Abstract
Fifty-seven compounds were purified from the stems of Tinospora sinensis, including three new compounds characterized as a lignan (1), a pyrrole alkaloid (11), and a benzenoid (17), respectively. Their structures were elucidated and established by various spectroscopic and spectrometric analytical methods. Among the isolates, fifteen compounds were examined for their anti-inflammatory potential in vitro. The results showed that several compounds displayed moderate inhibition of N-formyl-methionyl-leucyl-phenylalanine/cytochalasin B (fMLP/CB)-induced superoxide anion generation and elastase release.
1. Introduction
Inflammation is the first response of the immune system to infection or irritation. Neutrophils play an important role in eliminating most of the exogenous pathogens. Various autoimmune diseases are linked to neutrophil overexpression, such as rheumatoid arthritis, ischemia, and asthma, etc. [1,2,3]. According to response of diverse stimuli, activated neutrophils will secrete a series of cytotoxins. The superoxide anions and neutrophil elastase are the major secreted products of stimulated neutrophils in infected tissues and organs, which contribute to the destruction of tissue in chronic inflammatory diseases [4,5,6]. Therefore, inhibition of superoxide anion generation and elastase release by natural compounds is considered to be an effective screening platform to evaluate anti-inflammatory drug candidates.
The genus Tinospora, belonging to family Menispermaceae, is composed of more than 20 species all over the tropical regions of the Eastern Hemisphere [7]. This genus is traditionally medical used in Southeast Asian countries for treating malaria, skin diseases, gout, and diabetes [8]. The majority of scientific reports of this genus state their physiological activities including antioxidation, anti-inflammation, and cytotoxicity, especially with the most extensively explored hypoglycemic activity [9,10,11,12,13]. However, the bioactive principles of T. sinensis remained poorly understood. Therefore, this plant was selected for study to discover novel anti-inflammatory lead compounds due to their relieving rigidity of muscles and activating collaterals effects in long-term folk medicine usage, which may be related to anti-inflammatory bioactivity. According to the preliminary screening results, the methanol extract of T. sinensis collected from Vietnam displayed half maximal inhibitory concentration (IC50) values of 6.66 μg/mL and 4.68 μg/mL in the inhibition of superoxide anion generation and elastase release, respectively (Table S1). Further chromatography purification resulted in the characterization of nine lignans (1–9), six pyrrole alkaloids (10–15), seventeen benzenoids (16–32), ten terpenoids (33–42), eight steroids (43–50), four amides (51–54), one coumarin (55), and two others (56–57), respectively. The chemical structures of new compounds 1, 11, and 17 (Figure 1) were established on the basis of nuclear magnetic resonance (NMR) and mass spectrometric analyses. Some of these purified compounds were examined for inhibition of superoxide anion generation and elastase release, thereby evaluating their in vitro anti-inflammatory potentials.
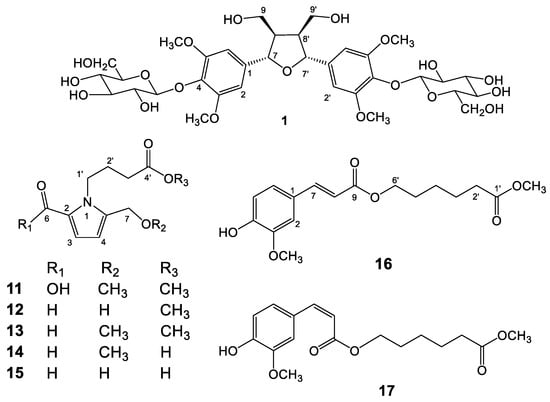
Figure 1.
Structures of compounds 1 and 11–17.
2. Results and Discussion
The dried stems of T. sinensis were refluxed with methanol and the obtained extract was divided into chloroform (CHCl3) and water (H2O) soluble fractions by liquid–liquid partition. Further purification over silica gel column and preparative thin layer chromatography (pTLC) resulted in the isolation of fifty-seven compounds. Among the isolated compounds, 1, 11, and 17 were new compounds. The other fifty-four known compounds were identified, including eight lignans, (+)-pinoresinol (2) [14], syringaresinol (3) [15], medioresinol (4) [16], (+)-epi-syringaresinol (5) [15], (+)-pinoresinol monomethyl ether (6) [17], (+)-glaberide I (7) [18], sesamin (8) [19], and sesamolin (9) [20]; five pyrrole alkaloids, 5-(hydroxymethyl)-1H-pyrrole-2-carbaldehyde (10) [21], methyl 4-[formyl-5-(hydroxymethyl)-1H-pyrrol-1-yl] butanoate (12) [22,23], methyl 4-[formyl-5-(methoxymethyl)-1H-pyrrol-1-yl] butanoate (13) [22,23], 4-[formyl-5-(methoxymethyl)-1H-pyrrol-1-yl] butanoic acid (14) [22,23], and 4-[formyl-5-(hydroxymethyl)-1H-pyrrol-1-yl] butanoic acid (15) [23]; seventeen benzenoids, rhodiolate (16) [24], methyl ferulate (18) [25], β-hydroxypropiovanillone (19) [26], 2-methyl-4,5-dimethoxybenzoic acid (20) [27], vanillic acid (21) [28], p-hydroxyl phenethanol (22) [29], tachioside (23) [30], icariside D2 (24) [31], salidroside (25) [32], syringin (26) [33], cordifolioside A (27) [34], p-hydroxybenzoic acid (28) [35], 4-(2-hydroxyethyl)benzoic acid (29) [36], syringic acid-4-O-α-l-rhamnoside (30) [37], isovanillic acid (31) [38], syringic acid (32) [39]; ten terpenoids, loliolide (33) [40], abscisic acid (34) [41], 3(17)-phytene 1,2-diol (35) [42], malabarolide (36) [43], lupeol (37) [44], 3-O-acetyloleanolic acid (38) [45], cycloeucalenol (39) [46], cycloabyssinone (40) [47], cycloartane-3β,25-diol (41) [48], and cycloart-22-ene-3β,25-diol (42) [49]; eight steroids, β-sitosterol (43) [50], stigmasterol (44) [50], 7α-hydroxysitosterol (45) [51], 7α-hydroxystigmasterol (46) [51], 6β-hydroxystigmast-4-en-3-one (47) [52], 6β-hydroxystigmasta-4,22-dien-3-one (48) [52], 7-ketositosterol (49) [53], and 3β-hydroxy-stigmasta-5,22-dien-7-one (50) [53]; four amides, 5,6-dimethoxy-N-methylphthalimide (51) [54], N-trans-feruloyldopamine (52) [55], N-trans-feruloyltyramine (53) [56], N-cis-feruloyltyramine (54) [57]; and one coumarin, scopoletin (55) [58]; and two others, lichexanthone (56) [59] and 2,6-dimethoxy-p-quinone (57) [60], respectively. The chemical structures of these new constituents were determined on the basis of 1D and 2D NMR and mass spectrometric analyses elucidated as follow.
