Abstract
Herbal medicines are widely used for treating liver diseases and generally regarded as safe due to their extensive use in Traditional Chinese Medicine practice for thousands of years. However, in recent years, there have been increased concerns regarding the long-term risk of Herb-Induced Liver Injury (HILI) in patients with liver dysfunction. Herein, two representative Chinese herbal medicines: one—Xiao-Chai-Hu-Tang (XCHT)—a composite formula, and the other—Radix Polygoni Multiflori (Heshouwu)—a single herb, were analyzed by network pharmacology study. Based on the network pharmacology framework, we exploited the potential HILI effects of XCHT and Heshouwu by predicting the molecular mechanisms of HILI and identified the potential hepatotoxic ingredients in XCHT and Heshouwu. According to our network results, kaempferol and thymol in XCHT and rhein in Heshouwu exhibit the largest number of liver injury target connections, whereby CASP3, PPARG and MCL1 may be potential liver injury targets for these herbal medicines. This network pharmacology assay might serve as a useful tool to explore the underlying molecular mechanism of HILI. Based on the theoretical predictions, further experimental verification should be performed to validate the accuracy of the predicted interactions between herbal ingredients and protein targets in the future.
1. Introduction
Herb-Induced Liver injury (HILI) refers to herbal drug-driven liver injuries which are a cause of acute and chronic liver disease. Thousands of kinds of medicines have been implicated in causing various liver injuries and this is the most common reason for a drug to be withdrawn from the market [1].
Xiao-chai-hu-tang (XCHT), a Chinese herbal formula, is commonly used for treatment of chronic hepatitis and liver fibrosis in East Asian countries such as China, Japan and Korea. According to previous studies, XCHT exhibits various pharmacological properties including anti-inflammation, antioxidant and anti-hepatic fibrosis [2,3]. In addition, XCHT has shown strong hepatoprotective effects in both basic and clinical studies by regulating the immune response in the context of hepatitis C viral infection [4,5]. There are currently ongoing clinical trials of XCHT for hepatitis C and liver cirrhosis at the University of California, at San Diego and the Memorial Sloan-Kettering Cancer Center in the USA [5]. In spite of its multiple pharmacological effects, several systematic studies have examined and challenged its clinical safety. In 1995, four patients in Japan treated with XCHT by oral administration exhibited acute drug-induced liver injury [6]. In 2006, a case report in Taiwan also reminded us of the probable HILI potential of XCHT [7]. In 2000, the Japanese Ministry of Health Department completely forbade patients with hepatitis, liver cirrhosis and liver carcinoma to take XCHT [8]. The HILI of Radix Polygoni Multiflori (Heshouwu in Chinese) has also aroused widespread concern in recent years. Heshouwu has been used as a hepatoprotective and anti-aging tonic in Traditional Chinese Medicine (TCM) for thousands of years. In previous pharmacological studies, Heshouwu showed hepatoprotective effects against liver inflammation, oxidation stress, nonalcoholic fatty liver disease (NAFLD), liver fibrosis and cirrhosis as well as hepatic cancer [9,10]. However, increasing evidence also showed that Heshouwu might lead to significant adverse drug reactions in liver. Many case reports have revealed that long-term use of Heshouwu may induce toxic hepatitis or other severe liver injury, and even death [11,12,13]. Recent study has evaluated the “dose-time-toxicity” relationship of the HILI caused by Heshouwu in mice. The water-extracted components (from 5.5 to 30.75 g/kg) and the ethanol-extracted components (from 8.5 to 24.5 g/kg) caused obvious damage to the liver organization in a dose-dependent manner, resulting in increased serum alanine transaminase (ALT) and aspartate transaminase (AST) levels [14,15]. A compilation of liver injury cases have established causality for 28/57 different TCM herbal medicines, including Heshouwu and XCHT [14]. Although these studies have indicated that Heshouwu and XCHT may cause side effects on the liver, there has been insufficient scientific evidence to determine the HILI mechanisms of Heshouwu and XCHT.
In recent years, network pharmacology has made a significant contribution to investigating the molecular mechanisms of action of Chinese herbal medicines through chemical pharmacokinetic absorption, distribution, metabolism, excretion (ADME) property evaluation, target prediction and network/pathway analysis. Network pharmacology analysis plays an important role in the effective use of Chinese herbal medicines by predicating the potential toxic ingredients of candidate herbal drugs. Herein, based on the network pharmacology framework, we analyzed the potential HILI mechanisms of XCHT and Heshouwu. This network pharmacology technique could be of great help in predicting the safety of traditional herbal medicines and promoting the herbal medicine-based drug discovery.
