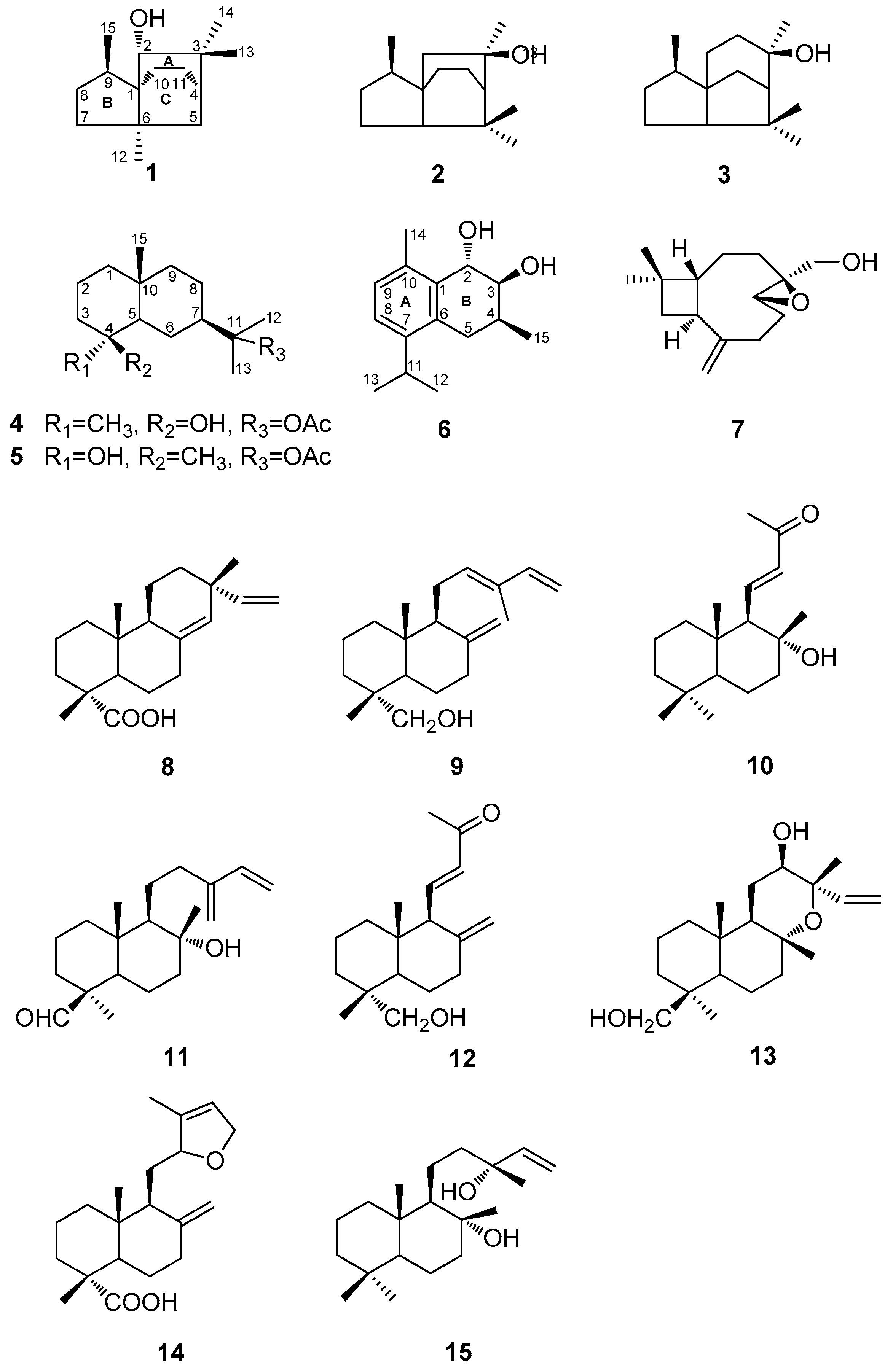
Abstract
Three new sesquiterpenoids, 2α-hydroxy-3,3,6α,9β-tetramethyltricyclo[4,3,21,4]undecane (1), 11-acetoxyeudesman-4β-ol (4), and 2α,3β-dihydroxy-4β-methyl-6,8,10-cadinatriene (6), four known sesquiterpenoids (2, 3, 5, and 7), together with eight known diterpenoids (8–15), were isolated from the wood of Cunninghamia konishii. Their structures were determined by detailed analysis of spectroscopic data and comparison with the data of known analogues. Four sesquiterpenoids (1, 4, 5, and 6) and all the diterpenoids (8–15) were evaluated for inhibition of nitric oxide production in lipopolysaccharides (LPS)-activated RAW 264.7 macrophages and the results showed that compounds 10 and 15 exhibited moderate inhibitory activities against nitric oxide production.
