Abstract
A novel method for cleavage of the dithiine ring in 5,12-(dimethyl)-thioqinantrenium bis-chloride 1 “via” reaction with sodium hydrosulfide leads to 1-methyl-3-mercaptoquinoline-4(1H)-thione 2. Further transformation of thiol and thione functions of compound 2 leads to a series of sulfide and disulfide derivatives of quinolinium salts 4 and 6. 1-Methyl-4-chloro-3-benzylthioquinoline chloride 8 was obtained by N-alkylating 4-chloro-3-benzylthioquinoline using dimethyl sulfate. Antimicrobial activity of the obtained compounds was investigated using six Gram-positive and six Gram-negative bacterial strains, as well as Candida albicans yeast. Greater activity was demonstrated towards Gram-positive strains. MIC values for compounds and with benzylthio 4d and benzoylthio 4f substituents in 3-quinoline position were found to be in the 0.5–1 μg/mL range, at a level similar to that of ciprofloxacin (reference). Compounds 4d and 4f also demonstrated interesting antifungal properties (MIC = 1).
1. Introduction
Increasing bacterial resistance to antibiotics has become a very serious medical problem for health care systems worldwide [1,2]. This situation requires continued efforts in searching for novel classes of compounds with antimicrobial activities. The rising number of reports concerning microbial resistance to antibiotics has led to the expression of concerns over imminent incurability of some pathogen-caused diseases. Quinoline stands out prominently among pharmacologically active compounds [3]. Its structural motif is present in many naturally-occurring compounds that show interesting biological properties. Quinine, the first effective antimalarial drug isolated from cinchona tree bark, provides a good example. Based on its established structure novel synthetic antimalarial drugs were obtained, such as chloroquine, primaquine and mefloquine [4]. Other examples of similar compounds include streptonigrin, produced by Streptomyces flocculus, an aminoquinone antitumor and antibacterial antibiotic [5] and camptothecin, a cytotoxic quinoline alkaloid isolated from the bark and stem of Camptotheca acuminata (Camptotheca, happy tree). Derivatives of the latter, topotecan and irinotecan, are anticancer drugs [6]. An important group of chemotherapeutics with quinoline motif are 4-quinolone derivatives effective against Gram-negative bacteria [7,8]. Quinoline ring systems have often been included when designing novel synthetic or semi-synthetic compounds with different pharmacological properties. Numerous quinoline derivatives, offering a broad spectrum of activity, have been reported. They include compounds with anticancer [9,10,11], antimycobacterial [12,13], antimicrobial [14,15], anticonvulsant [16,17], antiinflamatory [17,18] and cardiovascular [19,20] activity.
Substantial antimicrobial activity was demonstrated by 4-aminoquinoline salts [21,22,23]. There have been also reports concerning such activity in cases of sulfide derivatives of quinoline [24]. In our previous papers, we described the synthesis of 1,3,4-trisubstituted derivatives of quinoline salts [25,26]. Since initial trials demonstrated their strong antimicrobial properties we decided to investigate and report in here the activity of such derivatives against a wider panel of reference strains; we also report synthesis of novel derivatives.
2. Results and Discussion
2.1. Chemistry
5,12-Dialkylthioquintrenediinium bis-salts 1 were obtained by alkylating thioquinantrene (1,4-dithiino[2,3-c,5,6-c′]diquinoline) [24]. The previously described reaction of thioquinantrene ring opening requires gaseous hydrogen sulfide. The novel method described herein based on using sodium hydrosulfide is safer, less cumbersome, and does not require disposition of excess hydrogen sulfide. Reactions of salts 1 with sodium hydrosulfide in pyridine solution at room temperature occur with complete consumption of salts 1. The reaction occurs by nucleophilic attack of hydrosulfide anion on aza-activated positions 7a and 14a of salts 1, which leads to the opening of the 1,4-dithiin ring of salts 1. As the result of this reaction, two molecules of 1-methyl-3-mercaptoquinoline-4(1H)-thione 2 are formed (Scheme 1). This compound was obtained previously via reaction of salt 1 with hydrogen sulfide [27], but its structure has not been confirmed spectroscopically.
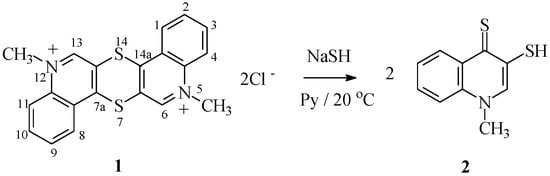
Scheme 1.
Synthesis of compounds 2.
Use of sodium hydrosulfide increases the speed of compound 1 dithiine ring cleavage. This is the result of the stronger nucleophilic properties of hydrosulfide anion as compared to the hydrogen sulfide molecule. The perquisite of reaction with hydrogen sulfide has been the use of pyridine as solvent (proton acceptor). Reaction with NaSH does not require the presence of a proton acceptor (it also proceeds in other solvents, e.g., ethanol, albeit much more slowly). The reaction products are easily soluble in pyridine. 1H-NMR spectrum of compound 2 in CDCl3 solution lacks a signal from thiol proton, which may be the result of its interaction with thiocarbonyl sulfur atom (C=S) in 4-quinoline position (Scheme 2). This is corroborated by the 13C-NMR spectrum of compound 2, in which C4quinolinyl signal occurs at δ = 134.64 ppm. The signal from thiocarbonyl carbon in 13C-NMR spectrum should be present above 180 ppm [28]. It appears that the hydrogen bond does not much affect the IR spectrum of this type of compound. A strong band corresponding to C=S stretching vibrations in compound 2 occurs at 1107 cm−1, whereas for compound 3a, which lacks such interactions, the band is present at 1112 cm−1. The elemental composition of compound 2 is confirmed by MS spectrum and the results of elemental analysis.
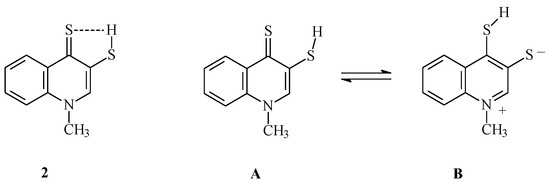
Scheme 2.
