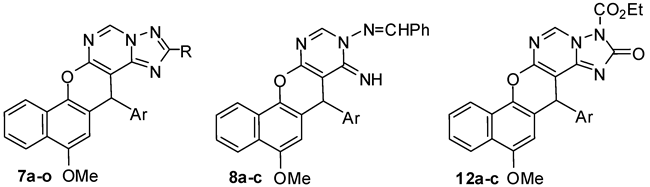
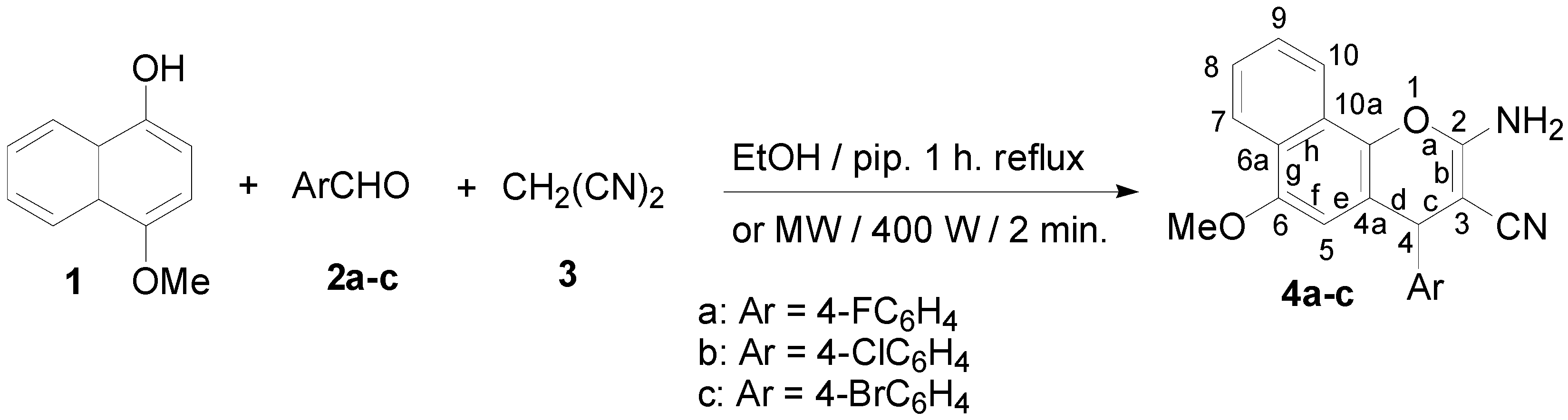Abstract
Three new series of chromene molecules have been synthesized in order to explore their antimicrobial activity. The series encompass 2-substituted 14-(4-halophenyl)-12-methoxy-14H-benzo[h]chromeno[3,2-e][1,2,4]-triazolo[1,5-c]pyrimidines 7a–o, 9-benzylideneamino-7-(4-halo-phenyl)-5-methoxy-8-imino-7H-benzo-[h]chromeno[2,3-d]pyrimidines 8a–b and 3-ethoxycarbonyl-14-(4-halophenyl)-12-methoxy-14H-benzo-[h]chromeno[3,2-e][1,2,4]triazolo[1,5-c]pyrimidine-2-one derivatives 12a–b. The structure of these novel compounds were confirmed using IR, 1H- and 13C-NMR as well as MS spectroscopy. The new compounds were evaluated in vitro for their antimicrobial activity and it was demonstrated that 7H-benzochromenopyrimidine and derivatives of 14H-benzochromenotriazolopyrimidine exhibited the most promising antibacterial activities compared to the reference antimicrobial agents. The structure activity relationship (SAR) studies of the target compounds agreed with the in vitro essays and confirmed higher potent antimicrobial activity against some of the tested microorganisms.
1. Introduction
The development of antimicrobial agents is an area of great activity due to the emergence of multidrug resistance in common pathogens and the appearance of new infections. Infectious diseases are considered the second-leading cause of death worldwide; consequently, tremendous efforts have been made to develop new antimicrobial agents that are active especially against the drug resistant strains.