2. Results and Discussion
2.1. Potential HILI Mechanisms of XCHT Predicted by Network Pharmacological Analysis
We have identified a total of 761 chemicals in XCHT. In multi-compound medicinal herbal mixtures like XCHT, many compounds in that lack appropriate pharmaceutical properties are believed to fail to reach the cellular targets, and thus exhibit little efficacy and should be neglected. The 438 XCHT ingredients with satisfactory pharmacokinetic properties were predicted through an in silico-based pharmacological method (Supplemental Table S1). The compounds and protein interaction analysis results showed that a total of 676 intracellular targets were predicted to interact with the 438 ingredients of XCHT. Among them, 51 are regarded as potential HILI targets. To further predict and illuminate the relationships between these potential active compounds and hepatotoxic target genes, a drug-target network were constructed (Figure 1). This network represents a global view of the potential compounds (red triangles) and targets (blue rectangles) in XCHT, and it comprised 74 nodes (23 potential compounds and 51 potential targets) and 131 edges (compound-target interactions). The degree of nodes is a key topological parameter that characterizes the most influential nodes in a network, and we used it to further determine the importance of active components and HILI targets [16]. Those high-degree nodes in the network, which had more compound-target interactions, are likely to play a more important role in HILI [17,18]. Our network analysis results showed that various candidate compounds in XCHT were linked to multiple targets, which may exhibit potential liver injury effects. Among the 23 candidate compounds, kaempferol and thymol exhibit the largest number of liver injury targets connections (degree = 6), followed by saikosaponin D (degree = 5), coumestrol (degree = 5), vanillin (degree = 5) and baicalein (degree = 5). For the 51 potential liver injury targets, the network showed CASP3 had the largest number of compound-target interactions (saikosaponin D, baicalin, nonenoic acid and mairin), followed by UGT1A10, UGT1A7, UGT1A8 (vanillin, thymol and coumestrol), and MMP9 (vanillin, wogolin and baicalein). The remaining 46 targets showed interactions with only two or one compounds. The information of 51 potential liver injury targets in XCHT can be found in Table 1, where all the data were manually collected and integrated from the STITCH, TTD, PharmGKB and CTD databases.
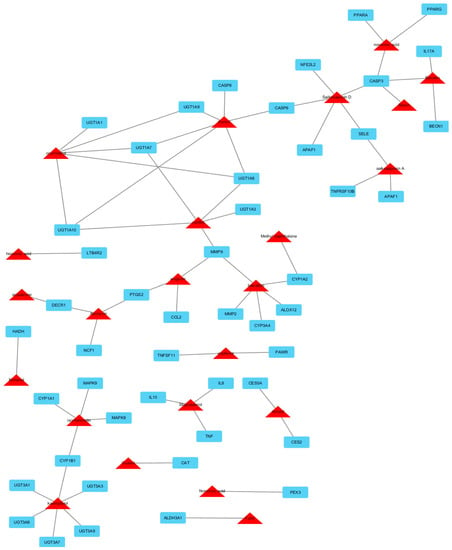
Figure 1.
Compound-target hepatotoxicity network of Xiao chai hu tang. This network represents a global view of the potential compounds (red triangles) and targets (blue rectangles) in XCHT, and it comprised 74 nodes (23 potential compounds and 51 potential targets) and 131 edges (compound-target interactions).

Table 1.
The information of 51 potential HILI targets in Xiao chai hu tang.
Caspase 3 (CASP3), which had the largest number of compound interactions in our network pharmacology study, was indicated as the potential key target of XCHT-induced liver injury. Previous studies have indicated that CASP3 played an important role in liver diseases [19,20]. The hepatocyte apoptosis induced by TNF-α is correlated with the activation of CASP3 [21,22]. Both in vitro and in vivo studies confirmed that pancreatitis-associated ascitic fluid can induce hepatocyte apoptosis and liver injury by activating CASP3 dependent pro-apoptotic pathways [23]. In addition, lower expression of CASP3 can aggravate hepatic steatosis in NAFLD rat model by inducing hepatocyte apoptosis [24]. Thus, we predicted that CASP3 might be a potential target for inducing liver injury by XCHT. Saikosaponin D, baicalin, nonenoic acid and mairin from Radix bupleuri and Radix scutellariae were expected as the possible liver injury ingredients in XCHT by targeting CASP3.
Uridine 5′-diphospho-glucuronosyltransferase (UGT) is a crucial enzyme family in liver for detoxification and removal of exogenous compounds (e.g., dietary substances, drugs, toxins) and endogenous substances (e.g., bilirubin, steroid hormones, and bile acids) [25,26,27]. In our compound-target network, UGT also plays a pivotal role in XCHT-induced liver injury. Literature review showed that suppression of UGTs such as UGT1A10, UGT1A7, UGT1A8 by various endogenous or exogenous factors not only reduced the ability of certain drugs’ glucuronidation and thus triggered clinical adverse drug-drug interaction (clinical HILI), but also resulted in acute liver injury by metabolism dysfunction of endogenous substances [28,29,30].
