An Accurate and Effective Method for Measuring Osimertinib by UPLC-TOF-MS and Its Pharmacokinetic Study in Rats
Abstract
1. Introduction
2. Results and Discussion
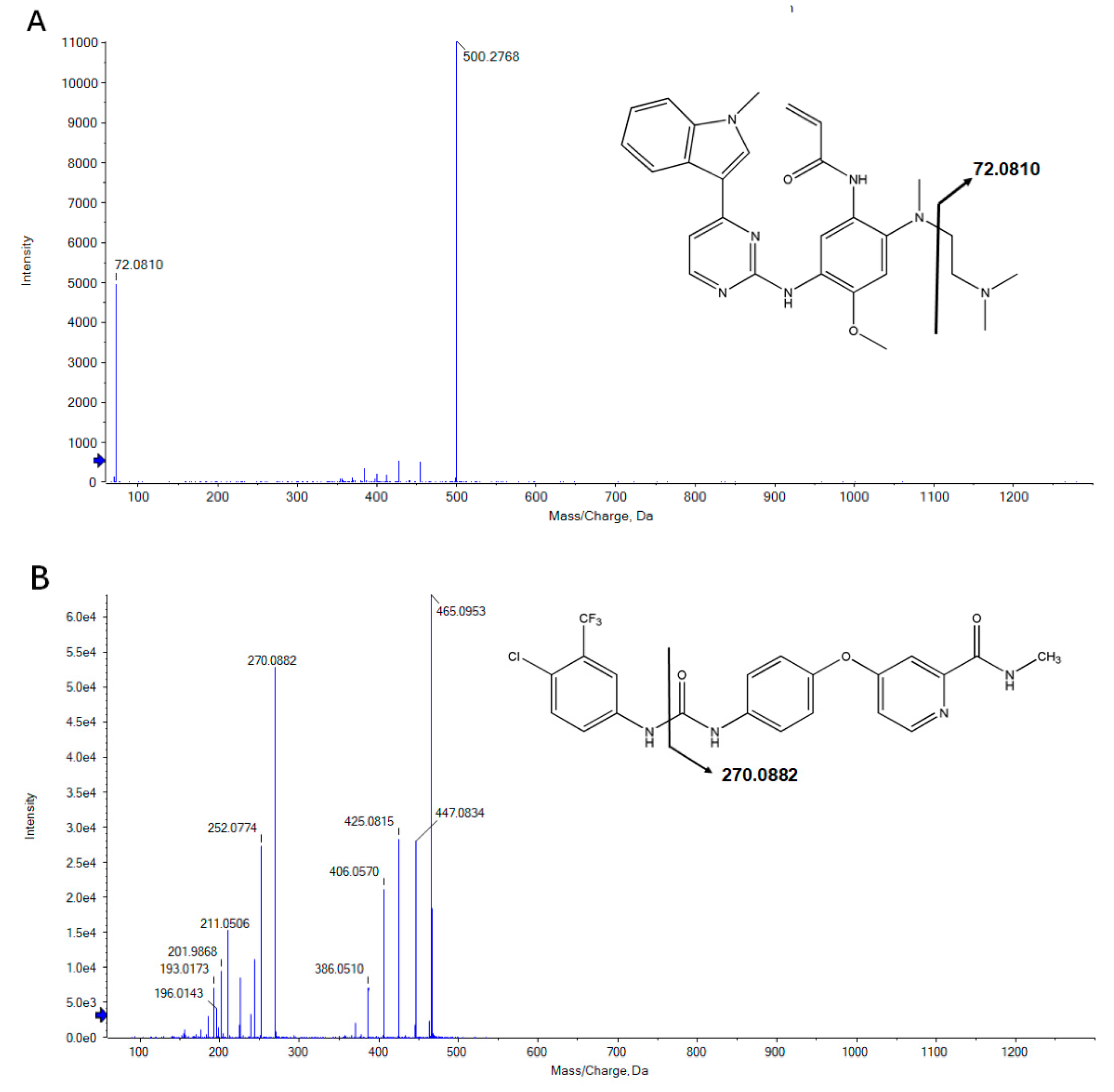
2.1. UPLC-TOF-MS Method Development
2.2. Method Validation
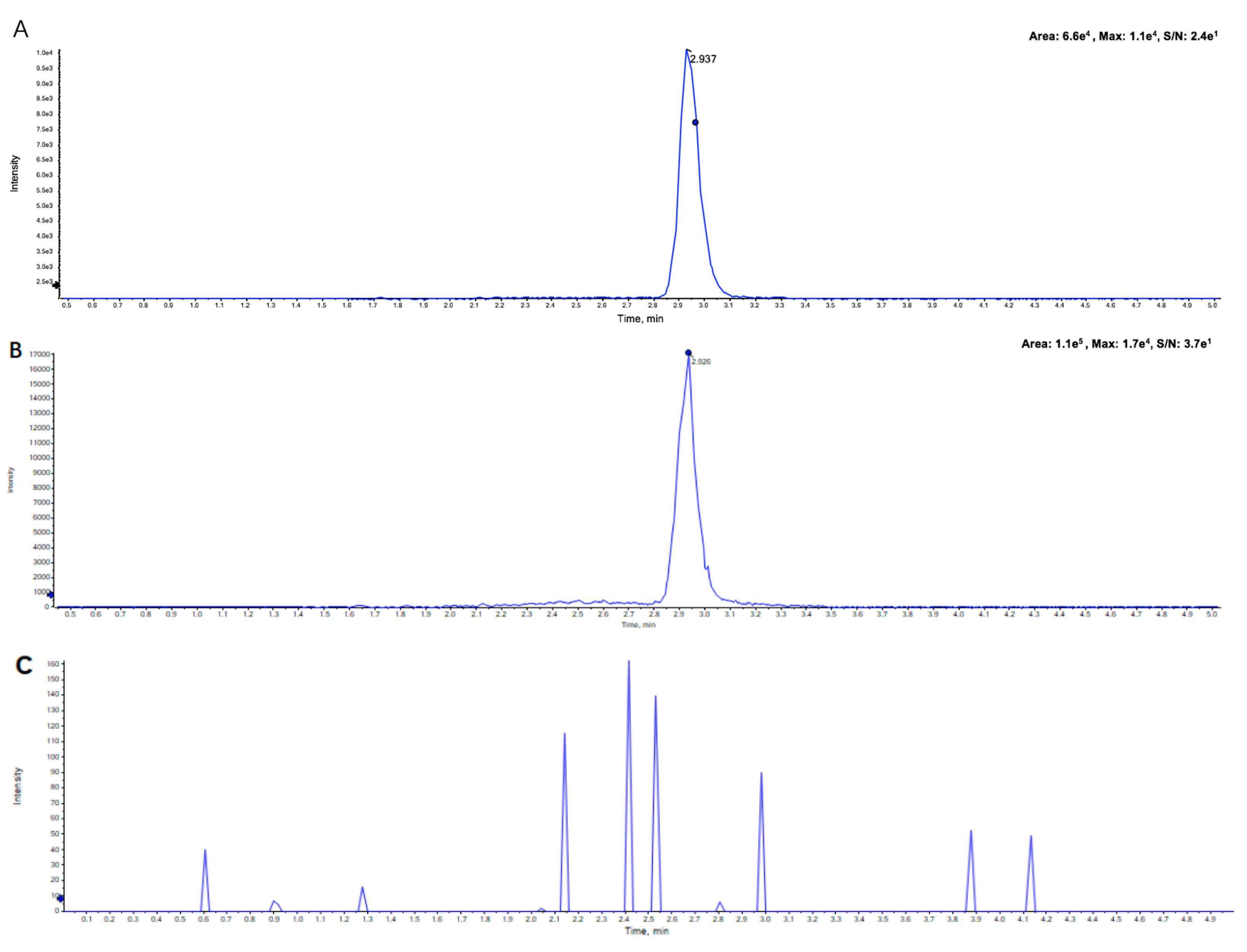
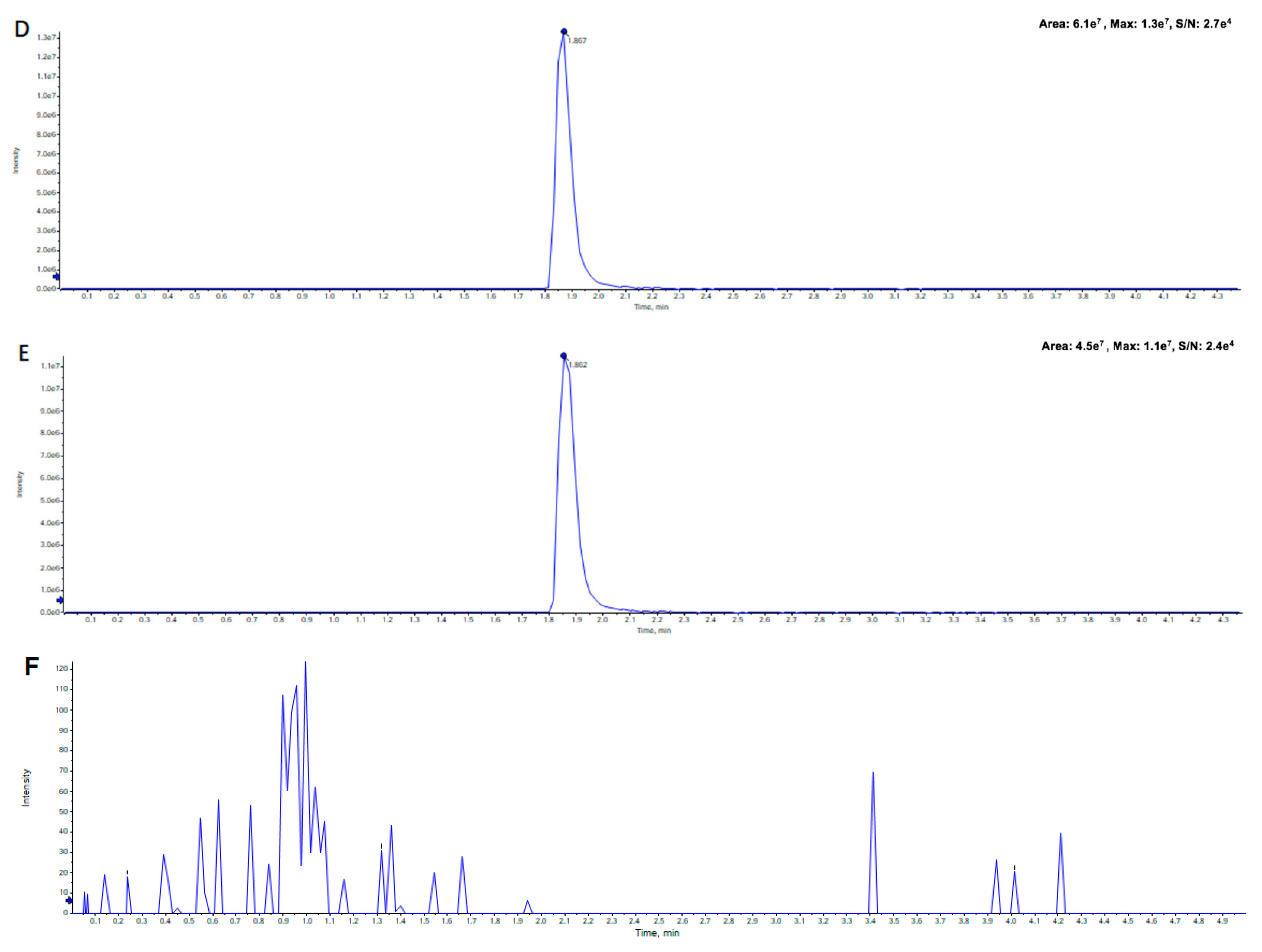
2.2.1. Specificity and Selectivity
2.2.2. Calibration and Lower Limit of Quantification (LLOQ)
2.2.3. Precision and Accuracy
2.2.4. Extraction Recovery and Matrix Effect
2.2.5. Stability
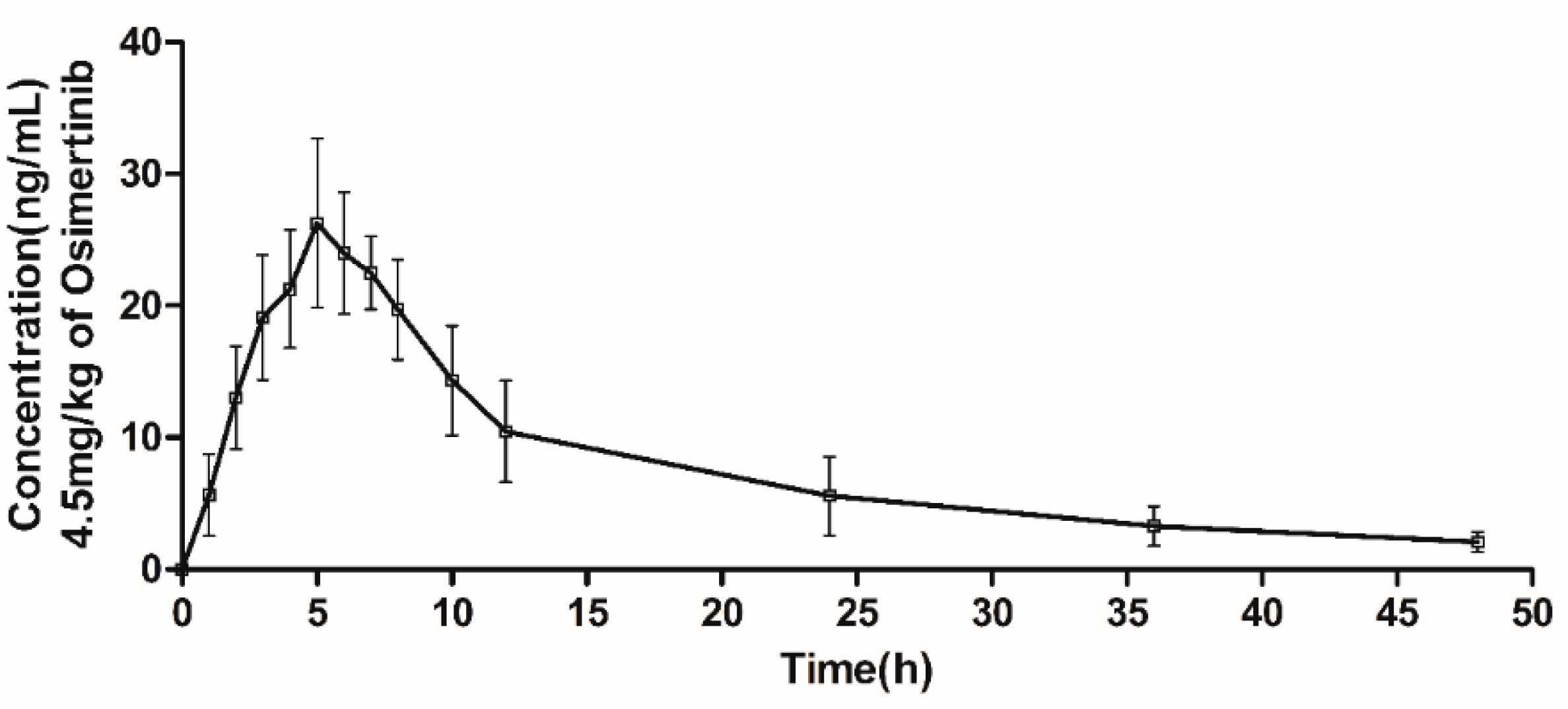
2.3. Pharmacokinetic Application
3. Materials and Methods
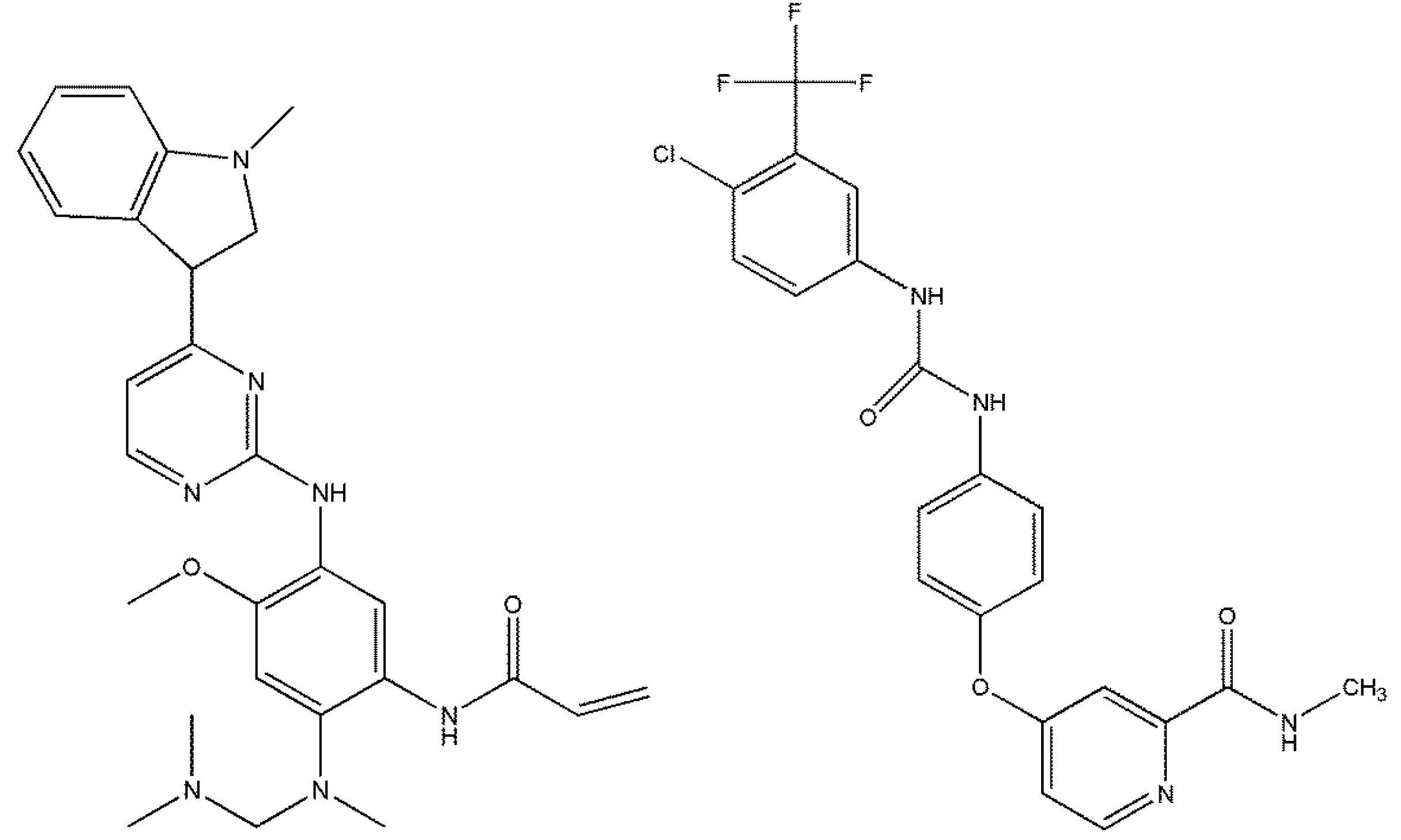
3.1. Drugs and Materials
3.2. Apparatus
3.3. Solution Preparation
3.4. UPLC-TOF-MS Condition
3.5. Pharmacokinetic Application
3.6. Sample Preparation
3.7. Method Validation
3.7.1. Selectivity and Specificity
3.7.2. Linearity and Sensitivity
3.7.3. Precision, Accuracy and Matrix Effect
3.7.4. Recovery
3.7.5. Stability
3.8. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of interest
References
- Chen, Z.; Chen, Y.; Xu, M.; Chen, L.; Zhang, X.; To, K.K.; Zhao, H.; Wang, F.; Xia, Z.; Chen, X.; et al. Osimertinib (AZD9291) enhanced the efficacy of chemotherapeutic agents in ABCB1- and ABCG2-overexpressing cells in vitro, in vivo, and ex vivo. Mol. Cancer Ther. 2016, 15, 1845–1858. [Google Scholar] [CrossRef] [PubMed]