1. Introduction
The genus Cunninghamia contains two species occurring in eastern Asia, Cunninghamia konishii and C. lanceolata. C. konishii, an endemic Taiwanese coniferous tree up to 50 m tall and with a 1–2.5 m trunk diameter, grows in the northern and central forests of Taiwan at elevations ranging from 1300 to 2700 m [1]. Its wood exhibits soft, lightweight, aromatic, and rot-resistant properties, and thus is one of the best building materials and wood products available in Taiwan. A series of monoterpenes, sesquiterpenes, diterpenes, and lignans were found in its wood [2,3,4,5,6,7,8,9,10,11,12], bark [13], leaf [8], and whole plant [14], some of which have been proven to possess anti-inflammatory [10], antifungal [8,9], and cytotoxic [14] activities. As part of our program to search for secondary metabolites from this plant, we had reported the isolation and structure elucidation of 27 diterpenoids and two lignans from the wood of this plant [6,7,10,11,12,15,16]. In our continuing study of new chemicals from the wood of C. konishii, three new sesquiterpenoids, 2α-hydroxy-3,3,6α,9β-tetramethyltricyclo[4,3,21,4]undecane (1), 11-acetoxyeudesman-4β-ol (4), and 2α,3β-dihydroxy-4β-methyl-6,8,10-cadinatriene (6), four known sesquiterpenoids (2, 3, 5, and 7), together with three known diterpenoids (8–10), were isolated (Figure 1). Herein, we reported the extraction, isolation, and structure elucidation of compounds 1, 4, and 6. Among these isolated compounds, four sesquiterpenoids (1, 4, 5, and 6) and eight diterpenoids (8–15), including five diterpenoids that we reported previously (11–15), were evaluated for their inhibitory effects on lipopolysaccharides (LPS)-induced nitric oxide production in RAW 264.7 cells.
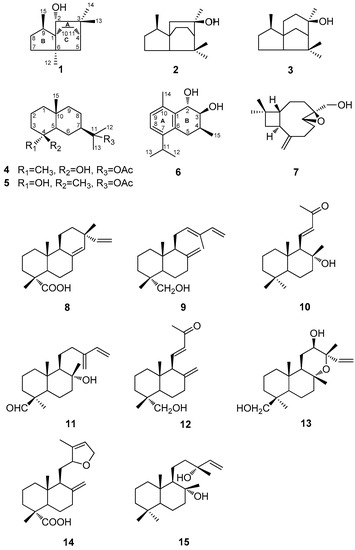
Figure 1.
The chemical structures of compounds 1–15 isolated from Cunninghamia konishii.
2. Results and Discussion
2.1. Isolation and Structural Elucidation
The MeOH extract of the wood of C. konishii was concentrated to give a brown residue, which was suspended in water and partitioned with EtOAc and n-BuOH, successively. The combined EtOAc soluble fraction was purified by repeated silica gel column chromatography and normal phase semipreparative high-performance liquid chromatography (HPLC) to obtain three new sesquiterpenoids, 2α-hydroxy-3,3,6α,9β-tetramethyltricyclo[4,3,21,4]undecane (1), 11-acetoxyeudesman-4β-ol (4), and 2α,3β-dihydroxy-4β-methyl-6,8,10-cadinatriene (6), four known sesquiterpenoids, 3,7,7,9-tetramethyloctahydro-3a,6-ethanoinden-9-ol (2) [17], cedrol (3) [18], 11-acetoxyeudesman-4α-ol (5) [19], and 4β,5β-epoxy-14-hydroxy-9-epi-β-caryophyllene (7) [20], in addition to three known compounds, sandaracopimaric acid (8) [21], elliotinol (9) [22], and 8α-hydroxy-15,16-bisnorlabda-11-en-13-one (10) [23] (Figure 1). The identification of the known compounds was established through direct comparison with the published physical and spectral data (IR (infrared), UV (ultraviolet), MS (mass spectrum), and NMR (nuclear magnetic resonance)).
