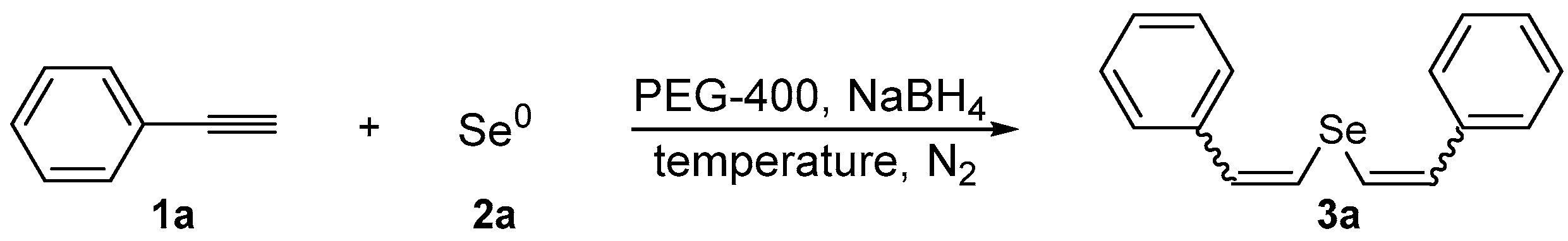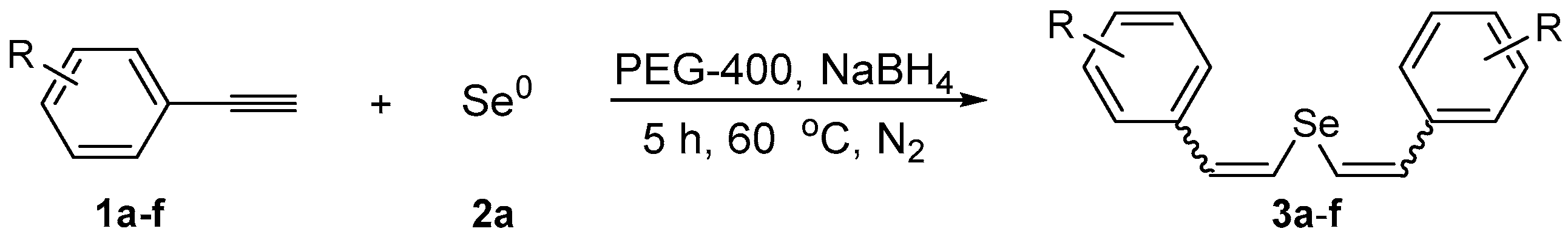Abstract
A simple and efficient protocol to prepare divinyl selenides has been developed by the regio- and stereoselective addition of sodium selenide species to aryl alkynes. The nucleophilic species was generates in situ, from the reaction of elemental selenium with NaBH4, utilizing PEG-400 as the solvent. Several divinyl selenides were obtained in moderate to excellent yields with selectivity for the (Z,Z)-isomer by a one-step procedure that was carried out at 60 °C in short reaction times. The methodology was extended to tellurium, giving the desired divinyl tellurides in good yields. Furthermore, the Fe-catalyzed cross-coupling reaction of bis(3,5-dimethoxystyryl) selenide 3f with (4-methoxyphenyl)magnesium bromide 5 afforded resveratrol trimethyl ether 6 in 57% yield.
1. Introduction
Chalcogen alkenes are attractive key intermediates in the synthesis of important compounds. They are useful synthons to access conjugated or isolated olefins with total control in the geometry of the double bond [1,2,3,4,5]. For instance, the iron- or nickel-catalyzed cross-coupling of divinyl chalcogenides with Grignard reagents [6], the direct coupling with terminal alkynes in the presence of a nickel/CuI catalyst [7], or similar transformations [8] are useful procedures to access functionalized alkenes. The synthesis of novel selenium heterocycles based on the reaction of selenium dichloride with divinyl sulfide [9,10,11] or divinyl selenide [12] was also reported.
Divinyl selenides however, are less studied compared to synthetic intermediates, probably due to the limited number of general methods to prepare them in a selective way. They can be prepared by a stereoconservative three-step reaction of in situ generated vinyl selenide anions with (E)- or (Z)-bromo styrenes (Scheme 1A) [13]. Divinyl selenides of (E,E)-configuration were predominantly formed by the Wittig–Horner reaction of chalcogen diphosphonate derivatives with aldehydes [14,15,16] (Scheme 1B). Both approaches are very robust, despite the low atom efficiency and a high E factor [17]. A more atom-economic approach of preparing divinyl selenides and tellurides involves the electrophilic addition of chalcogen halides, such as selenium dichloride or dibromide and tellurium tetrachloride, to alkynes (Scheme 1C). In these reactions, however, halo-substituted products are obtained and a reduction step is necessary to prepare the halogen-free divinyl chalcogenides [18,19,20,21,22]. Z,Z-bis(acylvinyl) selenides were exclusively obtained in moderate yields by the reaction of sodium selenide with ethynyl ketones derived from aromatic ketones. Only four examples were prepared and when bromoethynyl ketones were used, 1,3-diselenetanes were obtained instead of the respective divinyl selenides [23].
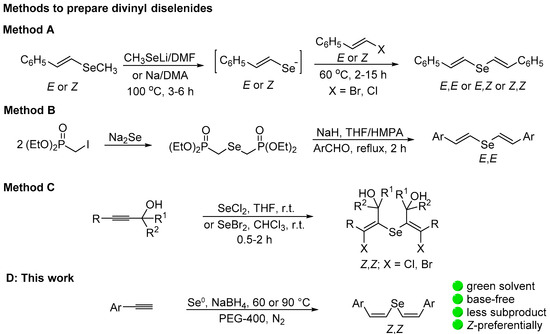
Scheme 1.
