Abstract
The three-point adsorption of tripod-shaped molecules enables the formation of robust self-assembled monolayers (SAMs) on solid surfaces, where the component molecules are fixed in a strictly upright orientation. In the present study, SAMs of a rigid molecular tripod consisting of an adamantane core and three CH2SH groups were employed to arrange ferrocene on a gold surface through oligo(p-phenyleneethynylene) linkers. Cyclic voltammetry of the monolayers demonstrated high surface coverage of ferrocene, yet the molecular interaction among adjacent ferrocene units was negligible. This was because of the extended intermolecular distance caused by the bulky tripod framework. The rates of electron transfer from the ferrocene to the gold surface through different linker lengths were determined by electrochemical measurements, from which the decay factor for oligo(p-phenyleneethynylene) wire was evaluated.
1. Introduction
Self-assembled monolayer (SAM) of thiolates on a gold surface, produced by the tight Au–S bonding, creates a secure molecule–metal junction [1,2,3,4]. The thermodynamically favored chemisorption of molecules enables monolayer formation simply by forcing thiols to make contact with a clean gold surface. The resultant dense SAMs have been applied to sensors [5,6], molecular machines [7,8], and molecular electronic devices [9,10,11]. In particular, a SAM of electron-transporting molecules could serve as an excellent junction between the device molecule and a metal electrode.
The component molecules in a densely packed SAM, however, are subjected to severe steric and electrostatic interactions, which can exert an undesirable mutual intermolecular influence that can potentially alter the structural and electronic features of the original molecules in their isolated state. This would hamper the design and utilization of the single-molecule functionalities of the monolayers.
The use of tripod-shaped anchors may be, due to their surface-demanding nature, effective in avoiding such interactions by isolating the molecules within a SAM. A variety of SAMs composed of tripodal trithiols have been reported using the sp3-hybridized carbon [10,12,13] or silicon [14] atom as a central tetrahedral core. Other tripods have utilized the rigid carbon framework of adamantane, in which the four bridgehead bonds extend in the tetrahedral direction [15,16,17,18,19,20,21]. A cyclohexane ring, which has a partial structure of adamantane, was also used as a core carbon framework for three thiol legs [22,23]. Previously, we reported a tripod molecule 1 [16,17,18,19,21] and its ferrocenyl derivative 2a [18,19,20], consisting of a rigid adamantane core and three CH2SH legs (Figure 1). X-ray photoelectron spectroscopy (XPS) studies supported the three-point adsorption of these molecules on the Au(111) surface [20], and a perpendicular orientation of the molecule was shown by DFT optimization [21].
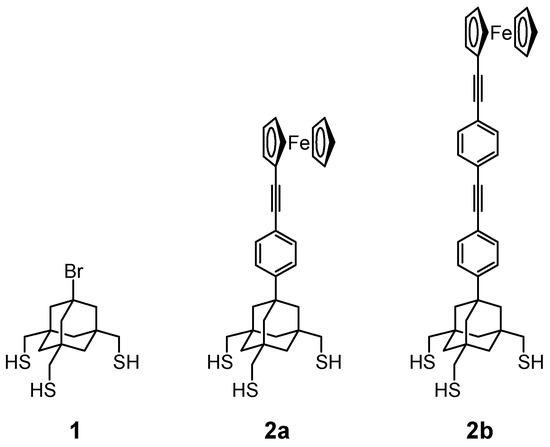
Figure 1.
Tripod-shaped trithiols with an adamantane core.
Based on the results of scanning tunneling microscopy (STM) studies, the SAM of 1 is hexagonally arranged with a closest molecular distance of 8.7 Å (Figure 2a) [16]. This distance is significantly larger than that normally observed for the SAMs of linear alkanethiols (5.0 Å) [2,4,24] and allows molecule 2a to arrange in the same density, wherein neighboring ferrocenyl groups are isolated from each other (Figure 2b) [20]. This feature is highly desirable for the study of the electron transport properties of π-conjugated molecular wires, such as oligo(p-phenyleneethynylene) or –(C6H4-C≡C)n–. The connection of the straight, rod-like structure of this wire to the adamantane tripod is of particular interest, since the wire may be kept upright in the SAM without intermolecular contact.