Compound 1 was isolated as a light yellow oil. A high resolution electron impact mass spectrometry (HR-EI-MS) molecular [M]+ ion at m/z 222.1980 ([M]+, calcd 222.1985) indicated the molecular formula of 1 to be C15H26O, showing three degrees of unsaturation. The IR spectrum demonstrated the presence of hydroxyl (3432 cm−1) functionality. Fifteen carbon signals were observed in the 13C-NMR spectrum of 1 (Table 1) and were assigned by the distortionless enhancement by polarization transfer (DEPT) experiments as four aliphatic methyl, five aliphatic methylene, two aliphatic methine, three aliphatic quaternary, and one oxygenated methine carbons. Its 1H-NMR spectrum (Table 1) revealed signals of the presence of one oxygenated methine (δH 3.04 (s)), three singlet methyls (δH 0.98 (s), 0.99 (s), and 1.12 (s)), and one characteristic Me-15 doublet methyl of cedrane sesquiterpenoid (δH 0.85 (d, 7.2)) [24]. From the above evidence, compound 1 was tentatively assigned as a functionalized tricycloundecane framework such as the cedrane derivative. The heteronuclear multiple bond coherence (HMBC) correlations between Me-15 (δH 0.85)/C-1 (δC 54.1 (s)) and C-9 (δC 40.7 (d)); Me-12 (δH 1.12)/C-1 and C-6 (δC 47.0 (s)); and H-8 (δH 1.17)/C-1, C-6, and C-9 help to confirm that ring B was a five-membered ring. Me-15 attached on C-9, and C-1 served as the bridgehead carbon of rings A, B, and C. The HMBC correlations between H-2 (δH 3.04)/C-4 (δC 49.7 (d)), C-6, C-9 and C-13 (δC 24.6 (q)); Me-12/C-1, C-5 (δC 34.6 (t)), C-6, and C-7 (δC 22.9 (t)); Me-13 (δH 0.99)/C-2 (δC 84.2 (d)), C-3 (δC 37.3 (s)), C-4, and C-14 (δC 29.9 (q)) suggested that ring A was a six-membered ring. The hydroxyl group located at C-2; Me-12 attached on C-6; two germinal methyls, Me-13 and 14, attached on C-3; and C-4 served as the bridgehead carbon of rings A and C. The remaining two carbon signals, together with the HMBC correlations H-10 (δH 1.51)/C-1 and C-4 and H-5 (δH 1.36)/C-11 (δC 31.2 (t)), hinted that ring C was also a six-membered ring (Figure 2). The nuclear Overhauser enhancement spectroscopy (NOESY) correlation between H-2/Hβ-7 (δH 1.13) and Me-13 indicated that the hydroxyl group at C-2 was in α orientation. The significant NOESY correlations between Me-12/Hα-7 (δH 1.58) and Hα-10 (δH 1.51) and Hβ-8 (δH 1.75)/Hβ-7 and Me-15 hinted that Me-12 and Me-15 were in α and β orientation, respectively. Therefore, compound 1 was determined as 2α-hydroxy-3,3,6α,9β-tetramethyltricyclo[4,3,21,4]undecane with a new sesquiterpene skeleton. Complete 1H- and 13C-NMR chemical shifts were established by 1H-1H correlated spectroscopy (1H-1H COSY), heteronuclear multiple-quantum coherence (HMQC), HMBC, and NOESY spectra (see Figures S3–S6 for more details).

Table 1.
NMR (nuclear magnetic resonance) data (CDCl3) of compound 1; δ in ppm, J in Hz.
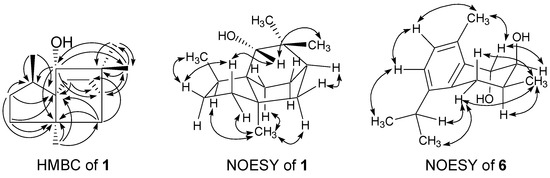
Figure 2.
Significant HMBC (one-headed arrows) and NOESY (two-headed arrows) correlations of compounds 1 and 6.
Compound 4 was also obtained as a yellow oil, and the high resolution electron impact mass spectrometry (HR-EI-MS) data determined the molecular formula to be C17H30O3 (m/z 282.2195 ([M]+, calcd 282.2184)), indicating three degrees of unsaturation. The IR spectrum displayed the presence of carbonyl (1731 cm−1) and hydroxyl (3432 cm−1) functionalities. The 1H- and 13C-NMR spectra of 4 (Table 2) revealed resonances for an acetyl group (δH 1.95 (s); δC 22.5 (q) and 170.5 (s)), and four singlet methyls (δH 1.00 (s), 1.14 (s), 1.42 (s), and 1.43 (s)). Seventeen carbon signals including two carbon signals of the acetyl group were observed in the 13C-NMR spectrum of 4 and were assigned by DEPT experiments as four alphatic methyl, six alphatic methylene, two alphatic methine, one aliphatic quaternary, two quaternary oxygenated, one ester carbonyl, and one acetylic methyl carbons. Take out one degree of unsaturation contributed from the carbonyl group, and the remaining two degrees of unsaturation, along with the information of the 15 carbon skeleton, hinted 4 would be a sesquiterpenoid derivative with a bicyclic structure. Compound 4 was thus tentatively proposed to be a eudesmane sesquiterpenoid. Comparison of the 1H- and 13C-NMR data with those of the known compound, 4-epicryptomeridiol [25], indicated that both compounds exhibited identical structure in the eudesmane skeleton, with the only difference occurring in the signals of an acetoxy group at C-11 in 4 instead of that of a hydroxyl group in 4-epicryptomeridiol (4a). After the 10% KOH alkaline hydrolysis, 4 could be transferred to 4-epicryptomeridiol. Compound 4 was accordingly determined to be 11-acetoxyeudesman-4β-ol.