- Cross, D.A.; Ashton, S.E.; Ghiorghiu, S.; Eberlein, C.; Nebhan, C.A.; Spitzler, P.J.; Orme, J.P.; Finlay, M.R.; Ward, R.A.; Mellor, M.J.; et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014, 4, 1046–1061. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Tong, X.; Xu, X.; Zheng, E.; Ni, J.; Li, J.; Yan, J.; Shao, Y.W.; Zhao, G. Case Report: Osimertinib achieved remarkable and sustained disease control in an advanced non-small-cell lung cancer harboring EGFR H773L/V774M mutation complex. Lung Cancer 2018, 121, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Le, X.; Costa, D.B. The safety and efficacy of osimertinib for the treatment of EGFR T790M mutation positive non-small-cell lung cancer. Expert Rev. Anticancer Ther. 2016, 16, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Ricciuti, B.; Chiari, R.; Chiarini, P.; Crino, L.; Maiettini, D.; Ludovini, V.; Metro, G. Osimertinib (AZD9291) and CNS response in two radiotherapy-naive patients with EGFR-Mutant and T790M-Positive advanced non-small cell lung cancer. Clin. Drug Investing. 2016, 36, 683–686. [Google Scholar] [CrossRef] [PubMed]
- Uchino, J.; Nakao, A.; Tamiya, N.; Kaneko, Y.; Yamada, T.; Yoshimura, K.; Fujita, M.; Takayama, K. Treatment rationale and design of the SPIRAL study: A phase II trial of osimertinib in elderly epidermal growth factor receptor T790M-positive nonsmall-cell lung cancer patients who progressed during prior EGFR-TKI treatment. Medicine 2018, 97, e11081. [Google Scholar] [CrossRef] [PubMed]
- Masuhiro, K.; Shiroyama, T.; Suzuki, H.; Takata, S.O.; Nasu, S.; Takada, H.; Morita, S.; Tanaka, A.; Morishita, N.; Okamoto, N.; et al. Impact of pleural effusion on outcomes of patients receiving Osimertinib for NSCLC harboring EGFR T790M. Anticancer Res. 2018, 38, 3567–3571. [Google Scholar] [CrossRef] [PubMed]
- Mountzios, G. Making progress in epidermal growth factor receptor (EGFR)-mutant non-small cell lung cancer by surpassing resistance: Third-generation EGFR tyrosine kinase inhibitors (EGFR-TKIs). Ann. Transl. Med. 2018, 6, 140. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, P.N., Jr.; Haaland, B.; Park, W.; San Tan, P.; Del Giglio, A.; de Lima Lopes, G., Jr. Cost-effectiveness of Osimertinib in the First-Line treatment of patients with EGFR-Mutated advanced non-small cell lung cancer. JAMA Oncol. 2018, 4, 1080–1084. [Google Scholar] [CrossRef] [PubMed]