Previous general methods to prepare divinyl selenides and the present work.
(Z,Z)-Divinyl tellurides were regio- and stereoselectively prepared by the hydrochalcogenation of alkynes via the anti-addition of Na2Te, generated in situ from Te0/NaBH4/EtOH in the presence of aqueous NaOH [24,25,26]. Alternatively, K2Te was used as nucleophile in the reaction with acetylene. The telluride anion was generated by heating a mixture of elemental tellurium and KOH in HMPA/H2O as the solvent at 110–120 °C under pressure [27]. These methods, however, are restricted to divinyl tellurides and require heating for a long time in the presence of a strong base (refluxing for up to 48 h), which is a problem with sensible substrates.
In recent years, new synthetic methodologies have been developed aiming to increase the efficiency and yields under mild reaction conditions [28,29]. In this regard, polyethylene glycol (PEG) has been extensively used as a thermally stable, nontoxic, cheap, and non-volatile solvent in the synthesis of organochalcogen compounds [30]. Some of us have recently described the in situ generation of chalcogenolate anions using the system RYYR/NaBH4/PEG-400 (Y = S, Se, Te) and their use in selective Michael additions, the hydrochalcogenation of alkynes [31,32,33], and the ring-opening of non-activated aziridines [34].
On the other hand, resveratrol (3,4′,5-trihydroxystilbene) is a stilbene-type polyphenolic product that is present in grapes, peanuts, mulberries, and particularly in red wine [35]. Resveratrol and methoxylated analogues are well known due to their multiple biological activities, including powerful antioxidant properties, which are associated with anti-inflammatory [36], anticancer [37], antibacterial [38] and neuroprotective effects [39]. However, these compounds are obtained only in small amounts by the extraction of plants; therefore, the development of general and simple synthetic strategies for the preparation of stilbenoids has become an important task [40,41,42,43].
As mentioned above, despite the high versatility of divinyl selenides in organic synthesis, the methods of preparing them in a selective way are limited and atom-intensive. Thus, the need for efficient and selective methods to access this class of compounds is imperative. Aiming to contribute to fill this gap, we present here our full results on the hydroselenation of aryl alkynes using the system Se0/NaBH4/PEG-400 to prepare (Z,Z)-divinyl selenides (Scheme 1D). The possibility of exploring the use of divinyl selenide in the synthesis of resveratrol trimethyl ether was also investigated.
2. Results and Discussion
In our preliminary experiments, we chose phenylacetylene 1a and selenium powder 2a as standard starting materials to perform the optimization studies under a N2 atmosphere and using PEG-400 as the solvent (Table 1). We evaluated the influence of temperature and the reaction time. Firstly, a mixture of selenium 2a (0.5 mmol) and NaBH4 (0.8 mmol) in PEG-400 (3.0 mL) was stirred at room temperature to generate in situ the nucleophilic selenium species. The selenium reduction was accompanied by a change in the color of the reaction mixture, from grey to colorless (30 min of stirring). After this time, phenylacetylene 1a (1.0 mmol) was added in the reaction vessel and the desired product 3a was obtained in 12% yield after 12 h at room temperature (Table 1, Entry 1). By increasing the temperature to 40 °C, the reaction proceeds smoothly and the desired product 3a was obtained in 42% yield in 12 h (Table 1, Entry 2). The temperature had a positive influence in the reaction of the sodium selenide with phenyl acetylene, and a similar 40% yield of 3a was obtained in 2 h at 60 °C (Table 1, Entry 3). Next, we investigated the reaction time and observed that 5 h gave the best result, with the desired divinyl selenide 3a being isolated as a mixture of (Z,Z)- and (Z,E)-isomers (Z,Z:Z,E ratio of 80:20) in 92% yield (Table 1, Entries 4 and 5). An additional test was performed, setting a time of 5 h and increasing the temperature to 80 °C; however, the yield was lower than that under milder conditions (Table 1, Entry 6). Thus, analyzing the results showed in Table 1, we established the best reaction conditions as being the previous reaction of selenium 2a (0.5 mmol) and NaBH4 (0.8 mmol) in PEG-400 (3.0 mL) at 60 °C under N2 for 30 min. Afterward, phenylacetylene 1a (1.0 mmol) is added dropwise and the resulting mixture is stirred for additional 5 h at the same temperature.

Table 1.
Optimization of the synthesis of divinyl selenide 3a a.
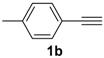
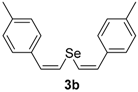
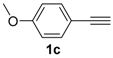
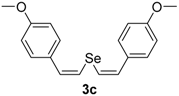
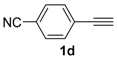
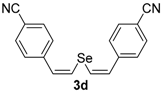
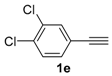
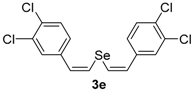
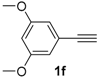
In order to demonstrate the efficiency of this protocol, we attempted to apply the designed method to other aryl alkynes and the results are depicted in Table 2. A closer inspection of Table 2 reveals that our protocol worked well for a range of substrates, delivering products 3a–f in good to excellent yields and high selectivity to the (Z,Z)-isomer. The reactions are sensitive to the electronic effect of the aromatic ring in the alkynes. The presence of strong electron-releasing group CH3O- in acetylenes 1c and 1f reduced the reactivity and a higher temperature (90 °C) was necessary to give good yields of the respective divinyl selenides 3c,f (Table 2, Entries 3 and 6). This deactivating effect was less pronounced in 4-tolyl acetylene 1b, which reacted under the standard conditions to afford the desired divinyl selenide 3b after 5 h in 82% yield. In this example, a small amount of the (E,E)-isomer was detected in addition to (Z,Z)- and (Z,E)-ones (Table 2, Entry 2). The reaction is less affected by the presence of electron-withdrawing groups. 3,4-Dichlorophenyl acetylene 1e afforded the respective (Z,Z)-divinyl selenide 3e in 93% yield as the only isomer (Table 2, Entry 5), while 4-ethynylbenzonitrile 1d produced 3d in 74% yield as a mixture of (Z,Z)-, (Z,E)- and (E,E)-isomers in a 53:43:4 ratio (Table 2, Entry 4). In additional studies, the performance of the reaction using 1,1-dimethylpropargyl alcohol 1g and hept-1-yne 1h was studied; however, there was no formation of the expected products and the starting materials were recovered (Table 2, Entries 7 and 8).

