Abstract
Antibiotic resistance is one of the most serious public health problems. Among bacterial resistance, β-lactam antibiotic resistance is the most prevailing and threatening area. Antibiotic resistance is thought to originate in antibiotic-producing bacteria such as Streptomyces. In this review, β-lactamases and penicillin-binding proteins (PBPs) in Streptomyces are explored mainly by phylogenetic analyses from the viewpoint of self-resistance. Although PBPs are more important than β-lactamases in self-resistance, phylogenetically diverse β-lactamases exist in Streptomyces. While class A β-lactamases are mostly detected in their enzyme activity, over two to five times more classes B and C β-lactamase genes are identified at the whole genomic level. These genes can subsequently be transferred to pathogenic bacteria. As for PBPs, two pairs of low affinity PBPs protect Streptomyces from the attack of self-producing and other environmental β-lactam antibiotics. PBPs with PASTA domains are detectable only in class A PBPs in Actinobacteria with the exception of Streptomyces. None of the Streptomyces has PBPs with PASTA domains. However, one of class B PBPs without PASTA domain and a serine/threonine protein kinase with four PASTA domains are located in adjacent positions in most Streptomyces. These class B type PBPs are involved in the spore wall synthesizing complex and probably in self-resistance. Lastly, this paper emphasizes that the resistance mechanisms in Streptomyces are very hard to deal with, despite great efforts in finding new antibiotics.
1. Introduction
The first antibiotic, penicillin, was discovered in 1929 by Fleming [1] and rediscovered in 1940 by Chain et al. [2] and in 1941 by Abraham et al. [3]. Surprisingly, even before the rediscovery of penicillin, a bacterial penicillinase (β-lactamase) was reported [4] and only few years later, penicillin-resistant Staphylococcus aureus [5,6] and Streptococcus pneumoniae [7] were already described, suggesting strongly that the resistant trait was intrinsically present in their genomes of these bacteria and, in addition, penicillin-inactivating substances (β-lactamases?) were speculated to be involved in the resistance [8]. Gram-negative bacteria such as Neisseria gonorrhoeae [9], Neisseria meningitides [10] and Escherichia coli [11] were intrinsically resistant. The same phenomenon was also observed with streptomycin [12]. That the resistance traits were inherently present in the genomes of Streptomyces was supported by the fact that the percentage production of β-lactamases in newly isolated Streptomyces strains and those isolated thirty years ago from soil was not so different with each other at the time in 1978 [13]. If the selective pressure of the use of β-lactam antibiotics would affect the resistant trait, the relative frequencies of β-lactamase producing Streptomyces strains newly isolated from soil would be much more, indicating that the selective pressure at that time is not so strong as at the present time. Recently, however, D’Costa et al. demonstrated that genes conferring resistance to tetracyclines, vancomycin, aminoglycosides, β-lactams and macrolides existed in 30,000-year old Beringian permafrost and that the amino acid sequences of the β-lactamases showed identities between 53% and 84% with those of known determinants, suggesting that antibiotic resistance is a natural phenomenon that predates the modern selective pressure of clinical antibiotic use [14].
Drug resistance is one of the greatest challenges in modern medicine. Among drug resistance, antibiotic resistance is the most prevailing and threatening topic in public health. According to the information of the Centers for Disease Control and Prevention (CDC), at least 2 million people become infected with bacteria that are resistant to antibiotics and at least 23,000 people die each year as a direct result of these infections in the United States each year [15], and in 2009 the Antibiotic Resistance Genes Database (ARDB) contained resistance information for 13,293 genes [16]. These resistance genes are hypothesized to originate in the antibiotic-producing bacteria such as Streptomyces [17,18,19]. Surprisingly, however, when Dantas et al. cultured clonal isolates from 11 different soils that could use one of 18 different antibiotics as their sole carbon source, phylogenetic profiling of the clonal isolates revealed a diverse set of species consisting of orders Burkholderiales (41%), Pseudomonadales (24%) and Actinomycetales (7%) and others and, indicated that pathogenic microbes can more readily use resistance genes originating from bacteria subsisting on antibiotics than the resistance gene from more distantly related antibiotic producer bacteria [20]. Burkholderiales and Pseudomonadales belong to the proteobacteria and are known to function as scavengers, capable of using a large variety of single carbon sources as food. However, horizontal gene transfer is possible from proteobacteria such as Burkholderiales and Pseudomonadales to Actinomycetales including antibiotic-producing Streptomyces in soil and then to pathogenic bacteria. From the result of a metagenomic analysis to isolate antibiotic resistance genes from soil, Riesenfeld et al. concluded that soil bacteria are a reservoir of antibiotic resistance genes with greater diversity than previously recognized [21]. However, D’Costa et al. described that soil dwelling bacteria produce and encounter a myriad of antibiotics and are a reservoir of resistance determinants (resistome) [22], although they used strains in soil samples collected from diverse locations such as urban, agricultural field, and forest but resembled actinomycetes both morphologically and microscopically [23]. Using multidrug-resistant proteobacteria from the soil, Forsberg et al. isolated 110 resistance genes. Of the 110 resistance genes, 18 had 100% amino acid identity to entries in GenBank and another 32 were highly similar. Interestingly, of 110 resistance genes, 55 were β-lactamase genes highly divergent from those of the antibiotic-producing Streptomyces. From these results they indicated ancient evolutionary relationships between β-lactamases from the soil bacteria and those of the antibiotic-producing bacteria [24]. However, β-lactamases from proteobacteria belong mainly to class C β-lactamases, while those from Streptomyces are class A β-lactamases [19]. So, it is not surprising that these two β-lactamases are only distantly related as described below. In any way, it is more reasonable to speculate that the resistance genes originated in antibiotic producing microorganisms and these resistance genes were developed from natural sources to clinic, human, animal, and other environments by transduction, transformation and conjugation to make the enormous diversity of antibiotic resistance genes and no barriers among the microbial compartments in the microbial world at the present time [25,26,27,28,29].
The phylum Actinobacteria constitute one of the largest phyla within the bacteria [30,31]. Streptomyces species belonging to the Actinobacteria dwell in the soil and are high guanine + cytosine (G + C)-content Gram positive bacteria. They are characterized by their peculiar morphology to undergo complex cellular differentiation like filamentous fungi [32] and by their production of a wide variety of secondary metabolites including β-lactam antibiotics [33,34] such as thienamycin [35] and clavulanic acid [36]. However, unlike penicillin- and cephalosporin-producing fungi, Streptomyces species are prokaryotic microorganisms so they must protect themselves from the attack of antibiotics to avoid suicide, that is, they have to have self-resistance mechanisms. In addition, β-lactam antibiotics have been most widely used for chemotherapy of infectious diseases even about 90 years after penicillin’s discovery, even though the chemical structures were extensively modified. β-Lactam antibiotic resistance is caused mainly by two mechanisms: antibiotic-degrading enzymes, β-lactamases and modification of target sites, penicillin-binding proteins (PBPs). Streptomyces species are known to be highly resistant to benzyl penicillin, although they are Gram-positive bacteria, so it is interesting to know the self-resistance mechanisms in Streptomyces and the relationships between PBPs, β-lactams and β-lactamases [19,37]. This review discusses the self-resistance in Streptomyces, focusing primarily on β-lactam antibiotics.
2. Self-resistance mechanisms
As described above, the self-resistance in antibiotic-producing bacteria such as Streptomyces is at least one of the origins of multi-drug resistance prevailing and threatening in various environments at the present time. Major biochemical mechanisms of drug resistance are detoxication or inactivation of the drug, changing the target site, blocking the transport of the drug into the cell and the efflux pump system [19,38]. These mechanisms are true with the self-resistance mechanisms or, bacteria use very similar or the same mechanisms for self-resistance in the antibiotic-producing bacteria and antibiotic resistance in pathogenic bacteria. While the major resistance mechanism in pathogenic bacteria is β-lactamases, that in Streptomyces is supposed to be penicillin-binding proteins [19]. A number of excellent review papers and databases have been published on antibacterial multidrug resistance and self-resistance [19,39,40,41,42,43].
2.1. β-Lactamases
β-Lactamases are the enzymes which catalyze the hydrolysis of the β-lactam antibiotics to produce antimicrobiologically inactive compounds. Because of this, β-lactamases are responsible for β-lactam resistance in many pathogenic bacteria [44]. These enzymes, however, are also produced by nonpathogenic bacteria such as Streptomyces [13,45], Bacillus [46] and the cyanobacteria [47,48]. In addition, recent whole genome analyses expand the β-lactamase world to β-lactamase superfamily proteins [49,50], β-lactamase domain-containing proteins [51] and RNA-metabolizing metallo-β-lactamases [52,53]. Consequently, β-lactamase-producing organisms include a thermobacterium (Meiothermus ruber DSM 1279) (GenBank accession number, ADD27888, the same hereafter, class C β-lactamase), a green sulfur bacterium (Pelodictyon phaeoclathratiforme BU-1, ACF43212, a metallo-β-lactamase), a green non-sulfur bacterium (Herpetosiphon aurantiacus DSM 785, ABX07048, a metallo-β-lactamase), a methylotrophic bacterium [54] (Methylovorus glucosetrophus SIP3-4, ACT50532, a metallo-β-lactamase), a fungus (Schizosaccharomyces pombe, CAA93219, a tRNA processing endonuclease, a metallo-β-lactamase) and even a plant [55] (Arabidopsis thaliana, AAC49866, a glyoxalase II mitochondrial enzyme) and mammals [56,57] (Rattus norvegicus and Homo sapiens, EDL84248 “a serine β-lactamase-like protein” and NP_116246 “a serine β-lactamase-like mitochondria protein”, respectively). Furthermore, an alkalophilic and halotolerant Gram-positive bacterium Oceanobacillus iheyensis isolated from the bottom of the Pacific Ocean at a depth of 1050 m [58] and Pseudomonas fluorescens isolated from a remote Antarctic coastal area [59] also produce β-lactamases. Moreover, screening of metagenomic libraries from soil bacterial communities for clones that confer antibiotic resistance gave new types of β-lactamases such as oligomeric [60] and bifunctional β-lactamases [61,62]. A bifunctional β-lactamase was also isolated from a pathogen Mycobacterium tuberculosis H37Rv, an Actinobacterium [63,64]. Therefore, various kinds of β-lactamases or β-lactamase superfamily are distributed in a variety of organisms, but in this review β-lactamases are described in a more classical sense.
β-Lactamases are grouped into four classes on the basis of their amino acid sequences [65,66,67,68,69,70,71,72]. This classification reflects more or less the substrate specificity: class A (penicillin-hydrolyzing), class C (cephalosporin-hydrolyzing) and class D (oxacillin-hydrolyzing) β-lactamases are active site serine enzymes and class B β-lactamases require one or two zinc ions for their activity and are called as metallo-β-lactamases [69,73,74,75,76]. A previous phylogenetic study indicated that class A β-lactamases could be divided into three subgroups [77]. β-Lactamases are not essential bacterial proteins in themselves, but are speculated to have evolved from the essential PBPs in some β-lactam-producing bacteria such as Streptomyces or related bacteria, because these bacteria have to have some self-protective strategies against β-lactam antibiotics [19,48]. PBPs are involved in peptidoglycan biosynthesis in prokaryotic microorganisms. Therefore, it is interesting to know the relationships of β-lactam antibiotics, β-lactamases and PBPs in Streptomyces, β-lactam-producing microorganisms. In addition, the relationship of β-lactamases in Streptomyces and those in pathogenic bacteria should be clarified. In this section, the phylogenetic relationships were analyzed among β-lactamases in Streptomyces and a couple of pathogenic bacteria, whose genome sequences were wholly clarified.
Previously, I investigated the characteristics of 59 β-lactamase candidate sequences among 529 putative β-lactamase and PBP sequences in 19 Actinobacteria [78,79], which were collected from the NCBI database [80]. There were some characteristic features in the 59 candidate sequences. First, β-lactamase candidates were not distributed uniformly in the strains: while Saccharomonospora viridis, Streptomyces avermitilis, Streptomyces scabiei and Streptosporangium roseum possess as many as five, twelve, five and five candidate genes, respectively, Mycobacterium leprae, Nocardioides sp. JS614 and Rhodococcus erythropolis had no candidate genes. In general, Streptomyces species produced β-lactamase candidates at higher rates. However, the numbers of candidate sequences were not necessarily related to the taxonomic classification. For example, although Streptosporangium and Nocardioides were closely related taxonomically [81], the numbers of β-lactamase candidates in Streptosporangium were five but those in Nocardioides were zero. Even in the same genus, M. tuberculosis produced four but M. leprae produced none. β-Lactamases detected by their enzymatic activity also distributed independently of actinobacterial taxonomy [82]. These results indicate strongly that β-lactamases evolve and are produced independent of taxonomy.