The majority of antimicrobial agents are diverse five- and six-membered heterocyclic molecules that play a crucial role in the metabolism of all living cells [1]. Moreover, a great deal of interest has been directed toward condensed ring systems due to their various types of physiological activities and the success in utilizing them as privileged medicinal scaffolds. In particular, fused chromene and benzochromene molecules have become some of the best potential candidates for pharmacological purposes due to their antimicrobial [2,3], antileishmanial [4,5], anticancer [6,7], antiproliferative [8], antioxidant [9,10], hypertensive [11], antitumor [12,13,14,15] effects and activities, as well as for the treatment of Alzheimer’s disease [16] and schizophrenia disorders [17].
Fused chromene ring systems also displayed blood platelet antiaggregating [18], antihistaminic [19], analgesic [20,21,22], hypolipidemic [23], DNA breaking and mutagenicity activities [24]. Furthermore, several reports have shown that 7H-benzo[h]chromeno[2,3-d]pyrimidine and 14H-benzo[h]chromeno[3,2-e][1,2,4]triazolo[1,5-c]pyrimidine moieties are promising and attractive scaffolds for the development of potent antimicrobial agents [4,25,26,27,28]. In addition, 7H-benzo[h]-chromeno[2,3-d]pyrimidine derivatives exhibit anticancer activities [29,30,31,32,33].
In continuation of the previous works [15,25,26,27,28,29,30,31,34,35,36,37], it seemed interesting to synthesize some new 7H-benzo[h]chromeno[2,3-d]-pyrimidine and 14H-benzo-14H-benzo[h]chromeno[3,2-e][1,2,4]-triazolo[1,5-c]pyrimidine derivatives and to appraise their antimicrobial activities. The structure-activity relationships (SAR) are discussed in this work to correlate between the substituent effects and the activities that aid in drug design.
2. Results and Discussion
2.1. Chemistry
The first series of the new chromene compounds was synthesized via multi component reactions of 4-methoxy-1-naphthol (1) with 4-halobenzaldehydes 2a–c and malononitrile (3) in ethanolic piperidine solution. The solution was refluxed for 1 h and gave the corresponding 2-amino-4-(4-halophenyl)-6-methoxy-4H-benzo[h]chromene-3-carbonitriles 4a–c. The desired compounds were also obtained from the same precursors using microwave irradiation for 2 min. at 140 °C. These results are depicted in Scheme 1.
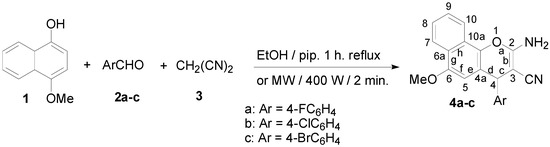
Scheme 1.
Synthesis of 2-amino-4-(4-halophenyl)-6-methoxy-4H-benzo[h]chromene-3-carbonitriles 4a–c.
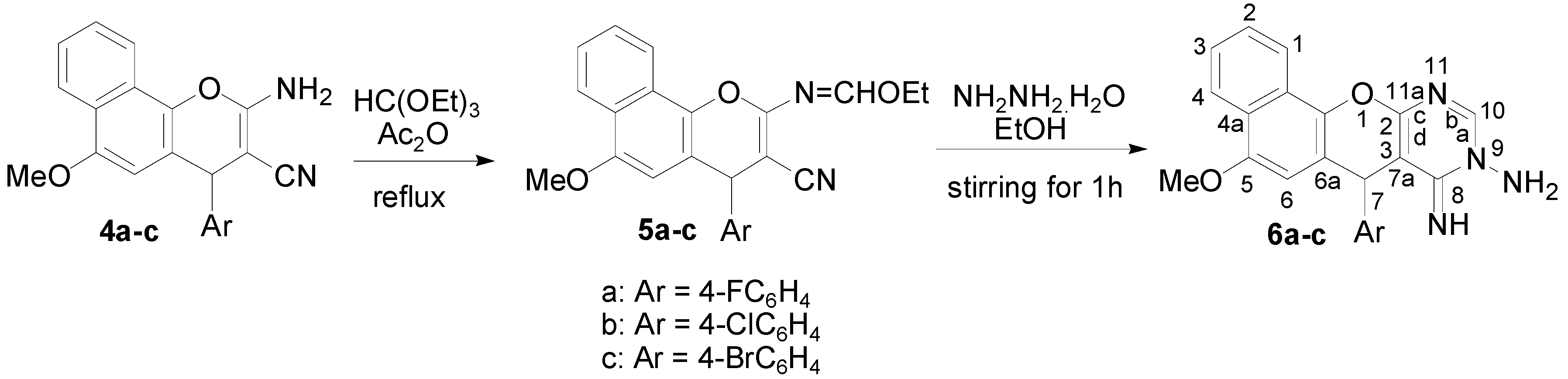
Treatment of compounds 4a–c with triethyl orthoformate in acetic anhydride at reflux gave the 2-ethoxymethyleneamino-4-(4-halophenyl)-6-methoxy-4H-benzo[h]chromene-3-carbonitriles 5a–c. Subsequent hydrazinolysis of the latter in ethanol under stirring at room temperature gave 9-amino-7-(4-halophenyl)-5-methoxy-8-imino-7H-benzo[h]chromeno[2,3-d] pyrimidines 6a–c, as illustrated in Scheme 2.