- Rood, J.J.M.; van Bussel, M.T.J.; Schellens, J.H.M.; Beijnen, J.H.; Sparidans, R.W. Liquid chromatography-tandem mass spectrometric assay for the T790M mutant EGFR inhibitor osimertinib (AZD9291) in human plasma. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2016, 1031, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Deng, Z.; Sun, P.; Mu, Y.; Xue, M. Development and Validation of a Rapid and Sensitive LC-MS/MS Method for the Pharmacokinetic Study of Osimertinib in Rats. J. AOAC Int. 2017, 100, 1771–1775. [Google Scholar] [CrossRef] [PubMed]
- Reis, R.; Labat, L.; Allard, M.; Boudou-Rouquette, P.; Chapron, J.; Bellesoeur, A.; Thomas-Schoemann, A.; Arrondeau, J.; Giraud, F.; Alexandre, J.; et al. Liquid chromatography-tandem mass spectrometric assay for therapeutic drug monitoring of the EGFR inhibitors afatinib, erlotinib and osimertinib, the ALK inhibitor crizotinib and the VEGFR inhibitor nintedanib in human plasma from non-small cell lung cancer patients. J. Pharm. Biomed. Anal. 2018, 158, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wang, W.; Zhang, Y.; Ma, Y.; Zhao, H.; Hu, P.; Jiang, J. Development and validation of a UPLC-MS/MS method for quantification of osimertinib (AZD9291) and its metabolite AZ5104 in human plasma. Biomed. Chromatogr. 2018, 17, e4365. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Park, J.S.; Choi, D.S.; Jung, M.Y. Characterization and quantitation of anthocyanins in purple-fleshed sweet potatoes cultivated in Korea by HPLC-DAD and HPLC-ESI-QTOF-MS/MS. J. Agric. Food Chem. 2013, 61, 3148–3158. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Jung, M.Y. Development of high-performance liquid chromatography-time-of-flight mass spectrometry for the simultaneous characterization and quantitative analysis of gingerol-related compounds in ginger products. J. Agric. Food Chem. 2012, 60, 10015–10026. [Google Scholar] [CrossRef] [PubMed]
- Shintani-Ishida, K.; Kakiuchi, Y.; Ikegaya, H. Successful quantification of 4’-methyl-alpha-pyrrolidinohexanophenone (MPHP) in human urine using LC-TOF-MS in an autopsy case. Forensic Toxicol. 2016, 34, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Wille, K.; Kiebooms, J.A.; Claessens, M.; Rappe, K.; Vanden Bussche, J.; Noppe, H.; Van Praet, N.; De Wulf, E.; Van Caeter, P.; Janssen, C.R.; et al. Development of analytical strategies using U-HPLC-MS/MS and LC-ToF-MS for the quantification of micropollutants in marine organisms. Anal. Bioanal. Chem. 2011, 400, 1459–1472. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Henion, J. Comparison between liquid chromatography-mass spectrometry for quantitative determination of idocifene in human plasma. J. Chromatogr. B Biomed. Sci. Appl. 2001, 5, 151–159. [Google Scholar] [CrossRef]
