Quorum Sensing Inhibition and Structure–Activity Relationships of β-Keto Esters
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental
3.1. Test Compounds
3.2. Disc Diffusion Bioassay
3.3. Dose–Response Bioassay
3.4. Competitive Antagonism Bioassay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| QS | Quorum sensing |
| AHL | N-acylated homoserine lactone |
| HSL | Homoserine lactone |
| MIC | Minimum inhibitory concentration |
| GFP | green fluorescent protein |
| OHHL | 3-oxo-hexanoyl-HSL |
| YP | yeast peptone media |
References
- Cabello, F.C. Heavy use of prophylactic antibiotics in aquaculture: A growing problem for human and animal health and for the environment. Environ. Microbiol. 2006, 8, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Ginocchio, F.; Mikulska, M. New treatment options against Gram-negative organisms. Crit. Care 2011, 15, 215. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.B.; Bassler, B.L. Quorum sensing in bacteria. Annu. Rev. Microbiol. 2001, 55, 165–199. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, N.A.; Barnard, A.M.; Slater, H.; Simpson, N.J.; Salmond, G.P. Quorum-sensing in Gram-negative bacteria. FEMS Microbiol. Rev. 2001, 25, 365–404. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.S.; Iglewski, B.H.P. Aeruginosa quorum-sensing systems and virulence. Curr. Opin. Microbiol. 2003, 6, 56–60. [Google Scholar] [CrossRef]
- Gerdt, J.P.; Blackwell, H.E. Competition studies confirm two major barriers that can preclude the spread of resistance to quorum-sensing inhibitors in bacteria. ACS Chem. Biol. 2014, 9, 2291–2299. [Google Scholar] [CrossRef] [PubMed]