Interaction between thiol proton and thione sulfur atom and the tautomeric structures of compound 2.
Reactions of 2 in aqueous sodium hydroxide with alkylating or acylating agents (allyl chloride, benzyl chloride and benzoyl chloride) occurred, as expected, on 3-quinoline thiolate sulfur, and led to their respective 1-methyl-3-thioquinoline-4(1H)-thione derivatives 3 with 73%–83% yield. The 13C-NMR spectra for compounds 3 show signal from the C4quinolinyl carbon occurring in the characteristic range for thiocarbonyl carbon, and this additionally confirms the structure of 2, the substrate of these reactions. Alkylating of thione function in the 4-quinoline position of compound 3 was carried out at 70 °C, using excess of alkylating agents (dimethyl sulfate, butyl bromide or benzyl chloride) as reaction milieu (Scheme 3). The reactions led to the respective quinoline salts 4 with quantitative yield.
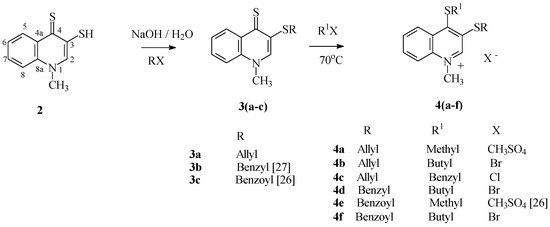
Scheme 3.
Synthesis of compounds 3 and 4.
1-Methyl-3-mercaptoquinoline-4(1H)-thione 2 in substance is stable, whereas in solution the thiol function is easily oxidized. Compounds 2 were transformed into di(1-methylquinoline-4(1H)-thione-3-yl) disulfide 5 by reacting (2) in DMF with atmospheric oxygen or ethanolic iodine (Scheme 4). Both procedures gave disulfide 5 with quantitative yield.
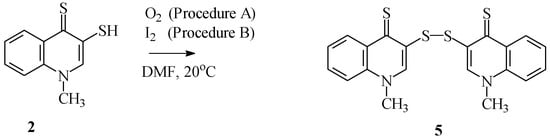
Scheme 4.
Synthesis of disulfide 5.
Alkylating disulfide 5 occurred as S-alkylation of both thione groups and led to bis-salts 6 (Scheme 5). The reaction speed is temperature-dependent. At room temperature, only trace reaction is observed. Reaction mixes showed no sign of mono-alkylation products, i.e., resulting from alkylation of a single thione group. As in the case of compounds 3, the bis-salts 6 were obtained with quantitative yield when reactions were performed at 80 °C and with excess of alkylating agent (dimethyl sulfate) as the reaction milieu.
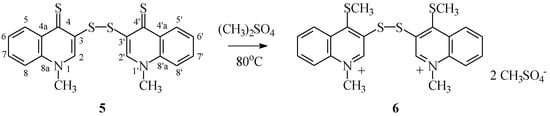
Scheme 5.
Synthesis of bis-methyl sulfide 6.
Salt 8 with chlorine atoms instead of thioalkyl groups in the 4-quinoline position was obtained by N-alkylating 4-chloro-3-thiobenzylquinoline 7 derivatives using dimethyl sulfate (Scheme 6). The reaction proceeds easily at 60 °C, leading to salts 8 with quantitative yield.
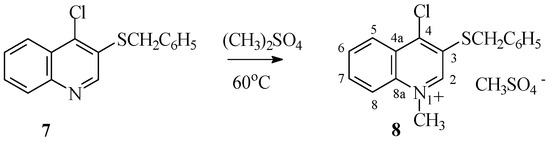
Scheme 6.
Synthesis of salt 8.
2.2. Biological Activity
The examined derivatives of quinolinium salts demonstrated activity (0.5–512 μg/mL concentration range) against both Gram-positive and Gram-negative bacteria, as well as against Candida albicans, a yeast-like fungus. However, greater activity was found against Gram-positive bacteria such as Staphylococcus, Streptococcus, Micrococcus and Bacillus. In almost all cases, the tested compounds were more active against Staphylococcus aureus methicillin-sensitive strains, although activity also remained very high for methicillin-resistant strains (MRSA). Most likely, the high activity of the tested compounds results from structural features of the bacterial wall. In the case of Gram-negative bacteria, the examined compounds were active in the 8–512 μg/mL range. As in the case of Gram-positive bacteria, the highest activity against Gram-negative strains was demonstrated by derivatives 4d and 4f. The lowest activity of sulfur derivatives of quinolinium salts was found for Gram-negative bacteria of Pseudomonas genus. Compounds 4d and 4f were also highly active against yeast-like fungus Candida albicans (MIC = 1). In order to examine the effect of thioalkyl group on the activity of the tested compounds their structure was modified via introduction of chlorine atoms instead of thioalkyl groups into the 4-quinoline position (compound 6), as well as introducing disulfide groups in the 3-quinoline position (compound 8), respectively. The obtained results demonstrate that the lack of the thioalkyl group in the 4-quinoline position considerably decreases antimicrobial activity, whereas the compound with the disulfide group shows activity comparable to those of compounds 4(a–c), i.e., derivatives containing the allylthio group in the 3-position.
We previously reported the activity of 1,3,4-trisubstituted 4-aminoquinoline salts [22,23]. These compounds also demonstrated activity against Gram-positive and Gram-negative bacteria. However, the structural modification achieved by introducing thioalkyl substituents instead of alkylamine ones in the 4-quinoline position markedly increased their antibacterial and antifungal activity. The MIC values reported here for Gram-positive bacteria are comparable to those of ciprofloxacin (reference). This may suggest the need for a continuing search effort for novel types of sulfur derivatives of quinoline salts, as well as the assessment of antibacterial activity using not only standard strains, but also clinically relevant ones.