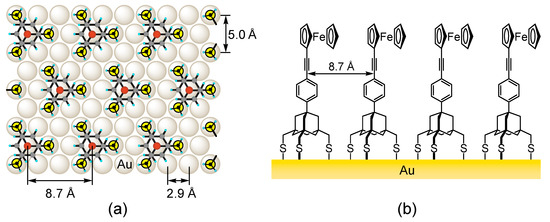
Figure 2.
(a) Proposed molecular orientation of the SAMs of 1 on the Au(111) surface; and (b) the schematic diagram of the SAM of ferrocene-terminated trithiol 2a on a gold surface.
In this paper, we report the electron transfer behavior of p-phenyleneethynylene bridges joining ferrocene and gold substrate using the SAMs of 2a and 2b via electrochemical techniques. Reliable single-molecule properties were expected, owing to the independence of each component molecule from neighboring molecules. The decay of the electron transfer rate through the molecule was discussed by comparing the results from the two SAMs.
2. Results and Discussion
2.1. Reductive Desorption
The SAMs were formed by immersing a Au(111) substrate, prepared by vacuum deposition of gold on mica, into a dichloromethane solution of 2a or 2b at ambient temperature. Thiol–gold SAMs generally undergo desorption of thiolate ion by electrochemical reduction according to Equation (1) [25,26,27,28,29,30,31].
where Au(s) represents the gold atom on the solid surface.
RS–Au(s) + e− → RS− + Au(s)
Previously, we reported that the SAMs of 1 and 2a show a reductive desorption peak in cyclic voltammetry [16,20]. The cyclic voltammetry of the SAM of 2b at negative potentials in aqueous KOH also showed an irreversible reduction peak at −1.024 V versus Ag/AgCl (Figure 3). The peak potential (Ep), charge density of the reductive wave (Qred), and full width at half-maximum (ΔEfwhm) of related SAMs are summarized in Table 1.
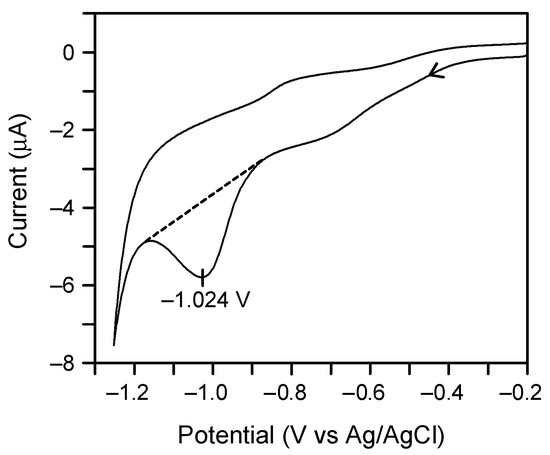
Figure 3.
Reductive desorption of the SAM of 2b on Au(111), as observed by cyclic voltammetry using the surface-modified gold substrate as a working electrode in 0.5 M aqueous KOH. The scan rate was 0.02 V/s. The geometric area of the working electrode was 0.152 cm2. The charge for reductive desorption was calculated from the area below the dotted line.

Table 1.
Peak potential (Ep), charge density (Qred), and full width at half-maximum (ΔEfwhm) for the electrochemical reductive desorption of SAMs derived from trithiols and dodecanethiol on Au(111). a
The SAMs of long-chain n-alkanethiols, such as n-dodecanethiol, are known to show a large negative Ep (<−1.0 V) because the adsorbed molecules resist desorption due to strong attractive interactions between closely neighbored, van der Waals-contacting alkyl groups [30]. Although such interactions are small in the SAMs of tripod molecules 2a and 2b, large negative reduction potentials were similarly observed for these molecules. This can be attributed to tighter binding of the molecules to the substrate by the three S–Au bonds.