- Fuqua, C.; Winans, S.C.; Greenberg, E.P. Census and consensus in bacterial ecosystems: The LuxR-LuxI family of quorum-sensing transcriptional regulators. Annu. Rev. Microbiol. 1996, 50, 727–751. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, T.B.; Givskov, M. Quorum-sensing inhibitors as anti-pathogenic drugs. Int. J. Med. Microbiol. 2006, 296, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Geske, G.D.; O’Neill, J.C.; Blackwell, H.E. Expanding dialogues: from natural autoinducers to non-natural analogues that modulate quorum sensing in Gram-negative bacteria. Chem. Soc. Rev. 2008, 37, 1432–1447. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.G.; Meighen, E.A. Purification and structural identification of an autoinducer for the luminescence system of Vibrio harveyi. J. Biol. Chem. 1989, 264, 21670–21676. [Google Scholar] [PubMed]
- Chhabra, S.R.; Stead, P.; Bainton, N.J.; Salmond, G.P.; Stewart, G.S.; Williams, P.; Bycroft, B.W. Autoregulation of carbapenem biosynthesis in Erwinia carotovora by analogues of N-(3-oxohexanoyl)-l-homoserine lactone. J. Antibiot. (Tokyo) 1993, 46, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Murphy, P.J.; Kerr, A.; Tate, M.E. Agrobacterium conjugation and gene regulation by N-acyl-l-homoserine lactones. Nature 1993, 362, 446–448. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, H.; Liang, W.; Hay, A.J.; Zhong, Z.; Kan, B.; Zhu, J. Quorum sensing regulatory cascades control Vibrio fluvialis pathogenesis. J. Bacteriol. 2013, 195, 3583–3589. [Google Scholar] [CrossRef] [PubMed]
- Pearson, J.P.; Gray, K.M.; Passador, L.; Tucker, K.D.; Eberhard, A.; Iglewski, B.H.; Greenberg, E.P. Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc. Natl. Acad. Sci. USA 1994, 91, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Wang, L.-H.; Wang, J.; Dong, Y.-H.; Hu, J.Y.; Zhang, L.