Matrix metalloproteinase 9 (MMP9) also showed multiple target-compound interactions in our network study. Our in silico study showed MMP9 might play an important role in XCHT-induced liver injury by inducing interstitial fibrosis in liver. Previous studies showed that MMP9 could be prognostic markers for liver fibrosis [31]. MMP9 could modulate inflammation and extracellular matrix (ECM) remodeling in liver. In vitro studies showed that MMP9 activity was critical for TGFβ2-induced matrix contraction, and could promote hepatic fibrosis [32]. Down-regulation of MMP9 expression and the TGF-β1/Smad signaling pathways could relieve liver fibrosis in vivo [33]. Further mechanism studies demonstrated that during HILI process, Interleukin-1 produced by Kupffer cells or liver myofibroblast cells might play a pivotal role in remodeling of hepatic fibrosis through an either p38-dependent or p38-independent pathway to modulate the expression of MMP9 [34]. The above literature review studies can partly support our network pharmacology analysis results of XCHT.
2.2. Potential HILI Mechanisms of Heshouwu Predicted by Network Pharmacological Analysis
We totally identified 73 major chemicals in Heshouwu. The sixteen ingredients with satisfactory pharmacokinetic properties of Heshouwu were predicted through an in silico-based pharmacologic method (Table 2). The compounds and proteins interaction analysis results showed that a total of 114 intracellular targets had potential interactions with the 16 ingredients of Heshouwu. Among them, 39 are regarded as potential HILI targets. To further predict and illuminate the relationship between these potential liver injury compounds and HILI target genes, a drug-target network was constructed (Figure 2). This network represents the interactions of the potential compounds (red triangles) and targets (blue rectangles) in Heshouwu, which comprised 43 nodes (13 candidate compounds and 30 potential targets) and 62 edges (compound-target interactions). Our network analysis results showed that various candidate compounds in Heshouwu were linked to multiple targets, which may exhibit multiple liver injury effects. Among the 13 candidate compounds, rhein exhibits the largest number of liver injury targets connections (degree = 7), followed by emodin (degree = 6) and aloe-emodin (degree = 5). For the 30 potential liver injury targets, the network showed that PPARG had the largest number of compound-target interactions (chrysazin, emodin anthrone, rhein and butanedioic acid), followed by CASP3 (aloe-emodin, β-sitosterol and luteolin), and MCL1 (emodin, rhein and quercetin). The remaining 27 targets showed interactions with only one or two compounds. The information of the 30 potential liver injury targets in Heshouwu is shown in Table 3, where all the data were manually collected and integrated from the STITCH, TTD, PharmGKB and CTD databases.

Table 2.
Compounds with satisfactory pharmacokinetic properties of Heshouwu.

Figure 2.
Compound-target network of Heshouwu that are associated with HILI. The red triangles are active compounds from Heshouwu and the blue rectangles represent potential hepatotoxic target genes, the grey lines represent the compound-target interaction. This network comprises 43 nodes (13 candidate compounds and 30 potential targets) and 62 edges (compound-target interactions).

Table 3.
The information of 39 potential HILI targets in Heshouwu.
Interestingly, like XCHT, Heshouwu might also induce liver injury by targeting CASP3 according to our network analysis. In addition, peroxisome proliferator-activated receptor gamma (PPARG) and myeloid cell leukemia-1 (MCL1) are other two potential targets for Heshouwu-induced liver injury. PPARG, also known as the glitazone receptor, could regulate fatty acid storage and glucose metabolism. Inefficient fatty acid oxidation in mitochondria and increased oxidative damage are features of NAFLD. In rodent models and patients with NAFLD, hepatic expression of PPARG coactivator 1α (PPARGC1A) is inversely correlated with liver fat and disease severity. In mice, loss of estrogen signaling contributes to oxidative damage caused by low levels of PPARGC1A in liver, exacerbating steatohepatitis associated with high fructose and fat diets [35]. Furthermore, a clinical study also found that genetic variation in PPARG was associated with NAFLD, and the minor alleles haplotype was associated with inflammatory and fibrotic changes that denoted histologically advanced NAFLD [36]. MCL1, belonging to the Bcl-2 family, can enhance cell survival via inhibiting apoptosis [37]. Previous studies found that suppressing MCL1 could activate the mitochondrial apoptotic pathway in hepatocytes and induce tumorigenesis [38]. In addition, knockout of MCL1 can increase hepatic apoptosis in mice deregulating the expression of MCL1 may contribute to HILI [39]. The above statements partly support our network pharmacology analysis results of Heshouwu.
3. Materials and Methods
The protocol of the integrated network pharmacology approach includes four main steps as follows (Figure 3).