- Abdelhameed, A.S.; Attwa, M.W.; Kadi, A.A. An LC-MS/MS method for ripad and sensitive high-throughput simultaneous determination of various protein kinase inhibitors in human plasma. Biomed. Chromatogr. 2017, 31. [Google Scholar] [CrossRef]
- Smeraglia, J.; McDougall, S.; Elsby, K.; Companjen, A.; White, S.; Golob, M.; Brudny-Kloeppel, M.; Amsterdam, P.; Timmerman, P. Conference report: AAPS and US FDA Crystal City V meeting on Quantitative Bioanalytical Method Validation and Implementation: Feedback from the EBF. Bioanalysis 2014, 6, 729–732. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
| Concentration (ng/mL) | Intra-Day (n = 7) | Inter-Day (n = 7) | ||||
|---|---|---|---|---|---|---|
| Measured Conc. (ng/mL) | Precision, RSD (%) | Accuracy (%) | Measured Conc. (ng/mL) | Precision RSD (%) | Accuracy (%) | |
| 400 | 395.12 ± 21.27 | 5.38 | 98.78 | 401.13 ± 24.15 | 6.02 | 100.28 |
| 20 | 21.08 ± 1.42 | 6.74 | 105.40 | 19.34 ± 1.83 | 9.46 | 96.70 |
| 2 | 2.05 ± 0.20 | 9.76 | 102.50 | 1.98 ± 0.17 | 8.59 | 99.00 |
| Analyte | Concentration (ng/mL) | Extraction Recovery (%) | |
|---|---|---|---|