Second, most of the β-lactamases detected so far by their enzymatic activity in Gram-positive bacteria belong to class A and a few belong to class B. However, this fact was not the case with whole genomic level.
Third, it is intriguing that Thermomonospora curvata, a high G + C Gram-positive soil thermophilic Actinobacteria, produced two class B and one class D β-lactamase candidates but no class A and class C enzymes. Usually, Gram-positive bacteria produce class A and class B β-lactamases, but not class C and class D. In addition, T. curvata DSM43183 Tcur2040 β-lactamase is closely related to Gram-negative class D β-lactamases, especially OXA-5 and OXA-27 type β-lactamase [79], although T. curvata is a Gram positive bacteria. Hereafter, genetic analyses are extended to β-lactamases of other 12 Streptomyces species listed in Table 1.

Table 1.
Classification of β-lactamases in 12 Streptomyces species.
2.1.1. Class A β-lactamases
The amino acid sequences of putative 25 class A β-lactamases together with a reference sequence (TEM-1, Klebsiella penumoniae plasmid, YP_005351445) were aligned by using the Muscle and T-coffee programs [83,84]. The results showed that all the sequences in Table 2 possessed main residues involved in the catalytic reaction (Table 3), except SCAT_1418, SCAT_4581, SRIM_07318, and F750_2387 (Table S1). These four sequences are defective in S70XXK73 and S130DN132. Furthermore, SCAT_1418, SCAT_4581 and F750_2387 do not carry K234T/SG236, indicating that these candidates do not function as β-lactamases (Table 2).

Table 2.
Presence or absence of active site residues in class A β-lactamases.

Table 3.
The list of active site residues discussed in this paper.
Although some other sequences are also deficient or possess incompletely identical signature amino acid sequences, they are supposed to operate as β-lactamases even if the catalytic efficiency is not perfect. GWG166 in SSCG_00160, GWG166 in F750_6336, IWE166 in B446_02285, IWE166 in B466_33010, RVI166 in STSU_15177, RGI166 in SCAT_0807, HYE166 in SCAT_5692, QYE166 in SSCG_00130, and RWS166 in F750_2387 are examples. In fact, blast analysis with SSCG_00160 as a query, putative β-lactamases from Saccharomonospora viridis (KHF44103), Amycolicicoccus subflavus DQS3-9A1 (AEF33802) and Rhodococcus sp. B7740 (AJW42765) were identified.
In addition blast analysis using SCAT_1418 as a query, metallo-dependent hydrolase of Streptomyces violaceusniger (WP_014060235) and metallo-dependent hydrolase of Streptomyces himastatinicus (WP_00971837) were identified. Actually, SCAT_1418 possesses some class B β-lactamase signature residues. Likewise, SCAT_4581 is highly similar to Zn-dependent hydrolases from Actinobacteria, so that they may be class B β-lactamases.
SRIM_07318 is too short to function as a β-lactamase, but may be a part of class A β-lactamase, as its amino acid sequence of the C-terminal part is very similar to that of class A β-lactamases from Streptomyces albus (WP_060729068) and Rhodococcus rhodnii (WP_037260801). F750_2387 is a large membrane protein and belongs to metallo-β-lactamase superfamily. The fact that SCAT_1418, SCAT_4581 and F750_2387 belong to metallo-β-lactamases but not to class A reflects on large molecular distances between these β-lactamases and others such as TEM-1 and SHV-1 (Table S2). Interestingly, the amino acid sequence of SRIM_10531 is completely identical with that of Streptomyces cellulosae (D12653). This is one example of the horizontal gene transfer. Another interesting thing is that the amino acid sequences of B446_02285 and B446_33010 in class A β-lactamases, and B446_02210 and B446_33085, and B446_02000 and B446_33290 in class B β-lactamases, and B446_01720 and B446_33570 in β-lactamase-like sequences and B446_01315 and B446_33975 in class C β-lactamases are also completely identical, indicating that about 7.7 Mb region including these genes were duplicated in the chromosome in the past. In fact, two transposases exist at both sides in reverse direction from 7,984,885-7,985,573n and 287,352-288,040n in Streptomyces collinus genome, respectively, suggesting that these transposases acted in the transposition or the duplication.
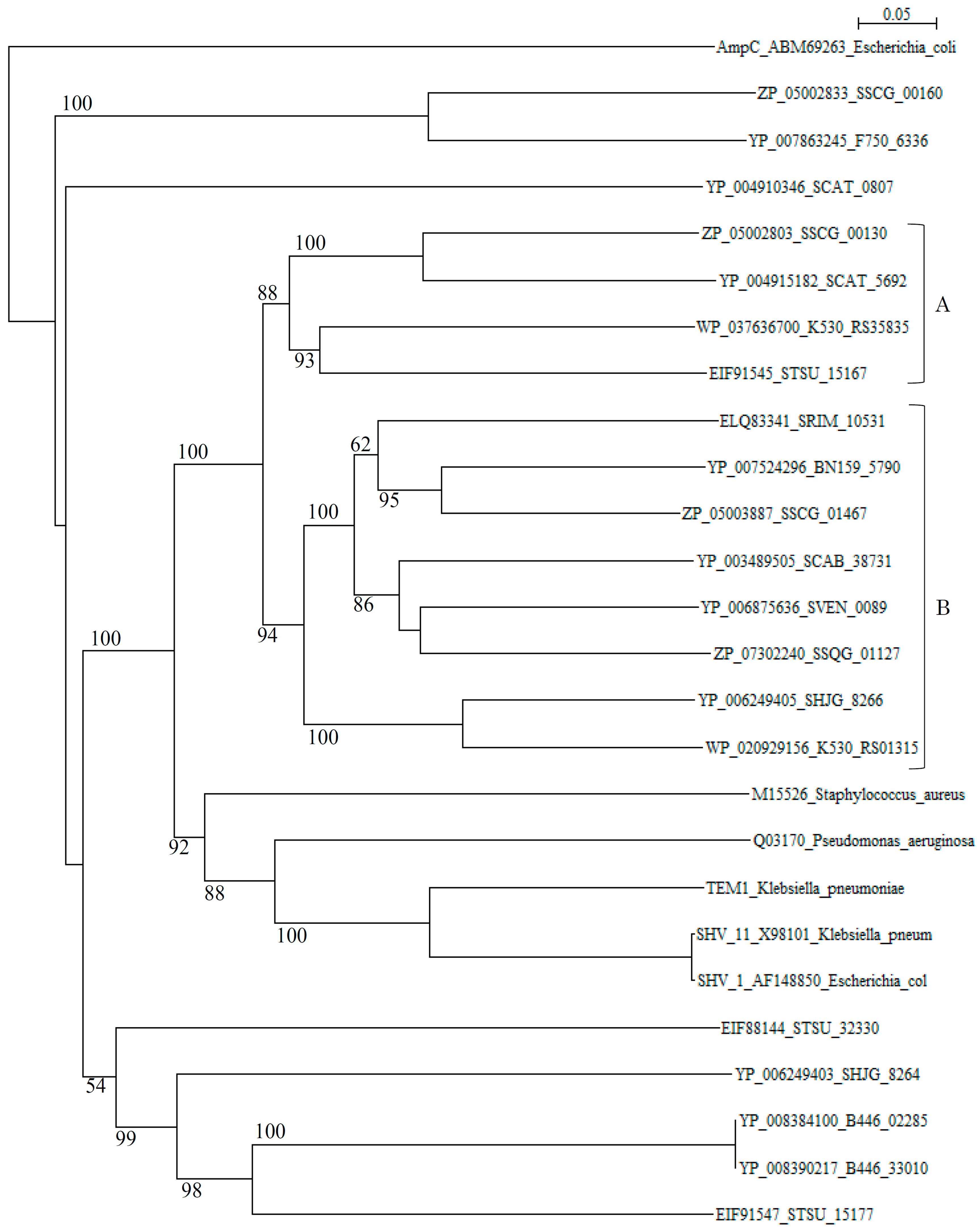
A phylogenetic tree of putative 20 class A β-lactamases constructed with those of S. aureus (M15526), P. aeruginosa (Q03170), K. pneumoniae (TEM-1, YP_005351445), K. pneumoniae (SHV-11, X98101), and E. coli (SHV-1, AF148850) as reference sequences disclosed that although these reference sequences are originated from quite different species, they form only one cluster (cluster C in Figure 1). In contrast, β-lactamases of Streptomyces are scattered in various branches, indicating that only a few β-lactamase genes of Streptomyces species have been transferred to pathogenic bacteria at the present time.
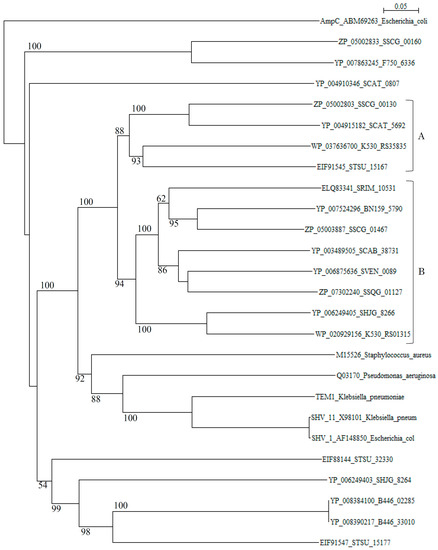
Figure 1.
Phylogenetic tree of putative 20 class A β-lactamases on the basis of amino acid sequences with those of Staphylococcus aureus (M15526), Pseudomonas aeruginosa (Q03170), Klebsiella pneumoniae (TEM-1, YP_005351445), Klebsiella pneumoniae (SHV-11, X98101), and Escherichia coli (SHV-1, AF148850) as reference sequences. The tree was constructed by using ClustalX 2 [88] and E. coli AmpC (ABM69263) as outgroup. Clusters A and B show blue-dextran/NADP+-non-binding and binding β-lactamases from Streptomyces, respectively and, cluster C shows those from pathogenic bacteria used as reference sequences. The bootstrap probabilities are shown at branching nodes.
However, it is possible that other β-lactamase genes of Streptomyces species may transfer to pathogenic bacteria in the near future, although many threatening class A genes may be evolved through mutation but not transferred to pathogenic bacteria, as it is seen in TEM-type and SHV-type β-lactamases [89]. Actually, molecular distances determined by the protdist program [90] between most class A β-lactamases of Streptomyces and those of pathogenic bacteria (Table S2) are not so large as in class B and class C β-lactamases, indicating that these class A β-lactamases are related closely with each other and with those of pathogenic bacteria than class B and class C enzymes. It is speculated, therefore, that the horizontal gene transfer of class A β-lactamase genes is easier than for class B and class C β-lactamases. The β-lactamases of Streptomyces can be classified into blue-dextran/NADP+-binding and non-binding groups [91,92]. Blue-dextran/NADP+-non-binding β-lactamases belong to cluster A and those of binding β-lactamases go to cluster B in the phylogenetic tree (Figure 1). However, the role of NADP+-binding of β-lactamases remains to be clarified.
As for the relationship between class A β-lactamases and the β-lactam biosynthetic gene cluster, SSCG_00130 (corresponding to SCLAV_4216, partial stop and SCAT_5692) is located in the terminal region of the cephamycin biosynthetic gene cluster and SSCG_00160 (corresponding to SCLAV_4187) is located within the clavulanic acid biosynthetic gene cluster in S. clavuligerus (Figure S1). These two clusters exist in the adjacent position. SSCG_01467 (corresponding to SCLAV_2436) is not related to β-lactam biosynthetic genes [78]. Similarly, SCAT_5692 is present in the terminal region of the cephamycin biosynthetic gene cluster in S. cattleya (Figure S2). However, a gene corresponding to SSCG_00160 in S. clavuligerus is missing in S. cattleya in accord with the absence of clavulanic acid biosynthesis. Therefore, it is suggested strongly that class A β-lactamases and β-lactam biosynthesis are closely related with each other. However, it remains to be clarified whether these β-lactamases are involved in the self-resistance in the Streptomyces species. SSCG_00160 (SCLAV_4187) is also thought to be involved in clavulanic acid biosynthesis [93,94].