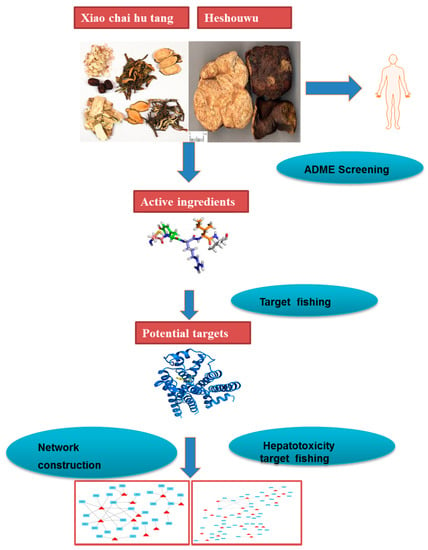
Figure 3.
Network pharmacology approach workflow in this study.
3.1. Molecular Database Construction
All of the known ingredients of XCHT or Heshouwu were manually collected from related literature and two phytochemical databases: Traditional Chinese Medicine Systems Pharmacology Database (TCMSP, http://ibts.hkbu.edu.hk/LSP/tcmsp.php) and TCM Database@Taiwan (http://tcm.cmu.edu.tw/). The ingredients for these herbal medicines were retrieved from the above databases by using the following search terms: Heshouwu, Radix Polygoni Multiflori, xiao chai hu tang, Sho-saiko-to.
3.2. Pharmacokinetic ADME Evaluation
In this step, an in silico integrative model~ADME was used to select the ingredients with favorable pharmacokinetics properties. The ADME system used in this study including predict oral bioavailability (PreOB) and predict Caco-2 permeability (PreCaco-2). Oral bioavailability (OB) is one of the most vital pharmacokinetic properties of orally administered drugs as it plays an important role for the efficiency of the drug delivery to the systemic circulation [40,41] Here, a reliable in silico screening model (OBioavail 1.1) was employed in OB value calculation of the constituents in XCHT and Heshouwu. This model was constructed based on 805 structurally diverse drugs and drug-like molecules. Multiple linear regression, partial least square and support vector machine methods were applied during this model building, ending up with determination coefficient (R2) = 0.80 and standard error of estimate (SEE) = 0.31 for test sets [42,43]. In addition, for orally administered drugs, another pivotal problem is their movement across the intestinal epithelial barrier, which determines the rate and extent of human absorption and ultimately affects its bioavailability [44]. Thus, a preCaco-2 model was used to predict the drug absorption. The phytochemical information of the compounds with their Caco-2 permeability properties were explored using the TCMSP database, the detailed parameters’ information, screening criteria and calculation can be obtained from TCMSP website (http://ibts.hkbu.edu.hk/LSP/tcmsp.php). Finally, compounds with OB ≥ 33% and Caco2 ≥ 0.4 cm/s were regarded as active ingredients for further study. It is worth noting that the OB values of saikosaponin d and saikosaponin A are lower than 33%, but both of them are widely expected to induce liver injury in vitro and in vivo, thus, these two additional compounds were also regarded as candidate compounds [45].
3.3. Identification Targets for Potential HILI
In order to build the compound-target interaction profiles in XCHT and Heshouwu, we analyzed the chemical compounds and protein interactions integrated from the Search Tool for Interactions of Chemicals and Proteins (STITCH) 5.0 database (http://stitch.embl.de/) and Herbal Ingredients’ Targets (HIT) Database (http://lifecenter.sgst.cn/hit/), which are both databases of known and predicted interactions between chemicals and proteins. These databases were based on text mining and molecular docking technology to predict compound-protein interaction, which has been applied in the research of TCM for discovering potential active ingredients and interpreting molecular mechanisms of herbal medicines [46,47]. The STITCH 5.0 database can be used to study potential interactions between 300,000 phytochemicals and 2.6 million proteins curated from 1133 organisms. In this database, the approximate probability of a predicted association for a chemical–protein interaction is determined by the confidence score, with a higher score indicating a stronger interaction (low confidence score ~0.2; medium confidence score ~0.5; high confidence score ~0.75; highest confidence score ~0.95, provided by STITCH 5.0 database). The HIT database is a publicly available research resource that includes more than 116,000 interactions between 9300 chemicals and 13,300 genes. Both databases were searched independently by two researchers to minimize any bias. Then, to better define the HILI of XCHT or Heshouwu, Therapeutic Target Database (TTD, http://bidd.nus.edu.sg/group/ttd/), PharmGKB (http://www.pharmgkb.org) and Comparative Toxicogenomics Database (CTD, http://ctdbase.org/) [48,49,50] were employed to eliminate the noise and exclude irrelevant targets obtained from above steps. The targets for HILI were retrieved from above database by using the following search terms: Liver injury, hepatic damage, hepatic toxicity, hepatic drug metabolism, fatty liver, steatosis, lipid metabolism, liver fibrosis, oxidative damage, oxidative stress, liver inflammation, hepatocyte apoptosis, hepatocellular necrosis and hepatogenic jaundice. Only the targets of Homo sapiens were kept for further analysis.