-H. Quorum quenching enzyme activity is widely conserved in the sera of mammalian species. FEBS Lett. 2005, 579, 3713–3717. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.M.; Bu, Y.; Suga, H. Library screening for synthetic agonists and antagonists of a Pseudomonas aeruginosa autoinducer. Chem. Biol. 2003, 10, 563–571. [Google Scholar] [CrossRef]
- Marsden, D.M.; Nicholson, R.L.; Skindersoe, M.E.; Galloway, W.R.J.D.; Sore, H.F.; Givskov, M.; Salmond, G.P.C.; Ladlow, M.; Welch, M.; Spring, D.R. Discovery of a quorum sensing modulator pharmacophore by 3D small-molecule microarray screening. Org. Biomol. Chem. 2010, 8, 5313–5323. [Google Scholar] [CrossRef] [PubMed]
- Boukraa, M.; Sabbah, M.; Soulère, L.; El Efrit, M.L.; Queneau, Y.; Doutheau, A. AHL-dependent quorum sensing inhibition: Synthesis and biological evaluation of α-(N-alkyl-carboxamide)-γ-butyrolactones and α-(N-alkyl-sulfonamide)-γ-butyrolactones. Bioorg. Med. Chem. Lett. 2011, 21, 6876–6879. [Google Scholar] [CrossRef] [PubMed]
- Frezza, M.; Soulère, L.; Reverchon, S.; Guiliani, N.; Jerez, C.; Queneau, Y.; Doutheau, A. Synthetic homoserine lactone-derived sulfonylureas as inhibitors of Vibrio fischeri quorum sensing regulator. Bioorg. Med. Chem. 2008, 16, 3550–3556. [Google Scholar] [CrossRef] [PubMed]
- Frezza, M.; Castang, S.; Estephane, J.; Soulère, L.; Deshayes, C.; Chantegrel, B.; Nasser, W.; Queneau, Y.; Reverchon, S.; Doutheau, A. Synthesis and biological evaluation of homoserine lactone derived ureas as antagonists of bacterial quorum sensing. Bioorg. Med. Chem. 2006, 14, 4781–4791. [Google Scholar] [CrossRef] [PubMed]