3. Materials and Methods
3.1. Chemistry
Melting points are uncorrected. NMR spectra were recorded using a Bruker Ascend 600 spectrometer (Bruker, Billerica, MA, USA). To assign the structures, the following 2D experiments were employed: 1H-13C gradient selected HSQC and HMBC sequences. Standard experimental conditions and standard Bruker programs were used. The 1H- and 13C-NMR spectral data are given relative to the TMS signal at 0.0 ppm. HR mass spectra were recorded with Bruker Impact II (Bruker, Billerica, MA, USA). Solid-state infrared spectra were recorded in the range of 4000–1000 cm−1 using the Shimadzu IRAffinity-1 FTIR spectrometer (Shimadzu, Kyoto, Japan) and KBr pellet method. CHNS elemental analysis was performed using EuroVector 3018 analyser (EuroVector, Pavia, Italy). Total halides were determined using titration (after mineralization) with mercuric nitrate solution.
3.1.1. Synthesis of 1-Methyl-3-(mercapto)quinoline-4(1H)-thione 2
Sodium hydrosulfide hydrate (2.5 mmol) was added to the mixture of bis-chloride (1) (0.419 g, 1 mmol) in 10 mL of dry pyridine, and the whole was mixed at 20 °C for 1 h. The mixture was poured into 100 mL of water. The formed precipitate was filtered off, washed with water and dried over calcium chloride in desiccator with concomitant argon atmosphere.
1-Methyl-3-(mercapto)quinoline-4(1H)-thione (2). Yield 87%; m.p. 258 °C; IR (KBr, cm−1): νmax: 1107 (C=S); (CDCl3), 600 MHz) δ (ppm): 4.01 (s, 3H, NCH3), 7.50–7.54 (m, 1H, H6), 7.54–7.58 (m, 1H, H8), 7.68–7.72 (m, 1H, H7), 8.06 (s, 1H, H2), 8.97–9.01 (m, 1H, H5); 13C-NMR (DMSOd-6, 150.9 MHz) δ (ppm): 42.30 (NCH3), 116.16 (C8), 126.37 (C6), 126.89 (C3), 130.82 (8a), 131.36 (C7), 131.64 (4a), 132.37 (C5), 133.20 (C2), 134.64 (C4); ESI-HRMS Calcd for C10H10NS2 ([M + H]+): 208.0254, Found: 208.0254; Anal. Calcd for C10H9NS2: C 57.94, H 4.38, N 6.76, S 30.93. Found: C 57.87, H 4.31, N 6.73, S 30.85.
3.1.2. General Procedure for Synthesis of 1-Methyl-3-thioquinoline-4(1H)-thione Derivatives 3
1 mmol (0.207 g) 1-methyl-3-mercaptoquinoline-4(1H)-thione (2) was added dropwise with stirring into 50 mL of 5% aqueous sodium hydroxide. An alkylating agent or acylating (1.25 mmol) was added dropwise with stirring for 1 h. The formed precipitate was filtered off, washed with water and dried over calcium chloride in vacuum desiccator. The raw product was purified through recrystallization from ethanol.
1-Methyl-3-(allylthio)quinoline-4(1H)-thione (3a). Yield 78%; m.p. 212–214 °C; 1H-NMR (DMSOd-6, 600 MHz) δ (ppm): 3.66 (d, J = 6.6 Hz, 2H, SCH2CH), 4.08 (s, 3H, NCH3), 5.10-5-18 (m, 1H, CH=CH2), 5.23–5.35 (m, 1H, CH=CH2), 5,78–6.00 (m, 1H, CH=CH2), 7.48–7.58 (m, 1H, H6), 7.75–7.82 (m, H, H7), 7.83–7.88 (m, 1H, H8), 8.02 (s, 1H, H2), 8.76–8.83 (m, 1H, H5); 13C-NMR (DMSOd-6, 150.9 MHz) δ: 34.46 (SCH2), 42.17 (NCH3), 118.23 (C8), 118.62 (CH2=CH), 126.17 (C6), 129.31 (C5), 131.54 (C4a), 132.07 (CH2=CH), 132.11 (C7), 134.05 (C3), 135.31 (C8a) 136.12 (C2), 187.09 (C4); ESI-HRMS Calcd for C13H14NS2 ([M + H]+): 248.0567, Found: 248.0564; Anal. Calcd for C13H13NS2: C 63.12, H 5.30, N 5.66, S 25.92. Found: C 63.07, H 5.24, N 5.58, S 25.89.
1-Methyl-3-(benzylthio)quinoline-4(1H)-thione (3b). Yield 83%; m.p. 204–206 °C; 1H-NMR (DMSOd-6, 600 MHz) δ (ppm): 4.07 (s, 3H, NCH3), 4.21 (s, 2H, SCH2), 7.32–7.37 (m, 3H, Harom), 7.41–7.44 (m, 2H, Harom), 7.53–7.58 (m, 1H, H6), 7.79–7.83 (m, 1H, H7), 7.85–7.89 (m, 1H, H8), 8.12 (s, 1H, H2), 8.79–8.83 (m, 1H, H5); 13C-NMR (DMSOd-6, 150.9 MHz) δ (ppm): 36.23 (CH2), 42.18 (NCH3), 118.28 (C8), 126.21 (C6), 127.60 (H1benzyl), 128.98 (C2,C6benzyl), 129.32 (C5), 129.60 (C3,C5benzyl), 132.03 (C4benzyl), 132.132.15 (C7), 132.46 (C4a), 135.34 (C8a), (C2), 137.50 (C3), 186.65 (C4); ESI-HRMS Calcd for C17H15NS2 ([M + H]+): 298.0724, Found: 298.0714; Anal. Calcd for C17H15NS2: C 68.65, H 5.08, N 4.71, S 21.56. Found: C 68.54, H 5.01, N 4.65, S 21.50.