3.4. Network Construction and Analzysis
To further explore the relationships between the compounds and targets associated with hepatotoxic effects, the Compound–Target network plotting was generated by Cytoscape 3.4.0 (http://www.cytoscape.org/) [51]. In the graphical network plot, nodes represent the compounds or proteins, and edges encode the compound–target interactions. In order to specify the importance of a node and how this node influences the communication between two nodes, all the properties of the network were analyzed by Network Analysis plugin.
4. Conclusions
Chinese herbal medicines are multi-component synergistic systems, which might play both therapeutic and toxic roles in humans. The HILI and quality control of herbal formulas or single herbs for public consumption are one of increasing concerns these days due to the lack of scientific evidence on their safety. In this paper, two drug-target networks of HILI of XCHT and Heshouwu were constructed through network pharmacology assays. The network predicted the potential underlying HILI mechanism of XCHT and Heshouwu and elucidated the interrelationship between hepatic toxicity and Chinese herbal medicine interventions. Therefore, network pharmacology is a useful tool to reveal the potential mechanism of HILI of Chinese herbal medicines and potential toxic ingredients. These data in our study, if combined with further in vitro and in vivo studies, should facilitate construction of a potential herb-induced liver injury network and improve the specificity of toxicity prediction for future drug candidates. Notwithstanding the advances in recent network pharmacology research, there are some crucial technical issues to be addressed and improved for data collection of herb-induced liver injury. First of all, the inventory of herbal products remains incomplete and new chemical structures are continuously being discovered. Secondly, previous researchers have explored and identified only a small part of the hepatotoxic target genes. Last but not least, stringently assessing the relationships between compounds and corresponding targets and obtaining accurate action modes such as activated drug-target interactions or inhibited drug-target interactions are still a challenge for present network pharmacology study. Despite these technical issues, for the multiple components-multiple targets interaction model of herbal medicines, conventional experimental research faces a situation of long-term investment to investigate the complex interaction mechanisms. Thus, our network pharmacology study which integrates the systems biology and in-silico technologies may offer a direction for the mechanism study of HILI. The high degree nodes, which may play hub roles in the compound-target network, will provide potential key object for next experimental study. Our results not only provide new insights for a deeper understanding of the molecular basis of potential HILI but also demonstrate a promising method for assessing the drug’s safety from herbal medicine. As further steps, herbal compound libraries should be established and further enriched to better correlate compound functions with structures. Experimental verification is also urgently needed to validate the accurate interactions between the predicted active ingredients and target proteins.
Supplementary Materials
Supplementary materials are available online. Table S1: Compounds with satisfactory pharmacokinetic properties of XCHT.
Acknowledgments
The study was financially supported by grants from the research council of the University of Hong Kong (Project Codes: 104003422 and 104004092), the Research Grants Committee (RGC) of Hong Kong, HKSAR (Project Code: 106160190), Wong’s Donation on Modern Oncology of Chinese Medicine (Project Code: 200006276), Gala Family Trust (Project Code: 200007008) and Government-Matching Grant Scheme (Project Code: 207060411).
Author Contributions
Y.F. designed the study; M.H. and N.W. retrieved and analyzed the data; M.H., S.L., H.Y.T., F.C., J.H. and N.W. wrote and revised the manuscript; Y.F. revised and finalized the manuscript. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare Jno conflict of interest.