The molecular formula of compound 1 was determined as C34H48O19 by high resolution electrospray ionization mass spectrometry (HR-ESI-MS) which showed a quasi-molecular ion peak [M − H − H2O]− at m/z 741.2612. The 1H and 13C-NMR spectra (Table 1) revealed the presence of two sets of 1,3,4,5-tetrasubstituted symmetrical aromatic rings [δH 6.66 (H-2, 6, 2′, 6′) and δC 133.7 (C-1, 1′), 104.2 (C-2, 6, 2′, 6′), 152.6 (C-3, 5, 3′, 5′), 137.1 (C-4, 4′)], two oxymethylenes [δH 4.18 (dd, J = 9.0, 6.7 Hz), 3.84 (dd, J = 9.0, 3.2 Hz) and δC 71.3 (C-9, 9′)], two methines [δH 3.09 (m, H-8, 8′) and δC 53.6 (C-8, 8′)], two oxymethines [δH 4.66 (brd, J = 3.8, H-7, 7′) and δC 85.0 (C-9, 9′)], and two methoxy groups (δH 3.76 and δC 56.4). The correlation spectroscopy (COSY) spectrum provided key correlations between H-7 (δH 4.66) and H-8 (δH 3.09), and between H-8 (δH 3.09) and methylene H-9 protons (δH 4.18 and 3.84). Its heteronuclear multiple bond correlation (HMBC) spectrum provided further correlations from H-7 to C-1, C-2, C-6, and C-8 suggested the aromatic ring was attached to C-7 (Figure 2). From these spectral information, 1 was indicated as a 2,5-diaryl tetrahydrofuranoid type lignan. Two sets of β-glucopyranosyl unit [δH 4.90 (br d, J = 5.2 Hz) and δC 102.6 (G-1, 1′), δH 3.59, 3.40 and δC 102.6 (G-6, 6′)] were also observed. The glucosylation shifts at C-9, -9′ (δC 71.3) and C-8, -8′ (δC 53.6) constructed the location of the glucosyl units at C-9 and C-9′ of the aglycone, when compared with unbound C-9 (δC 61.2) and C-8 (δC 54.9) reported in the literature [61]. The relative configurations between C-7 and C-8 (also C-7′ and C-8′) were established as trans-configurations due to no nuclear Overhauser effect (NOE) correlations between H-7 and H-8 (also H-7′ and H-8′) in the nuclear Overhauser enhancement spectroscopy (NOESY) experiment (Figure 2). Thus, the structure of compound 1 was determined as dihydroxymethylbis(3,5-dimethoxy-4-hydroxyphenyl)tetrahydrofuran-9,9′-O-β-diglucopyranoside and named trivially as tinosporide A.

Table 1.
NMR Spectroscopic Data of Compounds 1, 16, and 17.
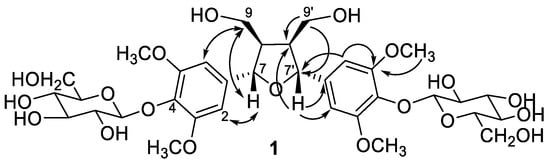
Figure 2.
Diagnostic HMBC (→) and NOESY (↔) correlations of compound 1.
Compounds 11–15 all exhibited similar ultraviolet (UV) and infrared (IR) absorption characteristics. Their UV spectra all displayed absorption maxima close to 293 nm, which are characteristic of the pyrrole-2-carbonyl basic skeleton [62]. The 1H-NMR spectrum (Table 2) exhibited signals for two methine protons at δH 6.16 (d, J = 3.9 Hz, H-4) and 7.01 (d, J = 3.9 Hz, H-3). Chemical shifts at δC 110.8 (C-4), 119.0 (C-3), 121.6 (C-2), and 136.9 (C-5) in 13C-NMR spectrum implied the occurrence of a heterocyclic ring containing a nitrogen atom and their proton coupling constants also indicated the 2,5 di-substituted pyrrole ring (Table 2). The 1H and 13C-NMR spectra of 11 also evidenced the presence of a butanoic acid moiety which appeared at δH 4.37 (br t, J = 7.6 Hz, H-1′), 2.36 (t, J = 7.3 Hz, H-3′), and 2.04 (m, H-2′), confirmed by HMBC correlations from H-3′ and H-2′ to a carbonyl carbon (δC 173.4, C-4′). The connection of the butanoic acid moiety on the nitrogen atom was suggested by observing long range correlation peaks from δH 4.37 (H-1′) to δC 136.9 (C-5) and δC 121.6 (C-2) in the HMBC spectrum (Figure 3). These spectral data clearly determined that a butanoic acid moiety was attached to N-1 of the pyrrole ring. An oxomethylene group connected to C-5 of pyrrole ring was proved by the HMBC correlation of δH 4.43 (H-7) and δC 136.9 (C-5). Two additional methoxy groups (δH 3.34, δC 51.6; δH 3.67, δC 57.7) were also observed and deduced to be located at C-7 and C-4′ by HMBC analysis (Figure 3). However, the HR-ESI-MS analytical data was unavailable due to the sample lability. Therefore, the molecular formula of 11 was proposed as C12H17NO5 according to the above-mentioned NMR spectral analysis and gas chromatograph–mass spectrometer (GC–MS) analytical results which exhibited a molecular ion peak at m/z 255 (see Supplementary Materials). On the basis of these data, the structure of 11 was determined as 1-(4-methoxy-4-oxobutyl)-5-(methoxymethyl)-1H-pyrrole-2-carboxylic acid and named trivially as tinosporin A.

Table 2.
NMR spectroscopic data of compounds 11–15.
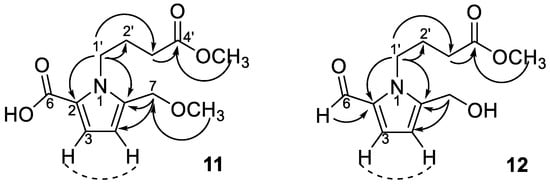
Figure 3.
Diagnostic HMBC (→)/COSY (---) correlations of compounds 11 and 12.