Table 2.
Synthesis of divinyl selenides 3 by the hydroselenation of aryl alkynes 1 a.
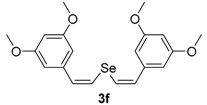
Afterward, we examined the possibility of using our protocol to prepare the divinyl tellurides analogues 4. Firstly, we performed the reaction tellurium powder (0.5 mmol) with NaBH4 (0.8 mmol) in PEG-400 (3.0 mL) at 60 °C under N2 atmosphere until consumption of the tellurium (30 min). Then, phenylacetylene 1a (1.0 mmol) was added and the mixture was stirred under N2 at the same temperature for an additional 5 h, affording the desired divinyl telluride 4a in 40% yield (Z,Z-isomer, only). Interested in the excellent selectivity and aiming to improve the yield, the reaction was repeated in the presence of NaOH (1.1 equiv), similarly described by Tucci and co-workers in a closely related reaction [24], affording product 4a in 82% yield after 5 h at 60 °C. By using the same strategy, bis(3,5-dimethoxystyryl) telluride 4b was obtained in 83% yield from 3,5-dimethoxyphenylacetylene 1f after 5 h at 90 °C (Scheme 2).
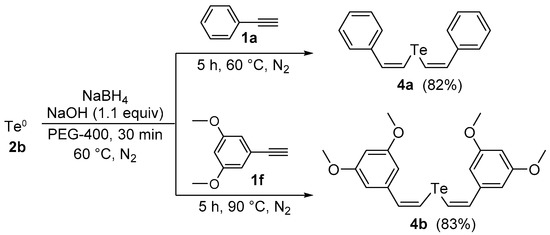
Scheme 2.
Synthesis of divinyl tellurides by hydrotelluration of aryl alkynes.
In order to demonstrate the synthetic versatility of divinyl selenides, the Fe-catalyzed cross-coupling of bis(3,5-dimethoxystyryl) selenide 3f with (4-methoxyphenyl)magnesium bromide 5 was investigated for the synthesis of resveratrol trimethyl ether 6. Following the synthesis and purification of 3f, it was reacted with Grignard’s reagent 5 in the presence of catalytic Fe(acac)3 at room temperature to afford, after 4 h, the expected resveratrol trimethyl ether 6 in 57% yield (Scheme 3). The reaction was stereoconservative, with compound 6 being obtained as a Z/E mixture (Z:E ratio of 73:27). The sequence shown in Scheme 3 is a straightforward way to polymethoxylated stilbenes, which are themselves biologically active and pharmaceutically relevant precursors (Scheme 3).
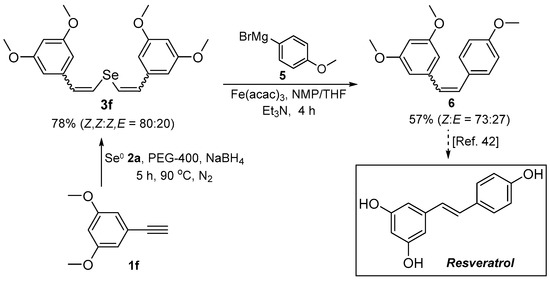
Scheme 3.
Synthesis of resveratrol trimethyl ether 6.
3. Materials and Methods
3.1. General Information
The reactions were monitored by thin layer chromatography (TLC), which were performed using Merck (Merck, Darmstadt, Germany) silica gel (60 F254), with a 0.25 mm thickness. For visualization, TLC plates were either exposed to UV light, or stained with iodine vapor or in a 5% vanillin solution in 10% aqueous H2SO4 and heat. Column chromatography was performed using Merck Silica Gel (230–400 mesh). High-resolution mass spectra (HRMS) were recorded in positive ion mode (ESI) using a Bruker microQTOF spectrometer (Bruker, Billerica, MA, USA). Low-resolution mass spectra were obtained with a Shimadzu GC-MS-QP2010 mass spectrometer (Shimadzu Corporation, Kyoto, Japan). NMR spectra were recorded with Bruker DPX (Bruker). (1H-NMR = 400 and 500 MHz; 13C-NMR = 100 and 126 MHz) instruments using CDCl3 as solvent and calibrated using tetramethylsilane (TMS) as internal standard. Coupling constants (J) were reported in Hertz and chemical shifts (δ) in ppm. The NMR spectra are found in the Supplementary Materials. The reagents (substituted alkynes, sodium tetrahydroborate, elemental chalcogen) and PEG-400 were purchased from Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO, USA).