References
- Shehu, A.I.; Ma, X.; Venkataramanan, R. Mechanisms of drug-induced hepatotoxicity. Clin. Liver. Dis. 2017, 21, 35–54. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.H.; Lin, C.C.; Lai, H.C.; Chiang, J.H.; Lin, J.G.; Li, T.C. Adjunctive traditional Chinese medicine therapy improves survival of liver cancer patients. Liver Int. 2015, 35, 2595–2602. [Google Scholar] [CrossRef] [PubMed]
- Amagaya, S.; Hayakawa, M.; Ogihara, Y.; Ohta, Y.; Fujiwara, K.; Oka, H.; Oshio, H.; Kishi, T. Treatment of chronic liver injury in mice by oral administration of xiao-chai-hu-tang. J. Ethnopharmacol. 1989, 25, 181–187. [Google Scholar] [PubMed]
- Tajiri, H.; Kozaiwa, K.; Ozaki, Y.; Miki, K.; Shimuzu, K.; Okada, S. Effect of sho-saiko-to(xiao-chai-hu-tang) on HBeAg clearance in children with chronic hepatitis B virus infection and with sustained liver disease. Am. J. Chin. Med. 1991, 19, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Kurtz, R.C.; Vickers, A.; Lau, N.; Yeung, K.S.; Shia, J.; Cassileth, B. A single arm phase II study of a Far-Eastern traditional herbal formulation (sho-sai-ko-to or xiao-chai-hu-tang) in chronic hepatitis C patients. J. Ethnopharmacol. 2011, 136, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Itoh, S.; Marutani, K.; Nishijima, T.; Matsuo, S.; Itabashi, M. Liver injuries induced by herbal medicine, syo-saiko-to (xiao-chai-hu-tang). Dig. Dis. Sci. 1995, 40, 1845–1848. [Google Scholar] [CrossRef] [PubMed]
- Hsu, L.M.; Huang, Y.S.; Tsay, S.H.; Chang, F.Y.; Lee, S.D. Acute hepatitis induced by Chinese hepatoprotective herb, xiao-chai-hu-tang. J. Chin. Med. Assoc. 2006, 69, 86–88. [Google Scholar] [CrossRef]
- Liu, Z.; Xiong, M.; Zhang, H. Experimental study on inhibitory effect of xiaochaihu decoction on duck hepatitis B virus. Zhongguo Zhong Xi Yi Jie He Za Zhi 2000, 20, 853–855. [Google Scholar] [PubMed]
- Sun, Y.N.; Li, W.; Song, S.B.; Yan, X.T.; Yang, S.Y.; Kim, Y.H. Nuclear factor Kappa B activation and peroxisome proliferator-activated receptor transactivational effects of chemical components of the roots of Polygonum multiflorum. Pharmacogn. Mag. 2016, 12, 31–35. [Google Scholar] [PubMed]
- Lin, L.; Ni, B.; Lin, H.; Zhang, M.; Li, X.; Yin, X.; Qu, C.; Ni, J. Traditional usages, botany, phytochemistry, pharmacology and toxicology of Polygonum multiflorum Thunb.: A review. J. Ethnopharmacol. 2015, 159, 158–183. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.A.; Min, H.J.; Yoo, S.S.; Kim, H.J.; Choi, S.N.; Ha, C.Y.; Kim, H.J.; Kim, T.H.; Jung, W.T.; Lee, O.J.; et al. Drug-induced liver injury: Twenty five cases of acute hepatitis following ingestion of Polygonum multiflorum Thunb. Gut. Liver 2011, 5, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Chen, J.; Ren, J.; Li, Y.; Zhai, J.; Mu, W.; Zhang, L.; Zheng, W.; Tian, G.; Shang, H. Liver damage associated with Polygonum multiflorum Thunb.: A systematic review of case reports and case series. Evid. Based Complement. Alternat. Med. 2015, 2015, 459749. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, X.; Liu, Y.; Pan, D.; Wang, Y.; Yang, N.; Xiang, L.; Cai, X.; Feng, Y. Hepatoprotection and hepatotoxicity of Heshouwu, a Chinese medicinal herb: Context of the paradoxical effect. Food Chem. Toxicol. 2016. [Google Scholar]
- Wu, X.; Chen, X.; Huang, Q.; Fang, D.; Li, G.; Zhang, G. Toxicity of raw and processed roots of Polygonum multiflorum. Fitoterapia 2012, 83, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Huang, Y.Y.; Duh, P.D.; Wu, S.C. Hepatoprotection of emodin and Polygonum multiflorum against CCl4-induced liver injury. Pharm. Biol. 2012, 50, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Ke, Z.P.; Zhang, X.Z.; Ding, Y.; Cao, L.; Li, N.; Ding, G.; Wang, Z.Z.; Xiao, W. Study on effective substance basis and molecular mechanism of Qigui Tongfeng tablet using network pharmacology method. Zhongguo Zhong Yao Za Zhi 2015, 40, 2837–2842. [Google Scholar] [PubMed]
- James, L.P.; Wilson, J.T.; Simar, R.; Farrar, H.C.; Kearns, G.L.; Simpson, P.M.; Hinson, J.A.; Pumford, N.R.; Pediatric Pharmacology Research Unit Network. Evaluation of occult acetaminophen hepatotoxicity in hospitalized children receiving acetaminophen. Clin. Pediatr. 2001, 40, 243–248. [Google Scholar]