Table 2.
NMR (nuclear magnetic resonance) data (CDCl3) of compounds 4 and 6; δ in ppm, J in Hz.
Compound 6 was obtained as a light yellow oil. The IR spectrum of 6 showed bands that were attributable to hydroxyl (3416 cm−1) and aromatic (1640 and 1460 cm−1) functionalities. The HR-EI-MS of 6 showed a molecular ion at m/z 234.1622, which corresponded to the molecular formula C15H22O2, indicating five degrees of unsaturation. The 1H- and 13C-NMR spectra of 6 (Table 2) revealed resonances for an isopropyl group [δH 1.19 (3H, d, J = 6.8 Hz), 1.20 (3H, d, J = 6.8 Hz), 3.15 (1H, sept, J = 6.8 Hz); δC 23.1 (q), 23.7 (q), 28.0 (d)], a benzylic methylene [δH 2.45 (1H, dd, J = 17.2 and 10.4 Hz), 2.80 (1H, d, J = 17.2 and 6.0 Hz); δC 28.7 (t)] and a benzylic methyl [δH 2.37 (3H, s); δC 18.7 (q)], two ortho-coupled aromatic protons [δH 7.07 (1H, d, J = 8.0 Hz), 7.15 (1H, d, J = 8.0 Hz); δC 124.8 (d), 128.9 (d)], and two oxymethines [δH 3.93 (1H, dd, J = 3.6 and 2.0 Hz), 4.79 (1H, d, J = 3.6 Hz); δC 73.6 (d), 69.8 (d)]. According to the above spectral characteristics, compound 6 exhibited a bicyclic sesquiterpenoid with a tetrasubstituted benzene ring, and was thus tentatively proposed to be a cadinatriene derivative. The position of the substituents in ring A and the relative configurations of the sterogenic C-atoms in ring B were determined by significant NOE correlations between Hα-5 (δH 2.80)/H-11 (δH 3.15) and Me-15 (δH 1.17); Me-12 (δH 1.20)/H-8 (δH 7.15); Me-14 (δH 2.37)/H-2 (δH 4.79) and H-9 (δH 7.07); and Me-15/Hβ-5 (δH 2.45) in the NOESY spectrum (Figure 2). The smaller coupling constants of two oxymethines [δH 3.93 (1H, dd, J = 3.6 and 2.0 Hz), 4.79 (1H, d, J = 3.6 Hz)] hinted that the two neighboring hydroxyl groups in ring B were all in axial orientation. The spectral data of 6 was in good agreement with those reported for the known compound konishiol [14], except for an incorrect published 1H-NMR data of Hα-5 (δH 2.45, instead of δH 2.80 in the literature). The specific optical rotation of 6 was +9.7, compared to −8.9 of konishiol, and 6 was thus determined as the enantiomer of konishiol, 2α,3β-dihydroxy-4β-methyl-6,8,10-cadinatriene, namely as ent-konishiol.
2.2. Inhibitory Activity against Nitric Oxide Production
Nitric oxide (NO) is derived from the oxidation of l-arginine by NO synthase (NOS) and is recognized as a mediator and regulator in biological actions, especially in inflammatory responses [26]. In inflammation and carcinogenesis conditions, there is an increased production of NO by inducible NO synthase (iNOS) [27]. Thus, inhibitors of NO might be of therapeutic importance in preventing pathological conditions catalyzed by inflammation. Macrophages contain various chemical mediators that may be responsible for several inflammatory stages and have been expected to be an origin of inflammation [28]. iNOS mainly exists in macrophages and can be induced by pro-inflammatory agents lipopolysaccharides (LPS). LPS can significantly increase the level of nitric oxide (NO) in macrophages through activation of iNOS [29]. In this study, the inhibitory activity toward NO production of four sesquiterpenoids (1, 4, 5, and 6) and eight diterpenoids (8–15) was evaluated by measurement of nitrite/nitrate in LPS-stimulated RAW 264.7 cells. To search for the appropriate concentrations for the above assay, these 12 compounds were first tested their cytotoxic activity against the RAW 264.7 cells, and no significant cytotoxic activities were observed under all tested concentrations (Table 3). Furthermore, compounds 10 and 15 exhibited moderate inhibitory effects on lipopolysaccharides (LPS)-induced nitric oxide production in RAW264.7 cells with IC50 values of 11.44 and 13.07 μg/mL, respectively (Table 3). Indomethacin is related to the inhibition of the cyclooxgenase 2 enzyme which synthesizes prostaglandin and was determined as a positive control (IC50 value of 65.4 μg/mL).