The small ΔEfwhm for n-dodecanethiol can also be ascribed to the strong attractive interaction between neighboring alkyl chains [30], while the much larger values observed for tripod trithiols indicate the insignificance of such interactions. The Qred of the SAM of 2b is comparable to that of 2a, indicating that, despite the extended molecular length of 2b, its SAM is as densely packed as in the case of 2a.
2.2. Oxidation of the Ferrocenyl Group
The cyclic voltammograms of the SAMs of 2a and 2b at positive potentials showed reversible redox waves at approximately 0.4 V versus Ag/AgNO3, owing to the single-electron oxidation of the ferrocenyl group. The redox charges, calculated from the mean value of oxidation and reduction peak areas, were Qox = 24 ± 2 μC/cm2 for both SAMs. This corresponds to a surface coverage (Γ = Qox/F, where F is the Faraday constant) of 2.5 × 10–10 mol/cm2 and indicates that the molecules were packed as densely as in the SAM of 1. If each of the 2a and 2b molecules are adsorbed by three sulfur atoms, the reductive desorption charge is expected to be 3FΓ = 72 μC/cm2. Considering that approximately 30% of the additional reductive charge is often observed upon desorption of S–Au SAMs due to the change in double layer capacitance, a Qred value of 94 μC/cm2 is expected. The observed values for Qred for the SAM of 2a and 2b approximate this value, which supports a secure three-point adsorption.
At sufficiently low scan rates (<0.1 V/s), the full width at half-maximum (ΔEfwhm) and the cathodic to anodic peak separation (ΔEpp) approximated those predicted for an ideal Nernstian redox system (ΔEfwhm = 90.6 mV and ΔEpp = 0 mV) [32], which indicated that each ferrocenyl group in these SAMs was well isolated from its neighboring molecules. In this scan rate region, ΔEpp was not affected by the scan rate variation.
Upon increasing the scan rate over 0.1 V/s, both SAMs showed a gradual increase in ΔEpp (Figure 4), which is typical of a kinetic outcome involving the rate of electron transfer through adsorbed material. Figure 5 illustrates the plots of the anodic and cathodic peak potentials relative to the formal potential E0 on the natural logarithm of the scan rate. Based on the increases in ΔEp as a function of the scan rate, the rate constant of electron transfer (kET) was estimated using Laviron’s Equation (2) [33], resulting in values of 40 ± 5 and 28 ± 4 s–1 for the SAMs of 2a and 2b, respectively.
where n is the number of electrons transferred per molecule (n = 1 in the present case), F is the Faraday constant, R is the gas constant, and T is the temperature (298 K). v0 is the scan rate at the intercept of the fit line (dashed lines in Figure 5) with the horizontal line Ep − E0 = 0. The transfer coefficient, α, was set to 0.5.
kET = αnFv0/RT
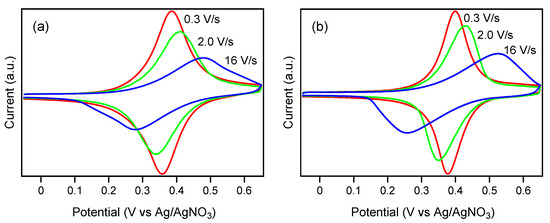
Figure 4.
Cyclic voltammograms of the SAM of (a) 2a and (b) 2b recorded at different scan rates in CH2Cl2 containing 0.1 M TBAP as a supporting electrolyte. The geometric area of the working electrode was 0.152 cm2.
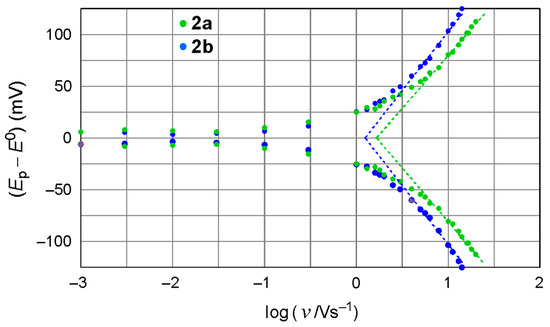
Figure 5.