- Danhof, M. Systems pharmacology-Towards the modeling of network interactions. Eur. J. Pharm. Sci. 2016, 94, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Linton, S.D.; Aja, T.; Armstrong, R.A.; Bai, X.; Chen, L.S.; Chen, N.; Ching, B.; Contreras, P.; Diaz, J.L.; Fisher, C.D.; et al. First-in-class pan caspase inhibitor developed for the treatment of liver disease. J. Med. Chem. 2005, 48, 6779–6782. [Google Scholar] [CrossRef] [PubMed]
- Schrieber, S.J.; Wen, Z.; Vourvahis, M.; Smith, P.C.; Fried, M.W.; Kashuba, A.D.; Hawke, R.L. The pharmacokinetics of silymarin is altered in patients with hepatitis C virus and nonalcoholic Fatty liver disease and correlates with plasma caspase-3/7 activity. Drug Metab. Dispos. 2008, 36, 1909–1916. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.M.; Feng, G.H.; Huang, F.; Li, Y.; Zhao, G.Z. Tumor necrosis factor-α, caspase-3 expression and hepatocyte apoptosis in fulminanting hepatic failure. Zhonghua Nei Ke Za Zhi 2003, 42, 566–570. [Google Scholar] [PubMed]
- Liang, B.; Guo, X.L.; Jin, J.; Ma, Y.C.; Feng, Z.Q. Glycyrrhizic acid inhibits apoptosis and fibrosis in carbon-tetrachloride-induced rat liver injury. World J. Gastroenterol. 2015, 21, 5271–5280. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Fier, A.; Carter, Y.; Liu, G.; Epling-Burnette, P.K.; Bai, F.; Loughran, T.P.; Mastorides, S., Jr.; Norman, J.G.; Murr, M.M. Liver injury during acute pancreatitis: The role of pancreatitis-associated ascitic fluid (PAAF), p38-MAPK, and caspase-3 in inducing hepatocyte apoptosis. J. Gastrointest. Surg. 2003, 7, 200–207. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, J.; Zheng, Q.; Dong, J.; Jiang, J. Effects of fenofibrate on hepatocyte apoptosis in nonalcoholic fatty liver. Zhonghua Gan Zang Bing Za Zhi 2015, 23, 688–693. [Google Scholar] [PubMed]
- Oda, S.; Shirai, Y.; Akai, S.; Nakajima, A.; Tsuneyama, K.; Yokoi, T. Toxicological role of an acyl glucuronide metabolite in diclofenac-induced acute liver injury in mice. J. Appl. Toxicol. 2016, 37, 545–553. [Google Scholar] [CrossRef] [PubMed]
- De Morais, S.M.; Wells, P.G. Deficiency in bilirubin UDP-glucuronyl transferase as a genetic determinant of acetaminophen toxicity. J. Pharmacol. Exp. Ther. 1988, 247, 323–331. [Google Scholar] [PubMed]
- Li, J.; He, C.; Fang, L.; Yang, L.; Wang, Z. Identification of human UDP-glucuronosyltransferase 1A4 as the major isozyme responsible for the glucuronidation of 20(S)-protopanaxadiol in human liver microsomes. Int. J. Mol. Sci. 2016, 17, 205. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Wang, X.X.; Hou, J.; Fang, Z.Z.; Wu, J.J.; Cao, Y.F.; Liu, S.W.; Ge, G.B.; Yang, L. Comparison of the inhibitory effects of tolcapone and entacapone against human UDP-glucuronosyltransferases. Toxicol. Appl. Pharmacol. 2016, 301, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, R.; Sumida, K.; Kutsuno, Y.; Sakamoto, M.; Itoh, T. UDP-glucuronosyltransferase (UGT) 1A1 mainly contributes to the glucuronidation of trovafloxacin. Drug Metab. Pharmacokinet. 2015, 30, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Kessler, F.K.; Kessler, M.R.; Auyeung, D.J.; Ritter, J.K. Glucuronidation of acetaminophen catalyzed by multiple rat phenol UDP-glucuronosyltransferases. Drug Metab. Dispos. 2002, 30, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Latif, M.S. Plasma levels of Matrix Metalloproteinase (MMP)-2, MMP-9 and tumor necrosis factor-α in chronic hepatitis C virus patients. Open Microbiol. J. 2015, 9, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Eldred, J.A.; Hodgkinson, L.M.; Dawes, L.J.; Reddan, J.R.; Edwards, D.R.; Wormstone, I.M. MMP2 activity is critical for TGFβ2-induced matrix contraction--implications for fibrosis. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4085–4098. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Li, X.; Feng, Q.; Chen, L.; Xu, L.; Hu, Y. Anti-fibrotic effect of Cordyceps sinensis polysaccharide: Inhibiting HSC activation, TGF-β1/Smad signalling, MMPs and TIMPs. Exp. Biol. Med. 2013, 238, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Miau, L.H.; Chen, C.H.; Chiou, L.L.; Huang, G.T.; Yang, P.M.; Sheu, J.C. Differential role of p38 in IL-1α induction of MMP-9 and MMP-13 in an established liver myofibroblast cell line. J. Biomed. Sci. 2003, 10, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Besse-Patin, A.; Leveille, M.; Oropeza, D.; Nguyen, B.N.; Prat, A.; Estall, J.L. Estrogen signals through peroxisome proliferator-activated receptor-γ coactivator 1α to reduce oxidative damage associated with diet-induced fatty liver disease. Gastroenterology 2016, 152, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Gawrieh, S.; Marion, M.C.; Komorowski, R.; Wallace, J.; Charlton, M.; Kissebah, A.; Langefeld, C.D.; Olivier, M. Genetic variation in the peroxisome proliferator activated receptor-γ gene is associated with histologically advanced NAFLD. Dig. Dis. Sci. 2012, 57, 952–957. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Zhang, S.; Guo, W.; Cao, S.; Yan, B.; Lu, Y.; Li, J. Cobalt protoporphyrin protects the liver against apoptosis in rats of brain death. Clin. Res. Hepatol. Gastroenterol. 2015, 39, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Hikita, H.; Kodama, T.; Tanaka, S.; Saito, Y.; Nozaki, Y.; Nakabori, T.; Shimizu, S.; Hayashi, Y.; Li, W.; Shigekawa, M.; et al. Activation of the Mitochondrial Apoptotic Pathway Produces Reactive Oxygen Species and Oxidative Damage in Hepatocytes That Contribute to Liver Tumorigenesis. Cancer Prev. Res. 2015, 8, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Vick, B.; Weber, A.; Urbanik, T.; Maass, T.; Teufel, A.; Krammer, P.H.; Opferman, J.T.; Schuchmann, M.; Galle, P.R.; Schulze-Bergkamen, H. Knockout of myeloid cell leukemia-1 induces liver damage and increases apoptosis susceptibility of murine hepatocytes. Hepatology 2009, 49, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Li, Y.; Wang, J.; Zhang, J.; Hou, T. ADME evaluation in drug discovery. 9. Prediction of oral bioavailability in humans based on molecular properties and structural fingerprints. Mol. Pharm. 2011, 8, 841–851. [Google Scholar] [PubMed]
- Saghir, S.A. Determination of ADME and bioavailability following intravenous, oral, and dermal routes of exposure. Curr. Protoc. Toxicol. 2009. [Google Scholar] [CrossRef]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y.; et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, X.; Li, Y.; Li, X.; Tao, W.; Li, B.; Wang, Y.; Yang, L. Systems pharmacology uncovers Janus functions of botanical drugs: Activation of host defense system and inhibition of influenza virus replication. Integr. Biol. 2013, 5, 351–371. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.; Araujo, F.; Barrias, C.C.; Granja, P.L.; Sarmento, B. Dissecting stromal-epithelial interactions in a 3D in vitro cellularized intestinal model for permeability studies. Biomaterials 2015, 56, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.K.; Singh, A.; Swati, D. A knowledge-based approach for identification of drugs against vivapain-2 protein of Plasmodium vivax through pharmacophore-based virtual screening with comparative modelling. Appl. Biochem. Biotechnol. 2014, 173, 2174–2188. [Google Scholar] [CrossRef] [PubMed]
- May, B.H.; Deng, S.; Zhang, A.L.; Lu, C.; Xue, C.C. In silico database screening of potential targets and pathways of compounds contained in plants used for psoriasis vulgaris. Arch. Dermatol. Res. 2015, 307, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Calvert, S.; Tacutu, R.; Sharifi, S.; Teixeira, R.; Ghosh, P.; de Magalhaes, J.P. A network pharmacology approach reveals new candidate caloric restriction mimetics in C. elegans. Aging Cell 2016, 15, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, L.; Liu, W.; Jin, Y.; Chen, Q.; Wang, L.; Fan, X.; Li, Z.; Cheng, Y. A network pharmacology study of Chinese medicine QiShenYiQi to reveal its underlying multi-compound, multi-target, multi-pathway mode of action. PLoS ONE 2014, 9, e95004. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Duan, J.A.; Bai, G.; Su, S.L. Network pharmacology study on major active compounds of siwu decoction analogous formulae for treating primary dysmenorrhea of gynecology blood stasis syndrome. Zhongguo Zhong Yao Za Zhi 2014, 39, 113–120. [Google Scholar] [PubMed]
- Lv, Y.N.; Li, S.X.; Zhai, K.F.; Kou, J.P.; Yu, B.Y. Network pharmacology-based prediction and verification of the molecular targets and pathways for schisandrin against cerebrovascular disease. Chin. J. Nat. Med. 2014, 12, 251–258. [Google Scholar] [CrossRef]
- Yu, G.; Zhang, Y.; Ren, W.; Dong, L.; Li, J.; Geng, Y.; Zhang, Y.; Li, D.; Xu, H.; Yang, H. Network pharmacology-based identification of key pharmacological pathways of Yin-Huang-Qing-Fei capsule acting on chronic bronchitis. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 85–94. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).