| Mean ± SD | RSD | ||
| Osimertinib | 400 | 95.24 ± 3.01 | 3.16 |
| 20 | 96.14 ± 1.83 | 1.90 | |
| 2 | 84.31 ± 3.18 | 3.77 | |
| Sorafinib | 500 | 87.22 ± 4.23 | 4.85 |
| Analyte | Calibration Curve | R2 | Rslope | ||
|---|---|---|---|---|---|
| Min | Max | ΔR | |||
| Osimertinib | Y = 0.085X + 0.1102 | 0.9997 | 0.810 | 0.926 | 0.116 |
| Sorafinib | Y= 0.0781X + 0.2314 | 0.9996 | 0.798 | 0.934 | 0.136 |
| Storage Condition | Concentration (ng/L) | Mean ± SD | RSD% |
|---|---|---|---|
| Autosampler (4 °C) temperature for 24 h | 2 | 2.12 ± 0.23 | 10.85 |
| 20 | 21.45 ± 1.81 | 8.44 | |
| 400 | 406.81 ± 5.64 | 1.39 | |
| Room temperature (25 °C) for 24 h | 2 | 2.21 ± 0.26 | 3.66 |
| 20 | 22.45 ± 2.18 | 9.71 | |
| 400 | 407.28 ± 5.12 | 1.26 | |
| Storage temperature (−80 °C) for 30 days | 2 | 2.29 ± 0.25 | 10.92 |
| 20 | 21.33 ± 1.74 | 8.16 | |
| 400 | 406.34 ± 7.51 | 1.85 | |
| Three freeze–thaw cycles (each at −80 °C for 24 h) | 2 | 2.27 ± 0.19 | 8.37 |
| 20 | 22.20 ± 1.92 | 8.65 | |
| 400 | 406.17 ± 6.19 | 1.52 |
| Pharmacokinetic Parameter | Osimertinib |
|---|---|
| AUC(0–t), ng/mL·h | 382.00 ± 69.00 |
| AUC(0–∞), ng/mL·h | 426.01 ± 81.73 |
| MRT, h | 14.51 ± 1.91 |
| t1/2z, h | 14.96 ± 3.44 |
| tmax, h | 4.80 ± 1.10 |
| Cmax, ng/mL | 28.49 ± 3.97 |