Compound 12 displayed very similar 1H and 13C-NMR signals (Table 2) as those of 11 except an additional aldehyde signal (δH 9.42 (s, H-6) and δC 180.9 (C-6)) and one methoxy group (δH 3.66 (s, OCH3) and δC 52.2 (OCH3)). Its HMBC spectrum exhibited the correlations from methoxy protons to butanoic acid C-4′ (δC 175.1), as shown in Figure 3. The molecular formula of 12 was proposed as C11H15NO4 also based on the GC–MS analytical data of the molecular ion peak at m/z 225 (see Supplementary Materials). Accordingly, the structure of 12 was established as methyl 4-[formyl-5-(hydroxymethyl)-1H-pyrrol-1-yl] butanoate. Compound 13 was shown to possess the molecular formula of C12H17O4N by GC–MS measurement. An additional methoxy group (δH 3.36) was observed in 13 by comparison of its 1H-NMR spectra with that of 12. The structure of compound 13 was elucidated as a methyl 4-[formyl-5-(methoxymethyl)-1H-pyrrol-1-yl] butanoate. Furthermore, compounds 14 and 15 were determined as 4-[formyl-5-(methoxymethyl)-1H-pyrrol-1-yl] butanoic acid and 4-[formyl-5-(hydroxymethyl)-1H-pyrrol-1-yl] butanoic acid, respectively, by comparison of their spectral data with those reported [22,23]. According to the above results, pyrrole alkaloids 10–15 were reported from Tinospora genus for the first time.
Compounds 16 and 17 showed the same adduct ion peaks and were both assigned the same molecular formula C17H22O6. The 1H-NMR spectrum of 16 revealed the existence of an aromatic protons at δH 7.07 (dd, J = 8.2, 1.8 Hz, H-6), 7.04 (d, J = 1.8 Hz, H-2), and 6.92 (d, J = 8.2 Hz, H-5); five methylenes at δH 4.19 (t, J = 6.6 Hz, H-6′), 2.34 (t, J = 7.4 Hz, H-2′), 1.67 (m, H-3′, 5′), and 1.47 (m, H-4′); and two methoxy singlets at δH 3.95 and 3.67. Additional signals at δH 7.59 (d, J = 16.0 Hz, H-7) and 6.47 (d, J = 16.0 Hz, H-8) suggested the presence of a trans double bond. The 13C-NMR spectrum revealed the existence of seventeen carbon atoms included an aromatic ring (δC 109.3, 112.7, 123.1, 127.0, 146.6, and 147.9), five methylenes (δC 64.0, 33.8, 28.1, 25.3, and 24.5), two methoxyls (δC 55.9 and 51.5), two carbonyls (δC 173.9 and 167.5), and a pair of olefinic carbons (δC 144.8 and 115.5). A 3,4-disubstituted cinnamoyl group linked with a hexanoyl alcohol was deduced from the NMR data which described above (Table 1). This was further confirmed by the key HMBC correlations from δH 3.67 (OCH3) to 173.9 (C-1′), from δH 4.19 (H-6′) to δC 167.5 (C-9), and 28.1 (C-5′), as shown in Figure 4. Therefore, compound 16 was confirmed as rhodiolate by comparison of its spectral data with those reported [24]. Compound 17 displayed closely related 1D NMR spectroscopic and mass spectrometric characteristics to 16 and was determined to have a similar structure to 16. However, a pair of olefinic protons at δH 6.80 (d, J = 12.9 Hz, H-7) and 5.81 (d, J = 12.9 Hz, H-8) suggested the cis double bond feature. However, 2D NMR spectral analysis of 17 could not be furnished because of the rapid transformation of cis–trans double bond. Thus, the structure of compound 17 was concluded to be methyl 6-((Z)-3-(4-hydroxy-3-methoxyphenyl)acryloyloxy)-hexanoate and assigned the trivial name as tinosporin B.
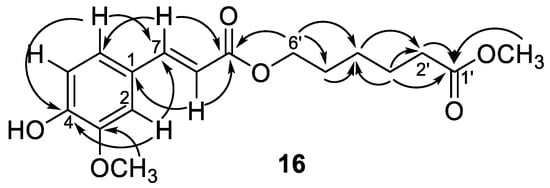
Figure 4.
Diagnostic HMBC (→) correlations of compound 16.
Fifteen purified compounds were examined for their inhibition bioactivity of superoxide anion generation and elastase release by human neutrophils in response to fMLP/CB (Table S2) [63,64]. However, most displayed weak inhibition percentages at the test concentration (10 μM). Among these, 1, 16, and 17 displayed higher inhibitions of superoxide anion generation at 10 μM with inhibition percentages ranged from 10.2 ± 7.1 to 20.2 ± 5.1%. In addition, compound 39 (10 μM) also exhibited inhibitory effect on elastase release with inhibition percentage of 22.3 ± 10.0% (Table S2). Columbin, an important furanoditerpenoid isolated from several Tinosporae Radix, exhibited significant anti-inflammatory activities in a dose-dependent manner [65]. However, based on our research data the related furanoid bisnorditerpenoid, malabarolide (36), was not the predominant component, maybe due to the different parts of plant materials. The conventional use of T. sinensis in traditional Chinese medicine is for relieving rigidity of muscles and activating collaterals, and the mechanism of action may be related to anti-inflammatory bioactivity. The present experimental data not only suggest that the extracts and purified compounds of the stems of T. sinensis have the potential to be developed as novel anti-inflammatory lead drugs or health foods, but also merit further investigation of the anti-inflammatory mechanism.
3. Materials and Methods
3.1. General Information
Optical rotations and UV spectra were measured using a Atago AP-300 digital polarimeter (Atago, Tokyo, Japan) and a GBC Cintra 101 spectrophotometer (GBC Scientific Equipment Ltd., Dandenong, Australia), respectively. IR spectra were obtained with a Shimadzu FT-IR Prestige-21 spectrophotometer (Shimadzu, Kyoto, Japan). 1H and 13C-NMR spectra were recorded on Bruker AV 700, AV 500, and Avance III 400 NMR spectrometers (Bruker, Billerica, MA, USA). Chemical shifts are shown in δ values (ppm) with tetramethylsilane as an internal standard. GC–MS were analyzed using a Shimadzu GC-2010 gas chromatograph/mass spectrometer equipped with a quadrupole mass analyzer (Shimadzu, Kyoto, Japan). The HR-ESI-MS were taken on a Bruker Daltonics micrOTOF orthogonal ESI-TOF mass spectrometer (Bruker, Billerica, MA, USA). Column chromatography (CC) was performed on silica (70–230 mesh and 230–400 mesh, Merck, Darmstadt, Germany) and Diaion HP-20 (Mitsubishi, Tokyo, Japan) gels, and preparative thin-layer chromatography (TLC) was conducted on Merck precoated silica gel 60 F254 plates (Merck, Darmstadt, Germany), using UV light to visualize the spots. Methanol, chloroform (GR grade), n-hexane, ethyl acetate, benzene, and acetone (ACS grade) were purchased from Merck (Darmstadt, Germany) and Mallinckrodt (St. Louis, MO, USA), respectively. DMSO-d6, CD3OD, and CDCl3 were purchased from Sigma-Aldrich (St. Louis, MO, USA).