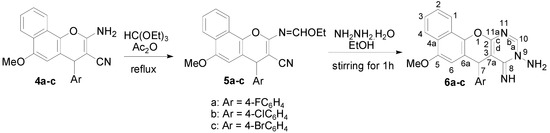
Scheme 2.
Synthesis of 2-ethoxymethyleneamino-4-(4-halophenyl)-6-methoxy-4H-benzo[h]chromene-3-carbonitriles (5a–c) and 9-amine-7-(4-halorophenyl)-5-methoxy-8-imino-7H-benzo[h]chromeno[2,3-d]pyrimidines (6a–c).
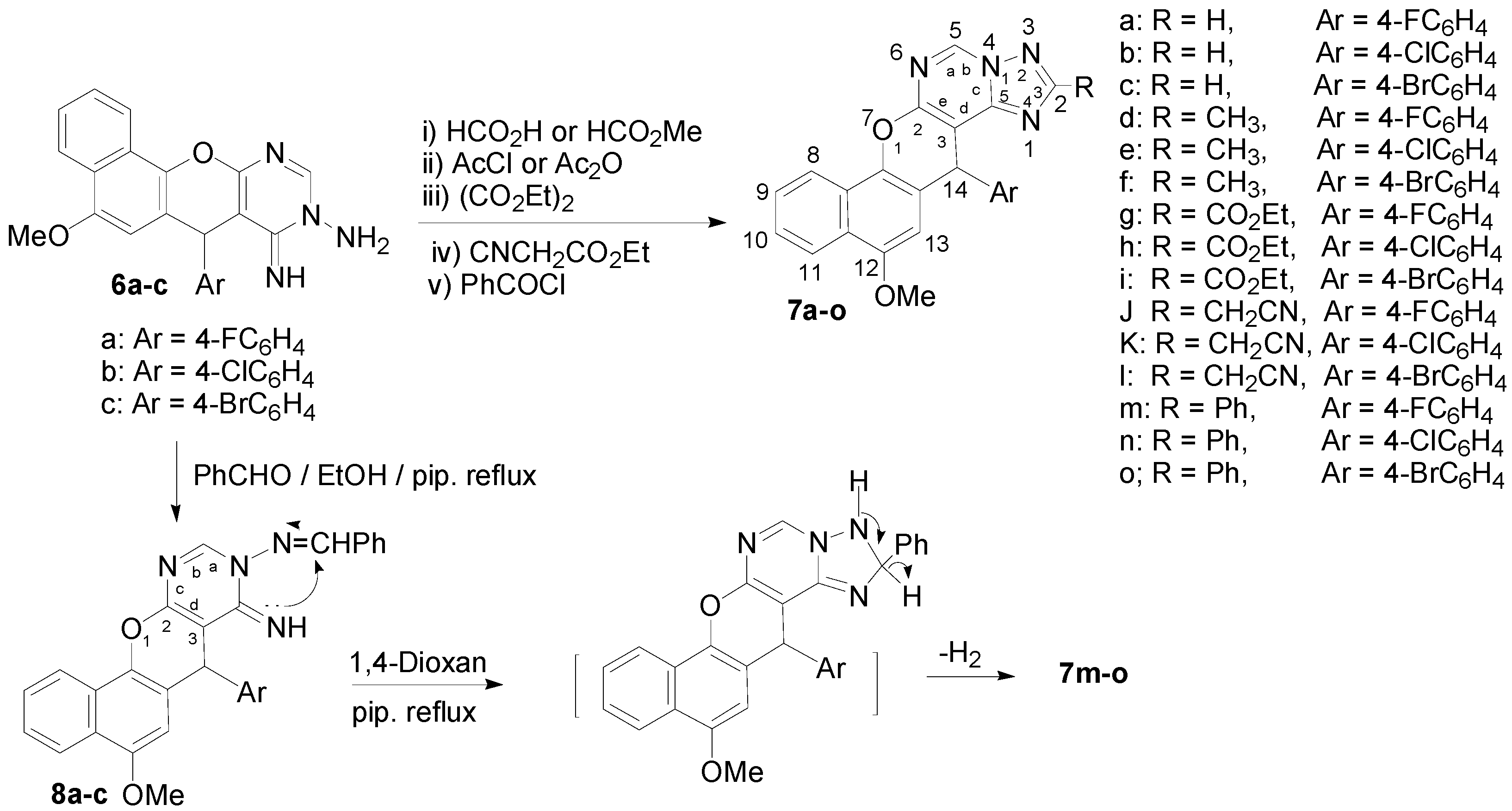
The aminoimino compounds 6 proved to be useful intermediates for the synthesis of a variety of 2-substituted 14H-benzo[h]chromeno[3,2-e][1,2,4]triazolo[1,5-c]pyrimidine derivatives. Thus, treatment of the aminoimino compounds 6a–c with either formic acid or methyl formate in benzene at reflux gave 14-(4-halophenyl)-12-methoxy-14H-benzo[h]chromeno[3,2-e]-[1,2,4]triazolo[1,5-c]-pyrimidines 7a–c, whilst acylation of compounds 6a–c with acetyl chloride or acetic anhydride gave the pentacyclic 2-methyltriazolopyrimidine derivatives 7d–f. On the other hand, condensation of compounds 6a–c with diethyl oxalate and ethyl cyanoacetate afforded 2-ethoxycarbonyltriazolo-pyrimidines 7g–i and 2-cyanomethyltriazolopyrimidine derivatives 7j–l, respectively, as shown in Scheme 3.