3.2. General Procedure for the Preparation of Divinyl Selenides 3a–f
In a two necked round bottomed flask under nitrogen atmosphere, sodium selenide was generated by reaction of elemental selenium (Se0, 0.5 mmol) with sodium tetrahydroborate (0.8 mmol, 0.030 g) in PEG-400 (3.0 mL) at 60 °C. After 30 min, a colorless solution was obtained and the corresponding alkyne was added (1.0 mmol). After stirring for 5 h at 60 or 90 °C, the reaction mixture was quenched with water (10.0 mL) and extracted with ethyl acetate (3 × 15.0 mL). The combined organic layers were dried over MgSO4, filtered and concentrated under vacuum. The residue was purified by column chromatography over silica gel eluting with hexanes to yield the products as an inseparable mixture of isomers. All compounds were properly characterized by MS, 1H-NMR, and 13C-NMR, and the isomers ratios were determined by GC and NMR.
Bis-(Z,Z)-styryl selenide 3a: Yield: 0.132 g (92%) [13]; Orange solid; 1H-NMR (CDCl3, 500 MHz) δ (ppm): 7.20–7.38 (m, 10H), 6.92 (d, J = 10.4 Hz, 2H), 6.64 (d, J = 10.4 Hz, 2H). MS: m/z (rel. int.) 286 (M+, 22.2), 205 (100), 102 (36.7), 91 (42.8), 77 (50.4). Z,E-3a: [13] 1H-NMR (CDCl3, 500 MHz) δ (ppm): 7.14 (d, J = 10.4 Hz, 1H), 7.08 (d, J = 15.4 Hz, 1H), 6.85 (d, J = 15.4 Hz, 1H), 6.75 (d, J = 10.4 Hz, 1H), other peaks were overlapped with those of Z,Z-3a. MS: m/z (rel. int.) 286 (M+, 25.0), 205 (100), 102 (35.3), 91 (41.4), 77 (44.1). 13C-NMR (125 MHz, CDCl3) δ (ppm): (Z,Z + Z,E) 137.0, 136.7, 134.7, 134.1, 130.5, 130.2, 128.61, 128.57, 128.4, 128.2, 127.9, 127.6, 127.3, 126.3, 125.9, 123.4, 121.3, 119.5. HRMS (ESI): m/z calcd. for C16H14Se [M + H]+: 287.0339, found: 287.0328.
Bis-(Z,Z)-4-methylstyryl selenide 3b: Yield: 0.129 g (82%); Orange solid; 1H-NMR (CDCl3, 400 MHz) δ (ppm): 7.10–7.50 (m, 8H), 6.91 (d, J = 10.4 Hz, 2H), 6.60 (d, J = 10.4 Hz, 2H), 2.34 (s, 6H). MS: m/z (rel. int.) 314 (M+, 38.8), 219 (100), 204 (25.9), 102 (20.4), 91 (32.4), 77 (6.6). Z,E-3b: 1H-NMR (CDCl3, 400 MHz) δ (ppm): 6.96 (d, J = 10.3 Hz, 1H), 6.86 (d, J = 15.6 Hz, 1H), 6.76 (d, J = 15.6 Hz, 1H), 6.69 (d, J = 10.3 Hz, 1H), other peaks were overlapped with those of E,E and Z,Z-3b. MS: m/z (rel. int.) 314 (M+, 32.6), 219 (100), 204 (23.7), 102 (18.6), 91 (28.0), 77 (6.2). E,E-3b: [15] 1H-NMR (CDCl3, 400 MHz) δ (ppm): 7.04 (d, J = 15.8 Hz, 2H), 6.84 (d, J = 15.8 Hz, 2H), other peaks were overlapped with those of Z,E and Z,Z-3b. MS: m/z (rel. int.) 314 (M+, 39.3), 219 (100), 204 (25.0), 102 (17.4), 91 (29.4), 77 (7.0). 13C-NMR (CDCl3, 100 MHz) δ (ppm): (Z,Z + Z,E + E,E) 138.0, 137.5, 137.13, 137.11, 134.31, 134.29, 134.25, 130.4, 130.1, 129.34, 129.3, 129.1, 128.22, 128.17, 127.2, 126.5, 126.3, 126.1, 125.91, 125.87, 122.5, 120.5, 118.2, 116.6, 21.3, 21.2. HRMS (ESI): m/z calcd. for C18H18Se [M + H]+: 315.0652, found: 315.0648.
Bis-(Z,Z)-4-methoxystyryl selenide 3c: Yield: 0.133 g (77%); Orange solid; 1H-NMR (CDCl3, 500 MHz) δ (ppm): 6.72–7.34 (m, 10H), 6.52 (d, J = 9.9 Hz, 2H), 3.80 (s, 6H). MS: m/z (rel. int.) 346 (M+, 13.9), 266 (100), 235 (36.7), 77 (10.2). Z,E-3c: 1H-NMR (CDCl3, 500 MHz) δ (ppm): 6.61 (d, J = 10.2 Hz, 1H), 3.78 (s, 6H), other peaks were overlapped with those of Z,Z-3c. MS: m/z (rel. int.) 346 (M+, 14.8), 266 (100), 235 (35.6), 77 (10.7). 13C-NMR (CDCl3, 100 MHz) δ (ppm): (Z,Z + Z,E) 159.2, 158.7, 134.6, 134.2, 129.9, 129.8, 129.7, 129.63, 129.6, 127.2, 127.1, 121.0, 119.2, 116.5, 114.2, 114.0, 113.8, 113.7, 55.2. HRMS (ESI): m/z calcd. for C18H18O2Se [M + H]+: 347.0550, found: 347.0545.