- Geske, G.D.; O’Neill, J.C.; Miller, D.M.; Wezeman, R.J.; Mattmann, M.E.; Lin, Q.; Blackwell, H.E. Comparative analyses of N-acylated homoserine lactones reveal unique structural features that dictate their ability to activate or inhibit quorum sensing. ChemBioChem 2008, 9, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Geske, G.D.; Mattmann, M.E.; Blackwell, H.E. Evaluation of a focused library of N-aryl l-homoserine lactones reveals a new set of potent quorum sensing modulators. Bioorg. Med. Chem. Lett. 2008, 18, 5978–5981. [Google Scholar] [CrossRef] [PubMed]
- Reverchon, S.; Chantegrel, B.; Deshayes, C.; Doutheau, A.; Cotte-Pattat, N. New synthetic analogues of N-acyl homoserine lactones as agonists or antagonists of transcriptional regulators involved in bacterial quorum sensing. Bioorg. Med. Chem. Lett. 2002, 12, 1153–1157. [Google Scholar] [CrossRef]
- Yang, Q.; Scheie, A.A.; Benneche, T.; Defoirdt, T. Specific quorum sensing-disrupting activity (A QSI) of thiophenones and their therapeutic potential. Sci. Rep. 2015, 5, 18033. [Google Scholar]
- Teasdale, M.E.; Liu, J.; Wallace, J.; Akhlaghi, F.; Rowley, D.C. Secondary metabolites produced by the marine bacterium Halobacillus salinus that inhibit quorum sensing-controlled phenotypes in gram-negative bacteria. Appl. Environ. Microbiol. 2009, 75, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.-L.; Bassler, B.L. Bacterial quorum-sensing network architectures. Annu. Rev. Genet. 2009, 43, 197–222. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.B.; Heydorn, A.; Hentzer, M.; Eberl, L.; Geisenberger, O.; Christensen, B.B.; Molin, S.; Givskov, M. gfp-Based N-Acyl Homoserine-Lactone Sensor Systems for Detection of Bacterial Communication. Appl. Environ. Microbiol. 2001, 67, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Manefield, M.; de Nys, R.; Naresh, K.; Roger, R.; Givskov, M.; Peter, S.; Kjelleberg, S. Evidence that halogenated furanones from Delisea pulchra inhibit acylated homoserine lactone (AHL)-mediated gene expression by displacing the AHL signal from its receptor protein. Microbiology 1999, 145, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 8–26 are available from the authors