| Vz/F, L/kg | 233.82 ± 66.68 |
| CLz/F, L/h/kg | 10.84 ± 1.94 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, S.-T.; Li, Y.; Yang, H.-T.; Wu, Y.; Li, Y.-J.; Ding, C.-Y.; Meng, L.; Dong, Z.-J.; Zhang, Y. An Accurate and Effective Method for Measuring Osimertinib by UPLC-TOF-MS and Its Pharmacokinetic Study in Rats. Molecules 2018, 23, 2894. https://doi.org/10.3390/molecules23112894
Dong S-T, Li Y, Yang H-T, Wu Y, Li Y-J, Ding C-Y, Meng L, Dong Z-J, Zhang Y. An Accurate and Effective Method for Measuring Osimertinib by UPLC-TOF-MS and Its Pharmacokinetic Study in Rats. Molecules. 2018; 23(11):2894. https://doi.org/10.3390/molecules23112894
Chicago/Turabian StyleDong, Song-Tao, Ying Li, Hao-Tian Yang, Yin Wu, Ya-Jing Li, Cong-Yang Ding, Lu Meng, Zhan-Jun Dong, and Yuan Zhang. 2018. "An Accurate and Effective Method for Measuring Osimertinib by UPLC-TOF-MS and Its Pharmacokinetic Study in Rats" Molecules 23, no. 11: 2894. https://doi.org/10.3390/molecules23112894
APA StyleDong, S.-T., Li, Y., Yang, H.-T., Wu, Y., Li, Y.-J., Ding, C.-Y., Meng, L., Dong, Z.-J., & Zhang, Y. (2018). An Accurate and Effective Method for Measuring Osimertinib by UPLC-TOF-MS and Its Pharmacokinetic Study in Rats. Molecules, 23(11), 2894. https://doi.org/10.3390/molecules23112894