Bis-(Z,Z)-4-cyanostyryl selenide 3d: Yield: 0.124 g (74%); Orange solid; 1H-NMR (DMSO-d6, 400 MHz) δ (ppm): 6.68–7.03 (m, 8H), 6.47 (d, J = 10.5 Hz, 2H), 6.30 (d, J = 10.5 Hz, 2H). Z,E-3d: 1H-NMR (DMSO-d6, 400 MHz) δ (ppm): 6.57 (d, J = 10.5 Hz, 1H), 6.52 (d, J = 15.1 Hz, 1H), 6.45 (d, J = 15.1 Hz, 1H), 6.19 (d, J = 10.5 Hz, 1H), other peaks were overlapped with those of E,E and Z,E-3d. E,E-3d: 1H-NMR (DMSO-d6, 400 MHz) δ (ppm): 6.08 (d, J = 15.6 Hz, 2H), other peaks were overlapped with those of Z,Z and Z,E-3d. 13C-NMR (DMSO-d6, 100 MHz) δ (ppm): (Z,Z + Z,E + E,E) 141.1, 141.0, 140.6, 132.8, 132.4, 132.33, 132.26, 130.7, 130.5, 128.9, 128.8, 128.7, 128.4, 127.2, 127.1, 126.8, 126.42, 126.36, 125.7, 125.5, 118.5, 118.4, 110.2, 109.9, 109.6, 109.4, 109.38. MS: m/z (rel. int.) 336 (M+, 19.0), 207 (100), 191 (13.8), 127 (40.4), 102 (9.0), 77 (11.6). HRMS (ESI): m/z calcd. for C18H12N2Se [M]+: 336.0166, found: 336.0150.
Bis-(Z,Z)-3,4-dichlorostyryl selenide 3e: Yield: 0.196 g (93%). Yellowish solid; m.p.: 152–155 °C. 1H-NMR (CDCl3, 400 MHz) δ (ppm): 7.43–7.45 (m, 4H), 7.21 (dd, J = 8.2 and 2.1 Hz, 2H), 6.86 (d, J = 10.4 Hz, 2H), 6.73 (d, J = 10.4 Hz, 2H). 13C-NMR (CDCl3, 100 MHz) δ (ppm): δ 136.8, 132.7, 131.4, 130.5, 130.1, 128.6, 127.2, 124.9. MS: m/z (rel. int.) 422 (M+, 6.2), 207 (83.8), 40 (100). HRMS (ESI): m/z calcd. for C16H10Cl4Se [M + H]+: 422.8780, found: 422.8758.
Bis-(Z,Z)-3,5-dimethoxystyryl selenide 3f: Yield: 0.158 g (78%); Yellowish solid; 1H-NMR (CDCl3, 500 MHz) δ (ppm): 6.88 (d, J = 10.3 Hz, 2H), 6.67 (d, J = 10.3 Hz, 2H), 6.54 (d, J = 2.3 Hz, 4H), 6.38 (t, J = 2.3 Hz, 2H), 3.79 (s, 12H). 13C-NMR (CDCl3, 125 MHz) δ (ppm): 160.7, 138.8, 130.2, 124.0, 106.1, 100.0, 55.3. Z,E-3f: 1H-NMR (CDCl3, 500 MHz) δ (ppm): 7.11 (d, J = 15.7 Hz, 1H), 6.93 (d, J = 10.3 Hz, 1H), 6.79 (d, J = 15.7 Hz, 1H), 6.77 (d, J = 10.3 Hz, 1H), 6.48 (d, J = 2.3 Hz, 4H), 6.34–6.35 (m, 2H), 3.81 (s, 12H). MS: m/z (rel. int.) 406 (M+, 2.8), 325 (100), 207 (45.9), 77 (15.0). HRMS (ESI): m/z calcd. for C20H22O4Se [M + H]+: 407.0762, found: 407.0740.
3.3. General Procedure for the Preparation of Divinyl Tellurides 4a,b
In a two-necked round bottomed flask under nitrogen atmosphere, sodium telluride was generated by reaction of elemental tellurium (Te0, 0.5 mmol) with sodium tetrahydroborate (0.8 mmol, 0.030 g) and NaOH (0.55 mmol, 0.022 g) in PEG-400 (3.0 mL) at 60 °C. After 30 min, a violet solution was obtained and the corresponding alkyne was added (1.0 mmol). After stirring for 5 h at 60 or 90 °C, the reaction mixture was quenched with water (10.0 mL) and extracted with ethyl acetate (3 × 15.0 mL). The combined organic layers were dried over MgSO4, filtered, and concentrated under vacuum. The residue was purified by column chromatography over silica gel eluting with hexanes to yield the products. All compounds were properly characterized by MS, 1H-NMR, and 13C-NMR.
Bis-(Z,Z)-styryl telluride 4a: Yield: 0.138 g (82%); White solid; m.p.: 44–46 °C (Lit. [26]: 46–47 °C). 1H-NMR (CDCl3, 400 MHz) δ (ppm): 7.43 (d, J = 10.6 Hz, 2H), 7.33–7.36 (m, 4H), 7.22–7.24 (m, 6H), 6.99 (d, J = 10.6 Hz, 2H). 13C-NMR (CDCl3, 100 MHz) δ (ppm): 138.8, 137.3, 128.4, 127.6, 127.4, 108.9. MS: m/z (rel. int.) 336 (M+, 17.2), 206 (100), 91 (71.4), 77(67.1).
Bis-(Z,Z)-3,5-dimethoxystyryl telluride 4b: Yield: 0.189 g (83%); Orange solid; m.p.: 76–77 °C. 1H-NMR (DMSO-d6, 400 MHz) δ (ppm): 7.45 (d, J = 10.5 Hz, 2H), 7.23 (d, J = 10.5 Hz, 2H), 6.42–6.47 (m, 6H), 3.76 (s, 12H). 13C-NMR (DMSO-d6, 100 MHz) δ (ppm): 160.6, 140.5, 136.9, 110.1, 105.0, 99.6, 55.2. MS: m/z (rel. int.) 456 (M+, 13.7), 325 (100), 175 (34.0). HRMS (ESI): m/z calcd. for C20H22O4Te [M + H]+: 457.0659, found: 457.0662.