1-Methyl-3-(benzoylthio)quinoline-4(1H)-thione (3c). Yield 73%; m.p. 210–212 °C; 1H-NMR (DMSOd-6, 600 MHz) δ (ppm): 4.07 (s, 3H, NCH3), 7.60–7.67 (m, 3H, Harom), 7.72–7.78 (m, 1H, Harom), 7.88–7.95 (m, 2H, Harom), 8.01–8.05 (m, 1H, H8), 8.68 (s, 1H, H2), 8.86–8.90 (m, 1H, H5); 13C-NMR (DMSOd-6, 150.9 MHz) δ (ppm): 41.94 (NCH3), 118.68 (C8), 121.41 (C1benzoyl), 127.07 (C4benzoyl), 127.56 (C3,C5benzoyl), 129.74 (C2,C6benzoyl), 130.73 (C5), 133.33 (C7), 133.45 (C3), 134.60 (C6), 136.39 (C4a), 136.66 (C8a), 145.68 (C2), 188.99 (CO), 193.19 (C4); ESI-HRMS Calcd for C17H14NOS2 ([M + H]+): 312.0517, Found: 312.0515; Anal. Calcd for C17H13NOS2: C 65.57, H 4.21, N 4.50, S 20.59. Found: C 65.51, H 4.14, N 4.47, S 20.55.
3.1.3. General Procedure for Synthesis of 1-Methyl-3-thio-4-thioquinolinium Derivatives Salts (4)
The mixture (suspension) of thione (3) (1 mmol) and alkylating agents (5 mmol) was stirred and heated at 80 °C for 3 h. Unreacted alkylating agents were removed by vacuum distillation. Then 5 mL of dry ethanol was added. The mixture was refluxed for 5 min and cooled down to room temperature. The solid was filtered off and washed with dry ethanol.
1-Methyl-3-(allylthio)-4-(methylthio)quinolinium methyl sulfate (4a). Yield 95%; oil; 1H-NMR (D2O, 600 MHz) δ (ppm): 2.53 (s, 3H, SCH3), 3.55 (s, 3H, OCH3), 3.67–3.74 (d, 2H, J = 6.6 Hz, SCH2CH), 4.41 (s, 3H, NCH3), 5.00–5.05 (m, 1H, CH=CH2), 5.09–5.17 (m 1H, CH=CH2), 5.74–5.85 (m, 1H, CH=CH2), 7.75–7.79 (m, 1H, H6), 7.93–7.98 (m, 1H, H7), 8.08–8.13 (m, 1H, H8), 8.44–8.48 (m, 1H, H5), 8.78 (s, 1H, H2); 13C-NMR (D2O, 150.9 MHz) δ (ppm): 19.38 (OCH3), 35.95 (SCH2), 45.07 (NCH3), 55.31 (SCH3), 118.81 (C8), 119.36 (CH=CH2),127.56 (C5), 130.03 (C4a), 130.41 (C6), 131.89 (CH=CH2), 134.71 (C7), 135.05 (C8a), 136.27 (C3), 146.48 (C2), 158.91 (C4); ESI-HRMS Calcd for C14H16NS2 ([M]+): 262.0724, Found: 262.0722; Anal. Calcd for C15H19NO4S3: C 48.24, H 5.13, N 3.75, S 25.75. Found: C 48.20, H 5.08, N 3.67, S 25.70.
1-Methyl-3-allylthio-4-(butylthio)quinolinium bromide (4b). Yield 91%; m.p. 168 °C dec.; 1H-NMR (D2O, 600 MHz) δ (ppm): 0.55–0.750 (t, J = 7.2 Hz, 3H, CH2CH3), 1.10–1.25 (m, 2H, CH2CH3), 1.25–1.40 (m, 2H, SCH2CH2), 3.00–3.12 (t, J = 7.2 Hz, SCH2CH2), 3.70–3.78 (d, 2H, J = 6.0 Hz, SCH2CH), 4.43 (s, 3H, NCH3), 5.03–5.08 (m, 1H, CH=CH2), 5.12–5.20 (m 1H, CH=CH2), 5.73–5.84 (m, 1H, CH=CH2), 7.73–7.82 (m, 1H, H6), 7.91–8.00 (m, 1H, H7), 8.08–8.12 (m, 1H, H8), 8.48–8.54 (m, 1H, H5), 8.79 (s, 1H, H2); 13C-NMR (D2O, 150.9 MHz) δ (ppm): 12.63 (CH3CH2), 21.03 (CH3CH2), 31.54 (SCH2CH2), 35.72 (SCH2CH), 37.06 (SCH2CH2), 45.22 (NCH3), 118.80 (C8), 119.45 (CH=CH2), 127.72 (C5), 130.53 (C6), 130.86 (C4a), 131.80 (CH=CH2), 134.65 (C7), 136.15 (C8a), 136.55 (C3), 145.84 (C2), 156.69 (C4); ESI-HRMS Calcd for C17H22NS2 ([M]+): 304.1193, Found: 304.1191; Anal. Calcd for C17H22BrNS2: C 53.12, H 5.77, Br 20.79, N 3.64, S 16.68. Found: C 53.05, H 5.71, Br 20.70, N 3.59, S 16.63.
1-Methyl-3-allylthio-4-(benzylthio)quinolinium chloride (4c). Yield 93%; m.p 183 °C dec; 1H-NMR (D2O, 600 MHz) δ (ppm): 3.62–3.68 (d, J = 6.6 Hz, 2H, SCH2CH), 4.02 (s, 2H, SCH2), 4.32 (s, 3H, NCH3), 5.02–5.09 (m, 1H, CH=CH2), 5.11–5.18 (m, 1H, CH=CH2), 5.64–5.75 (m, 1H CH=CH2), 6.71–6.76 (m, 2H, Hbenzyl), 6.81–6.90 (m, 3H, Hbenzyl), 7.58–7.64 (m, 1H, H6), 7.83–7.89 (m, 1H, H7), 7.96–8.01 (m, 1H, H8), 8.13–8.18 (m, 1H, H5), 8.65 (s, 1H, H2); 13C-NMR (D2O, 150.9 MHz) δ (ppm): 35.32 (SCH2CH), 40.23 (SCH2), 45.44 (NCH3), 118.73 (C8), 119.83 (CH=CH2), 127.42 (C4benzyl), 127.71 (C5), 128.41 (C2,C6benzyl), 128.76 (C3,C5benzyl), 128.86 (C1benzyl), 130.72 (C6), 131.09 (C4a), 131.47 (CH=CH2), 134.60 (C7), 135.79 (C8a), 138.25 (C3), 145.13 (C2), 152.90 (C4); ESI-HRMS Calcd for C20H20NS2 ([M]+): 338.1037, Found: 338.1037; Anal. Calcd for C20H20ClNS2: C 64.24, H 5.39, Cl 9.48, N 3.75, S 17.15. Found: C 64.17, H 5.35, Cl 9.41, N 3.69, S 17.11.