2.1.2. Class B β-lactamases
On the basis of phylogenetic and sequences analyses, 63 class B β-lactamase candidates were selected in 12 Streptomyces species. The amino acid sequence alignment using the Muscle and T-coffee programs [83,84] revealed that all the sequences contain the conserved active site residues (HXHXD120) except SSCG_05130, SHJG_8335, SCAT_4145, and SRIM_16845 (Table 4 and Table S3; In the case of AAA22276_Bacillus cereus reference sequence, the active site residues are shifted to downstream in Table S3).

Table 4.
Presence or absence of active site residues in class B β-lactamases.
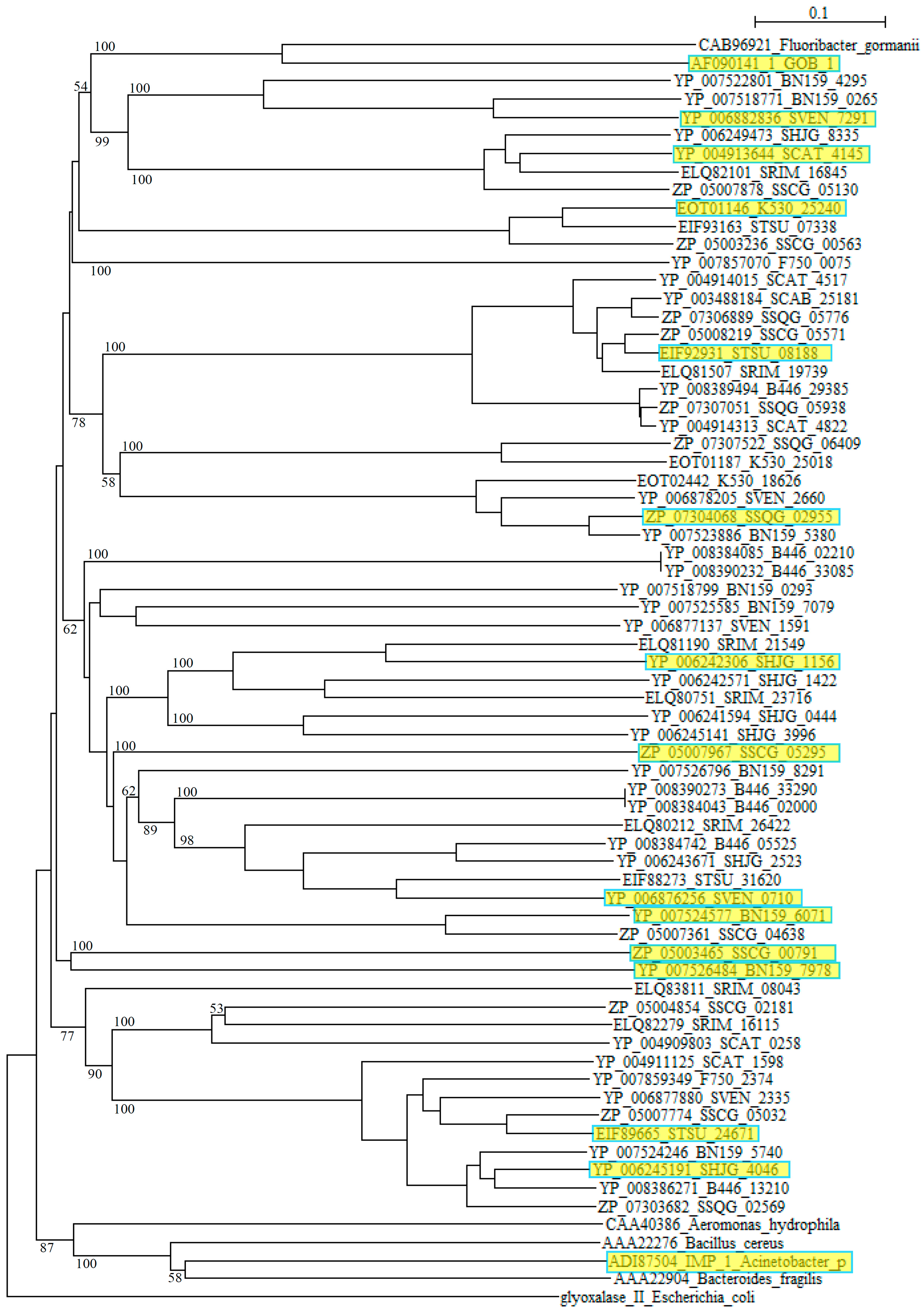
However, blast analyses using these sequences as queries clarified that they are closely related to αββα metallo β-lactamase (MBL) fold hydrolases from Streptomyces such as S. roseochrmogenus (WP_023553317), S. silaceus (WP_055701146), S. sclerotialus (WP_030612498), S. corchorusii (WP_059262406), S. hygroscopicus (WP_058083348), S. achromogenes (WP_030611171), S. bingchenggensis (WP_014181511), S. alboniger (WP_055536123), S. rimosus (WP_033031918), S. albus (WP_060732298), S. monomycini (WP_030021421) and others, indicating that these sequences belong to the metallo β-lactamase superfamily. As in the case of class A, while the class B β-lactamases of pathogenic bacteria form compact clusters in a phylogenetic tree, those from Streptomyces are dispersed quite extensively (Figure 2).
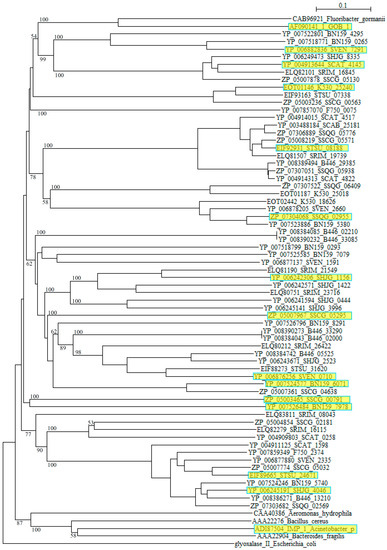
Figure 2.
Phylogenetic tree of putative 63 class B β-lactamases on the basis of amino acid sequences. The tree was constructed by using ClustalX 2 [88] and Escherichia coli glyoxalase II (CDZ19109) as outgroup. β-Lactamases from Fluoribacter gormanii (CAB96921), Elizabethkingia meningoseptica GOB-1 (AF090141_1), Aeromonas hydrophila (CAA40386), Bacillus cereus (AAA22276), Acinetobacter pittii IMP_1 (ADI87504), and Bacteroides fragilis (AAA22904) were used as reference sequences. The sequences marked with yellow boxes were used for the calculation of molecular distances (Table S4). The bootstrap probabilities are shown at branching nodes.
Moreover, comparing to Class A and class C β-lactamases, molecular distances calculated by using the protdist program [90] are mostly quite large between those of Streptomyces species with each other and those with pathogenic bacteria (Table S4), suggesting that the class B β-lactamase enzymes are assemblies of highly diverged proteins. Recently, new type of metallo-β-lactamases such as New Delhi metallo-β-lactamase (NDM) have emerged and swiftly spread worldwide. It is possible that various metallo-β-lactamases of Streptomyces are also transferred to pathogenic bacteria and pose a similar public health threat.
2.1.3. Class C β-lactamases
The amino acid sequence alignment of putative 94 class C β-lactamases is shown in Table S5. From this alignment, the presence or absence of the active site residues was inferred and the results was shown in Table 5. All the sequences possess active site residues shown in Table 3, with exceptions of SHJG_8639 (YP_006249777), SHJG_2828 (YP_006243975), SSCG_03668 (ZP_05006222), SRIM_31085 (ELQ79325), BN159_7932 (YP_007526438), SSQG_02491 (ZP_07303604), and SSQG_00225 (ZP_07301338). However, the N-terminal sequences of SHJG_2828, SSCG_03668, SRIM_31085, SSQG_02491 and SSQG_00225 are missing, so the numbers of amino acid residues are too short to compare. In fact, blast analysis shows that SHJG_8639 is similar to Zn-dependent hydrolases (l-ascorbic acid metabolism, UlaG) of Streptomyces corchorusii (WP_059265188), S. bingchenggensis (WP_043485943) , and Nonomuraea candida (WP_043633066); SHJG_2828 is similar to Zn-dependent hydrolases (glyoxylase, GloB) of multiple species of Streptomyces (WP_037824258, WP_037629085 and others), Streptomyces fulvoviolaceus (WP_03061340) , and S. avermitilis (WP_010988349); SSCG_03668 is related to serine hydrolases of Streptomyces aureus (WP_037619730), Actinokineospora spheciospongiae (WP_035277635) and Micromonospora parva (WP_030334201); SRIM_31085 is similar to PBPs of S. rimosus (WP_030645517), S. albus (WP_060729102) and S. yokosukanensis (KUN09412); BN_7932 is associated with serine hydrolases of Streptomyces venezuelae (WP_055639726), Streptomyces vietnamensis (WP_041132293), and Streptomyces antibioticus (WP_059193915); SSQS_02491 is similar to serine hydrolases of Streptomyces olindensis (KDN73989), Streptomyces acidiscabies (WP_059045061), and Streptomyces torulosus (WP_055716632); and SSQG_00225 is related to PBPs of Streptomyces chartreusis (WP_010033556), Streptomyces iakyrus (WP_051814832), and Streptomyces pactum (WP_055421147). Therefore, all of these sequences are members of the β-lactamase superfamily. The amino acid numbers of SHJG_2828, SSCG_03668, SRIM_31085, SSQG_02491 and SSQG_00225 are too short to function as β-lactamases, and the C-terminal residues in SSCG_03303 are missing.

Table 5.
Presence or absence of active site residues in class C β-lactamases.
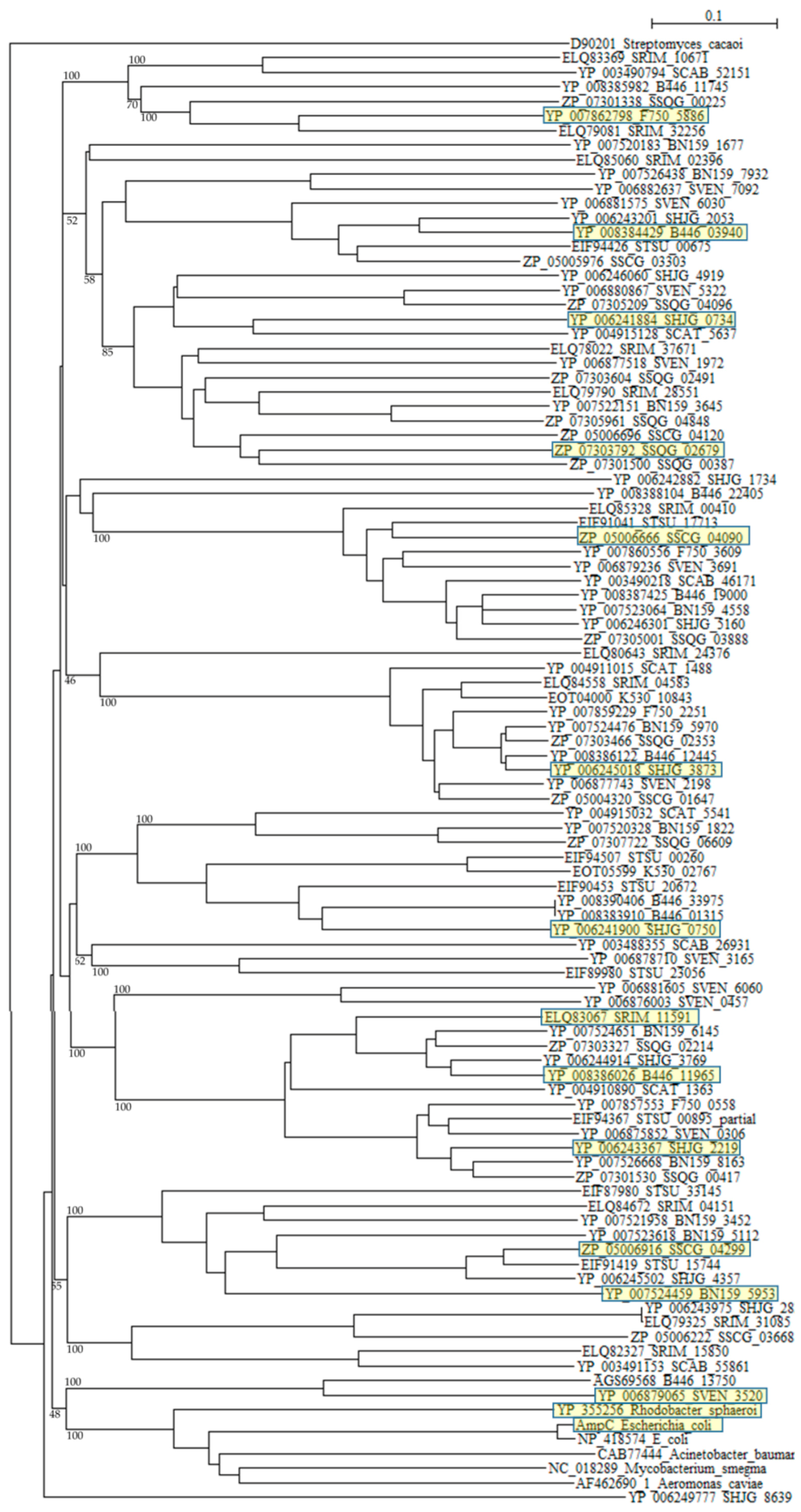
A phylogenetic tree was constructed with β-lactamases of Rhodobacter sphaeroides (YP_355265), Mycobacterium smegmatis (NC_018289), Acinetobacter baumannii (CAB77444), Aeromonas caviae (AF462690_1), and E. coli (ABM69263 and NP_418574) as reference sequences (Figure 3). Although six reference sequences are selected from various different species [95], they form only one cluster as described previously [77,78]. On the other hand, β-lactamases of Streptomyces are dispersed into numerous clusters, indicating that they originate from different kinds of proteins. A molecular distance analysis using the protdist program [90] confirmed this observation (Table S6). The molecular distances between Streptomyces β-lactamases and that of pathogenic bacteria are very large, but those of β-lactamases of Streptomyces are also large with each other, so if we continue to develop and use new, newer and the newest β-lactam antibiotics at the present rate, a dreadful microbial revolution may occur near future.