Plot of Ep − E0 versus the logarithm of scan rate for the SAMs of 2a and 2b on Au(111). E0 was 0.371 and 0.392 V versus Ag/Ag+ for 2a and 2b, respectively.
2.3. Dependence of the Electron Transfer Rate on Distance
In general, the electron transfer rate decays exponentially with distance, as follows.
where k0 is the preexponential factor and β is the decay constant. The distances (r), taken from the plane determined by the three sulfur atoms to the carbon atom in the ferrocene attached to the ethynyl carbon, were estimated to be 12.9 and 19.7 Å in the SAMs of 2a and 2b, respectively, from the DFT optimized structure of the trithiols (Figure 6). On the basis of the deceleration of the electron transfer from 40 s−1 (2a) to 28 s−1 (2b), the decay constant (β) was derived to be 0.05 Å−1.
kET= k0 exp(−βr)
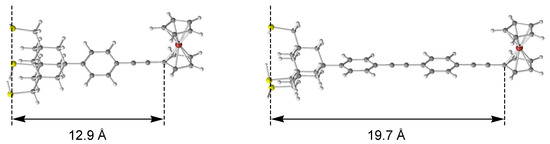
Figure 6.
Structures of trithiol 2a (left) [20] and 2b (right) optimized at the B3LYP/3-21G(d)-LANL2DZ level.
The β values for similar, but adamantane-free, oligo(p-phenyleneethynylene) wires substituted with methyl and propoxy groups were reported by Creager et al. (β = 0.36 Å−1, n = 3–6) [34] and by Sachs et al. (β = 0.57 Å−1, n = 2, 3) [35] using non-electroactive diluent thiols. The r–ln kET plot in Figure 7 shows their data, together with our results and those reported by Creager for linear alkyl chain wires, FcCONH(CH2)mS–Au(s) (m = 7–10 and 15) [36].
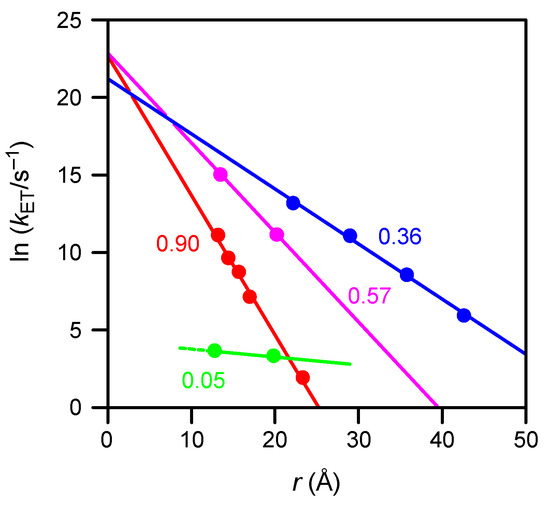
Figure 7.
Plots of ln kET versus distance for SAMs of molecular wires bridging ferrocene and gold via S–Au bonding. Green (this work), blue [34], and purple [35]: oligo(p-phenyleneethynylene) wire. Red [36]: linear alkylamide wire. Values of β are indicated.
A significant feature of our data is the extremely slow electron transfer, by a factor of approximately e–10 (<10–4), compared with that reported for adamantane-free oligo(p-phenyleneethynylene) wires. The reason could be the insertion of an aliphatic adamantyl group into our molecule. Another important point is that although all lines in Figure 7, except for that of the present work, converge to ln kET = 23 at r = 0, which corresponds to the common limit at zero distance, our data extrapolates to a much lower value, ln kET~4. This could also be caused by the presence of the adamantane tripod acting as a resistor between the end of the conjugated wire and the gold.
The cause of the very small β (0.05 Å−1) of our linker is unclear. A possible cause of the large difference between this value and other oligo(p-phenyleneethynylene) wires in Figure 7 could be the electrolyte used for our measurement (TBAP in CH2Cl2), which has a lower polarity than either aqueous HClO4 or NaClO4 that was used in other studies [34,35].