1-Methyl-3-benzylthio-4-(butylthio)quinolinium bromide (4d). Yield 90%; m.p. 140 °C dec.; 1H-NMR (DMSOd-6, 600 MHz) δ(ppm): 0.73–0.82 (t, J = 7.2 Hz, 3H, CH2CH3), 1.28–1.37 (m, 2H, CH2CH3), 1.39–1.46 (m, 2H, SCH2CH2), 3.15–3.20 (t, J = 7.2 Hz, 2H, SCH2CH2), 4.64 (s, 1H, SCH2), 4.67 (s, 3H, NCH3), 7.28–7.34 (m, 1H, H4benzyl), 7.34–7.40 (m, 2H, H3,H5benzyl), 7.45–7.48 (m, 2H, H2,H6benzyl), 8.05–8.10 (m, 1H, H6), 8.20–8.23 (m, 1H, H7), 8.47–8.51 (m, 1H, H8), 8.70–8.74 (m, 1H, H5), 9.56 (s, 1H, H2); 13C-NMR (DMSOd-6, 150.9 MHz) δ (ppm): 13.76 (CH3CH2), 21.56 (CH3CH2), 32.10 (SCH2CH2), 36.80 (SCH2), 37.43 (SCH2CH2) 45.81 (NCH3), 120.53 (C8), 127.68 (C5), 128.34 (C4benzyl), 129.32 (C3,C5benzyl), 129.68 (C2,C6benzyl), 130.92 (C4a), 131.35 (C6), 134.59 (C1benzyl), 135.93 (C7), 136.41 (C8a), 138.36 (C3), 147.06 (C2), 153.07 (C4); ESI-HRMS Calcd for C21H24NS2 ([M]+): 354.1350, Found: 354.1351; Anal. Calcd for C21H24BrNS2: C 58.06, H 5.57, Br 18.39, N 3.22, S 14.76. Found: C 57.91, H 5.50, Br 18.35, N 3.15, S 14.74.
1-Methyl-3-benzoylthio-4-(methylthio)quinolinium methyl sulfate (4e). Yield 95%; m.p. 98 °C dec.; 1H-NMR (D2O, 600 MHz) δ (ppm): 2.53 (s, 3H, SCH3), 3.51 (s, 3H, OCH3), 4.36 (s, 3H, NCH3), 7.25–7.31 (m, 2H, H3,H5benzoyl), 7.44–7.49 (m, 1H, H4benzoyl), 7.70–7.78 (m, 2H, H2,H6benzoyl), 7.80–7.86 (m, 1H, H6), 8.04–8.09 (m, 1H, H7), 8.12–8.17 (m, 1H, H8), 8.47–8.52 (m, 1H, H5), 9.03 (s, 1H, H2); 13C-NMR (D2O, 150.9 MHz) δ (ppm): 20.88 (SCH3), 44.93 NCH3), 55.28 (OCH3), 119.13 (C8), 125.11 (C3), 127.58 (C3,C5benzoyl), 128.60 (C5), 129.14 (C2,C6benzoyl), 129.66 (C4a), 130.63 (C6), 134.29 (C1benzoyl), 135.30 (C4benzoyl), 136.50 (C7), 137.39 (C8a), 151.46 (C2), 167.62 (C4), 189.08 (CO), ESI-HRMS Calcd for C18H16NOS2 ([M]+): 326.0673, Found: 326.0672; Anal. Calcd for C19H19NO5S3: C 52.16, H 4.38, N 3.20, S 21.98: Found: 52.08, H 4.31, N 3.15, S 21.95.
1-Methyl-3-benzoylthio-4-(butylthio)quinolinium bromide (4f). Yield 89%; m.p. 112 °C dec.; 1H-NMR (DMSOd-6, 600 MHz) δ (ppm): 0.45–0.53 (t, J = 7.2 Hz, 3H, CH2CH3), 1.00–1.11 (m, 2H, CH2CH3), 1.19–1.25 (m, 2H, SCH2CH2), 3.00–3.09 (t, J = 7.2 Hz, 2H, SCH2CH2), 4.43 (s, 3H, NCH3), 7.35–7.40 (m, 2H, H3,H5benzoyl), 7.53–7.57 (m, 1H, H4benzoyl), 7.80–7.84 (m, 2H, H2,H6benzoyl),. 7.84–7.88 (m, 1H, H6), 8.07–8.12 (m, 1H, H7), 8.18–8.23 (m, 1H, H8), 8.60–8.64 (m, 1H, H5), 9.14 (s, 1H, H2); 13C-NMR (DMSOd-6, 150.9 MHz) δ (ppm): 12.52 (CH2CH3), 20.91 (CH2CH3), 31.54 (SCH2CH2), 38.79 (SCH2CH2), 45.08 (NCH3), 119.16 (C8), 126.69 (C3), 127.64 (C3,C5benzoyl), 129.00 (C5), 129.24 (C2,C6benzoyl), 130.57 (C4a), 130.77 (C6), 134.42 (C1benzoyl), 135.39 (C4benzoyl), 136.53 (C7), 137.56 (C8a), 151.63 (C2), 166.03 (C4), 189.51 (CO); ESI-HRMS Calcd for C21H22NOS2 ([M]+): 368.1143, Found: 368.1134; Anal. Calcd for C21H22BrNOS2: C 56.25, H 4.94, Br 17.82, N 3.12, S 14.30. Found: C 56.14, H 4.86, Br 17.74, N 3.07, S 14.25.