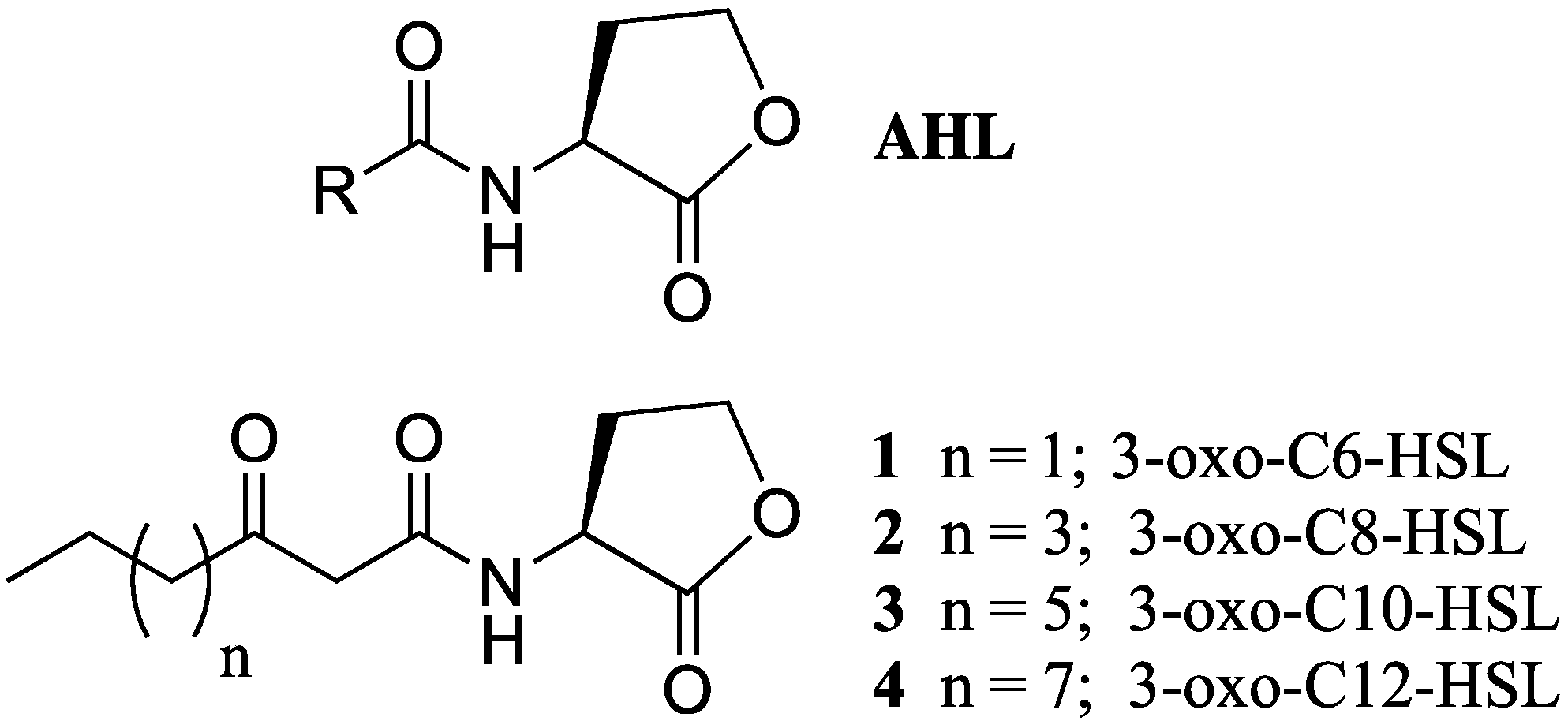

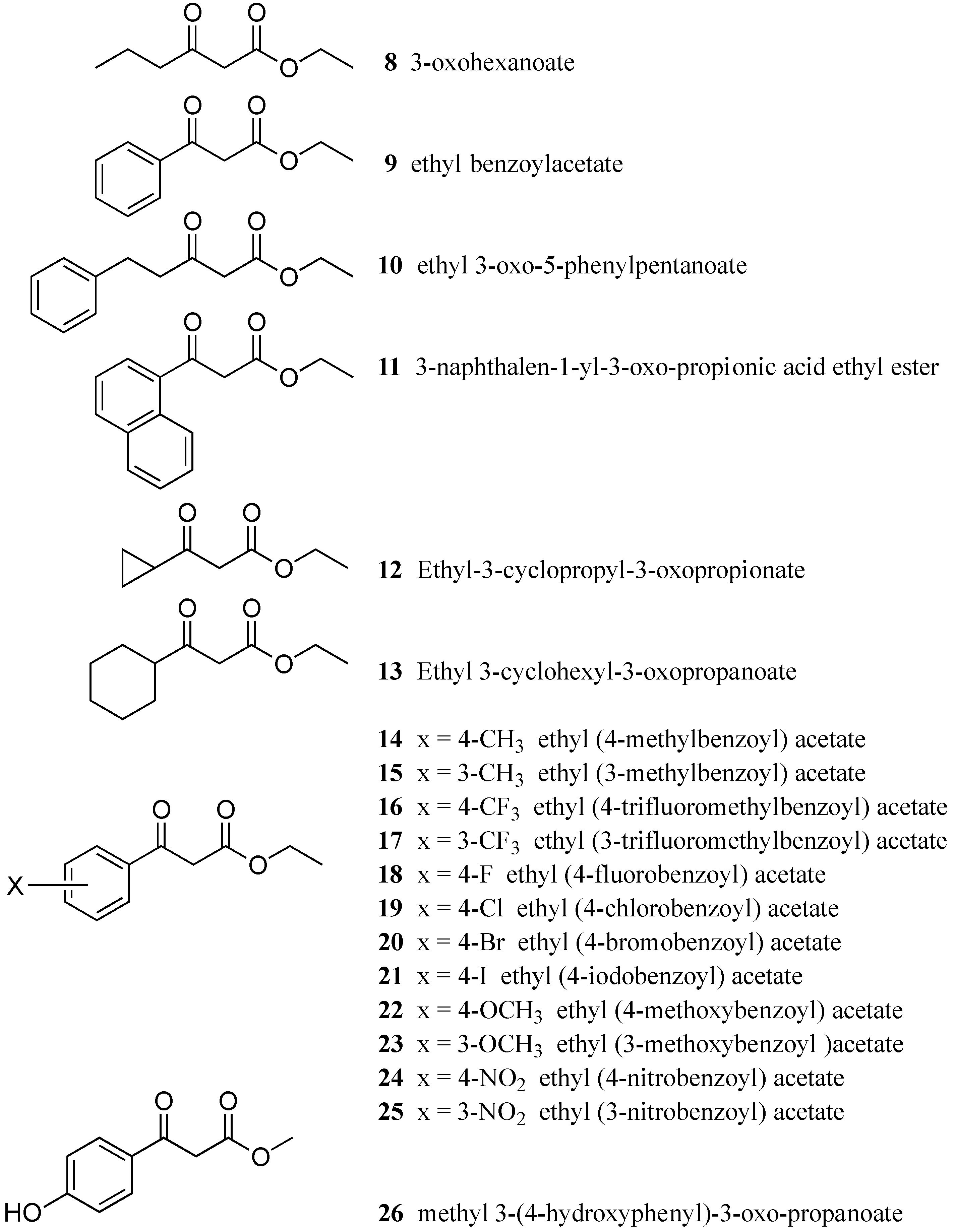
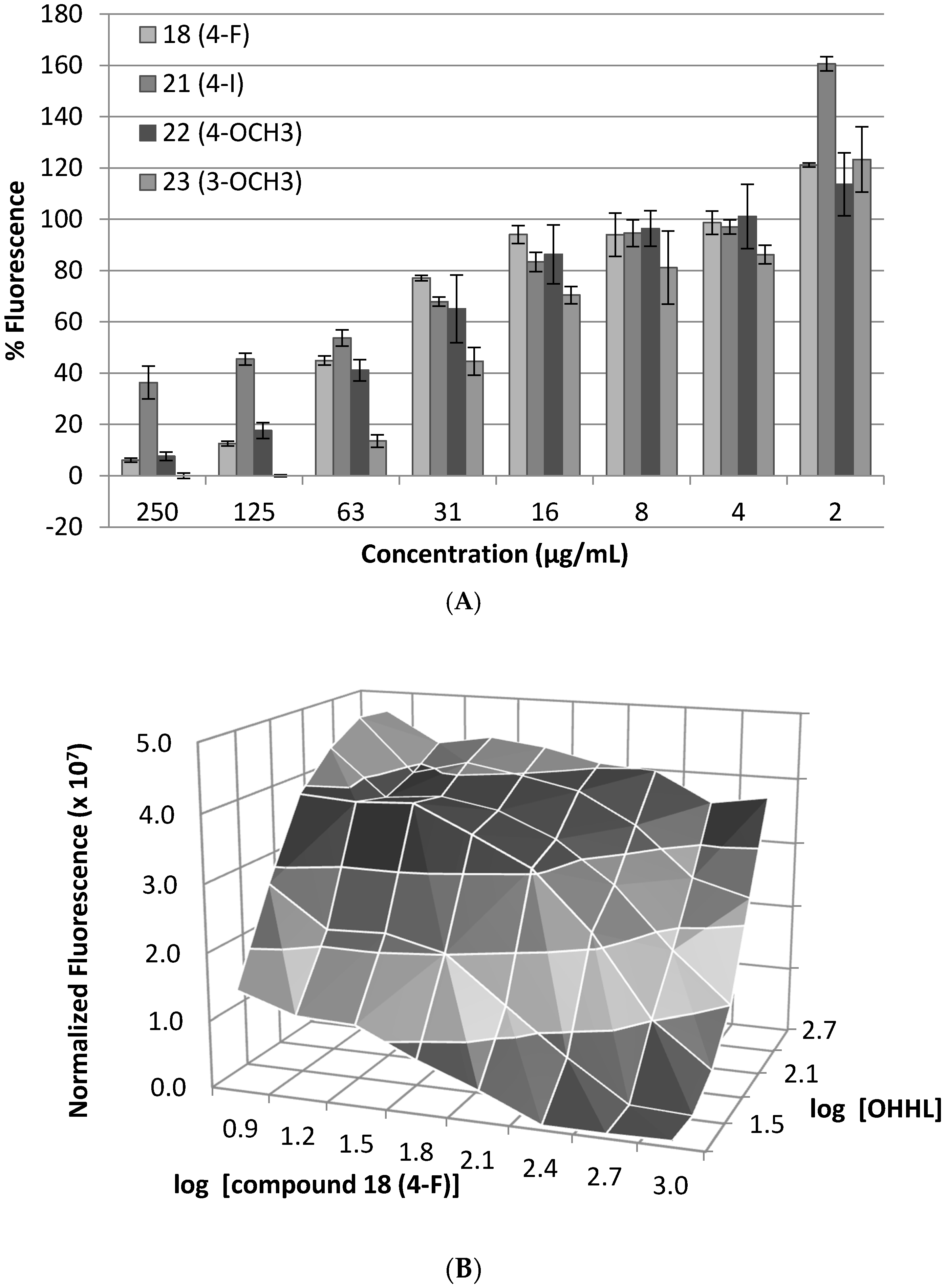
| Compound | IC50 (µM) | MIC (µM) | Compound | IC50 (µM) | MIC (µM) |
|---|---|---|---|---|---|
| 8 | inactive | >1000 | 18 | 23 | >1000 |
| 9 | 76 | >1000 | 19 | 53 | 1000 |
| 10 | inactive | >1000 | 20 | 81 | 500 |
| 11 | 87 | >1000 | 21 | 39 | 500 |
| 12 | inactive | >1000 | 22 | 36 | >1000 |
| 13 | inactive | >1000 | 23 | 41 | >1000 |
| 14 | 96 | >1000 | 24 | 53 | >1000 |
| 15 | 56 | >1000 | 25 | 71 | >1000 |
| 16 | inactive | 1000 | 26 | inactive | >1000 |
| 17 | 92 | 1000 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forschner-Dancause, S.; Poulin, E.; Meschwitz, S. Quorum Sensing Inhibition and Structure–Activity Relationships of β-Keto Esters. Molecules 2016, 21, 971. https://doi.org/10.3390/molecules21080971
Forschner-Dancause S, Poulin E, Meschwitz S. Quorum Sensing Inhibition and Structure–Activity Relationships of β-Keto Esters. Molecules. 2016; 21(8):971. https://doi.org/10.3390/molecules21080971
Chicago/Turabian StyleForschner-Dancause, Stephanie, Emily Poulin, and Susan Meschwitz. 2016. "Quorum Sensing Inhibition and Structure–Activity Relationships of β-Keto Esters" Molecules 21, no. 8: 971. https://doi.org/10.3390/molecules21080971
APA StyleForschner-Dancause, S., Poulin, E., & Meschwitz, S. (2016). Quorum Sensing Inhibition and Structure–Activity Relationships of β-Keto Esters. Molecules, 21(8), 971. https://doi.org/10.3390/molecules21080971