3. Materials and Methods
3.1. Materials
For electrochemical experiments, water was purified using a Millipore Simplicity 185 Water System (18 MΩ cm resistivity). Other solvents for electrolyte preparation and the rinsing of glassware were of the highest purity commercially available and were used without further purification. Gold (99.99%) was obtained as a 0.8-mm wire. Mica was purchased from Nilaco (Tokyo, Japan) in the form of a 0.40–0.45-mm natural sheet.
Trithiol 2a was prepared by the method we reported earlier [20]. Compound 2b was synthesized by Sonogashira coupling reaction of iodopheny-terminated trithioacetate 3 [16,20] and ferrocene derivative 4 [37] to give 5, followed by the methanolysis of the three AcS groups (Scheme 1). Details of the synthetic procedure are provided in the Supplementary Materials.
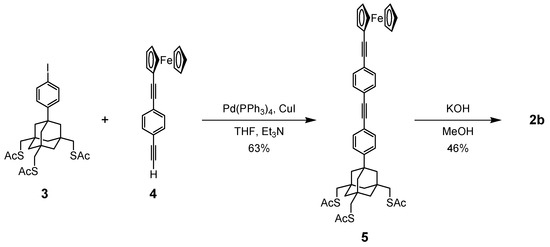
Scheme 1.
Synthetic route to elongated trithiol 2b.
3.2. Preparation of Self-Assembled Monolayers on Gold
Gold substrates with a (111) surface were prepared via vacuum vapor deposition of gold (99.99%) onto freshly cleaved mica sheets (0.05 mm thickness, 6 × 6 cm) under high vacuum (<10−3 Pa) at a substrate temperature of 580 °C. The deposition was performed at an evaporation rate of 1.0–1.5 nm/s until a gold thickness of 200 nm was reached. The obtained substrate was cut into 1 × 2 cm pieces and annealed at 530 °C in a furnace for 8 h under air to remove the surface contamination and to minimize defects. SAMs were formed by soaking the substrate in a 0.1 mM solution of trithiols in CH2Cl2 for at least 24 h.
3.3. Electrochemical Measurements
Glassware (cells and pipettes, etc.) used for electrochemical measurements were soaked in 10% potassium hydroxide in 2-propanol for 24 h to remove surface organic contaminants, washed thoroughly with deionized water, and dried under air at 100 °C before use. The surface-modified gold substrate was mounted at the bottom of a cone-shaped cell using an O-ring to serve as a working electrode. The area of the electrode exposed to the electrolyte was 0.152 cm2 (i.e., a 4.4 mm diameter circle). Reductive desorption was recorded with aqueous 0.5 M KOH using an Ag/AgCl/sat. KCl reference electrode (Supplementary Materials, Figure S5a). Redox waves of a ferrocenyl group were observed using a CH2Cl2 solution containing 0.1 M tetrabutylammonium perchlorate (TBAP) and an Ag/AgNO3 (0.01 M in CH3CN) reference electrode (Supplementary Materials Figure S5b). The electrolyte solution in the cell was de-aerated by bubbling argon for 10 min before scanning. Voltammograms were recorded using a ALS600C electrochemical analyzer (BAS, Tokyo, Japan).
3.4. Theoretical Calculations
Results of DFT calculations [38] for 2a were reported earlier [20]. Calculations for 2b were performed in a similar manner using the Gaussian 03 program [39]. Geometry optimization was carried out using the B3LYP method and general basis set (Gen keyword). The C, H, and S atoms were calculated with the 3-21G(d) basis set, whereas Fe was calculated with the LANL2DZ (5D, 7F) basis set. The results of optimization are shown in Supplementary Materials, Table S1. The obtained geometry was verified by frequency calculations to have no imaginary frequencies.