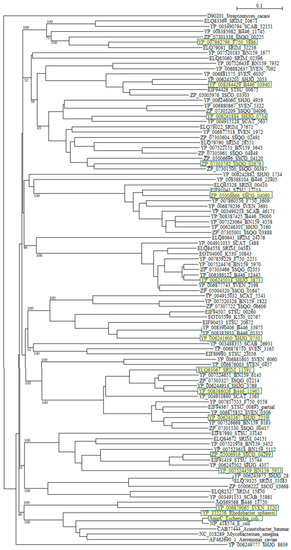
Figure 3.
Phylogenetic tree of putative 94 class C β-lactamases on the basis of amino acid sequences. The tree was constructed by using ClustalX 2 [88] and Streptomyces cacaoi class A β-lactamase (D90201) as outgroup. β-lactamases of Rhodobacter sphaeroides (YP_355265), Mycobacterium smegmatis (NC_018289), Acinetobacter baumannii (CAB77444), Aeromonas caviae (AF462690_1), and E. coli (ABM69263 and NP_418574) were used as reference sequences. The sequences marked with yellow boxes were used for the calculation of molecular distances (Table S6). The bootstrap probabilities are shown at branching nodes.
2.2. Penicillin-Binding Proteins (PBPs)
The bacterial cell wall peptidoglycan is a three-dimensional, covalently closed, net-like mesh called sacculus in which glycan strands are cross-linked by peptide chains. It maintains cell shape and provides mechanical strength to resist osmotic pressure [96,97]. The peptidoglycan chains are composed of alternating β-1,4-linked N-acetylglucosamine and N-acetylmuramic acid residues. The carboxyl groups of the N-acetylmuramic acid residues are involved in amide bond to terminal l-alanine residues of the peptide units l-alanyl-γ-d-glutamyl-l-diaminoacyl-d-alanine. The glycan chain is biosynthesized by catalysis of glycosyltransferases and the cross-linkage of the peptide chains between two adjacent glycan chains is catalyzed by transpeptidases [87,98]. The transpeptidases, also called penicillin-binding proteins (PBPs), were initially identified as their ability to bind penicillins [99,100,101]. Depending on the structure and the catalytic activity of their N-terminal domain, they are classified into class A, class B and class C PBPs [87,96,101,102]. Both class A and class B PBPs have the transpeptidase activity in the C-terminal region. However, the N-terminal domain in class A PBPs is responsible for their glycosyltransferase activity, while the glycosyltransferase domain is lacking in class B PBPs. Instead, the PBP dimer domain (Pfam03717) is present at the N-terminal region in class B PBPs. However the detailed functional role of the dimer domain is yet to be fully explicated. Class C PBPs are also called low molecular weight PBPs and, having the carboxypeptidase activity, are responsible for the maturation and recycling of the peptidoglycan [96], but they are not essential and are excluded from further study. There are many excellent review paper on PBPs [87,96,97,98,100,101,102], and the comprehensive review on the PBPs in Actinobacteria in general was already published [103], so this review describes the PBPs in Streptomyces, especially emphasizing their roles in self-resistance.
2.2.1. Class A PBPs
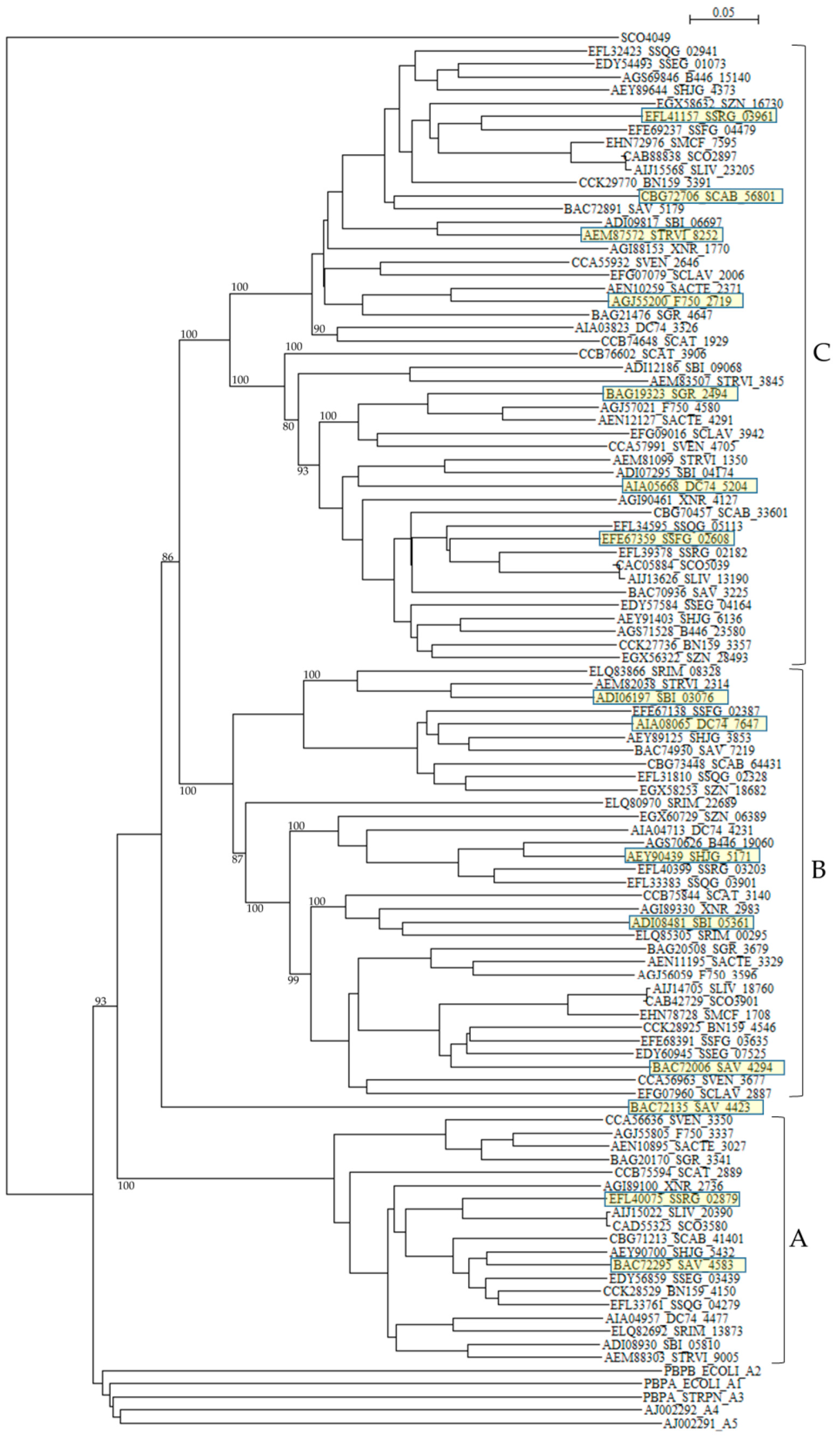
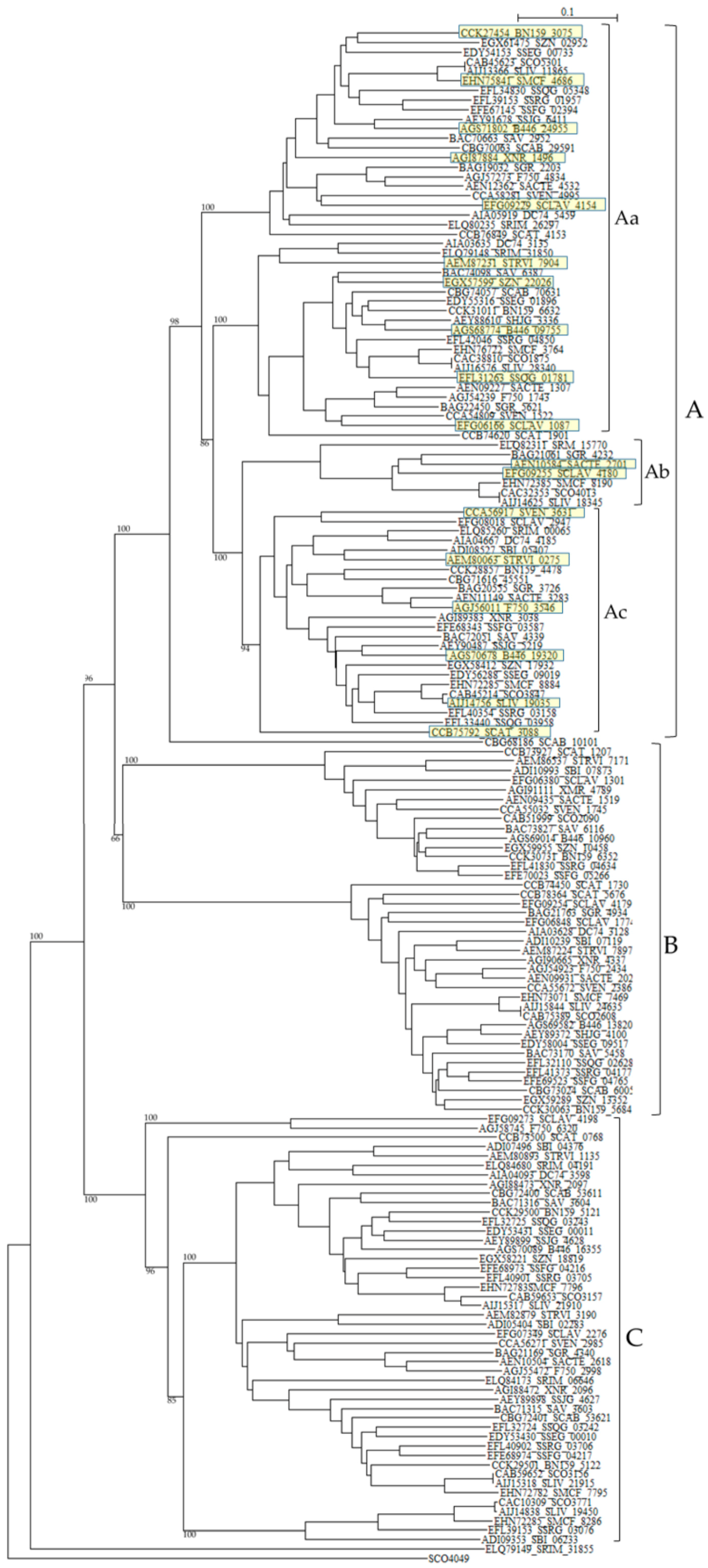
The numbers and types of putative PBPs in 24 Streptomyces are summarized in Table 6. The amino acid sequences of putative 100 class A PBPs in 24 Streptomyces species together with a reference sequence (Q04704/CVP62105, S. pneumoniae) were aligned by the Muscle and T-coffee programs (Table S7). All sequences analyzed possess active site S465XXK468 residues, excluding SAV_4423, SBI_09068, STRVI_3845, SCAB_64431, SZN_18682, DC74_7647, SSQG_02328, SSFG_02387, SAV_7219, SHJG_3853, SRIM_08328, SBI_03076, and STRVI_2314. However, active site residues S524XN546, and K716TG718 are mostly conserved. In fact, blast analysis of STRVI_3845 as a query showed that glycosyltransferases of S. iranensis (WP_044567878), S. hygroscopicus (WP_060948597) and S. bingchenggensis (WP_014181633) are identified and, that of SSQG_02328 as a query, PBPs of S. afghaniensis (WP_037667886), S. iakyrus (WP_033306427), and S. caeruleatus (KUN93289) are identified, and that of SHJG_3853 as a query, PBPs of S. antibioticus (WP_053211715), S. reticuli (CUW28219), and S. achromogenes (WP_030604457) are identified. In addition, the glycosyltransferase and transpeptidase domains are preserved in these sequences, indicating that these sequences can function as PBPs. A phylogenetic tree was constructed by using SCO4049 (putative penicillin acylase) as outgroup (Figure 4). The class A PBPs are clearly divided to three groups: A, B and C. SAV_4423 is a lone sequence. The PBPs in these groups are obviously corresponding to those in clusters A, B and C in the amino acid alignment (Table S7).