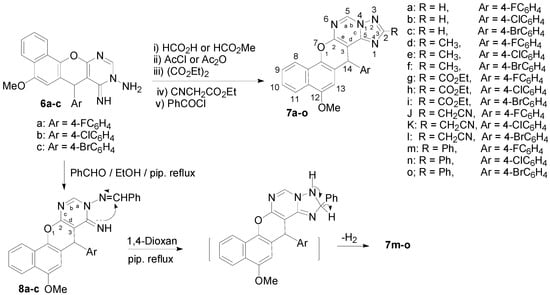
Scheme 3.
2-substituted 14-(4-halophenyl)-12-methoxy-14H-benzo[h]chromeno[3,2-e][1,2,4]triazolo[1,5-c]pyrimidines (7a–o) and 9-benzylideneamino-7-(4-halophenyl)-5-methoxy-8-imino-7H-benzo[h]chromeno[2,3-d]pyrimidines (8a–c).
Aroylation of compounds 6a–c with benzoyl chloride in refluxing dry benzene proceeded readily to give the 2-phenyltriazolopyrimidine derivatives 7m–o, Scheme 3. Condensation of the aminoimino compounds 6a–c with benzaldehyde in ethanolic piperidine solution under reflux gave the open chain product 9-benzylideneamino-7-(4-halophenyl)-5-methoxy-8-imino-7H-benzo[h]-chromeno[2,3-d]pyrimidines 8a–c, Scheme 3. Compounds 7m–o were also prepared by cyclization of compounds 8a–c in 1,4-dioxane/piperdine solution under reflux [26] as confirmed by the m.p., mixed m.p., and their identical IR and MS spectra, Scheme 3.