3.2. Materials
The stems of T. sinensis were collected from Vietnam in August 2009, and the plant material was identified and authenticated by Assoc. Prof. Dr. Vu Xuan Phuong, Institute of Ecology and Biological Resources, Vietnamese Academy of Science and Technology. A voucher specimen (Viet-TSWu-2009-1801-001) was deposited in the herbarium of the Institute of Ecology and Biological Resources, Vietnamese Academy of Science and Technology, Hanoi, Vietnam.
3.3. Extraction and Isolation
The dried stems of T. sinensis (10 kg) was refluxed with methanol (30 L × 8 × 8 h) and then filtered and concentrated under reduced pressure to obtain the methanol extract (400 g). The extract was suspended in distilled water and successively partitioned with chloroform to yield a chloroform layer (60 g) and water soluble (340 g). The chloroform layer was chromatographed directly on silica gel and eluted with a gradient of n-hexane and acetone to afford 10 fractions (CF 1-10). Fractions CF 1, 2, and 4 did not show any significant spots under TLC check and therefore were not purified further. Fraction CF 3 was isolated by CC on silica gel with a step gradient with benzene and acetone mixtures and the subfraction CF 3-6 was further purified by TLC using n-hexane-ethyl acetate (50:1) to yield cycloabyssinone (40, 3 mg). Fraction CF 5 was purified using silica gel CC eluted with gradient mixtures of n-hexane and acetone to afford thirteen subfractions (CF 5-1 to 5-13). CF 5-2 was fractionated by silica gel CC eluted with benzene ethyl acetate and then lupeol (37, 8 mg), cycloeucalenol (39, 15 mg), and a mixture of β-sitosterol (43) and stigmasterol (44) (364 mg), respectively, was purified from the minor fractions by TLC using n-hexane-ethyl acetate (50:1). CF 5-5 was performed on silica gel CC with gradient mixtures of hexane and acetone to produce ten minor fractions. One minor fraction CF 5-5-7 was purified by silica gel CC with mixture of benzene and acetone and further purification by TLC using chlorofrom-acetone (9:1) yielded a mixture of 7α-hydroxysitosterol (45) and 7α-hydroxystigmasterol (46) (6 mg). CF 5-7 was subjected to silica gel CC eluted with a gradient mixture of benzene ethyl acetate to afford ten minor fractions. CF 5-7-4 was further isolated by silica gel CC, eluted with hexane ethyl acetate and subsequent TLC using hexane ethyl acetate (6:1) to afford 3-O-acetyloleanolic acid (38, 4 mg).
Fraction CF 6 was isolated by silica gel CC by gradient elution with mixture of n-hexane and ethyl acetate to result in eleven subfractions (CF 6-1 to 6-11). CF 6-4 was further purified by silica gel CC eluted with n-hexane-acetone to produce eight minor fractions (CF 6-4-1 to 6-4-8). Lichexanthone (56, 4 mg) was purified by TLC using chloroform-ethyl acetate (100:1) from CF 6-4-3. CF 6-4-4 was subjected to silica gel CC eluted by benzene-acetone gradient mixtures and further purified by TLC using chloroform:acetone (10:1) to afford 2-methyl-4,5-dimethoxybenzoic acid (20, 4 mg). CF 6-5 was subjected to silica gel CC with chloroform and methanol gradient mixtures to afford five minor fractions. CF 6-5-2 was isolated by silica gel CC eluted by chloroform:ethyl acetate gradient mixtures and subsequent TLC using hexane-ethyl acetate (10:1) to produce tinosporin A (11, 1 mg), 3(17)-phytene 1,2-diol (35, 3 mg), cycloart-22-ene-3β,25-diol (42, 4 mg), 5,6-dimethoxy- N-methyl-phthalimide (51, 8 mg), respectively. CF 6-6 was isolated by silica gel CC with chloroform and methanol gradient mixtures and further purified by TLC using hexane:acetone (10:1) to yield methyl 4-[formyl-5-(methoxymethyl)-1H-pyrrol-1-yl] butanoate (13, 2 mg).
Fraction CF 7 was chromatographed on silica gel column eluted with gradient mixtures of chloroform and ethyl acetate to afford seven subfractions (CF 7-1 to 7-7). CF 7-2 was purified by silica gel CC successively eluted with hexane:acetone, hexane ethyl acetate, and chloroform ethyl acetate and one minor fraction (CF 7-2-5-3) to afford methyl ferulate (18, 5 mg). Another minor fraction CF 7-2-5-4 was further isolated by silica gel CC with gradient elution of benzene and acetone, and subsequent purification by TLC using hexane ethyl acetate (5:1) to give rhodiolate (16, 2 mg) and tinosporin B (17, 2 mg). CF 7-3 was also performed silica gel CC eluted with hexane ethyl acetate to afford ten minor fractions, and CF 7-3-7 was further isolated by silica gel CC eluted with hexane-ethyl acetate and subsequent TLC using benzene ethyl acetate (30:1) to afford (+)-pinoresinol monomethyl ether (6, 3 mg). CF 7-4 was isolated by silica gel CC eluted with hexane ethyl acetate to yield ten minor fractions. Of these, CF 7-4-5 was further purified by silica gel CC (hexane-acetone mixing eluents) and subsequent TLC using chloroform:acetone (20:1) to afford cycloartane-3β,25-diol (41, 16 mg). CF 7-4-6 was also subjected into silica gel CC (hexane:acetone mixing eluents) to give seven minor fractions. Further purification of CF 7-4-6-4, CF 7-4-6-5, and CF 7-4-6-6 by silica gel CC eluted with chloroform:acetone (9:1) to yield loliolide (33, 5 mg), a mixture of 6β-hydroxystigmast-4-en-3-one (47) and 6β-hydroxystigmasta-4,22-dien-3-one (48) (2 mg), and a mixture of 7-ketositosterol (49) and 3β-hydroxystigmasta-5,22-dien-7-one (50) (6 mg), respectively.