Table 3.
Cell viability and in vitro decrease of nitric oxide production of compounds 1, 4, 5, 6 and 8–15 in LPS-stimulated RAW 264.7 cells.
3. Experimental Section
3.1. Chemicals
LPS (endotoxin from Escherichia coli, serotype 0127:B8), indomethacin, MTT(3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) and other chemicals were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
3.2. General
The UV spectra were obtained on a Shimadzu UV-1601PC spectrophotometer (Shimadzu Corp., Kyoto, Japan). Optical rotations were measured with a JASCO DIP-180 digital spectropolarimeter (JASCO Inc., Tokyo, Japan). The IR spectra were recorded on a Nicolet 510P FT-IR spectrometer (Thermo Scientific Inc., Waltham, MA, USA). The 1D- and 2D-NMR spectra were measured with a Varian-Unity-Plus-400 spectrometer (Varian Inc., Palo Alto, CA, USA). Chemical shift values are given in ppm with reference to solvent (TMS as standard) and coupling constants (J) are given in Hz. The 2D-NMR spectra were recorded by using standard pulse sequences. EI-MS and HR-EI-MS were recorded on a JEOL SX-102A mass spectrometer (JEOL Ltd., Tokyo, Japan). Column chromatography was carried out on Merck Si gel (230–400 mesh ASTM, Merck, Darmstadt, Germany). TLC (thin-layer chromatography) analysis was carried out using aluminum pre-coated Si plates (Silica Gel 60 F-254; Merck) and the spots were detected by spraying with 5% H2SO4 and then heating at 100 °C. Semi-preparative HPLC was performed using a normal phase column (LiChrosorb Si 60, 7 μm, 250 × 10 mm; Merck & Co., Inc.) on a LDC Analytical-III system.
3.3. Plant Material
The wood of C. konishii was collected at Luantashan, Nantau County, Taiwan, in December 1996. The material was identified by Prof. Shao-Shun Ying, Department of Forestry, National Taiwan University. A voucher specimen (013492) has been deposited at the Herbarium of the Department of Botany, National Taiwan University, Taipei, Taiwan.
3.4. Extraction and Isolation
Dried wood (6.5 kg) of C. konishii was crushed into pieces and extracted by immersing in MeOH (60 L × 3) at r.t. for seven days each time. The combined MeOH extract was evaporated under reduced pressure at 45 °C to afford a brown crude viscous residue (60.2 g), which was suspended in H2O (500 mL), and then partitioned sequentially, using hexane (500 mL × 3), EtOAc (500 mL × 4), and BuOH (500 mL × 3) as solvent. The EtOAc fraction (15.6 g) was chromatographed on silica gel (450 g; 4.5 × 60 cm) using n-hexane–EtOAc (10:0, 9:1, 4:1, 7:3, 3:2, 1:1, 2:3, 3:7, 1:4, and 0:10) and EtOAc–MeOH (5:1) mixtures as solvent systems to obtain 11 fractions. Fr. 2 (150 mg) from n-hexane–EtOAc (9:1) elution was identified as a mixture of 1–3. Further purification by semi-preparative HPLC (hexane/CH2Cl2/EtOAc 10:5:1) gave 1 (2.2 mg), 2 (1.8 mg), and 3 (3.2 mg). Fr. 4 (320 mg) from n-hexane–EtOAc (7:3) elution was identified as a crude 9. Further purification by semi-preparative HPLC (hexane/CH2Cl2/EtOAc/iPrOH 8:2:1:0.2) gave 9 (1.2 mg). Fr. 6 (270 mg) from n-hexane–EtOAc (1:1) elution was identified as a mixture of 4, 5, 7, and 8. Further purification by semi-preparative HPLC (hexane/CH2Cl2/EtOAc/iPrOH 10:5:1:0.2) gave 4 (3.1 mg), 5 (2.2 mg), 7 (1.3 mg), and 8 (2.0 mg). Fr. 8 (310 mg) from n-hexane–EtOAc (3:7) elution was identified as a mixture of 6 and 10. Further purification by semi-preparative HPLC (hexane/EtOAc/iPrOH 3:1:0.3) gave 6 (2.1 mg) and 10 (1.6 mg).