Table 6.
The numbers and types of putative PBP and protein kinase genes.
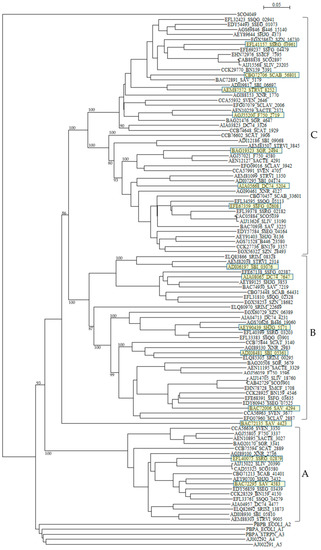
Figure 4.
Phylogenetic tree of putative 100 class A PBPs on the basis of amino acid sequences. The tree was constructed by using ClustalX 2 [88] and S. coelicolor putative penicillin acylase (SCO4049, CAC32320) as outgroup. E. coli PBP1A (subclass A1, PBPA_ECOLI), E. coli PBP1B (subclass A2, PBPB_ECOLI), S. pneumoniae PBP1A (subclass A3, PBPA_STRPN), S. pneumoniae PBP2A (subclass A4, AJ002292), and S. pneumoniae PBP1B (subclass A5, AJ002291) were used as reference sequences. The sequences marked with yellow boxes were used for the calculation of molecular distances (Table S8). The PBPs in groups A, B, and C are corresponding to those in clusters A, B, and C in the amino acid alignment shown in Table S7. The bootstrap probabilities are shown at branching nodes.
According to the penicillin-binding cores of PBPs, class A PBPs are classified into two subclasses in Gram-negative bacteria and three subclasses in Gram-positive bacteria [104]: A1 (whose prototype is Escherichia coli PBP1A, PBPA_ECOLI), A2 (whose prototype is E. coli PBP1B, PBPB_ECOLI), A3 (whose prototype is Streptococcus pneumoniae 1A, PBPA_STRPN), A4 (whose prototype is S. pneumoniae 2A, AJ002292), and A5 (whose prototype is S. pneumoniae 1B, AJ002291). Interesting enough, all class A PBPs from Streptomyces analyzed in this paper form a completely different cluster in the phylogenetic tree from these five subclasses (Figure 4), where E values (number of alignments expected by chance) between PBPs in subclasses A1 to A5 and those in Streptomyces are in the range of 3.4 × 10–16 to 4.9 × 10–31, indicating low similarities. These results suggests strongly that the gene transfer and/or gene conversion occurred rarely between PBPs in Streptomyces and those in Gram-positive and Gram-negative bacteria. However, the molecular distances are not so large except PBP of Helicobacter pylori and PBPs of Streptomyces, indicating that these PBPs are not so different as speculated by the phylogenetic tree with each other (Table S8), and it is possible that the horizontal gene transfer of these genes may take place from Streptomyces to pathogenic bacteria near future.
2.2.2. Class B PBPs
One hundred sixty one class B PBPs in 24 Streptomyces listed in Table 6 were analyzed. Intriguingly, more than half of Streptomyces species carry two successive class B PBPs in tandem. For example, XNR_2096 and XNR_2097 (E value is 5.4 × 10–58, the same hereafter), SAV_3603 and SAV_3604 (3 × 10–68), SCLAV_4178 and SCLAV_4180 (1.5 × 10–11), SMCF_7795 and SMCF_7796 (3.3 × 10–60), SCO3156 and SCO3157 (2.8 × 10–47), BN159_5121 and BN159_5122 (1.4 × 10–62), SSFG_04216 and SSFG_04217 (1.3 × 10–56), SSRG_03705 and SSRG_03706 (2.7 × 10–62), SHJG_4627 and SHJG_4628 (2.8 × 10–69), SLIV_21910 and SLIV_21915 (4.3 × 10–58), SCAB_53611 and SCAB_53621 (6.8 × 10–53), SSEG_00010 and SSEG_00011 (3.8 × 10–63), and SSQG_03242 and SSQG_03243 (5.5 × 10–65). These amino acid sequences are not only very similar to each other, but also all the sequences belong to the same cluster in a phylogenetic tree constructed by using SCO4049 as outgroup (cluster C in Figure 5). In fact, the amino-acid sequence identity and similarity of PBPs in cluster C in the phylogenetic tree are in the range of 49.2%–51.8% and 71.8%–77.8%, respectively. Moreover, the nucleotide sequences of each pair are arrayed in the same direction, indicating that they were duplicated and transferred to each other very recently. Interestingly enough, the pair of a cephamycin and clavulanic acid producer, that is, S. clavuligerus SCLAV_4179 and SCLAV_4180 is an exception.
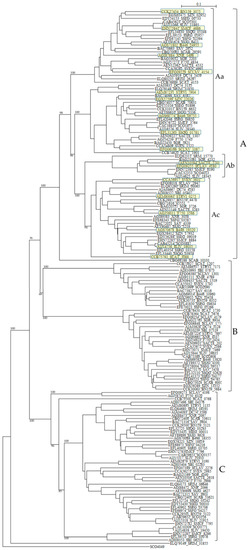
Figure 5.
Phylogenetic tree of putative 161 class B PBPs on the basis of amino acid sequences. The tree was constructed by using ClustalX 2 [88] and S. coelicolor putative penicillin acylase (SCO4049, CAC32320) as outgroup. The sequences marked with yellow boxes were used for the calculation of molecular distances (Table S9). The bootstrap probabilities are shown at branching nodes. The phylogenetic tree were arbitrary divided to three groups, A, B, and C.
SCLAV_4179 belong to cluster B and SCLAV_4180 pertain to cluster A in the phylogenetic tree and the similarity of the amino-acid sequences is very low (E-value is 1.5 × 10–11). This character may be related to β-lactam production and these two PBPs seem to behave independently, because these two sequences are aligned in the reverse direction, i.e., SCLAV_4180 (pbpA) acts together with the clavulanic acid biosynthetic gene cluster, while SCLAV_4179 (pbp2) goes on with SCLAV_4178 (cytochrome P450, partial start) and SCLAV_4177 (RNA polymerase) or independently [78]. Moreover, PBP SCLAV_4179 in S. clavuligerus is reported to have a low affinity to β-lactam antibiotics and is essential to the growth [105]. Therefore, it is assumed that PBP SCLAV_4179 is involved in the self-resistance. SCLAV_4198 (class BPBP, pcbR) is located in the border between cephamycin and clavulanic acid biosynthetic gene clusters but behaves independently from these clusters, considering the gene direction. In S. cattleya which produces only cephamycin but not clavulanic acid, genes corresponding to SCLAV_4180 and SCLAV_4198 are deleted, but a gene (SCAT_5676) corresponding to SCLAV_4179 and responsible for self-resistance is retained [78]. This fact supports the above ideas (Figure S2).
Related to self-resistance, Ogawara and Horikawa reported nearly 40 years ago that β-lactam-producing Streptomyces species such as S. clavuligerus possessed PBPs of very low affinity to benzylpenicillin [106]. Later, two PBPs in S. clavuligerus, that is, SCLAV_4179 and SCLAV_4198 were reported to have low affinity to penicillins [105,107]. A mutant disrupted in SCLAV_4198 gene exhibited a significant decrease in its resistance to benzylpenicillin and cephalosporins [107]. Moreover, a probe containing SCLAV_4198 hybridized to genomic DNAs from β-lactam producers, S. jumonjinensis NRRL 5741, S. griseus NRRL 3851 and S. lipmanii NRRL 3584, suggesting that SCLAV_4198-like sequences and SCLAV_4198-mediated resistance mechanisms are likely to be present in these β-lactam-producing species. Likewise, most Streptomyces species have two PBPs in SCLAV_4198-belonging cluster C in the tree.
These characters are moderately similar with each other: SRIM_04191 and SRIM_06646 (1.4 × 10–59), SCLAV_4198 and SCLAV_2276 (8.1 × 10–27), F750_6320 and F750_2998 (5.6 × 10–29), STRVI_1135 and STRVI_3190 (1.2 × 10–61), and SBI_04376 and SBI_06233 (7.7 × 10–36) are examples. Together with the fact that tandem two genes in most Streptomyces species are associated with cluster C as described above, these results clearly indicate that most Streptomyces species are firmly defended by the presence of two low-affinity PBPs. Moreover, cluster B PBPs, to which SCLAV_4179 belong, reconfirm the self-resistance [103], that is, two pairs of low affinity PBPs (e.g., SCLAV_4198 and SCLAV_2276 in cluster C in Figure 5, and SCLAV_4179 and SCLAV_1774 in cluster B in Figure 5) guard Streptomyces from the attack of β-lactam antibiotics.
3. PASTA Domains: Connector between Penicillin-Binding Proteins and Protein Kinases
Protein phosphorylation was first described as a major regulatory mechanism in eukaryotes [108,109]. Later, the regulation by phosphorylation with serine/threonine protein kinases (STPKs) in particular has been expanded to prokaryotes [110,111,112,113]. While investigating the S. coelicolor homolog of PknB, a STPK, Yeats et al. identified a definitive domain in its C-terminus. This domain is also found in C-terminus of high molecular weight PBPs, so that it is termed as the PASTA domain (penicillin-binding protein and serine/threonine kinase-associated domain) [114]. PASTA domains are not found in eukaryotes and are restricted to firmicutes and Actinobacteria. Interestingly, some PASTA domains in PBPs such as PBP2x of S. pneumoniae bind peptidoglycan and β-lactam antibiotics [115], but others such as PonA2 of M. tuberculosis do not [116], indicating that PASTA domains in PBPs operate in a species-specific manner. On the other hand, PASTA domains in STPKs bind peptidoglycan and β-lactam antibiotics, and STPKs with PASTA domains are reported to play important roles in the regulation of bacterial cell division, and morphogenesis [111,112,114,117,118]. Previously, I described the distribution of PASTA domains in Actinobacteria [119], so in this review I discuss the interaction of PASTA domains in PBPs and STPK from the point of view of self-resistance.
In sharp contrast to other bacteria such as B. subtilis, Clostridium perfringens and S. pneumonia, PBPs with PASTA domains are detected only in class A PBPs but not in class B PBPs of Actinobacteria. In addition, none of Streptomyces species has PBPs with PASTA domain. Class A PBPs have both transglycosylase and transpeptidase domains, whereas class B PBPs have only transpeptidase domain. Therefore, it is interesting to know the interaction between transglycosylase and PASTA domains in Actinobacteria. However, it is not interpreted yet. Related to this fact, one of class B PBPs without PASTA domain and a STPK with four PASTA domains are located in adjacent position in most Streptomyces species (Table 6). For example, DC74_4185 and DC74_4186, XNR_3038 and XNR_3037, SAV_4339 and SAV_4338, SBI_05407 and SBI_05406, SCO3847 and SCO3848, and others. These PBPs belong to cluster Ac in a phylogenetic tree (Figure 5). The genomic structures in PBP-STPK regions of S. coelicolor, S. clavuligerus, and S. griseus are very similar and suggest that STPK, PBP and FtsW/RodA family protein form an operon (Table 7). In S. coelicolor, this family of PBP (i.e., SCO3847) is reported to form the Streptomyces spore wall synthesizing complex (SSSC) [120,121]. Although they are supposed to be transported to peptidoglycan biosynthesis machinery through the PASTA domains of STPK (i.e., SCO3848) and be involved in the peptidoglycan biosynthesis and/or morphogenesis coupled with PBPs and FtsW/RodA family proteins, Jones et al. reported that SCO3848 (PknB) regulates the timing of development and TCA cycle favoring antibiotic production together with two forkhead-associated proteins (FHA, SCO3843 and SCO3844) [122]. In addition, SCO3848 (PknB) are supposed to play coordinately with the protein phosphatase (i.e., SCO3845) inside the operon. It is very interesting to know how self-resistance is implicated in this reaction, because the identity and the similarity of PBPs of this group (cluster Ac in Figure 5) to SCLAV_4180 are in the range of 40%–91%, and 70%–95%, respectively and those of SCLAV_2947 (a low affinity PBP) and SCLAV_4180 are 53.3% and 80.2% in 497 amino acid residues, respectively (E value is 1.7 × 10–102). The indication that clusters Aa, Ab, and Ac PBPs in Figure 5 are close together with each other is confirmed by the molecular distance determination (Table S9). Cluster A PBPs in Figure 5 are also closely related with PBPs of pathogenic bacteria (Table S9). Unfortunately, however, the role of cluster A PBPs in self-resistance remains to be elucidated.