3.1.4. Synthesis of Di(1-methylquinoline-4(1H)-thione-3-yl) Disulfide (5)
Procedure (A). Air was passed through the solution of 1-methyl-3-mercaptoquinoline-4(1H)-thione (2) (1 mmol, 0.207g) in dry DMF (50 mL) at rt over 1 h. The solid product was filtered off and washed with dry ether. The mixture of raw product and 10 mL dry ethanol was refluxed for 5 min and cooled down to room temperature. The solid product was filtered off and washed with dry ethanol.
Procedure (B). To the solution of 1-methyl-3-mercaptoquinoline-4(1H)-thione (2) (1 mmol, 0.207 g) in dry DMF (50 mL) a solution of 1.5 mmol (0.381 g) of iodine in 5 mL of ethanol was added over 1 h with stirring (rt). The solid product was filtered off and washed with dry ether. The mixture of raw product and 10 mL dry ethanol was refluxed for 5 min and cooled down to room temperature. The solid product was filtered off and washed with dry ethanol.
Di(1-methylquinoline-4(1H)-thione-3-yl) disulfide (5). Yield: Procedure A: 92%, Procedure B: 95%; m.p. 301–302 °C; 1H-NMR (DMSOd-6, 600 MHz) δ (ppm): 4.05 (s, 6H, NCH3, N′CH3), 7.60–7.65 (m, 2H, H6, H6′), 7.83–7.87 (m, 2H, H7, H7′), 7.90–7.94 (m, 2H, H8, H8′), 8.34 (s, 2H, H2,H2′), 8.76–8.80 (m, 2H, H5,H5′); ESI-HRMS Calcd for C20H16N2S4 ([M]2+): 206.0098, Found: 206.0092; Anal. Calcd for C20H16N2S4: C 58.22, H 3.91, N 6.79, S 31.08. Found: C 58.17, H 3.86, N 6.74, S 31.05.
3.1.5. Synthesis of Bis-Methyl Sulfate di(1-methyl-4-(methylthio)quinolinium-3-yl) Disulfide 6
The mixture of compounds (5) (1 mmol) and dimethyl sulfate (5 mmol) was stirred and heated at 80 °C for 2 h. Then, 10 mL of dry ethanol was added. The mixture was refluxed for 5 min and cooled down to room temperature. The solid was filtered off and washed with dry ethanol. The raw product was purified through recrystallization from ethanol.
Bis-methyl sulfate di(1-methyl-4-(methylthio)quinolinium-3-yl) disulfide (6) Yield 94%; m.p. 120 °C dec.; 1H-NMR (DMSOd-6, 600 MHz) δ (ppm): 2.80 (s, 6H, OCH3), 3.31 (s, 6H, SCH3,S′CH3), 4.62 (s, 6H, NCH3,N′CH3), 8.12–8.15 (m, 2H, H6,H′6), 8.29–8.33 (m, 2H, H7,H′7), 8.53–8.56 (m, 2H, H8,H′8), 8.72–8.76 (m, 2H, H5,H′5), 9.62 (s, 2H, H2,H′2);.13C-NMR (DMSOd-6, 150.9 MHz) δ (ppm): 21.24 (C), 46.14 (C), 53.24 (C), 120.77 (C8), 128.15 (C5), 130.02 (C4a), 131.58 (C6), 135.14 (C7), 135.89 (C3), 137.65 (C8a), 148.46 (C2), 158.13 (C4); ESI-HRMS Calcd for C22H22N2S4 ([M]2+): 221.0333, Found: 221.0322; Anal. Calcd for C24H28N2O8S6: C 43.36, H 4.24, N 4.21, S 28.93, Found: C 43.27, H 4.19, N 4.17, S 28.90.
3.1.6. Synthesis of 1-Methyl-4-chloro-3-(benzylthio)quinolinium Methyl Sulfate (8)
The mixture (suspension) of compounds (7) (1 mmol) and dimethyl sulfate (5 mmol) was stirred and heated at 80 °C for 2 h. Then, 5mL of dry ethanol was added. The mixture was refluxed for 5 min and cooled down to the room temperature. The solid was filtered off and washed with dry ethanol. The raw product was purified through recrystallization from ethanol.
1-Methyl-4-chloro-3-(benzylthio)quinolinium methyl sulfate (8). Yield 89%; m.p. 204 °C dec.; 1H-NMR (DMSOd-6, 600 MHz) δ (ppm): 3.70 (s, 3H, OCH3.), 4.64 (s,2H, CH2), 4.67 (s, 3H, NCH3), 7.26–7.32 (m, 3H, H4benzyl), 7.32–7.43 (m, 2H, H3,H5benzyl), 7.43–7.50 (m, 2H, H2,H6benzyl) 8.10–8.18 (m, 1H, H6), 8.23–8.27 (m, 1H, H7), 8.48–8.59 (m, 2H, H5,H8), 9.68 (s, 1H, H2), 13C-NMR (DMSOd-6, 150.9 MHz) δ ppm): 37.32 (CH2), 45.95 (NCH3), 53.29 (OCH3), 120.59 (C5), 126.02 (C8), 127.22 (C4a), 128.43 (C4benzyl), 129.38 (C3,C5benzyl), 129.70 (C2,C6benzyl), 132.21 (C6), 132.29 (C1benzyl), 135.54 (C7), 135.63 (C3), 137.46 (C8a), 148.67 (C4), 149.46 (C2); ESI-HRMS Calcd for C18H18ClNS ([M]+): 300.0614, Found: 300.0612; Anal. Calcd for C18H18ClNO4S2: C 52.49, H 4.40, Cl 8.61, N 3.40, S 15.57. Found: C 52.38, H 4.36, Cl 8.54, N 3.37, S 15.51.