2α-Hydroxy-3,3,6α,9β-tetramethyltricyclo[4,3,21,4]undecane (1). Light yellow oil; = −38.7 (c = 0.20, CHCl3); EI-MS (70 eV) m/z (rel. int.%): 222 ([M]+, 4), 204 ([M − H2O]+, 38), 203 (68), 189 (98), 183 (57), 161 (100); HR-EI-MS m/z: 222.1980 [M]+ (calcd for C15H26O, 222.1985); IR (KBr) νmax: 3432, 1460, 1376, 1015 cm−1; 1H-NMR and 13C-NMR (400/100 MHz, in CDCl3): see Table 1.
11-Acetoxyeudesman-4β-ol (4). Light yellow oil; = +3.8 (c = 0.21, CHCl3); EI-MS (70 eV) m/z (rel. int.%): 282 ([M]+, 3), 259 ([M − CH3COOH]+, 25), 207 (28), 204 (100), 189 (24); HR-EI-MS m/z: 282.2195 [M]+ (calcd for C17H30O3, 282.2184); IR (KBr) νmax: 3432, 1731, 1454, 1370, 1260, 1125, 1015 cm−1; 1H-NMR and 13C-NMR (400/100 MHz, in CDCl3): see Table 2.
2α,3β-Dihydroxy-4β-methyl-6,8,10-cadinatriene (6). Light yellow oil; = +9.7 (c = 0.19, CHCl3); EI-MS (70 eV) m/z (rel. int.%): 234 ([M]+, 64), 216 (44), 201 (43), 187 (42), 173 (41), 161 (62); HR-EI-MS m/z: 234.1622 [M]+ (calcd for C15H22O2, 234.1621); UVmax (CH3OH): 204, 261 nm; IR (KBr) νmax: 3416, 1640, 1467, 1387, 1049, 997 cm−1; 1H-NMR and 13C-NMR (400/100 MHz, in CDCl3): see Table 2.
3.5. Cell Culture
A murine macrophage cell line RAW264.7 (BCRC No. 60001) was obtained from the Bioresources Collection and Research Center (BCRC) of the Food Industry Research and Development Institute (Hsinchu, Taiwan). Cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM, Sigma, St. Louis, MO, USA) supplemented with 10% fetal bovine serum (FBS, Sigma). Cells were cultured in a humidified atmosphere with 5% CO2 at 37 °C and subcultured every three days at a dilution of 1:5 using 0.05% trypsin-0.02% EDTA in Ca2+-, Mg2+-free phosphate-buffered saline (DPBS).
3.6. Measurement of Nitric Oxide/Nitrite
The anti-inflammatory activity of compounds was evaluated by using a nitric oxide (NO) inhibitory activity assay. As a stable NO metabolite, production of NO was indirectly determined by Griess reaction to measure the concentration of nitrite in the culture medium. The cells were incubated with test compounds (0, 2.5, 5, 10, 20 and 40 μg/mL) in the presence of LPS (100 ng/mL) at 37 °C for 24 h. Then, cells were dispensed into 96-well plates, and 100 μL of each supernatant was reacted with an equal volume of Griess reagent (1% sulfanilamide, 0.1% naphthyl ethylenediamine dihydrochloride and 5% phosphoric acid) and incubated at room temperature for 10 min. Absorbance was then measured at 540 nm using a Micro-Reader (Molecular Devices Orleans Drive, Sunnyvale, CA, USA). A standard curve was generated, using freshly prepared 0–100 μM potassium nitrate dissolved in assay buffer, to quantitate unknown nitrite in samples.
3.7. Cell Viability
Cells (2 × 105) were cultured in 96-well plate containing DMEM supplemented with 10% FBS. After 24 h of cells incubation, cells were cultured with test compounds in the presence of 100 ng/mL LPS (lipopolysaccharide) for 24 h. Untreated cells served as the control. After that, the cells were washed twice with DPBS and 100 μL of 0.5 mg/mL MTT was added to each well for further 2 h incubation at 37 °C. The medium was then discarded, and the colored crystals of produced formazan were dissolved in 100 μL dimethyl sulfoxide (DMSO). After 30 min incubation, the absorbance was measured at 570 nm on a microplate reader (Molecular Devices).
3.8. Statistical Analysis
The data is expressed as means ± standard errors (SE). The IC50 values were calculated from the dose curves using a non-linear regression algorithm (SigmaPlot 8.0; SPSS Inc., Chicago, IL, USA, 2002). Statistical evaluation was carried out by one-way analysis of variance (ANOVA followed by Scheffe’s multiple range tests).