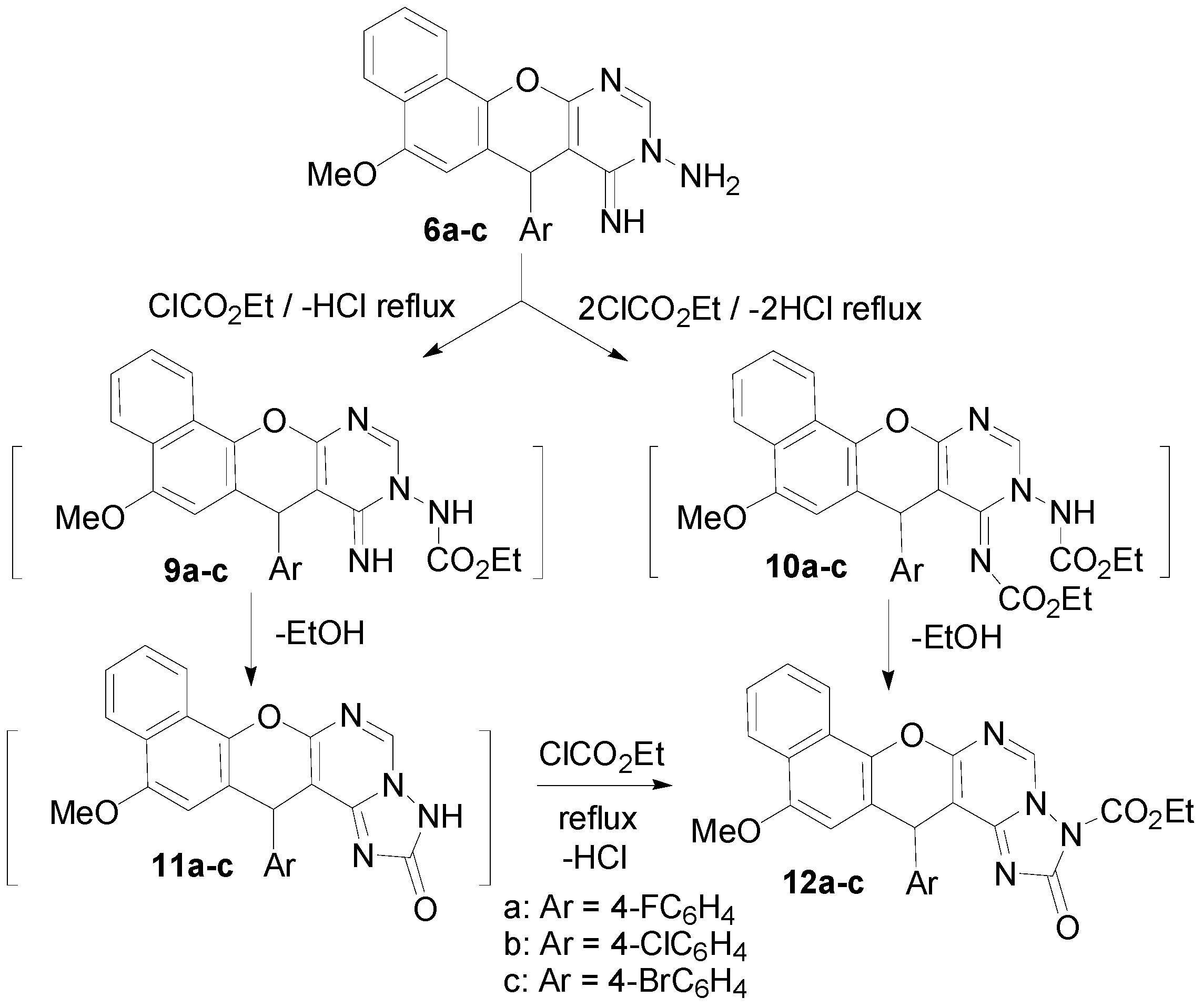
Interaction of the aminoimino compounds 6a–c with ethyl chloroformate in dry benzene at reflux gave the corresponding 1:2 adducts, the 3-ethoxycarbonyl-14-(4-halophenyl)-12-methoxy-14H-benzo[h]-chromeno[3,2-e]-[1,2,4]triazolo[1,5-c]pyrimidine-2-ones 12a–c instead of the 1:1 adducts, the triazolopyrimidin-2-one derivatives 11a–c. These results are depicted in Scheme 4. The formation of 12a–c is assumed to proceed via interaction of 6a–c with one mole of ethyl chloroformate and elimination of HCl to yield the intermediates 9a–c, which then cyclized to the non-isolable compounds 11a–c via elimination of EtOH. The intermediates 11a–c then reacted with another mole of ethyl chloroformate to eliminate HCl and give 12a–c. Alternatively, interaction of 6a–c with two moles of ethyl chloroformate could eliminate two HCl molecules and yield the intermediate bis-(ethoxycarbonyl) derivatives 10a–c, which then cyclized to 12a–c with elimination of an ethanol molecule (Scheme 4). The structures of 7, 8 and 12 were established on the basis of IR, 1H-NMR, 13C-NMR and MS data. The 7-position of compounds 8 and the 14-position of compounds 7 and 12 are chiral centers, and all the reactions were monitored using the TLC technique.