3.2. Biological Assays
Analysis of antimicrobial activity was performed using the serial microdilution method and Muelller-Hinton broth (MHB). Thirteen standard strains from ATTC collection were used (Staphylococcus aureus ATCC 25923 (SA25923), Staphylococcus aureus ATCC 43300 (SA43300), Staphylococccus epidermidis ATCC 12228 (SE12228), Enterococcus faecalis ATCC 29212 (E29212), Micrococcus luteus ATCC 10240 (ML10240), Bacillus subtilis ATCC 11774 (BS11774), Escherichia coli ATCC 11776 (EC11776), Klebsiella pneumoniae ATCC 27736 (KP27736), Proteus mirabilis ATCC 7002 (PM7002), Pseudomonas aeruginosa ATCC 27853 (PA27853), Acinetobacter baumannii ATCC 19606 (AB19606), Serratia marcescens ATCC 8100 (SM8100) and Candida albicans ATCC 60193 (CA60193). An inoculum density of 5 × 105 cfu/mL was used. Antimicrobial activity was examined using 96-well titration plates. The concentration range of the examined sulfur derivatives of quinolinium salts was 0.5–512 μg/mL As control, ciprofloxacin (CIP) was used (0.001–2 μg/mL range). MIC determination was performed for 20-h incubation periods at 37 °C. Tests were performed in accordance with EUCAST recommendations [29]. The results are presented in Table 1.

Table 1.
Evaluation of the minimum inhibitory concentration (MIC) (μg/mL) of quinolinium salt sulfur derivatives against tested bacterial strains.
4. Conclusions
Reaction of thioquinanthrenium salts 1 with sodium hydrosulfide leads to cleavage of the dithiin ring and the formation of 1-methyl-3-mercaptoquinoline-4(1H)-thione 2. Subsequent reactions of the thiol and thione groups in compound 2 allow the series of sulfide and disulfide derivatives of quinolinium salts 4 and 6 to be obtained. 1-Methyl-4-chloro-3-(benzyllthio)quinoline salt 8 can be synthesized by N-alkylating of 4-chloro-3-(benzylthio)quinoline using dimethyl sulfate. The obtained compounds demonstrated greater activity against Gram-negative bacteria than Gram-positive ones. Introduction of sulfur substituents into 4-quinoline position markedly increased activity compared to previously reported 4-aminoquinoline derivatives. Compounds 4d and 4f with thiobenzyl and thiobenzoyl substituents in the 3-quinoline position demonstrated activity comparable to that of ciprofloxacine, used as reference.
The MIC values exhibited by these compounds warrant the continuing search for novel 1,3,4-trisubstituted quinoline salts with antimicrobial activity.
Acknowledgments
This work was supported by the Medical University of Silesia in Katowice, Poland. Grant No. KNW-1-015/K/7/O.
Author Contributions
A.Z. and R.D.W. developed the concept of the work. A.Z., A.E. and E.K. carried out the synthetic work. R.D.W., M.K. and D.I. conducted a study of the biological activity. A.Z., R.D.W., A.S. and T.J.W. analyzed the data and wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Emori, T.G.; Culver, D.H.; Horan, T.C.; Jarvis, W.R.; White, J.W.; Olson, D.R.; Banerjee, S.; Edwards, J.R.; Martone, W.J.; Gaynes, R.P.; et al. National Nosocomial Infections Surveillance System (NNIS): Description of Surveillance Methods. Am. J. Infect. Control. 2004, 32, 470–485. [Google Scholar] [CrossRef]
- CDC. Staphylococcus aureus resistant to vancomycin—United States. Morb. Mortal. Wkly. Rep. 2002, 51, 565–567. [Google Scholar]
- Kumar, S.; Bawa, S.; Gupta, H. Biological Activities of Quinoline Derivatives. Mini-Rev. Med. Chem. 2009, 9, 1648–1654. [Google Scholar] [CrossRef] [PubMed]
- Delgado, J.N.; Remers, W.A. (Eds.) Wilson and Gisvold’s Textbook of Organic Medicinal and Pharmaceutical Chemistry, 10th ed.; Lippincott-Raven Publishers: Philadelphia, Pennsylvania, 1998; pp. 235–252. ISBN 978-0-397-51583-7. [Google Scholar]
- Bringmann, G.; Reichert, Y.; Kane, V. The total synthesis of streptonigrin and related antitumor antibiotic natural products. Tetrahedron 2004, 60, 3539–3574. [Google Scholar] [CrossRef]
- Wall, M.E.; Wani, M.C.; Cook, C.E.; Palmer, K.H.; McPhail, A.I.; Sim, G.A. Plant antitumor agents. I. The isolation and structure of camptothecin, a novel alkaloidal leukemia and tumor inhibitor from camptotheca acuminate. J. Am. Chem. Soc. 1966, 88, 3888–3890. [Google Scholar] [CrossRef]
- Sharma, P.C.; Jain, A.; Jain, S. Fluoroquinolone antibacterials: A review on chemistry, microbiology and therapeutic prospects. Acta Poloniae Pharm. Drug Res. 2009, 66, 587–604. [Google Scholar]
- Patrick, G.L. Antibacterial agents. In An Introduction to Medicinal Chemistry, 2nd ed.; Oxford University Press: Oxford, UK, 2003; pp. 379–435. ISBN 978-0-19-969739-7. [Google Scholar]
- Vittorio, C.; Guyen, B.; Opoku-Boahen, Y.; Mann, J.; Gowan, S.M.; Lloyd, M.; Martin, A.R.; Stephen, N. A novel inhibitor of human telomerase derived from 10H-indolo[3,2-b]quinoline. Bioorg. Med. Chem. Lett. 2000, 10, 2063–2066. [Google Scholar] [CrossRef]
- Mikata, Y.; Mika, Y.; Shun-Ichiro, O.; Ichiro, O.; Kawasaki, M.; Maeda, M.; Shigenobu, Y. Effect of side chain location in (2-aminoethyl)aminomethyl-2-phenylquinolines as antitumor agents. Bioorg. Med. Chem. Lett. 1998, 8, 1243–1248. [Google Scholar] [CrossRef]