Table 7.
Comparison of genomic structures in PBP-STPK region of three Streptomyces species.
4. Relationship between β-Lactam Biosynthetic Gene, β-Lactamase and PBP: Additional Comments
The relationship between β-lactam biosynthetic gene clusters, β-lactamases, and PBPs of S. clavuligerus and S. cattleya was shown in Figure S1 [33,123,124,125,126,127]. The genetic organization of clavulanic acid biosynthetic gene cluster of S. clavuligerus was published [93,128]. Comparison of cephamycin gene clusters in S. clavuligerus and S. cattleya indicates clearly that clavulanic acid gene cluster from SCLAV_4180 to SCLAC_4198 in S. clavuligerus was inserted between SCAT_5676 and SCAT_5678 of S. cattleya (Figure S2). SCAT_5677 is missing. Interestingly, both sides of the clavulanic acid gene cluster are occupied by PBPs (SCLAV_4180 and SCLAV_4198), indicating that these PBPs behave together with clavulanic acid/cephamycin gene cluster and are involved in the self-resistance of S. clavuligerus. Similar gene constructs for cephamycin biosynthesis are observed in Nocardia lactamdurans, Lysobacter lactamgenus, Penicillium chrysogenum, and Acremonium chrysogenum [129,130,131,132]. However, β-lactamase and PBP genes can be detected neither in β-lactam biosynthetic gene clusters nor in whole genomes of P. chrysogenum, and A. chrysogenum. On the other hand, the organizations of clavulanic acid gene clusters of three Streptomyces species, S. clavuligerus, S. flavogirseus, and S. viridis are also similar with each other. Unfortunately, however, relationship between β-lactam biosynthesis, β-lactamases, and PBPs remains to be clarified, although β-lactamase was reported to be expressed during the active growth phase, prior to the formation of β-lactam antibiotics [129]. Recently, an interesting paper on taxonomy, physiology, and natural products of Actinobacteria was published [133].
5. Conclusions
Although antibiotics still play a key role for the prevention of microbial infections as remaining treasures from the twentieth century, antibiotic resistance is prevailing and putting us in a critical situation. Furthermore, it is said that the post-antibiotic era is coming soon [134]. As described in this paper, the β-lactamases in antibiotic-producing Streptomyces are diverse in their characteristics, and the PBPs are multiplexed in their guard systems. In addition, as antibiotic resistance mechanisms are supposed to originate and evolve in their producing microbes, and be transferred to pathogenic bacteria by transformation, transduction, transfection and/or conjugation, the public health crisis will be getting worse and worse. β-Lactamases and PBPs are two major resistance mechanisms in pathogenic bacteria against β-lactam antibiotics, the most frequently used antibiotics for infectious diseases at the present time. Moreover, from about 40 years ago, the rate of discovery of new antibiotics has declined rapidly and many large pharmaceutical companies have abandoned research and development on antibiotics [135]. To avoid this situation, therefore, it is urgently needed to make the public and the government recognize the situation. In addition, new antibiotics should be screened by using various technologies such as activation of cryptic gene clusters for antibiotic biosynthesis, metagenomics mining, combinatorial biosynthesis, target-directed computer-aided chemical synthesis, systems biology, genetic manipulation, omics, and so on [136,137,138,139,140,141]. Furthermore, combination therapies with monoclonal antibodies and vaccines are also required. When these requirements are attained, “New Golden Age” is possible [141]. However, it is worth mentioning again that the resistance mechanisms in Streptomyces species are very hard to deal with as described in this paper.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/21/5/605/s1.
Conflicts of Interest
The author declares no conflict of interest.
References
- Fleming, A. On the antibacterial action of cultures of penicillium, with special reference to their use in the isolation of B. influenzae. Br. J. Exp. Pathol. 1929, 10, 226–236. [Google Scholar] [CrossRef]
- Chain, E.; Florey, H.W.; Gardner, A.D.; Heatley, N.G.; Jennings, M.A.; Orr-Ewing, J.; Sanders, A.G. Penicillin as a chemotherapeutic agent. Lancet 1940, 236, 226–228. [Google Scholar] [CrossRef]
- Abraham, E.P.; Chain, E.; Fletcher, C.M.; Florey, H.W.; Gardner, A.D.; Heatley, N.G.; Jennings, M.A. Further observations in penicillin. Lancet 1941, 238, 177–189. [Google Scholar] [CrossRef]
- Abraham, E.P.; Chain, E. An enzyme from bacteria able to destroy penicillin. Rev. Infect. Dis. 1940, 10, 677–678. [Google Scholar] [CrossRef]
- Plough, H.H. Penicillin resistance of Staphylococcus aureus and its clinical implications. Am. J. Clin. Pathol. 1945, 15, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Bondi, A., Jr.; Dietz, C.C. Penicillin resistant staphylococci. Proc. Soc. Exp. Biol. Med. 1945, 60, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, K.R. Studies on induced resistance to penicillin in pneumococcus type I. Acta Pathol. Microbiol. Scand. 1945, 22, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Gots, J.S. Production of extracellular penicillin-inactivating substances associated with penicillin resistance in Staphylococcus aureus. Proc. Soc. Exp. Biol. Med. 1945, 60, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.P.; Bohnhoff, M. Studies on the action of penicillin; development of penicillin resistance by gonococcus. Proc. Soc. Exp. Biol. Med. 1945, 60, 354–356. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.P.; Bohnhoff, M. Studies on action of penicillin; virulence of penicillin resistant strains of meningococcus. Proc. Soc. Exp. Biol. Med. 1945, 60, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Shwartzman, G. Studies on the nature of resistance of Gram-negative bacilli to penicillin. J. Exp. Med. 1946, 83, 65–88. [Google Scholar] [CrossRef] [PubMed]
- Waksman, S.A.; Reilly, H.C.; Schatz, A. Strain specificity and production of antibiotic substances. V. Strain resistance of bacteria to antibiotic substances, especially streptomycin. Proc. Natl. Acad. Sci. USA 1945, 31, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Ogawara, H.; Horikawa, S.; Shimada-Miyoshi, S.; Yasuzawa, K. Production and property of beta-lactamases in Streptomyces: Comparison of the strains isolated newly and thirty years ago. Antimicrob. Agents Chemother. 1978, 13, 865–870. [Google Scholar] [CrossRef] [PubMed]
- D’Costa, V.M.; King, C.E.; Kalan, L.; Morar, M.; Sung, W.W.; Schwarz, C.; Froese, D.; Zazula, G.; Calmels, F.; Debruyne, R.; et al. Antibiotic resistance is ancient. Nature 2011, 477, 457–461. [Google Scholar] [CrossRef] [PubMed]
- About antimicrobial resistance. Available online: http://www.cdc.gov/drugresistance/ (accessed on 20 March 2016).
- Liu, B.; Pop, M. ARDB—Antibiotic resistance genes database. Nucleic Acids Res. 2009, 37, D443–D447. Available online: http://ardb.cbcb.umd.edu/ (accessed on 20 March 2016). [Google Scholar] [CrossRef] [PubMed]
- Walker, M.S.; Walker, J.B. Streptomycin biosynthesis and metabolism. Enzymatic phosphorylation of dihydrostreptobiosamine moieties of dihydrostreptomycin-(streptidino) phosphate and dihydrostreptomycin by Streptomyces extracts. J. Biol. Chem. 1970, 245, 6683–6689. [Google Scholar] [PubMed]
- Benveniste, R.; Davies, J. Aminoglycoside antibiotic-inactivating enzymes in actinomycetes similar to those present in clinical isolates of antibiotic-resistant bacteria. Proc. Natl. Acad. Sci. USA 1973, 70, 2276–2280. [Google Scholar] [CrossRef] [PubMed]
- Ogawara, H. Antibiotic resistance in pathogenic and producing bacteria, with special reference to β-lactam antibiotics. Microbiol. Rev. 1981, 45, 591–619. [Google Scholar] [PubMed]
- Dantas, G.; Sommer, M.O.; Oluwasegun, R.D.; Church, G.M. Bacteria subsisting on antibiotics. Science 2008, 320, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Riesenfeld, C.S.; Goodman, R.M.; Handelsman, J. Uncultured soil bacteria are a reservoir of new antibiotic resistance genes. Environ. Microbiol. 2004, 6, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.D. The antibiotic resistome: The nexus of chemical and genetic diversity. Nat. Rev. Microbiol. 2007, 5, 175–186. [Google Scholar] [CrossRef] [PubMed]
- D’Costa, V.M.; McGrann, K.M.; Hughes, D.W.; Wright, G.D. Sampling the antibiotic resistome. Science 2006, 311, 374–377. [Google Scholar] [CrossRef] [PubMed]
- Forsberg, K.J.; Reyes, A.; Wang, B.; Selleck, E.M.; Sommer, M.O.; Dantas, G. The shared antibiotic resistome of soil bacteria and human pathogens. Science 2012, 337, 1107–1111. [Google Scholar] [CrossRef] [PubMed]
- Mole, B. MRSA: Farming up trouble. Nature 2013, 499, 398–400. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.L. Antibiotics and antibiotic resistance genes in natural environments. Science 2008, 321, 365–367. [Google Scholar] [CrossRef] [PubMed]
- Aminov, R.I. Horizontal gene exchange in environmental microbiota. Front. Microl. 2011, 2. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.A.; Wright, G.D. The antibiotic resistance “mobilome”: Searching for the link between environment and clinic. Front. Microbiol. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Francino, M.P. Antibiotics and the human gut microbiome: Dysbioses and accumulation of resistance. Front. Micribiol. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Gupta, R.S. Phylogenetic framework and molecular signatures for the main clades of the phylum Actinobacteria. Microbiol. Mol. Biol. Rev. 2012, 76, 66–112. [Google Scholar] [CrossRef] [PubMed]
- Ventura, M.; Canchaya, C.; Tauch, A.; Chandra, G.; Fitzgerald, G.F.; Chater, K.F.; van Sinderen, D. Genomics of Actinobacteria: Tracing the evolutionary history of an ancient phylum. Microbiol. Mol. Biol. Rev. 2007, 71, 495–548. [Google Scholar] [CrossRef] [PubMed]
- Chater, K.F.; Biró, S.; Lee, K.J.; Palmer, T.; Schrempf, H. The complex extracellular biology of Streptomyces. FEMS Microbiol. Rev. 2010, 34, 171–198. [Google Scholar] [CrossRef] [PubMed]
- Liras, P.; Martin, J.F. Gene clusters for β-lactam antibiotics and control of their expression: Why have clusters evolved, and from where did they originate? Int. Microbiol. 2006, 9, 9–19. [Google Scholar] [PubMed]
- Liu, G.; Chater, K.F.; Chandra, G.; Niu, G.; Tan, H. Molecular regulation of antibiotic biosynthesis in streptomyces. Microbiol. Mol. Biol. Rev. 2013, 77, 112–143. [Google Scholar] [CrossRef] [PubMed]
- Kahan, J.S.; Kahan, F.M.; Goegelman, R.; Currie, S.A.; Jackson, M.; Stapley, E.; Miller, T.W.; Miller, A.K.; Hendlin, D.; Mochales, S.; et al. Thienamycin, a new beta-lactam antibiotic. I. Discovery, taxonomy, isolation and physical properties. J. Antibiot. 1979, 32, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Reading, C.; Cole, M. Clavulanic acid: A beta-lactamase-inhiting beta-lactam from Streptomyces clavuligerus. Antimicrob. Agents Chemother. 1977, 11, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.L. Penicillin binding proteins, β-lactams, and lactamases: Offensives, attacks, and defensive countermeasures. Crit. Rev. Microbiol. 2000, 26, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.D.; Maas, W.K. Analysis of the biochemical mechanism of drug resistance in certain bacterial mutants. Proc. Natl. Acad. Sci. USA 1952, 38, 775–785. [Google Scholar] [CrossRef] [PubMed]
- The Comprehensive Antibiotic Resistance Database. Available online: http://arpcard.mcmaster.ca/ (accessed on 20 March 2016).