4. Conclusions
Tripod-shaped trithiols, 2a and 2b, bearing a ferrocenyl group via mono and bis(p-phenyleneethynylene) linker formed SAMs on Au(111) surface that featured negligible interactions among neighboring ferrocenyl groups, regardless of the high coverage. These SAMs showed kETs [40 ± 5 s–1 (2a) and 28 ± 4 s–1 (2b)] that are more than four orders of magnitude smaller than the values reported for linkers of the same type and length. A very small decay constant, β = 0.05 Å−1, was also observed. These findings can be explained by the fact that a saturated framework of adamantane insulates conjugated p-phenyleneethynylene wire and gold. Another possible reason is the low polarity of the electrolyte we employed, compared with those used in other works. Studies on the electron transfer properties of further elongated oligo(p-phenyleneethynylene) linkers are in progress. Also, it would be valuable to elucidate the nature of the “resistance” posed by the adamantane framework by the use of directly linked ferrocene–adamantane tripod.
Supplementary Materials
Supplementary materials including synthetic procedures, figures of NMR spectra and cyclic voltammetry cells, and the results of DFT calculations are available online.
Author Contributions
T.K. (Toshikazu Kitagawa) conceived and designed the experiments. T.K. (Takashi Kawano), T.H., and I.H. conducted the experiments. T.K. (Toshikazu Kitagawa) and K.H. analyzed the data. T.O. performed theoretical calculations. T.K. (Toshikazu Kitagawa) wrote the paper.
Funding
This research was funded by MEXT KAKENHI Grant Number JP20027006 and JSPS KAKENHI Grant Number JP15K05474. We also thank the Izumi Science and Technology Foundation for supporting our study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ulman, A. Formation and Structure of Self-Assembled Monolayers. Chem. Rev. 1996, 96, 1533–1554. [Google Scholar] [CrossRef] [PubMed]
- Poirier, G.E. Characterization of Organosulfur Molecular Monolayers on Au(111) using Scanning Tunneling Microscopy. Chem. Rev. 1997, 97, 1117–1127. [Google Scholar] [CrossRef] [PubMed]
- Love, J.C.; Estroff, L.A.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-Assembled Monolayers of Thiolates on Metals as a Form of Nanotechnology. Chem. Rev. 2005, 105, 1103–1169. [Google Scholar] [CrossRef] [PubMed]
- Kind, M.; Wöll, C. Organic surfaces exposed by self-assembled organothiol monolayers: Preparation, characterization, and application. Prog. Surf. Sci. 2009, 84, 230–278. [Google Scholar] [CrossRef]
- Gooding, J.J.; Mearns, F.; Yang, W.; Liu, J. Self-Assembled Monolayers into the 21st Century: Recent Advances and Applications. Electroanalysis 2003, 15, 81–96. [Google Scholar] [CrossRef]
- Bertin, P.A.; Ahrens, M.J.; Bhavsar, K.; Georganopoulou, D.; Wunder, M.; Blackburn, G.F.; Meade, T.J. Ferrocene and Maleimide-Functionalized Disulfide Scaffolds for Self-Assembled Monolayers on Gold. Org. Lett. 2010, 12, 3372–3375. [Google Scholar] [CrossRef] [PubMed]
- Van Delden, R.A.; ter Wiel, M.K.J.; Pollard, M.M.; Vicario, J.; Koumura, N.; Feringa, B.L. Unidirectional molecular motor on a gold surface. Nature 2005, 437, 1337–1340. [Google Scholar] [CrossRef] [PubMed]
- Kay, E.R.; Leigh, D.A.; Zerbetto, F. Synthetic Molecular Motors and Mechanical Machines. Angew. Chem. Int. Ed. 2007, 46, 72–191. [Google Scholar] [CrossRef] [PubMed]