- Sharples, D.; Spengler, C.; Molnar, J.; Zsuzsanna, A.; Molnar, A.; Kiss, J.T.; Szabo, J.A.; Hilgeroth, A.; Gallo, S.; Mohamoud, A.; et al. The interaction between resistance modifiers such as pyrido[3,2-g]quinoline, aza-oxafluorene and pregnane derivatives with DNA, plasmid DNA and tRNA. Eur. J. Med. Chem. 2005, 40, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, P.; Dinakaran, M.; Yogeeswari, P.; Sriram, D.; China, A.; Nagaraja, V. Synthesis and antimycobacterial activities of novel 6-nitroquinolone-3-carboxylic acids. Eur. J. Med. Chem. 2009, 44, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Nayyar, A.; Monga, V.; Malde, A.K.; Coutinho, E.; Jain, R. Synthesis, anti-tuberculosis activity, and 3D-QSAR study of 4-(adamantan-1-yl)-2-substituted quinolones. Bioorg. Med. Chem. 2007, 15, 626–640. [Google Scholar] [CrossRef] [PubMed]
- Sadana, A.K.; Mirza, Y.; Om, P. Hypervalent iodine mediated synthesis of 1-aryl/hetryl-1,2,4-triazolo[4,3-a] pyridines and 1-aryl/hetryl 5-methyl-1,2,4-triazolo[4,3-a]quinolines as antibacterial agents. Eur. J. Med. Chem. 2003, 38, 533–536. [Google Scholar] [CrossRef]
- Narender, P.; Srinivas, U.; Ravinder, M.; Rao, B.A.; Ramesh, C.; Harakishore, K.; Gangadasu, B.; Murthy, U.S.N.; Rao, V.J. Synthesis of multisubstituted quinolines from Baylis-Hillman adducts obtained from substituted 2-chloronicotinaldehydes and their antimicrobial activity. Bioorg. Med. Chem. 2006, 14, 4600–4609. [Google Scholar] [CrossRef] [PubMed]
- Murugananthamn, N.; Sivakumar, R.; Anbalagan, N.; Gunasekaran, V.; Leonard, J.T. Synthesis, anticonvulsant and antihypertensive activities of 8-substituted quinoline derivatives. Biol. Pharm. Bull. 2004, 27, 1683–1687. [Google Scholar] [CrossRef]
- Calhoun, W.; Carlson, R.P.; Crossley, R.; Datko, L.J.; Dietrich, S.; Healtheringtoj, K.; Marshall, L.A.; Meade, P.J.; Opalko, A.; Shephard, R.G. Synthesis and antiinflammatory activity of certain 5,6,7,8-tetrahydroquinolines and related compounds. J. Med. Chem. 1995, 38, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Kohno, Y.; Awano, K.; Miyashita, M.; Fujimori, S.; Kuriyama, K.; Sakoe, Y.; Kudoh, S.; Saito, K.; Kojima, E. Synthesis and antirheumatic activity of novel tetrahydroquinoline-8-carboxylic acid derivatives. Bioorg. Med. Chem. Lett. 1997, 7, 1515–1518. [Google Scholar] [CrossRef]
- McCall, J.M.; Brink, R.E.; Kamdar, B.V.; Ska-Letzky, L.L.; Perricone, S.C.; Piper, R.C.; Delehonty, P.J. 7-(Trifluoromethyl)-4-aminoquinoline hypotensives: Novel peripheral sympatholytics. J. Med. Chem. 1986, 29, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Sircar, I.; Haleen, S.J.; Burke, S.E.; Barth, H. Synthesis and biological activity of 4-(diphenylmethyl)-[(4-quinolinyloxy-)methyl]-1-piperazineethanol and related compounds. J. Med. Chem. 1992, 35, 4442–4449. [Google Scholar] [CrossRef] [PubMed]
- Zięba, A.; Wojtyczka, R.D.; Kępa, M.; Idzik, D. Antimicrobial activity of novel 1-methyl-3-thio-4-aminoquinolinium salts. Folia Microbiol. 2010, 55, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Zięba, A.; Wojtyczka, R.D.; Kępa, M.; Idzik, D. Synthesis and in vitro antimicrobial activity of 1-methyl-3-sulfothio-4-aminoquinolinium chlorides. Acta Poloniae Pharm. Drug Res. 2013, 70, 163–166. [Google Scholar]
- Wojtyczka, R.D.; Zięba, A.; Dziedzic, A.; Kępa, M.; Idzik, D. An activity of thioacyl derivatives of 4-aminoquinolinium salts towards biofilm producing and planktonic forms of coagulase-negative staphylococci. BioMed Res. Int. 2015, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hagrs, M.; Bayoumi, H.A.; El-Gamal, K.M.; Mayhoub, A.S.; Hamada, S.; Abulkhair, H.S. Synthesis and preliminary antimicrobial evaluation of some new 6-methoxyquinoline-3-carbonitrile derivatives. Beni-Suef Univ. J. Basic Appl. Sci. 2015, 4, 338–345. [Google Scholar] [CrossRef]
- Maślankiewicz, A.; Zięba, A. 1-Alkyl-3,4-di(alkylthio)quinolinium salts. Pol. J. Chem. 1994, 68, 1957–1971. [Google Scholar]
- Zięba, A. 3-Acylsulfanyl-1-methyl-4-methylsulfanyl-quinolinium salts and their transformation into 4-aminoquinolinium-3-thiolates and azaphenothiazine derivatives. Pol. J. Chem. 2008, 82, 1399–1402. [Google Scholar]
- Maślankiewicz, A.; Zięba, A. 5,12-Di-(1-alkyl)thioquinanthrenediinium bis-salts and 1-alkyl-3-alkylthio-1,4-dihydro-4-thiooxoquinolines. Heterocycles 1992, 34, 247–258. [Google Scholar] [CrossRef]
- Pretsch, E.; Bühlmann, P.; Badertscher, M. Structure determination of organic compounds. In Tables of Spectral Data, 4th ed.; Revised and Enlarged Edition; Springer: Berlin/Heidelberg, Germany, 2009; p. 127. ISBN 978-3-540-93810-1. [Google Scholar]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). Determination of minimum inhibitory concentration (MIC) of antibacterial agents by broth dilution. Clin. Microbiol. Infect. 2003, 9, ix–xv. [Google Scholar]
Sample Availability: Samples of the compounds described herein are available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).