Fraction CF 8 was isolated by silica gel CC eluted with gradient mixtures of hexane and acetone to afford six subfractions (CF 8-1 to 8-6). CF 8-4 was performed silica gel CC eluted with hexane ethyl acetate and further purified by TLC using benzene:acetone (20:1) to give N-trans-feruloyldopamine (52, 6 mg). Ten subfractions (CF 9-1 to 9-10) were obtained from CF 9 by silica gel CC eluted with gradient mixture of chloroform and acetone. CF 9-3 was further isolated by silica gel CC, eluted with benzene:ethyl acetate and, following TLC purification of minor fraction CF 9-3-6 using chloroform:acetone (30:1) to afford (+)-pinoresinol (2, 10 mg) and scopoletin (55, 3 mg), CF 9-3-7 was further purified by TLC using chloroform:acetone (10:1) to afford medioresinol (4, 4 mg), (+)-epi-syringaresinol (5, 3 mg), (+)-glaberide I (7, 3 mg), and 2,6-dimethoxy-p-quinone (57, 5 mg), respectively. CF 9-3-8 was isolated by silica gel CC eluted with gradient mixtures of chloroform-methanol and then purified by TLC using chloroform:methanol (300:1) to yield syringaresinol (3, 12 mg). CF 9-4 was divided to eight minor fractions by silica gel CC eluted with benzene:acetone solvent mixture. Of these, CF 9-4-5 was further fractionated by silica gel CC eluted with chloroform:acetone (30:1) to give β-hydroxypropiovanillone (19, 3 mg). CF 9-7 was isolated by silica gel CC (chloroform:acetone gradient mixture) to yield six minor fractions and one of these CF 9-7-4 was afforded N-trans-feruloyltyramine (53, 8 mg) and N-cis-feruloyltyramine (54, 5 mg) by further silica gel CC eluted with chloroform:acetone (30:1) and subsequent TLC using chloroform:methanol (50:1). The last fraction (CF 10) of the chloroform layer was also purified by silica gel CC eluted with gradient mixture of chloroform and acetone. The resulting subfraction CF 10-5 was divided to several minor fractions by silica gel CC eluted with chloroform:methanol (50:1) solvent mixture and further purified by TLC using chloroform:acetone (10:1) to give abscisic acid (34, 1 mg).
The water soluble fraction was subjected directly to Diaion HP-20 column chromatography, eluted by water and gradient with methanol, to afford seventeen fractions (WF 1-17). Fractions WF 1-5, 9, 11, and 14-16 did not show any significant spots under TLC check and therefore were not purified further. WF 6, 7, and 8 were purified by silica gel CC eluted with gradient mixture of chloroform and methanol and afforded tachioside (23, 10 mg); vanillic acid (21, 5 mg), p-hydroxyl phenethanol (22, 3 mg), icariside D2 (24, 10 mg); and salidroside (25, 10 mg), respectively.
Fraction WF 10 was chromatographed on silica gel column eluted with gradient mixtures of chloroform and methanol to afford six subfractions (WF 10-1 to 10-6). WF 10-2 was purified by silica gel CC eluted with chloroform and methanol and one minor fraction (WF 10-2-3) affording 4-(2-hydroxyethyl)benzoic acid (29, 2 mg). WF 10-3 was also performed silica gel CC eluted with chloroform and methanol solvent mixture to afford ten minor fractions, and WF 10-3-4 was further isolated by silica gel CC eluted with chloroform and acetone (10:1) to afford p-hydroxybenzoic acid (28, 5 mg). WF 10-4 was isolated by silica gel CC eluted with chloroform and methanol solvent mixture to yield ten minor fractions. Of these, WF 10-4-5 was further purified by silica gel CC (chloroform:acetone mixing eluents) and subsequent TLC using chloroform:acetone (10:1) to afford 5-(hydroxymethyl)-1H-pyrrole-2-carbaldehyde (10, 1 mg). Recrystallization of WF 10-4-7 and 10-4-9 by chloroform:acetone produced syringin (26, 25 mg) and cordifolioside A (27, 30 mg), respectively. WF 10-6 was isolated by silica gel CC eluted with chloroform and methanol solvent mixture to yield five minor fractions. Of these, WF 10-6-3 was further purified by silica gel CC eluted by chloroform and acetone (9:1) to afford syringic acid-4-O-α-l-rhamnoside (30, 8 mg).
Fractions WF 12, 13, and 17 were all chromatographed on silica gel column eluted with gradient mixtures of chloroform and methanol to produce several subfractions. WF 12-2 was purified by silica gel CC eluted with chloroform ethyl acetate to afford methyl 4-[formyl-5-(hydroxymethyl)-1H-pyrrol-1-yl] butanoate (12, 10 mg). Similarly, 4-[formyl-5-(methoxymethyl)-1H-pyrrol-1-yl] butanoic acid (15, 5 mg) and 4-[formyl-5-(methoxymethyl)-1H-pyrrol-1-yl] butanoic acid (14, 7 mg) resulted from the chromatographic elution of WF 12-5 and 12-12, respectively. WF 13-1 was isolated by silica gel CC eluted with chloroform and methanol solvent mixture to yield ten minor fractions. Of these, WF 13-1-7 was further purified by silica gel CC (chloroform-acetone mixing eluents) and subsequent TLC using chloroform:acetone (10:1) to syringic acid (32, 3 mg). Another subfraction WF 13-3 was further isolated by silica gel CC with gradient elution of chloroform and methanol, and subsequent purification by TLC using chloroform and methanol (9:1) to give isovanillic acid (31, 2 mg). Recrystallization of WF 13-4 and 13-13 by chloroform:acetone produced tinosporide A (1, 15 mg) and malabarolide (36, 10 mg), respectively. WF 17-2 was isolated by silica gel CC eluted with chloroform and methanol (9:1) and further purified by TLC using chloroform:acetone (20:1) to afford sesamin (8, 5 mg) and sesamolin (9, 2 mg).
Tinosporide A (1): colorless powder; UV (MeOH) λ max (log ε) 272 (2.87) nm; IR (neat) νmax 3258, 2862, 2358, 1592, 1457, 1418, 1235, 1131, 1045 cm−1; 1H-NMR (500 MHz, DMSO-d6) and 13C-NMR (125 MHz, DMSO-d6), see Table 1; HR-ESI-MS m/z 741.2612 ([M − H − H2O]−, calcd for C34H45O18, 741.2611).
Tinosporin A (11): Pale yellow syrup; UV (EtOH) λmax: 319, 293, 220 nm; 1H-NMR (700 MHz, CDCl3) and 13C-NMR (175 MHz, CDCl3), see Table 2; GC–MS m/z 255 ([M]+), 237, 210, 180, 136, 101, 59.
Tinosporin B (17): Colorless syrup; UV (MeOH) λmax (log ε): 323 (3.32), 299 (3.18, sh), 235(3.13), 218(3.20) nm; IR (neat) νmax: 3410, 2926, 2853, 1729, 1709, 1632, 1595, 1515, 1464, 1432, 1376, 1270, 1162, 1126, 1033 cm−1; 1H-NMR (400 MHz, CDCl3) see Table 1; HR-ESI-MS m/z 345.1311 ([M + Na]+, calcd for C17H22O6Na, 345.1309).