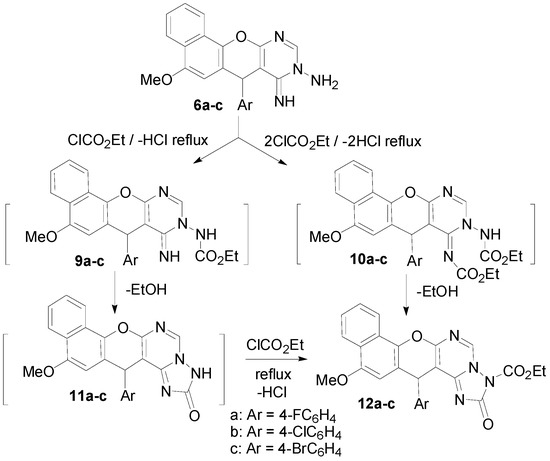
Scheme 4.
Synthesis of 3-ethoxycarbonyl-14-(4-halophenyl)-12-methoxy-14H-benzo[h]chromeno[3,2-e][1,2,4]triazolo[1,5-c]pyrimidine-2-ones (12a–c).
2.2. Antibacterial Evaluation
The target compounds 7a–o, 8a–c and 12a–c were tested in vitro for their antimicrobial activities by the agar diffusion method using Mueller-Hinton agar medium for bacteria and Sabouraud’s agar medium for fungi [32,33]. The tested microorganisms were obtained from the Regional Center for Mycology & Biotechnology (RCMP), Al-Azhar University.
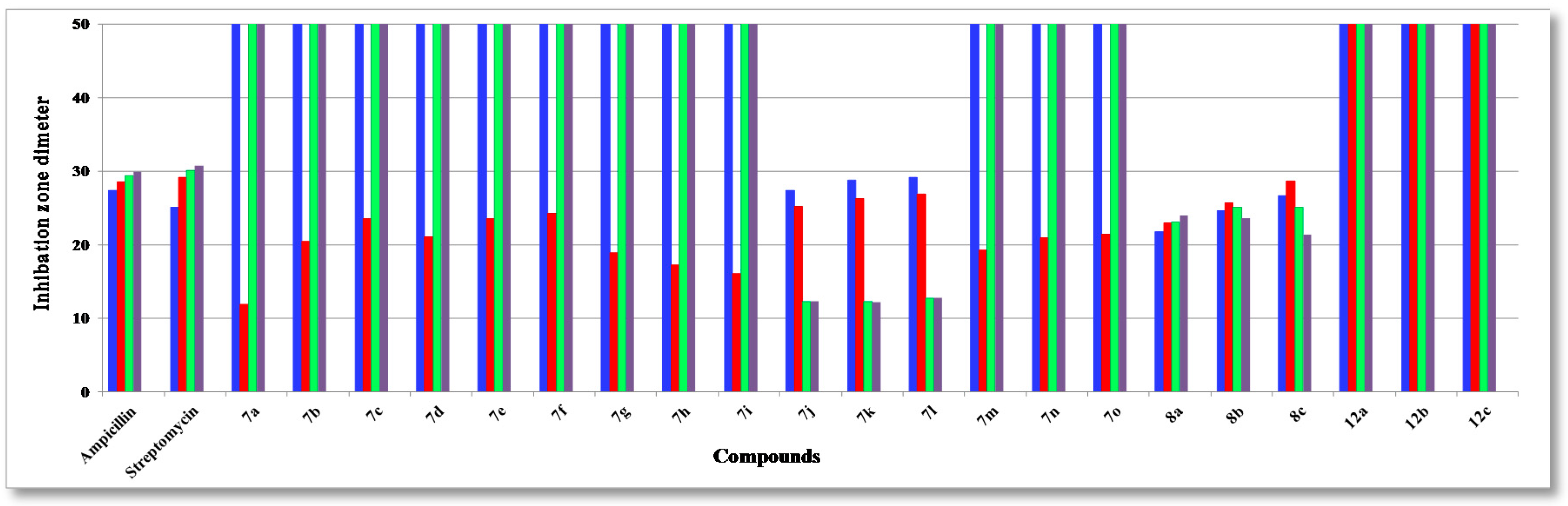
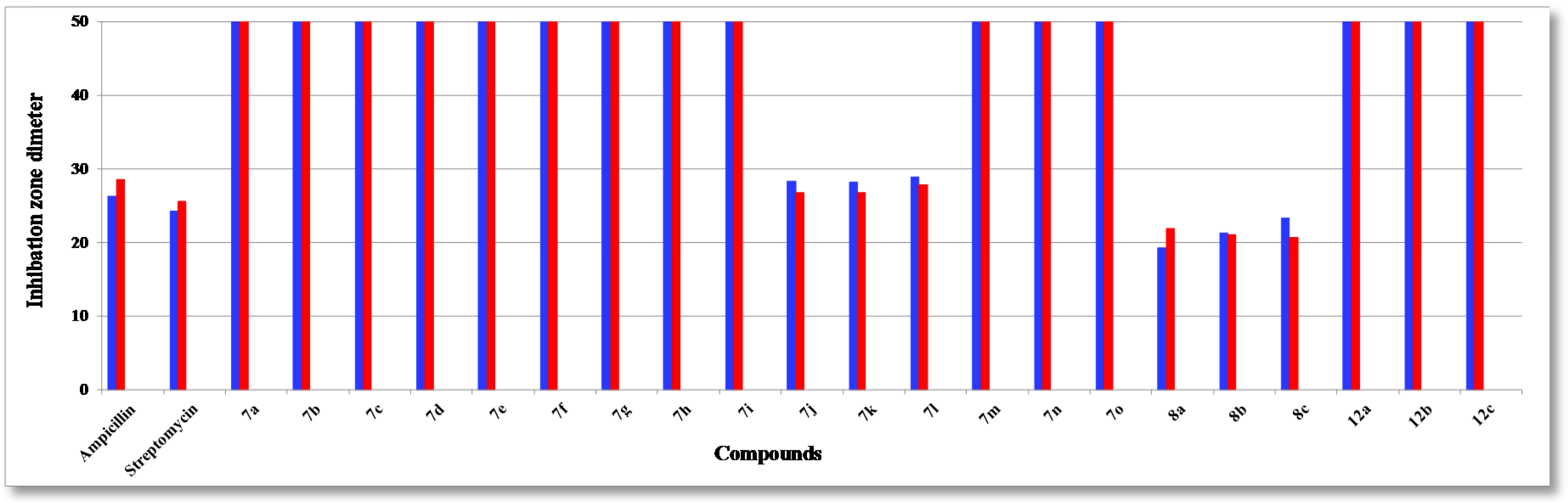
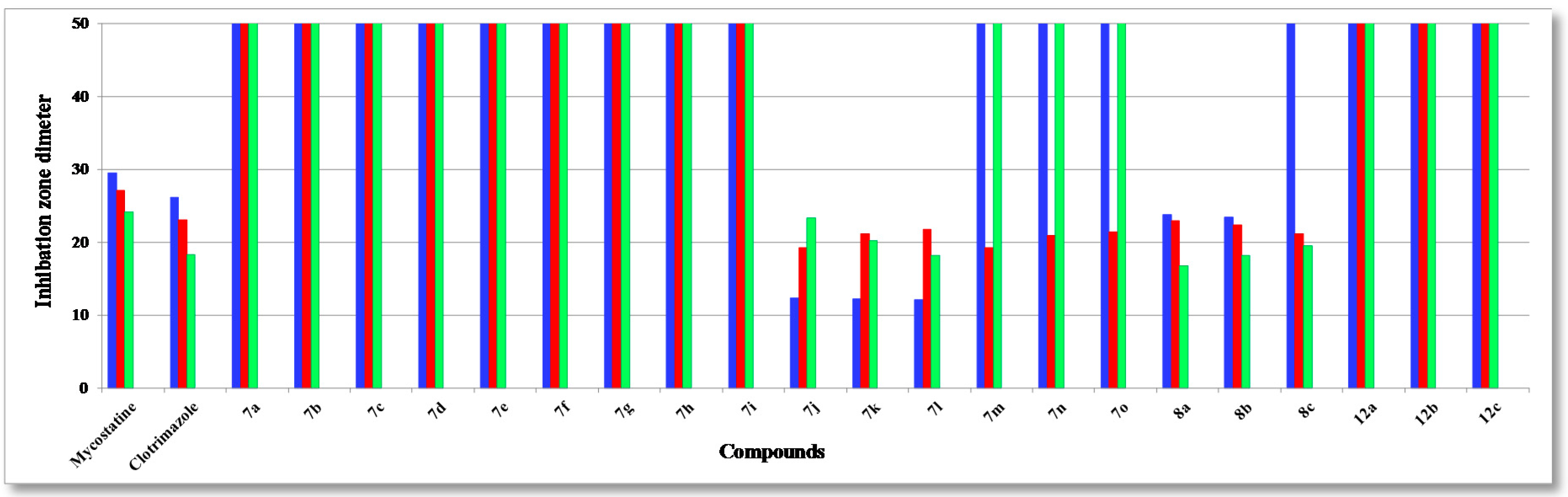
The assayed collection included four Gram-positive species of pathogenic bacteria: Staphylococcus aureus (RCMB 000106), Staphylococcus epidermidis (RCMB 000107), Bacillis subtilis (RCMB 000108), Bacillus pumilus (RCMB 000109), and two Gram-negative ones: Pseudomonas aeruginosa (RCMB 000102), Escherichia coli (RCMB 