- Tour, J.M. Molecular Electronics. Synthesis and Testing of Components. Acc. Chem. Res. 2000, 33, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, D.; Takimiya, K.; Aso, Y.; Otsubo, T.; Hasobe, T.; Yamada, H.; Imahori, H.; Fukuzumi, S.; Sakata, Y. Large Photocurrent Generation of Gold Electrodes Modified with [60]Fullerene-Linked Oligothiophenes Bearing a Tripodal Rigid Anchor. J. Am. Chem. Soc. 2002, 124, 532–533. [Google Scholar] [CrossRef] [PubMed]
- McCreery, R.L. Molecular Electronic Junctions. Chem. Mater. 2004, 16, 4477–4496. [Google Scholar] [CrossRef]
- Fox, M.A.; Whitesell, J.K.; McKerrow, A.J. Fluorescence and Redox Activity of Probes Anchored through an Aminotrithiol to Polycrystalline Gold. Langmuir 1998, 14, 816–820. [Google Scholar] [CrossRef]
- Wei, L.; Tiznado, H.; Liu, G.; Padmaja, K.; Lindsey, J.S.; Zaera, F.; Bocian, D.F. Adsorption Characteristics of Tripodal Thiol-Functionalized Porphyrins on Gold. J. Phys. Chem. B 2005, 109, 23963–23971. [Google Scholar] [CrossRef] [PubMed]
- Yam, C.M.; Cho, J.; Cai, C. Preparation, Characterization, and Heck Reaction of Multidentate Thiolate Films on Gold Surfaces. Langmuir 2003, 19, 6862. [Google Scholar] [CrossRef]
- Kittredge, K.W.; Minton, M.A.; Fox, M.A.; Whitesell, J.K. α-Helical polypeptide films grown from sulfide or thiol linkers on gold surfaces. Helv. Chim. Acta 2002, 85, 788–798. [Google Scholar] [CrossRef]
- Kitagawa, T.; Idomoto, Y.; Matsubara, H.; Hobara, D.; Kakiuchi, T.; Okazaki, T.; Komatsu, K. Rigid molecular tripod with an adamantane framework and thiol legs. synthesis and observation of an ordered monolayer on Au(111). J. Org. Chem. 2006, 71, 1362–1369. [Google Scholar] [CrossRef] [PubMed]
- Katano, S.; Kim, Y.; Matsubara, H.; Kitagawa, T.; Kawai, M. Hierarchical chiral framework based on a rigid adamantane tripod on Au(111). J. Am. Chem. Soc. 2007, 129, 2511–2515. [Google Scholar] [CrossRef] [PubMed]
- Katano, S.; Kim, Y.; Kitagawa, T.; Kawai, M. Self-assembly and scanning tunneling microscopy tip-induced motion of ferrocene adamantane trithiolate adsorbed on Au(111). Jpn. J. Appl. Phys. 2008, 47, 6156–6159. [Google Scholar] [CrossRef]
- Katano, S.; Kim, Y.; Kitagawa, T.; Kawai, M. Tailoring electronic states of a single molecule using adamantane-based molecular tripods. Phys. Chem. Chem. Phys. 2013, 15, 14229–14233. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, T.; Matsubara, H.; Komatsu, K.; Hirai, K.; Okazaki, T.; Hase, T. Ideal redox behavior of the high-density self-assembled monolayer of a molecular tripod on a Au(111) surface with a terminal ferrocene group. Langmuir 2013, 29, 4275–4282. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, T.; Nakanishi, S.; Mizuno, A.; Niwa, Y.; Tabata, H.; Hirai, K.; Okazaki, T. Tuning the coverage of self-assembled monolayer by introducing bulky substituents onto rigid adamantane tripod. ARKIVOC 2018, 2, 131–144. [Google Scholar] [CrossRef]
- Singhana, B.; Rittikulsittichai, S.; Lee, T.R. Tridentate adsorbates with cyclohexyl headgroups assembled on gold. Langmuir 2013, 29, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Singhana, B.; Jamison, A.C.; Hoang, J.; Lee, T.R. Self-assembled monolayer films derived from tridentate cyclohexyl adsorbates with alkyl tailgroups of increasing chain length. Langmuir 2013, 29, 14108–14116. [Google Scholar] [CrossRef] [PubMed]