3.4. Anti-inflammatory Bioactivity Examination
3.4.1. Preparation of Human Neutrophils
The use of human neutrophils was approved by the Institutional Review Board at Chang Gung Memorial Hospital, Taoyuan, Taiwan, and the study was conducted according to the Declaration of Helsinki (2013). Written informed consent was obtained from each healthy donor before blood was drawn. The details of the preparation of human neutrophils are provided in the Supplementary Materials.
3.4.2. Measurement of Superoxide Anion Generation and Elastase Release
The assay of the generation of superoxide anion was based on the superoxide dismutase (SOD)-inhibitable reduction of ferricytochrome c. Degranulation of azurophilic granules was determined by elastase release as described previously [63,64]. The details of measurement of superoxide anion generation and elastase release were provided in the Supplementary Materials.
Supplementary Materials
The following are available online. S1: Anti-inflammatory bioactivity experimental procedures; Tables S1 and S2: Inhibitory effects of extracts and compounds from T. sinensis; Figures S1–S16: NMR spectra of compounds 1, 11, 12, 16, and 17.
Author Contributions
Conceptualization, P.-C.K. and T.-S.W.; Data curation, S.-H.L.; Investigation, P.-H.C.; Methodology, T.-L.H. and C.-C.C.; Resources, T.-D.T.; Writing—original draft, S.-H.L. and H.-Y.H.; Writing—review & editing, P.-C.K. and T.-S.W. All authors read and approved the final manuscript.
Funding
This research is sponsored by the Ministry of Science and Technology (MOST), Taiwan granted to S.-H.L. and T.-S.W. The authors are also thankful for partial financial support from Chang Gung Memorial Hospital (CMRPD1B0281~3, CMRPF1D0442~3, CMRPF 1F0011~3, CMRPF1F0061~3 and BMRP450 granted to H.-L.H).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Witko-Sarsat, V.; Rieu, P.; Descamps-Latscha, B.; Lesavre, P.; Halbwachs-Mecarelli, L. Neutrophils: Molecules, functions and pathophysiological aspects. Lab. InvestIG. 2000, 80, 617–653. [Google Scholar] [CrossRef] [PubMed]
- Okajima, K.; Harada, N.; Uchiba, M. Ranitidine Reduces Ischemia/Reperfusion-Induced Liver Injury in Rats by Inhibiting Neutrophil Activation. J. Pharmacol. Exp. Ther. 2002, 301, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Ennis, M. Neutrophils in asthma pathophysiology. Curr. Allergy Asthma Rep. 2003, 3, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.L.; Li, G.L.; Lan, Y.H.; Chia, Y.C.; Hsieh, P.W.; Wu, Y.H.; Wu, Y.C. Potent inhibitors of superoxide anion production in activated human neutrophils by isopedicin, a bioactive component of the Chinese medicinal herb Fissistigma oldhamii. Free Radic. Biol. Med. 2009, 46, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Malech, H.L.; Gallin, J.I. Current concepts: Immunology: Neutrophils in human diseases. N. Engl. J. Med. 1987, 317, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Van Eeden, S.F.; Klut, M.E.; Walker, B.A.M.; Hogg, J.C. The use of flow cytometry to measure neutrophil function. J. Immunol. Methods 1999, 232, 23–43. [Google Scholar] [CrossRef]
- Editorial Committee of the Flora of Taiwan. Flora of Taiwan, 2nd ed.; Department of Botany, National Taiwan University: Taipei, Taiwan, 1996; Volume 2, p. 605. [Google Scholar]
- Krishna, K.L.; Jigar, B.; Jagruti, P. Guduchi (Tinospora cordifolia): Biological and Medicinal properties: A review. Int. J. Altern. Med. 2009, 6, 1–12. [Google Scholar]
- Mishra, A.; Kumar, S.; Bhargava, A.; Sharma, B.; Pandey, A.K. Studies on in vitro antioxidant and antistaphylococcal activities of some important medicinal plants. Cell Mol. Biol. 2011, 57, 16–25. [Google Scholar] [PubMed]
- Upadhyay, A.K.; Kumar, K.; Kumar, A.; Mishra, H.S. Tinospora cordifolia (Willd.) Hook. f. and Thoms. (Guduchi)–validation of the Ayurvedic pharmacology through experimental and clinical studies. Int. J. Ayurveda Res. 2010, 1, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Velazquez, E.A.; Kimura, D.; Torbati, D.; Ramachandran, C.; Totapally, B.R. Immunological response to (1,4)-α-D-glucan in the lung and spleen of endotoxin-stimulated juvenile rats. Basic Clin. Pharmacol. Toxicol. 2009, 105, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.H.; Ruan, C.T.; Hsieh, P.H.; Su, M.J.; Lee, S.S. Hypoglycemic Diterpenoids from Tinospora crispa. J. Nat. Prod. 2012, 75, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Ruan, C.T.; Lam, S.H.; Lee, S.S.; Su, M.J. Hypoglycemic action of borapetoside A from the plant Tinospora crispa in mice. Phytomedicine 2013, 20, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.H.; Akao, T.; Hamasaki, K.; Deyama, T.; Hattori, M. Biotransformation of pinoresinol diglucoside to mammalian lignans by human intestinal microflora, and isolation of Enterococcus faecalis strain PDG-1 responsible for the transformation of (+)-pinoresinol to (+)-lariciresinol. Chem. Pharm. Bull. 2003, 51, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Wu, T.Y.; Chang, F.R.; Wu, Y.C. Lignans and kauranes from the stems of Annona cherimola. J. Chin. Chem. Soc. 1998, 45, 629–634. [Google Scholar] [CrossRef]
- Deyama, T. The constituents of Eucommia ulmoides Oliv. I. Isolation of (+)-medioresinol di-O-β-D- glucopyranoside. Chem. Pharm. Bull. 1983, 31, 2993–2997. [Google Scholar] [CrossRef]