000103), using two standard antibiotics, ampicillin, and streptomycin (25 µg/mL) as reference drugs, and three fungi: Aspergillus fumigatus (RCMB 002003), Candida albicans (RCMB 005002) and Saccharomyces cerevisiae (RCMB 006002), using two standard antibiotics, mycostatine and clotrimazole (25 µg/mL) as reference drugs. The mean zone of inhibition in mm ± standard deviation beyond the well diameter (6 mm) was determined using a 25 µg/mL concentration of the tested compounds. The inhibitory effects of the synthetic compounds against these organisms are given in Figure 1, Figure 2, and Figure 3 and Table 1.
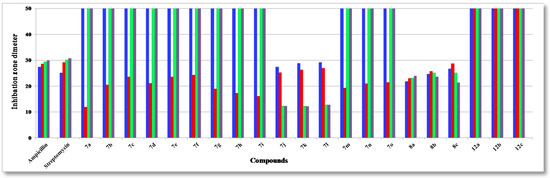
Figure 1.
Antibacterial activity (Gram-positive) of the tested compounds compared to ampicillin and streptomycin: S. aureus (blue); S. epidermidis (red); B.subtilis (green) and B. pumilus (pale purple).
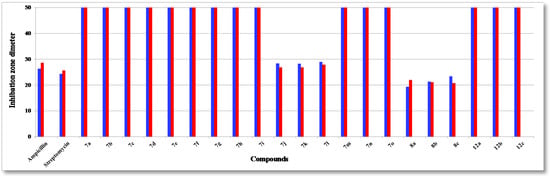
Figure 2.
Antibacterial activity (Gram-negative) of tested compounds compared to ampicillin and streptomycin: P. aeruginosa (blue) and E. coli (red).