3.4. Preparation of 3,4’,5-Trimethoxystilbene 6
To a two-necked 100 mL round bottomed flask under nitrogen, the dry solvent (NMP/THF 1:3, 20.0 mL), bis(3,5-dimethoxystyryl) selenide 3f (0.406 g, 1 mmol), Et3N (2.5 mL) and Fe(acac)3 (71 mg, 20 mol %) was added. The mixture was stirred at room temperature, and after 15 min a solution of the Grignard reagent from 1-bromo-4-methoxybenzene (5, 10 mmol; 10.0 mL of a 1 mol/L sol. in THF) was added dropwise. The reactions were monitored by TLC until total disappearance of the starting material 3f. After completion of the reaction, aqueous sat. NH4Cl (15.0 mL) was added and the reaction mixture was extracted with ethyl acetate (3 × 20.0 mL), the organic layer was washed with water and dried over MgSO4. After filtration, the solvent was removed under reduced pressure and the residue was purified by column chromatography on silica gel (ethyl acetate/hexanes, 10:90).
3,4’,5-Trimethoxystilbene 6: Yield: 0.155 g (57%) [42]; Whitish oil; Z-6: 1H-NMR (CDCl3, 400 MHz) δ (ppm): 7.11 (d, J = 8.7 Hz, 2H), 6.66 (d, J = 8.7 Hz, 2H), 6.42 (d, J = 12.0 Hz, 1H), 6.343 (d, J = 2.3 Hz, 2H), 6.337 (d, J = 12.0 Hz, 1H), 6.22 (t, J = 2.3 Hz, 1H), 3.66 (s, 3H), 3.56 (s, 6H). MS: m/z (rel. int.) 270 (M+, 100), 239 (23.6), 224 (15.6), 196 (7.9), 181 (4.9), 152 (7.8), 141 (4.7), 115 (6.0), 102 (1.2) 76 (2.9). E-6: 1H-NMR (CDCl3, 400 MHz) δ (ppm): 7.33 (d, J = 8.8 Hz, 2H), 6.94 (d, J = 16.2 Hz, 1H), 6.80 (d, J = 16.2 Hz, 1H), 6.78 (d, J = 8.8 Hz, 2H), 6.55 (d, J = 2.3 Hz, 2H), 6.28 (t, J = 2.3 Hz, 1H), 3.71 (s, 6H), 3.70 (s, 3H). MS: m/z (rel. int.) 270 (M+, 100), 239 (21.3), 224 (10.8), 196 (6.5), 181 (4.1), 152 (6.0), 141 (3.5), 115 (4.2), 102 (0.9) 76 (2.4). 13C-NMR (CDCl3, 100 MHz) δ (ppm): (Z + E) 160.9, 160.5, 159.3, 158.7, 139.6, 139.4, 130.2, 130.1, 129.8, 129.5, 128.65, 128.60, 127.7, 126.5, 114.1, 113.4, 106.5, 104.3, 99.8, 99.5, 55.22, 55.18, 55.1.
4. Conclusions
An efficient methodology to prepare divinyl selenides from elemental selenium and arylalkynes was developed. The nucleophilic species of selenium was generated in situ from elemental selenium and NaBH4/PEG-400. The reactions proceeded at a gentle heating of 60 °C for only 5 h, affording the corresponding divinyl selenides in good to excellent yields with high selectivity to the Z,Z-isomer. The same strategy was used to prepare (Z,Z)-divinyl tellurides analogues from elemental tellurium, but good results were obtained only when NaOH was present in the reaction media. Divinyl selenides with the appropriate substitution pattern was used in a Fe-catalyzed cross-coupling reaction with a Grignard reagent to afford 3,4’,5-trimethoxystilbene in moderate yield. Thus, this procedure could be used to access valuable polymethoxylated stilbenes.
Supplementary Materials
NMR spectra of synthesized compounds are available online.
Acknowledgments
The authors thank FAPERGS, CNPq, and CAPES for financial support. CNPq is also acknowledged for the fellowship for G.P., R.G.J., and E.J.L. This manuscript is part of the scientific activity of the international multidisciplinary “SeS Redox and Catalysis” network.