- Kitagawa, S.; Nishibe, S.; Benecke, R.; Thieme, H. Phenolic compounds from Forsythia leaves. II. Chem. Pharm. Bull. 1988, 36, 3667–3670. [Google Scholar] [CrossRef]
- Kinjo, J.; Higuchi, H.; Fukui, K.; Nohara, T. Lignoids from Albizziae cortex. II. A biodegradation pathway of syringaresinol. Chem. Pharm. Bull. 1991, 39, 2952–2955. [Google Scholar] [CrossRef]
- Jong, T.T.; Jean, M.Y. Constituents of Houttuynia cordata and the crystal structure of vomifoliol. J. Chin. Chem. Soc. 1993, 40, 399–402. [Google Scholar] [CrossRef]
- Haslam, E. The stereochemistry of sesamolin. J. Chem. Soc. C 1970, 17, 2332–2334. [Google Scholar] [CrossRef]
- Sudhakar, G.; Kadam, V.D.; Bayya, S.; Pranitha, G.; Jagadeesh, B. Total synthesis and stereochemical revision of acortatarins A and B. Org. Lett. 2011, 13, 5452–5455. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.B.; Chang, B.Y.; Hwang, B.Y.; Kim, S.Y.; Lee, M.K. Pyrrole alkaloids from the fruits of Morus alba. Bioorg. Med. Chem. Lett. 2014, 24, 5656–5659. [Google Scholar] [CrossRef] [PubMed]
- Chin, Y.W.; Lim, S.W.; Kim, S.H.; Shin, D.Y.; Suh, Y.G.; Kim, Y.B.; Kim, Y.C.; Kim, J. Hepatoprotective Pyrrole Derivatives of Lycium chinense Fruits. Bioorg. Med. Chem. Lett. 2003, 13, 79–81. [Google Scholar] [CrossRef]
- Zhou, J.T.; Li, C.Y.; Wang, C.H.; Wang, Y.F.; Wang, X.D.; Wang, H.T.; Zhu, Y.; Jiang, M.M.; Gao, X.M. Phenolic Compounds from the Roots of Rhodiola crenulata and Their Antioxidant and Inducing IFN-γ Production Activities. Molecules 2015, 20, 13725–13739. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, S.; Subbarao, G.V.; Nakahara, K.; Yoshihashi, T.; Ito, O.; Maeda, I.; Ono, H.; Yoshida, M. Nitrification inhibitors from the root tissues of Brachiaria humidicola, a tropical grass. J. Agric. Food Chem. 2007, 55, 1385–1388. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, E.; Suzumura, K.; Yamazaki, M. Pharmacologically active components of Todopon Puok (Fagraea racemosa), a medicinal plant from Borneo. Chem. Pharm. Bull. 1995, 43, 2200–2204. [Google Scholar] [CrossRef] [PubMed]
- Olesch, B.; Böhm, H. Abbau des 2-benzyl-isochinolin-alkaloids sendaverin. Arch. Pharm. 1972, 305, 222–229. [Google Scholar] [CrossRef]
- Lee, S.Y.; Choi, S.U.; Lee, J.H.; Lee, D.U.; Lee, K.R. A new phenylpropane glycoside from the rhizome of Sparganium stoloniferum. Arch. Pharm. Res. 2010, 33, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.M.; Liu, Y.L.; Li, X.R.; Feng, Y.L.; Yang, S.L. Two new phenylglycol derivatives isolated from Syringa reticulata var. mandshurica and their antifungal activities. Chem. Pharm. Bull. 2009, 57, 863–866. [Google Scholar] [PubMed]
- Zhong, X.N.; Otsuka, H.; Ide, T.; Hirata, E.; Takeda, Y. Hydroquinone diglycoside acyl esters from the leaves of Myrsine seguinii. Phytochemistry 1999, 52, 923–927. [Google Scholar] [CrossRef]
- Miyase, T.; Ueno, A.; Takizawa, N.; Kobayashi, H.; Oguchi, H. Ionone and lignan glycosides from Epimedium diphyllum. Phytochemistry 1989, 28, 3483–3485. [Google Scholar] [CrossRef]
- Kuwajima, H.; Takai, Y.; Takaishi, K.; Inoue, K. Synthesis of 13C-labeled possible intermediates in the biosynthesis of phenylethanoid derivatives, cornoside and rengyosides. Chem. Pharm. Bull. 1998, 46, 581–586. [Google Scholar] [CrossRef]
- Greca, M.D.; Ferrara, M.; Fiorentino, A.; Monaco, P.; Previtera, L. Antialgal compounds from Zantedeschia aethiopica. Phytochemistry 1998, 49, 1299–1304. [Google Scholar] [CrossRef]
- Maurya, R.; Wazir, V.; Tyagi, A.; Kapil, R.S. Cordifoliosides A and B, two new phenylpropene disaccharides from Tinospora cordifolia possessing immunostimulant activity. Nat. Prod. Lett. 1996, 8, 7–10. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chang, F.R.; Teng, C.M.; Wu, Y.C. Cheritamine, a new N-fatty acyl tryptamine and other constituents from the stems of Annona cherimola. J. Chin. Chem. Soc. 1999, 46, 77–86. [Google Scholar] [CrossRef]
- Li, A.; Mishra, Y.; Malik, M.; Wang, Q.; Li, S.; Taylor, M.; Reichert, D.E.; Luedtke, R.R.; Mach, R.H. Evaluation of N-phenyl homopiperazine analogs as potential dopamine D3 receptor selective ligands. Bioorg. Med. Chem. 2013, 21, 2988–2998. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Li, Y.J.; Wang, A.M.; He, X.; Liao, S.G.; Lan, Y.Y. Two new phenolic glycosides from Inula cappa. J. Asian Nat. Prod. Res. 2010, 12, 765–769. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.K.; Lee, P.H.; Kuo, Y.H. The chemical constituents from the aril of Cassia fistula L. J. Chin. Chem. Soc. 2001, 48, 1053–1058. [Google Scholar] [CrossRef]
- Chang, Y.C.; Chang, F.R.; Wu, Y.C. The constituents of Lindera glauca. J. Chin. Chem. Soc. 2000, 47, 373–380. [Google Scholar] [CrossRef]
- Wada, T. Structure of digiprolactone. Chem. Pharm. Bull. 1965, 13, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.; Tripathi, J.; Chatterjee, S.; Gautam, S. Natural predominance of abscisic acid in Pongammia pinnata ("Karanj") honey contributed to its strong antimutagenicity. J. Agric. Food Chem. 2017, 65, 4624–4633. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.D.; Acosta, A.L. New cembranoid diterpenes and a geranylgeraniol derivative from the common Caribbean sea whip Eunicea succinea. J. Nat. Prod. 1997, 60, 1134–1138. [Google Scholar] [CrossRef] [PubMed]
- Atta-ur-Rahman; Ahmad, S.; Rycroft, D.S.; Prknyi, L.; Choudhary, M.I.; Clardy, J. Malabarolide, a novel furanoid bisnorditerpenoid from Tinospora malabarica. Tetrahedron Lett. 1988, 29, 4241–4244. [Google Scholar] [CrossRef]
