]
- Tao, J.L.; Wang, Z.X. Pincer-Nickel-Catalyzed Cross-Coupling of Aryl Sulfamates with Arylzinc Chlorides. Eur. J. Org. Chem. 2015, 29, 6534–6540. [Google Scholar] [CrossRef]
- Rao, M.L.N.; Dhanorkar, R.J. Pd-catalyzed chemoselective threefold cross-coupling of triarylbismuths with benzylic bromides. RSC Adv. 2013, 3, 6794–6798. [Google Scholar] [CrossRef]
- Futamura, S.; Zong, Z.M. Photobromination of side-chain methyl groups on arenes with N-bromosuccinimide–convenient and selective syntheses of bis(bromomethyl)- and (bromomethyl) methylarenes. Bull. Chem. Soc. Jpn. 1992, 65, 345–348. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, M.T.; Lu, J.M. N-Heterocyclic carbene–palladium(II)–1-methylimidazole complex catalyzed Suzuki–Miyaura coupling of benzylic chlorides with arylboronic acids or potassium phenyltrifluoroborate in neat water. Org. Biomol. Chem. 2013, 11, 2266–2272. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).