- King, D.T.; Sobhanifar, S.; Strynadka, N.C. One ring to rule them all: Current trends in combating bacterial resistance to the β-lactams. Protein Sci. 2016. [Google Scholar] [CrossRef] [PubMed]
- Cundliffe, E.; Demain, A.L. Avoidance of suicide in antibiotic-producing microbes. J. Ind. Microbiol. Biotechnol. 2010, 37, 643–672. [Google Scholar] [CrossRef] [PubMed]
- McArthur, A.G.; Waglechner, N.; Nizam, F.; Yan, A.; Azad, M.A.; Baylay, A.J.; Bhullar, K.; Canova, M.J.; De Pascale, G.; Ejim, L.; et al. The comprehensive antibiotic resistance database. Antimicrob. Agents Chemother. 2013, 57, 3348–3357. [Google Scholar] [CrossRef] [PubMed]
- Gibson, M.K.; Forsberg, K.J.; Dantas, G. Improved annotation of antibiotic resistance determinants reveals microbial resistomes cluster by ecology. ISME J. 2015, 9, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Richmond, M.H.; Sykes, R.B. The β-lactamases of gram-negative bacteria and their possible physiological role. Adv. Microb. Physiol. 1973, 9, 31–88. [Google Scholar] [PubMed]
- Ogawara, H. Production and property of β-lactamases in Streptomyces. Antimicrob. Agents Chemother. 1975, 8, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Abraham, E.P.; Waley, S.G. Beta-Lactamases; Hamilton-Miller, J.M.T., Smith, J.T., Eds.; Academic Press: London, UK, 1979; p. 338. [Google Scholar]
- Kushner, D.J.; Breuil, C. Penicillinase (β-lactamase) formation by blue-green algae. Arch. Microbiol. 1977, 112, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Urbach, C.; Fastrez, J.; Soumillion, P. A new family of cyanobacterial penicillin-binding proteins. A missing link in the evolution of class A β-lactamases. J. Biol. Chem. 2008, 283, 32516–32526. [Google Scholar] [CrossRef] [PubMed]
- Podzelinska, K.; He, S.-M.; Wathier, M.; Yakunin, A.; Proudfoot, M.; Hove-Jensen, B.; Zechel, D.L.; Jia, Z. Structure of PhnP, a phosphodiesterase of the carbon-phosphorus lyase pathway for phosphonate degradation. J. Biol. Chem. 2009, 284, 17216–17226. [Google Scholar] [CrossRef] [PubMed]
- Sattler, S.A.; Wang, X.; Lewis, K.M.; DeHan, P.J.; Park, C.M.; Xin, Y.; Liu, H.; Xian, M.; Xun, L.; Kang, C. Characterizations of two bacterial persulfide dioxygenases of the metallo-β-lactamase superfamily. J. Biol. Chem. 2015, 290, 18914–18923. [Google Scholar] [CrossRef] [PubMed]
- Daiyasu, H.; Osaka, K.; Ishino, Y.; Toh, H. Expansion of the zinc metallo-hydrolase family of the β-lactamase fold. FEBS Lett. 2001, 503, 1–6. [Google Scholar] [CrossRef]
- Dominski, Z. Nucleases of the metallo-β-lactamase family and their role in DNA and RNA metabolism. Crit. Rev. Biochem. Mol. Biol. 2007, 42, 67–93. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Schilling, O.; Späth, B.; Marchfelder, A. The tRNase Z family of proteins: Physiological functions, substrate specificity and structural properties. Biol. Chem. 2005, 386, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Lapidus, A.; Clum, A.; Labutti, K.; Kaluzhnaya, M.G.; Lim, S.; Beck, D.A.; del Glavina Rio, T.; Nolan, M.; Mavromatis, K.; Huntemann, M.; et al. Genomes of three methylotrophs from a single niche reveal the genetic and metabolic divergence of the Methylophilaceae. J. Bacteriol. 2011, 193, 3757–3764. [Google Scholar] [CrossRef] [PubMed]
- Limphong, P.; Nimako, G.; Thomas, P.W.; Fast, W.; Makaroff, C.A.; Crowder, M.W. Arabidopsis thaliana mitochondrial glyoxalase 2-1 exhibits β-lactamase activity. Biochemistry 2009, 48, 8491–8493. [Google Scholar] [CrossRef] [PubMed]
- Florea, L.; Di Francesco, V.; Miller, J.; Turner, R.; Yao, A.; Harris, M.; Walenz, B.; Mobarry, C.; Merkulov, G.V.; Charlab, R.; et al. Gene and alternative splicing annotation with AIR. Genome Res. 2005, 15, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.S.; Southan, C.; Ellington, K.; Campbell, D.; Tew, D.G.; Debouck, C. Identification, genomic organization, and mRNA expression of LACTB, encoding a serine β-lactamase-like protein with an amino-terminal transmembrane domain. Genomics 2001, 78, 12–14. [Google Scholar] [CrossRef] [PubMed]
- Toth, M.; Smith, C.; Frase, H.; Mobashery, S.; Vakulenko, S. An antibiotic-resistance enzyme from a deep-sea bacterium. J. Am. Chem. Soc. 2010, 132, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Michaux, C.; Massant, J.; Kerff, F.; Frère, J.-M.; Docquier, J.D.; Vandenberghe, I.; Samyn, B.; Pierrard, A.; Feller, G.; Charlier, P.; et al. Crystal structure of a cold-adapted class C β-lactamase. FEBS J. 2008, 275, 1687–1697. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-B.; Joo, S.; Yoon, S.; Kim, S.; Moon, J.; Ryu, Y.; Kim, K.K.; Kim, T.D. Purification, crystallization and preliminary crystallographic analysis of Est-Y29: A novel oligomeric β-lactamase. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2009, 65, 310–312. [Google Scholar] [CrossRef] [PubMed]
- Allen, H.K.; Moe, L.; Rodbumrer, J.; Gaarder, A.; Handelsman, J. Functional metagenomics reveals diverse β-lactamases in a remote Alaskan soil. ISME J. 2009, 3, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Donato, J.J.; Moe, L.A.; Converse, B.J.; Smart, K.D.; Berklein, F.; McManus, P.S.; Handelsman, J. Metagenomic analysis of apple orchard soil reveals antibiotic resistance genes encoding predicted bifunctional proteins. Appl. Environ. Microbiol. 2010, 76, 4396–4401. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhang, L.; Zhang, H.; He, Z.G. Characterization of a bifunctional β-lactamase/ribonuclease and its interaction with a chaperone-like protein in the pathogen Mycobacterium tuberculosis H37Rv. Biochemistry 2011, 76, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Kumar, A.; Arya, S.; Gupta, U.D.; Singh, K.; Kaur, J. Characterization of a novel esterase Rv1497 of Mycobacterium tuberculosis H37Rv demonstrating β-lactamase activity. Enzyme Microb. Technol. 2016, 82, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Ambler, R.P. The structure of beta-lactamases. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1980, 289, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Bush, K.; Jacoby, G.A. Updated functional classification of β-lactamases. Antimicrob. Agents Chemother. 2010, 54, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Jaurin, B.; Grundström, T. ampC cephalosporinase of Escherichia coli K-12 has a different evolutionary origin from that of beta-lactamases of the penicillinase type. Proc. Natl. Acad. Sci. USA 1981, 78, 4897–4901. [Google Scholar] [CrossRef] [PubMed]
- Ouellette, M.; Bissonnette, L.; Roy, P.H. Precise insertion of antibiotic resistance determinants into Tn21-like transposons: Nucleotide sequence of the OXA-1 β-lactamase gene. Proc. Natl. Acad. Sci. USA 1987, 84, 7378–7382. [Google Scholar] [CrossRef] [PubMed]
- Palzkill, T. Metallo-β-lactamase structure and function. Ann. N. Y. Acad. Sci. 2013, 1277, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Frère, J.M.; Galleni, M.; Bush, K.; Dideberg, O. Is it necessary to change the classification of β-lactamases? J. Antimicrob. Chemother. 2005, 55, 1051–1053. [Google Scholar] [CrossRef] [PubMed]
- Bush, K.; Jacoby, G.A.; Medeiros, A.A. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob. Agents Chemother. 1995, 39, 1211–1233. [Google Scholar] [CrossRef] [PubMed]
- Bradford, P.A. Extended-spectrum β-lactamases in the 21st century: Characterization, epidemiology, and detection of this important resistance threat. Clin. Microbiol. Rev. 2001, 14, 933–951. [Google Scholar] [CrossRef] [PubMed]
- Pettinati, I.; Brem, J.; Lee, S.Y.; McHugh, P.J.; Schofield, C.J. The chemical biology of human metallo-β-lactamase fold proteins. Trends Biochem. Sci. 2016, 41, 338–355. [Google Scholar] [CrossRef] [PubMed]
- Bebrone, C. Metallo-β-lactamases (classification, activity, genetic organization, structure, zinc coordination) and their superfamily. Biochem. Pharmacol. 2007, 74, 1686–1701. [Google Scholar] [CrossRef] [PubMed]
- Garau, G.; Di Guilmi, A.M.; Hall, B.G. Structure-based phylogeny of the metallo-β-lactamases. Antimicrob. Agents Chemother. 2005, 49, 2778–2784. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.R.; Toleman, M.A.; Poirel, L.; Nordmann, P. Metallo-β-lactamases: The quiet before the storm? Clin. Microbiol. Rev. 2005, 18, 306–325. [Google Scholar] [CrossRef] [PubMed]
- Ogawara, H. Phylogenetic tree and sequence similarity of β-lactamases. Mol. Phylogenet. Evol. 1993, 2, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Ogawara, H. Self-resistance to β-lactam antibiotics in Streptomyces (in Japanese). Bull. Meiji Pharmaceut. Univ. 2014, 43, 1–20. [Google Scholar]
- Ogawara, H. Molecular phylogenetics of β-lactamases in Actinobacteria. Bull. Meiji Pharmaceut. Univ. 2013, 42, 1–18. [Google Scholar]
- BioProject. Available online: http://www.ncbi.nlm.nih.gov/guide/all/ (accessed on 10 September 2011).
- Stackebrandt, E.; Rainey, F.A.; Ward-Rainey, N.L. Proposal for a New Hierarchic Classification System, Actinobacteria classis nov. Int. J. Syst. Bacteriol. 1997, 47, 479–491. [Google Scholar] [CrossRef]
- Ogawara, H.; Kawamura, N.; Kudo, T.; Suzuki, K.I.; Nakase, T. Distribution of β-lactamases in actinomycetes. Antimicrob. Agents Chemother. 1999, 43, 3014–3017. [Google Scholar] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Notredame, C.; Higgins, D.G.; Heringa, J. T-Coffee: A novel method for fast and accurate multiple sequence alignment. J. Mol. Biol. 2000, 302, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Galleni, M.; Lamotte-Brasseur, J.; Rossolini, G.M.; Spencer, J.; Dideberg, O.; Frère, J.-M. Metallo-β-lactamases Working Group. Standard numbering scheme for class B beta-lactamases. Antimicrob. Agents Chemother. 2001, 45, 660–663. [Google Scholar] [CrossRef] [PubMed]
- Lobkovsky, E.; Moews, P.C.; Liu, H.; Zhao, H.; Frere, J.-M.; Knox, J.R. Evolution of an enzyme activity: Crystallographic structure at 2-A resolution of cephalosporinase from the ampC gene of Enterobacter cloacae P99 and comparison with a class A penicillinase. Proc. Natl. Acad. Sci. USA 1993, 90, 11257–11261. [Google Scholar] [CrossRef] [PubMed]
- Goffin, C.; Ghuysen, J.M. Biochemistry and comparative genomics of SxxK superfamily acyltransferases offer a clue to the mycobacterial paradox: Presence of penicillin-susceptible target proteins versus lack of efficiency of penicillin as therapeutic agent. Microbiol. Mol. Biol. Rev. 2002, 66, 702–738. [Google Scholar] [CrossRef] [PubMed]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed]
- β-Lactamase Classification and Amino Acid Sequences for TEM, SHV and OXA Extended-Spectrum and Inhibitor Resistant Enzymes. Available online: http://www.lahey.org/studies/ (accessed on 20 March 2016).