- Fotie, J.; Bohle, D.S.; Leimanis, M.L.; Georges, E.; Rukunga, G.; Nkengfack, A.E. Lupeol long-chain fatty acid esters with antimalarial activity from Holarrhena floribunda. J. Nat. Prod. 2006, 69, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.C.; Desjardins, A.E.; Wu, C.D.; Kinghorn, A.D. Activity of triterpenoid glycosides from the root bark of Mussaenda macrophylla against two oral pathogens. J. Nat. Prod. 1999, 62, 1379–1384. [Google Scholar] [CrossRef] [PubMed]
- Kocór, M.; St. Pyrek, J. Cyclotrichosantol, a new C31 31-nortriterpene. J. Org. Chem. 1973, 38, 3688–3690. [Google Scholar]
- Baldé, A.M.; Apers, S.; Claeys, M.; Pieters, L.; Vlietinck, A.J. Cycloabyssinone, a new cycloterpene from Harrisonia abyssinica. Fitoterapia 2001, 72, 438–440. [Google Scholar] [CrossRef]
- Kikuchi, T.; Toyoda, T.; Arimoto, M.; Takayama, M.; Yamano, M. Studies on the neutral constituents of Pachysandra terminalis Sieb. et Zucc. I. Isolation and characterization of sterols and triterpenes. Yakugaku Zasshi 1969, 89, 1358–1366. [Google Scholar] [CrossRef] [PubMed]
- Öksüz, S.; Shieh, H.L.; Pezzuto, J.M.; Özhatay, N.; Cordell, G.A. Biologically active compounds from the Euphorbiaceae; part 1. Triterpenoids of Euphorbia nicaeensis subsp. glareosa. Planta Med. 1993, 59, 472–473. [Google Scholar]
- Kuo, Y.H.; Li, Y.C. Constituents of the bark of Ficus microcarpa L. f. J. Chin. Chem. Soc. 1997, 44, 321–325. [Google Scholar] [CrossRef]
- Kimura, Y.; Yasukawa, K.; Takido, M.; Akihisa, T.; Tamura, T. Inhibitory effect of some oxygenated stigmastane-type sterols on 12-O-tetradecanoylphorbol-13-acetate-induced inflammation in mice. Biol. Pharm. Bull. 1995, 18, 1617–1619. [Google Scholar] [CrossRef] [PubMed]
- Katsui, N.; Matsue, H.; Hirata, T.; Masamune, T. Phytosterols and triterpenes in roots of the “kidney bean” (Phaseolus vulgaris L.). Bull. Chem. Soc. Jpn. 1972, 45, 223–226. [Google Scholar] [CrossRef]
- Zhang, X.; Geoffroy, P.; Miesch, M.; Julien-David, D.; Raul, F.; Aoudé-Werner, D.; Marchioni, E. Gram-scale chromatographic purification of beta-sitosterol. Synthesis and characterization of beta-sitosterol oxides. Steroids 2005, 70, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Tsai, I.L.; Ishikawa, T.; Wang, C.J.; Chen, I.S. Alkaloids from trunk bark of Hernandia nymphaeifolia. Phytochemistry 1996, 42, 1479–1484. [Google Scholar] [CrossRef]
- Tseng, C.F.; Iwakama, S.; Mikajiri, A.; Shibuya, M.; Hanaoka, F.; Ebizuka, Y.; Padmawinata, K.; Sankawa, U. Inhibition of in vitro prostaglandin and leukotriene biosyntheses by cinnamoyl-β-phenethylamine and N-acyldopamine derivatives. Chem. Pharm. Bull. 1992, 40, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.J.; Chang, F.R.; Wu, Y.C. The constituents of Cananga odorata. J. Chin. Chem. Soc. 1999, 46, 607–611. [Google Scholar] [CrossRef]
- Yoshihara, T.; Yamaguchi, K.; Takamatsu, S.; Sakamura, S. A new lignan amide, grossamide, from bell pepper (Capsicum annuum var. grossum). Agric. Biol. Chem. 1981, 45, 2593–2598. [Google Scholar]
- Bayoumi, S.A.L.; Rowan, M.G.; Beeching, J.R.; Blagbrough, I.S. Constituents and secondary metabolite natural products in fresh and deteriorated cassava roots. Phytochemistry 2010, 71, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Harris, T.M.; Hay, J.V. Biogenetically modeled syntheses of heptaacetate metabolites. Alternariol and lichexanthone. J. Am. Chem. Soc. 1977, 99, 1631–1636. [Google Scholar] [CrossRef]
- Nishina, A.; Hasegawa, K.; Uchibori, T.; Seino, H.; Osawa, T. 2,6-Dimethoxy-p-benzoquinone as an antibacterial substance in the bark of Phyllostachys heterocycla var. pubescens, a species of thick-stemmed bamboo. J. Agric. Food Chem. 1991, 39, 266–269. [Google Scholar] [CrossRef]
- Kanchanapoom, T.; Kamel, M.S.; Kasai, R.; Yamasaki, K.; Picheansoonthon, C.; Hiraga, Y. Lignan glucosides from Acanthus ilicifolius. Phytochemistry 2001, 56, 369–372. [Google Scholar] [CrossRef]
- Kim, S.B.; Chang, B.Y.; Jo, Y.H.; Lee, S.H.; Han, S.B.; Hwang, B.Y.; Kim, S.Y.; Lee, M.K. Macrophage activating activity of pyrrole alkaloids from Morus alba fruits. J. Ethnopharmacol. 2013, 145, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.C.; Chung, P.J.; Ho, C.M.; Kuo, C.Y.; Hung, M.F.; Huang, Y.T.; Chang, W.Y.; Chang, Y.W.; Chan, K.H.; Hwang, T.L. Propofol Inhibits Superoxide Production, Elastase Release, and Chemotaxis in Formyl Peptide–Activated Human Neutrophils by Blocking Formyl Peptide Receptor 1. J. Immunol. 2013, 190, 6511–6519. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.P.; Hsieh, P.W.; Chang, Y.J.; Chung, P.J.; Kuo, L.M.; Hwang, T.L. 2-(2-Fluoro-benzamido)benzoate ethyl ester (EFB-1) inhibits superoxide production by human neutrophils and attenuates hemorrhagic shock-induced organ dysfunction in rats. Free Radic. Biol. Med. 2011, 50, 1737–1748. [Google Scholar] [CrossRef] [PubMed]
- Moody, J.O.; Robert, V.A.; Connolly, J.D.; Houghton, P.J. Anti-inflammatory activities of the methanol extracts and an isolated furanoditerpene constituent of Sphenocentrum jollyanum Pierre (Menispermaceae). J. Ethnopharmacol. 2006, 104, 87–91. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of all the isolated compounds are available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).