Author Contributions
G.P., R.G.J., and E.J.L. conceived and designed the experiments; A.M.B., E.Q.L., and E.L.B. performed the experiments; A.M.B., E.Q.L., G.P., E.J.L., L.S., and C.S. analyzed the data; G.P., E.J.L., and C.S. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zeni, G.; Lüdtke, D.S.; Panatieri, R.B.; Braga, A.L. Vinylic Tellurides: From Preparation to Their Applicability in Organic Synthesis. Chem. Rev. 2006, 106, 1032–1076. [Google Scholar] [CrossRef] [PubMed]
- Perin, G.; Lenardão, E.J.; Jacob, R.G.; Panatieri, R.B. Synthesis of Vinyl Selenides. Chem Rev. 2009, 109, 1277–1301. [Google Scholar] [CrossRef] [PubMed]
- Menezes, P.H.; Zeni, G. Vinyl Selenides in Patai’s Chemistry of Functional Groups; John Wiley & Sons: New York, NY, USA, 2011. [Google Scholar]
- Alonso, F.; Beletskaya, I.P.; Yus, M. Transition-Metal-Catalyzed Addition of Heteroatom-Hydrogen Bonds to Alkynes. Chem. Rev. 2004, 104, 3079–3159. [Google Scholar] [CrossRef] [PubMed]
- Freudendahl, D.M.; Shahzad, S.A.; Wirth, T. Recent Advances in Organoselenium Chemistry. Eur. J. Org. Chem. 2009, 2009, 1649–1664. [Google Scholar] [CrossRef]
- Silveira, C.C.; Mendes, S.R.; Wolf, L. Iron-Catalyzed Coupling Reactions of Vinylic Chalcogenides with Grignard Reagents. J. Braz. Chem. Soc. 2010, 21, 2138–2145. [Google Scholar] [CrossRef]
- Silveira, C.C.; Braga, A.L.; Vieira, A.S.; Zeni, G. Stereoselective Synthesis of Enynes by Nickel-Catalyzed Cross-Coupling of Divinylic Chalcogenides with Alkynes. J. Org. Chem. 2003, 68, 662–665. [Google Scholar] [CrossRef] [PubMed]
- Uemura, S.; Takahashi, H.; Ohe, K. Styryl coupling vs. styryl acetate formation in reactions of styryl tellurides with palladium(II) salts. J. Organomet. Chem. 1992, 423, C9–Cl2. [Google Scholar] [CrossRef]
- Amosova, S.V.; Penzik, M.V.; Albanov, A.I.; Potapov, V.A. The reaction of selenium dichloride with divinyl sulfide. J. Organomet. Chem. 2009, 694, 3369–3372. [Google Scholar] [CrossRef]
- Amosova, S.V.; Penzik, M.V.; Albanov, A.I.; Potapov, V.A. Addition of selenium dibromide to divinyl sulfide: Spontaneous rearrangement of 2,6-dibromo-1,4-thiaselenane to 5-bromo-2-bromomethyl-1,3-thiaselenolane. Tetrahedron Lett. 2009, 50, 306–308. [Google Scholar] [CrossRef]
- Potapov, V.A.; Kurkutov, E.O.; Musalov, M.V.; Amosova, S.V. Reactions of selenium dichloride and dibromide with divinyl sulfone: Synthesis of novel four- and five-membered selenium heterocycles. Tetrahedron Lett. 2010, 51, 5258–5261. [Google Scholar] [CrossRef]
- Potapov, V.A.; Amosova, S.V.; Volkova, K.A.; Penzik, M.V.; Albanov, A.I. Reactions of selenium dichloride and dibromide with divinyl selenide: Synthesis of novel selenium heterocycles and rearrangement of 2,6-dihalo-1,4-diselenanes. Tetrahedron Lett. 2010, 51, 89–92. [Google Scholar] [CrossRef]
- Testaferri, L.; Tiecco, M.; Tingoli, U.; Chianelli, D. Stereospecific synthesis of divinyl selenides nucleophilic substitutions of unactivated vinyl halides by vinyl selenide anions. Tetrahedron 1986, 42, 63–69. [Google Scholar] [CrossRef]
- Comasseto, J.V.; Petragnani, N. The reaction of selenophosphonates with carbonyl compounds. Vinylic selenides. J. Organomet. Chem. 1978, 152, 295–304. [Google Scholar] [CrossRef]
- Silveira, C.C.; Santos, P.C.S.; Braga, A.L. Preparation and nickel-catalyzed coupling reactions of divinylic selenides. Tetrahedron Lett. 2002, 43, 7517–7520. [Google Scholar] [CrossRef]
- Silveira, C.C.; Rinaldi, F.; Guadagnin, R.C. Preparation and Reactivity of Chalcogenyl Phosphonates and Phosphane Oxides. Eur. J. Org. Chem. 2007, 2007, 4935–4939. [Google Scholar] [CrossRef]
- Sheldon, R.A. The E Factor: Fifteen years on. Green Chem. 2007, 9, 1273–1283. [Google Scholar] [CrossRef]
- Braverman, S.; Cherkinsky, M.; Jana, R.; Kalendar, Y.; Sprecher, M. Reaction of selenium and tellurium halides with propargyl alcohols. The regio- and stereoselectivity of addition to the triple bond. J. Phys. Org. Chem. 2010, 23, 1114–1120. [Google Scholar] [CrossRef]
- Potapov, V.A.; Khuriganova, O.I.; Musalov, M.V.; Larina, L.I.; Amosova, S.V. Stereospecific Synthesis of E,E-Bis(2-chlorovinyl)selenide. Russ. J. Gen. Chem. 2010, 80, 541–542. [Google Scholar] [CrossRef]
- Potapov, V.A.; Musalov, M.V.; Khuriganova, O.I.; Larina, L.I.; Amosova, S.V. Reactions of Stereoselective Addition of Selenium Dibromide and Monobromide to Acetylene. Russ. J. Org. Chem. 2010, 46, 753–754. [Google Scholar] [CrossRef]
- Braverman, S.; Pechenick-Azizi, T.; Gottlieb, H.E.; Sprecher, M. Synthesis and Reactivity of Divinylselenium Dichlorides and Dibromides. Synthesis 2011, 2011, 577–584. [Google Scholar] [CrossRef]
- Musalova, M.V.; Potapov, V.A.; Amosova, S.V. Synthesis of Novel E-2-Chlorovinyltellurium Compounds Based on the Stereospecific Anti-addition of Tellurium Tetrachloride to Acetylene. Molecules 2012, 17, 5770–5779. [Google Scholar] [CrossRef] [PubMed]