- Protdist-Program to Compute Distance Matrix from Protein Sequences. Available online: http://evolution.genetics.washington.edu/phylip/doc/protdist.html (accessed on 20 March 2016).
- Urabe, H.; Toyama, K.; Ogawara, H. Cloning from Streptomyces cellulosae of the gene encoding beta-lactamase, a blue-dextran binding protein. J. Antibiot. 1990, 43, 1483–1488. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ogawara, H. Sequence of a gene encoding β-lactamase from Streptomyces cellulosae. Gene 1993, 124, 111–114. [Google Scholar] [CrossRef]
- Jensen, S.E.; Paradkar, A.S.; Mosher, R.H.; Anders, C.; Beatty, P.H.; Brumlik, M.J.; Griffin, A.; Barton, B. Five additional genes are involved in clavulanic acid biosynthesis in Streptomyces clavuligerus. Antimicrob. Agents Chemother. 2004, 48, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Khaleeli, N.; Townsend, C.A. Expansion of the clavulanic acid gene cluster: Identification and in vivo functional analysis of three new genes required for biosynthesis of clavulanic acid by Streptomyces clavuligerus. J. Bacteriol. 2000, 182, 4087–4095. [Google Scholar] [CrossRef] [PubMed]
- Jacoby, G.A. AmpC β-lactamases. Clin. Microbiol. Rev. 2009, 22, 161–182. [Google Scholar] [CrossRef] [PubMed]
- Macheboeuf, P.; Contreras-Martel, C.; Job, V.; Dideberg, O.; Dessen, A. Penicillin binding proteins: Key players in bacterial cell cycle and drug resistance processes. FEMS Microbiol. Rev. 2006, 30, 673–691. [Google Scholar] [CrossRef] [PubMed]
- Typas, A.; Banzhaf, M.; Gross, C.A.; Vollmer, W. From the regulation of peptidoglycan synthesis to bacterial growth and morphology. Nat. Rev. Microbiol. 2012, 10, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Van Heijenoort, J. Lipid intermediates in the biosynthesis of bacterial peptidoglycan. Microbiol. Mol. Biol. Rev. 2007, 71, 620–635. [Google Scholar] [CrossRef] [PubMed]
- Suginaka, H.; Blumberg, P.M.; Strominger, J.L. Multiple penicillin-binding components in Bacillus subtilis, Bacillus cereus, Staphylococcus aureus, and Escherichia coli. J. Biol. Chem. 1972, 247, 5279–5288. [Google Scholar] [PubMed]
- Spratt, B.G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc. Natl. Acad. Sci. USA 1975, 72, 2999–3003. [Google Scholar] [CrossRef] [PubMed]
- Pratt, R.F. Substrate specificity of bacterial DD-peptidases (penicillin-binding proteins). Cell. Mol. Life Sci. 2008, 65, 2138–2155. [Google Scholar] [CrossRef] [PubMed]
- Sauvage, E.; Kerff, F.; Terrak, M.; Ayala, J.A.; Charlier, P. The penicillin-binding proteins: Structure and role in peptidoglycan biosynthesis. FEMS Microbiol. Rev. 2008, 32, 234–258. [Google Scholar] [CrossRef] [PubMed]
- Ogawara, H. Penicillin-binding proteins in Actinobacteria. J. Antib. 2015, 68, 223–245. [Google Scholar] [CrossRef] [PubMed]
- Goffin, C.; Ghuysen, J.M. Multimodular penicillin-binding proteins: An enigmatic family of orthologs and paralogs. Microbiol. Mol. Biol. Rev. 1998, 62, 1079–1093. [Google Scholar] [PubMed]
- Ishida, K.; Hung, T.V.; Liou, K.; Lee, H.C.; Shin, C.H.; Sohng, J.K. Characterization of pbpA and pbp2 encoding penicillin-binding proteins located on the downstream of clavulanic acid gene cluster in Streptomyces clavuligerus. Biotechnol. Lett. 2006, 28, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Ogawara, H.; Horikawa, S. Penicillin-binding proteins of Streptomyces cacaoi, Streptomyces olivaceus, and Streptomyces clavuligerus. Antimicrob. Agents Chemother. 1980, 17, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Paradkar, A.S.; Aidoo, K.A.; Wong, A.; Jensen, S.E. Molecular analysis of a β-lactam resistance gene encoded within the cephamycin gene cluster of Streptomyces clavuligerus. J. Bacteriol. 1996, 178, 6266–6274. [Google Scholar] [PubMed]
- Hanks, S.K.; Hunter, T. Protein kinases 6. The eukaryotic protein kinase superfamily: Kinase (catalytic) domain structure and classification. FASEB J. 1995, 9, 576–596. [Google Scholar] [PubMed]
- Hunter, T. Tyrosine phosphorylation: Thirty years and counting. Curr Opin. Cell Biol. 2009, 21, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Dorado, J.; Inouye, S.; Inouye, M. A gene encoding a protein serine/threonine kinase is required for normal development of M. xanthus, a gram-negative bacterium. Cell 1991, 67, 995–1006. [Google Scholar] [CrossRef]
- Pereira, S.F.; Goss, L.; Dworkin, J. Eukaryote-like serine/threonine kinases and phosphatases in bacteria. Microbiol. Mol. Biol. Rev. 2011, 75, 192–212. [Google Scholar] [CrossRef] [PubMed]
- Manuse, S.; Fleurie, A.; Zucchini, L.; Lesterlin, C.; Grangeasse, C. Role of eukaryotic-like serine/threonine kinases in bacterial cell division and morphogenesis. FEMS Microbiol. Rev. 2016, 40, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, L.E.; Koonin, E.V.; Zhulin, I.B. One-component systems dominate signal transduction in prokaryotes. Trends. Microbiol. 2005, 13, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Yeats, C.; Finn, R.D.; Bateman, A. The PASTA domain: A β-lactam-binding domain. Trends Biochem. Sci. 2002, 27, 438–440. [Google Scholar] [CrossRef]
- Gordon, E.; Mouz, N.; Duée, E.; Dideberg, O. The crystal structure of the penicillin-binding protein 2x from Streptococcus pneumoniae and its acyl-enzyme form: Implication in drug resistance. J. Mol. Biol. 2000, 299, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Calvanese, L.; Falcigno, L.; Maglione, C.; Marasco, D.; Ruggiero, A.; Squeglia, F.; Berisio, R.; D’Auria, G. Structural and binding properties of the PASTA domain of PonA2, a key penicillin binding protein from Mycobacterium tuberculosis. Biopolymers 2014, 101, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, A.; De Simone, P.; Smaldone, G.; Squeglia, F.; Berisio, R. Bacterial cell division regulation by Ser/Thr kinases: A structural perspective. Curr. Protein Pept. Sci. 2012, 13, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Peters, K.; Schweizer, I.; Beilharz, K.; Stahlmann, C.; Veening, J.W.; Hakenbeck, R.; Denapaite, D. Streptococcus pneumoniae PBP2x mid-cell localization requires the C-terminal PASTA domains and is essential for cell shape maintenance. Mol. Microbiol. 2014, 92, 733–755. [Google Scholar] [CrossRef] [PubMed]
- Ogawara, H. Distribution of PASTA domains in penicillin-binding proteins and serine/threonine kinases of Actinobacteria. J. Antibiot. 2016. [Google Scholar] [CrossRef] [PubMed]
- McCormick, J.R.; Flärdh, K. Signals and regulators that govern Streptomyces development. FEMS Micribiol. Rev. 2012, 36, 206–231. [Google Scholar] [CrossRef] [PubMed]
- Ladwig, N.; Franz-Wachtel, M.; Hezel, F.; Soufi, B.; Macek, B.; Wohlleben, W.; Muth, G. Control of morphological differentiation of Streptomyces coelicolor A3(2) by phosphorylation of MreC and PBP2. PLoS ONE 2015, 10, e0125425. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Del Sol, R.; Dudley, E.; Dyson, P. Forkhead-associated proteins genetically linked to the serine/threonine kinase PknB regulate carbon flux towards antibiotic biosynthesis in Streptomyces coelicolor. Microb. Biotechnol. 2011, 4, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Llarena, F.; Martín, J.F.; Galleni, M.; Coque, J.J.; Fuente, J.L.; Frère, J.M.; Liras, P. The bla gene of the cephamycin cluster of Streptomyces clavuligerus encodes a class A β-lactamase of low enzymatic activity. J. Bacteriol. 1997, 179, 6028–6034. [Google Scholar]
- Streptomyces clavuligerus ATCC 27064 chromosome, whole genome shotgun sequence. Available online: http://www.ncbi.nlm.nih.gov/nuccore/294323433?report=graph (accessed on 20 March 2016).
- Streptomyces cattleya NRRL 8057 = DSM 46488, complete genome. Available online: http://www.ncbi.nlm.nih.gov/nuccore/357397620?report=graph (accessed on 20 March 2016).
- Jensen, S.E. Biosynthesis of clavam metabolites. J. Ind. Microbiol. Biotechnol. 2012, 39, 1407–1419. [Google Scholar] [CrossRef] [PubMed]
- Paradkar, A. Clavulanic acid production by Streptomyces clavuligerus: Biogenesis, regulation and strain improvement. J. Antibiot. 2013, 66, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Mellado, E.; Lorenzana, L.M.; Rodríguez-Sáiz, M.; Díez, B.; Liras, P.; Barredo, J.L. The clavulanic acid biosynthetic cluster of Streptomyces clavuligerus: Genetic organization of the region upstream of the car gene. Microbiology 2002, 148, 1427–1438. [Google Scholar]
- Coque, J.J.; Liras, P.; Martín, J.F. Genes for a β-lactamase, a penicillin-binding protein and a transmembrane protein are clustered with the cephamycin biosynthetic genes in Nocardia lactamdurans. EMBO J. 1993, 12, 631–639. [Google Scholar] [PubMed]
- Kimura, H.; Izawa, M.; Sumino, Y. Molecular analysis of the gene cluster involved in cephalosporin biosynthesis from Lysobacter lactamgenus YK90. Appl. Microbiol. Biotechnol. 1996, 44, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.F. Clusters of genes for the biosynthesis of antibiotics: Regulatory genes and overproduction of pharmaceuticals. J. Ind. Microbiol. 1992, 9, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Brakhage, A.A.; Al-Abdallah, Q.; Tüncher, A.; Spröte, P. Evolution of β-lactam biosynthesis genes and recruitment of trans-acting factors. Phytochemistry 2005, 66, 1200–1210. [Google Scholar] [CrossRef] [PubMed]
- Barka, E.A.; Vatsa, P.; Sanchez, L.; Gaveau-Vaillant, N.; Jacquard, C.; Klenk, H.P.; Clément, C.; Ouhdouch, Y.; van Wezel, G.P. Taxonomy, Physiology, and Natural Products of Actinobacteria. Microbiol. Mol. Biol. Rev. 2016, 80. [Google Scholar] [CrossRef] [PubMed]
- Alanis, A.J. Resistance to antibiotics: Are we in the post-antibiotic era? Arch. Med. Res. 2005, 36, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.A.; Shlaes, D. Fix the antibiotics pipeline. Nature 2014, 472. [Google Scholar] [CrossRef] [PubMed]
- Rokem, J.S.; Lantz, A.E.; Nielsen, J. Systems biology of antibiotic production by microorganisms. Nat. Prod. Rep. 2007, 24, 1262–1287. [Google Scholar] [CrossRef] [PubMed]
- Bull, A.T.; Stach, J.E. Marine actinobacteria: New opportunities for natural product search and discovery. Trends Microbiol. 2007, 15, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Demain, A.L. Importance of microbial natural products and the need to revitalize their discovery. J. Ind. Microbiol. Biotechnol. 2014, 41, 185–201. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, B.O.; Van Lanen, S.G.; Baltz, R.H. Microbial genome mining for accelerated natural products discovery: Is a renaissance in the making? J. Ind. Microbiol. Biotechnol. 2014, 41, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Katz, L.; Baltz, R.H. Natural product discovery: Past, present, and future. J. Ind. Microbiol. Biotechnol. 2016, 43, 155–176. [Google Scholar] [CrossRef] [PubMed]
- Baltz, R.H. Genetic manipulation of secondary metabolite biosynthesis for improved production in Streptomyces and other actinomycetes. J. Ind. Microbiol. Biotechnol. 2016, 43, 343–370. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).