- Kakiuchi, T.; Iida, M.; Gon, N.; Hobara, D.; Imabayashi, S.; Niki, K. Miscibility of adsorbed 1-undecanethiol and 11-mercaptoundecanoic acid species in binary self-assembled monolayers on Au(111). Langmuir 2001, 17, 1599–1603. [Google Scholar] [CrossRef]
- Walczak, M.M.; Popenoe, D.D.; Deinhammer, R.S.; Lamp, B.D.; Chung, C.; Porter, M.D. Reductive desorption of alkanethiolate monolayers at gold: A measure of surface coverage. Langmuir 1991, 7, 2687–2693. [Google Scholar] [CrossRef]
- Widrig, C.A.; Chung, C.; Porter, M.D. The electrochemical desorption of n-alkanethiol monolayers from polycrystalline Au and Ag electrodes. J. Electroanal. Chem. 1991, 310, 335–359. [Google Scholar] [CrossRef]
- Yang, D.-F.; Wilde, C.P.; Morin, M. Studies of the electrochemical removal and efficient re-formation of a monolayer of hexadecanethiol self-assembled at an Au(111) single crystal in aqueous solutions. Langmuir 1997, 13, 243–249. [Google Scholar] [CrossRef]
- Imabayashi, S.; Iida, M.; Hobara, D.; Feng, Z.Q.; Niki, K.; Kakiuchi, T. Reductive desorption of carboxylic-acid-terminated alkanethiol monolayers from Au(111) surfaces. J. Electroanal. Chem. 1997, 428, 33–38. [Google Scholar] [CrossRef]
- Hobara, D.; Miyake, K.; Imabayashi, S.; Niki, K.; Kakiuchi, T. In-situ scanning tunneling microscopy imaging of the reductive desorption process of alkanethiols on Au(111). Langmuir 1998, 14, 3590–3596. [Google Scholar] [CrossRef]
- Kakiuchi, T.; Usui, H.; Hobara, D.; Yamamoto, M. Voltammetric properties of the reductive desorption of alkanethiol self-assembled monolayers from a metal surface. Langmuir 2002, 18, 5231–5238. [Google Scholar] [CrossRef]
- Eggers, P.K.; Zareie, H.M.; Paddon-Row, M.N.; Gooding, J.J. Structure and properties of redox active self-assembled monolayers formed from norbornylogous bridges. Langmuir 2009, 25, 11090–11096. [Google Scholar] [CrossRef] [PubMed]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley and Sons: New York, NY, USA, 2000; pp. 590–593. [Google Scholar]
- Laviron, E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Creager, S.; Yu, C.J.; Bamdad, C.; O’Connor, S.; MacLean, T.; Lam, E.; Chong, Y.; Olsen, G.T.; Luo, J.; Gozin, M.; et al. Electron transfer at electrodes through conjugated “molecular wire” bridges. J. Am. Chem. Soc. 1999, 121, 1059–1064. [Google Scholar] [CrossRef]
- Sachs, S.B.; Dudek, S.P.; Hsung, R.P.; Sita, L.R.; Smalley, J.F.; Newton, M.D.; Feldberg, S.W.; Chidsey, C.E.D. Rates of interfacial electron transfer through π-conjugated spacers. J. Am. Chem. Soc. 1997, 119, 10563–10564. [Google Scholar] [CrossRef]
- Weber, K.; Hockett, L.; Creager, S. Long-range electronic coupling between ferrocene and gold in alkanethiolate-based monolayers on electrodes. J. Phys. Chem. B 1997, 101, 8286–8291. [Google Scholar] [CrossRef]
- Hsung, R.P.; Chidsey, C.E.D.; Sita, L.R. Synthesis and characterization of unsymmetric ferrocene-terminated phenylethynyl oligomers Cp2Fe-[C≡C-C6H4]n-X (X = SH, SMe, SOMe, and SO2Me). Organometallics 1995, 14, 4808–4815. [Google Scholar] [CrossRef]
- Koch, W.; Holthausen, M.C. A Chemist’s Guide to Density Functional Theory, 2nd ed.; Wiley–VCH: Weinheim, Germany, 2000; ISBN 3-527-30372-3. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A., Jr.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 03; Revision E.01; Gaussian, Inc.: Wallingford, CT, USA, 2004. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).