4. Conclusions
Three new sesquiterpenoids, 2α-hydroxy-3,3,6α,9β-tetramethyltricyclo[4,3,21,4]undecane (1), 11-acetoxyeudesman-4β-ol (4), and 2α,3β-dihydroxy-4β-methyl-6,8,10-cadinatriene (6), four known sesquiterpenoids (2, 3, 5, and 7), together with eight known diterpenoids (8–15), were isolated from the wood of C. konishii. Among them, four sesquiterpenoids (1, 4, 5, and 6) and eight diterpenoids (8–15), five of which we reported previously (11–15), were evaluated for their anti-inflammatory activity and the results showed that compounds 10 and 15 exhibited moderate inhibitory effects on lipopolysaccharides (LPS)-induced nitric oxide production in RAW264.7 cells. This investigation of secondary metabolites may contribute to a better understanding of the chemical characteristics of C. konishii.
As to the biological activity, these 12 compounds (1, 4, 5, 6, and 8–15) exhibited no significant cytotoxic activity at all tested concentrations. Compounds 10 and 15 showed stronger NO production inhibition than the other diterpenoids (8, 9, 11, 12, 13, and 14). A hydroxyl group at C-8 served as the active site, derived from the hydration of a double bond at C-8 and C-17, and may play an important role in their inhibitory activities against NO production.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/21/4/490/s1.
Acknowledgments
Financial was supported from CMU under the Aim for Top University Plan of the Ministry of Education, Taiwan, and Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW104-TDU-B-212-113002).
Author Contributions
Chi-I Chang and Yen-Cheng Li performed the isolation and structure elucidation of the constituents, and manuscript writing. Chien-Chih Chen, Ping-Jyun Sung, and Sheng-Yang Wang contributed to the structure elucidation and also part of the preparation of the manuscript. Hsun-Shuo Chang, Che-Yi Chao, and Guan-Jhong Huang conducted the bioassay and analyzed the data. Yen-Cheng Li and Yueh-Hsiung Kuo planned, designed, and organized the whole research of this study and the preparation of the manuscript. All authors read and approved the final version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, H.L.; Keng, H. Flora of Taiwan, 2nd ed.; Editorial Committee of the Flora of Taiwan: Taipei, Taiwan, 1994; Volume 1, pp. 582–585. [Google Scholar]
- Ikeda, T.; Fujita, Y. Essential oils of Cunninghamia konishii Hayata. J. Chem. Soc. Jpn. 1929, 50, 32–45. [Google Scholar]
- Ikeda, T.; Fujita, Y. The pinene in Cunninghamia konishii Hayata. J. Chem. Soc. Jpn. 1929, 50, 66–70. [Google Scholar]
- Cheng, Y.S.; Lin, C.S. Study of the extractive constituents from the wood of Cunninghamia konishii Hayata. J. Chin. Chem. Soc. 1979, 26, 169–172. [Google Scholar] [CrossRef]
- Chang, S.T.; Yin, H.W. Identificition of the needle crystal appeared on the wood surface of Cunninghamia konishii Hyata. Bull. Taiwan For. Res. Inst. New Ser. 1991, 6, 57–63. [Google Scholar]
- Li, Y.C.; Kuo, Y.H. Five New Diterpenoids from the Wood of Cunninghamia konishii. J. Nat. Prod. 1998, 61, 997–1000. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Kuo, Y.H. Labdane-type diterpenoids from the wood of Cunninghamia konishii. Chem. Pharm. Bull. 2002, 50, 498–500. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.S.; Lin, C.Y.; Gu, H.J.; Chang, S.T. Antifungal activities and chemical composition of wood and leaf essential oils from Cunninghamia konishii. J. Wood Chem. Technol. 2011, 31, 204–217. [Google Scholar] [CrossRef]
- Cheng, S.S.; Chung, M.J.; Lin, C.Y.; Wang, Y.N.; Chang, S.T. Phytochemicals from Cunninghamia konishii Hayata act as antifungal agents. J. Agric. Food Chem. 2012, 60, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Li, Y.C.; You, B.J.; Chang, W.T.; Chao, L.K.; Lo, L.C.; Wang, S.Y.; Huang, G.J.; Kuo, Y.H. Diterpenoids with anti-inflammatory activity from the wood of Cunninghamia konishii. Molecules 2013, 18, 682–689. [Google Scholar] [PubMed]