- Potapov, V.A.; Elokhina, V.N.; Larina, L.I.; Yaroshenko, T.I.; Tatarinova, A.A.; Amosova, S.V. Reactions of sodium selenide with ethynyl and bromoethynyl ketones: Stereo- and regioselective synthesis of functionalized divinyl selenides and 1,3-diselenetanes. J. Organomet. Chem. 2009, 694, 3679–3682. [Google Scholar] [CrossRef]
- Tucci, F.C.; Chieffi, A.; Comasseto, J.V. Tellurium in Organic Synthesis. Preparation of Z-Vinylic Cuprates from Z-Vinylic Tellurides and Their Reaction with Enones and Epoxides. J. Org. Chem. 1996, 61, 4975–4989. [Google Scholar] [CrossRef]
- Barros, S.M.; Comasseto, J.V.; Berriel, J. Vinyllithiums from butyl-vinyl tellurides and bis-vinyl tellurides. Tetrahedron Lett. 1989, 30, 7353–7356. [Google Scholar] [CrossRef]
- Barros, S.M.; Dabdoub, M.J.; Dabdoub, V.M.B.; Comasseto, J.V. Hydrotelluration of Acetylenes: Synthesis of Vinylic Tellurides, Divinyl Tellurides, and l-(Organyltelluro)-l,3-butadienes. Organometallics 1989, 8, 1661–1665. [Google Scholar] [CrossRef]
- Trofimov, B.A.; Amosova, S.V.; Gurasova, N.K.; Musorin, G.K. Reactions of triads Se8-KOH-DMSO, Se8-KOH-DMSO, Te-KOH-HMPA with acetylenes. Tetrahedron 1982, 38, 713–718. [Google Scholar] [CrossRef]
- Kerton, F.M.; Marriott, R. RSC Green Chemistry Book Series—Alternative Solvents for Green Chemistry, 2nd ed.; RSC Publishing: Cambridge, UK, 2013. [Google Scholar]
- Reichardt, C.; Welton, T. Solvents and Solvent Effects in Organic Chemistry, 4th ed.; WILEY-VCH: Weinheim, Germany, 2011. [Google Scholar]
- Perin, G.; Alves, D.; Jacob, R.G.; Barcellos, A.M.; Soares, L.K.; Lenardão, E.J. Synthesis of Organochalcogen Compounds using Non-Conventional Reaction Media. Chem. Sel. 2016, 2, 205–258. [Google Scholar] [CrossRef]
- Perin, G.; Borges, E.L.; Alves, D. Highly stereoselective method to prepare bis-phenylchalcogen alkenes via addition of chalcogenolate to phenylseleno alkynes. Tetrahedron Lett. 2012, 53, 2066–2069. [Google Scholar] [CrossRef]
- Perin, G.; Borges, E.L.; Rosa, P.C.; Carvalho, P.N.; Lenardão, E.J. Simple cleavage of diorganyl diselenides with NaBH4/PEG-400 and direct Michael addition to electron-deficient alkenes. Tetrahedron Lett. 2013, 54, 1718–1721. [Google Scholar] [CrossRef]
- Perin, G.; Borges, E.L.; Peglow, T.J.; Lenardão, E.J. Direct Michael addition to electron-deficient alkenes using diorganyl dichalcogenides (Te/S) and NaBH4/PEG-400. Tetrahedron Lett. 2014, 55, 5652–5655. [Google Scholar] [CrossRef]
- Silva, P.C.; Borges, E.L.; Lima, D.B.; Jacob, R.G.; Lenardão, E.J.; Perin, G.; Silva, M.S. A simple and non-conventional method for the synthesis of selected β-arylalkylchalcogeno substituted alcohols, amines and carboxylic acids. ARKIVOC 2016, V, 376–389. [Google Scholar]
- Neves, A.R.; Lúcio, M.; Lima, J.L.C.; Reis, S. Resveratrol in Medicinal Chemistry: A Critical Review of its Pharmacokinetics, Drug-Delivery, and Membrane Interactions. Curr. Med. Chem. 2012, 19, 1663–1681. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.-L.; Chong, I.-W.; Lee, Y.-C.; Tsai, J.-R.; Wang, H.-M.; Hsieh, C.-C.; Kuo, H.-F.; Liu, W.-L.; Chen, Y.-H.; Chen, H.-L. Anti-inflammatory Effects of Resveratrol on Hypoxia/Reoxygenation-Induced Alveolar Epithelial Cell Dysfunction. J. Agric. Food Chem. 2015, 63, 9480–9487. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.; Jiang, W.; Dong, H.-M.; Qiu, S.-X.; Lu, Y. Synthesis and cytotoxic activities of E-resveratrol derivatives. Chin. J. Nat. Med. 2015, 13, 375–382. [Google Scholar] [CrossRef]
- Park, S.; Cha, S.-H.; Cho, I.; Park, S.; Park, Y.; Cho, S.; Park, Y. Antibacterial nanocarriers of resveratrol with gold and silver nanoparticles. Mater. Sci. Eng. C 2016, 58, 1160–1169. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.-H.; Lee, H.; Lee, S.-R. Protective effect of resveratrol against neuronal damage following transient global cerebral ischemia in mice. J. Nutr. Biochem. 2016, 27, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Guiso, M.; Marra, C.; Farina, A. A new efficient resveratrol synthesis. Tetrahedron Lett. 2002, 43, 597–598. [Google Scholar] [CrossRef]
- Botella, L.; Nájera, C. Synthesis of methylated resveratrol and analogues by Heck reactions in organic and aqueous solvents. Tetrahedron 2004, 60, 5563–5570. [Google Scholar] [CrossRef]
- Lara-Ochoa, F.; Sandoval-Minero, L.C.; Espinosa-Pérez, G. A new synthesis of resveratrol. Tetrahedron Lett. 2015, 56, 5977–5979. [Google Scholar] [CrossRef]
- Brown, J.W.; Jarenwattananon, N.N.; Otto, T.; Wang, J.L.; Glöggler, S.; Bouchard, L.-S. Heterogeneous Heck coupling in multivariate metal–organic frameworks for enhanced selectivity. Catal. Commun. 2015, 65, 105–107. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 3, 4 and 6 are available from the authors.
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).