- Chen, Y.C.; Li, Y.C.; Chiu, H.L.; Cheng, W.Y.; Hong, Y.H.; Sung, P.J.; Kuo, C.C.; Wu, B.T.; Kuo, Y.H. Diterpenoids from the wood of Cunninghamia konishii. Helv. Chim. Acta 2013, 96, 2282–2287. [Google Scholar] [CrossRef]
- Chang, C.I.; Li, Y.C.; Kuo, C.C.; Chao, C.Y.; Chang, H.S.; Wu, J.H.; Wang, S.Y.; Kuo, Y.H. Two new lignans from the wood of Cunninghamia konishii. Nat. Prod. Commun. 2013, 8, 805–806. [Google Scholar]
- Cheng, Y.S.; Tsai, M.D. Terpenes and sterols of Cunninghamia konishii. Phytochemistry 1972, 11, 2108–2109. [Google Scholar] [CrossRef]
- He, K.; Shi, G.; Zeng, L.; Ye, Q.; McLaughlin, J.L. Konishiol, A new sesquiterpene, and bioactive components from Cunninghamia konishii. Planta Med. 1997, 63, 158–160. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.I.; Li, Y.C.; Hong, Y.H.; Cheng, W.Y.; Chao, C.Y.; Tsuzuki, M.; Amagaya, S.; Huang, C.J.; Kuo, Y.H. Two new labdane-type diterpenes from the wood of Cunninghamia konishii. Phytochem. Lett. 2014, 7, 107–110. [Google Scholar] [CrossRef]
- Chang, C.I.; Li, Y.C.; Yang, C.S.; Chao, C.Y.; Li, Y.J.; Chang, H.S.; Huang, C.J.; Kuo, C.C.; Kuo, Y.H. Two new labdane-type diterpene acids from the wood of Cunninghamia konishii. Helv. Chim. Acta 2015, 98, 123–127. [Google Scholar] [CrossRef]
- Fang, J.M.; Chen, Y.C.; Wang, B.W.; Cheng, Y.S. Terpenes from heartwood of Juniperus chinensis. Phytochemistry 1996, 41, 1361–1366. [Google Scholar] [CrossRef]
- Stork, G.; Clarke, F.H. Cedrol: Stereochemistry and total synthesis. J. Am. Chem. Soc. 1961, 83, 3114–3125. [Google Scholar] [CrossRef]
- Su, W.C.; Fang, J.M.; Cheng, Y.S. Sesquiterpenes from leaves of Cryptomeria japonica. Phytochemistry 1995, 39, 603–607. [Google Scholar]
- Barrero, A.F.; Molina, J.; Oltra, J.E.; Altarejos, J.; Barragan, A.; Lara, A.; Segura, M. Stereochemistry of 14-hydroxy-β-caryophyllene and related compunds. Tetrahedron 1995, 51, 3813–3822. [Google Scholar] [CrossRef]
- Edwards, O.E.; Nicolson, A.; Rodger, M.N. The structure of sandaracopimaric acid. Can. J. Chem. 1960, 38, 663–667. [Google Scholar] [CrossRef]
- Bohlmann, F.; Jakupovic, J.; King, R.M.; Robinson, H. Neue ent-atisiren-und ent-kaurensäure-derivate aus Helianthus-arten. Phytochemistry 1980, 19, 863–868. [Google Scholar] [CrossRef]
- Wahlberg, I.; Eklund, A.M.; Nordfors, K.; Vogt, C.; Enzell, C.R.; Berg, J.E. Tobacco Chemistry. 69. Five new labdanic compounds from tobacco. Acta Chem. Scand. 1988, 42, 708–716. [Google Scholar]
- Brown, G.D.; Liang, G.Y.; Sy, L.K. Terpenoids from the seeds of Artemisia annua. Phytochemistry 2003, 64, 303–323. [Google Scholar] [CrossRef]
- Nanayakkara, D.N.P.; Kinghorn, A.D.; Farnsworth, N.R. New hydroxylated eudesmane sesquiterpenoids from Amanoa oblongifolia. J. Chem. Res. 1986, 12, 454–455. [Google Scholar]
- Geller, D.A.; Billiar, T.R. Molecular biology of nitric oxide synthases. Cancer Metastasis Rev. 1998, 17, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Moncada, S.; Palmer, R.M.; Higgs, E.A. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmcol. Rev. 1991, 43, 109–142. [Google Scholar]
- Luo, Y.; Liu, M.; Dai, Y.; Yao, X.; Xia, Y.; Chou, G.; Wang, Z. Norisoboldine inhibits the production of pro-inflammatory cytokines in lipopolysaccharide-stimulated RAW 264.7 cells by down-regulating the activation of MAPKs but not NF-κB. Inflammation 2010, 33, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.C.; Rimbach, G.; Saliou, C.; Valacchi, G.; Packer, L. Activity of monomeric, dimeric, and trimeric flavonoids on NO production, TNF-α secretion, and NF-κB-dependent gene expression in RAW264.7 macrophages. FEBS Lett. 2000, 465, 93–97. [Google Scholar] [CrossRef]
- Sample Availability: Samples of all the compounds, except for 1–4, 6, and 7, are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).