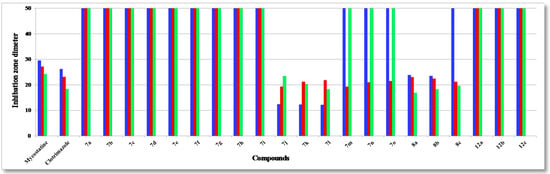
Figure 3.
Antifungal activity of tested compounds compared to mycostatine and clotrimazole: A. fumigatus (blue); C. albicans (red) and S. cerevisiae (green).

Table 1.
Antimicrobial screening for compounds 7a–o, 8a–c and 12a–c (25 µg/mL).
2.3. SAR Studies
The structure activity relationship (SAR) studies of compounds 7a–o, 8a–c and 12a–c revealed that compounds 8a–c and 7j with inhibitory effects of 21.8 ± 0.14, 24.6 ± 0.21, 26.6 ± 0.13 and 27.3 ± 0.47 µg/mL were 1.3, 1.1, 1.0, 1.0 times more active than the standard antibiotic ampicillin (27.4 ± 0.15 µg/mL), while compounds 8a, b were 1.2 and 1.0 times more active than the standard antibiotic streptomycin (25.1 ± 0.18 µg/mL) against S. aureus, respectively, and other compounds showed almost equipotent activities or were inactive, implying that the 7H-benzo[h]chromeno-[2,3-d]-pyrimidine nucleus was more active than the 14H-benzo[h]chromeno[3,2-e][1,2,4]triazolo-[1,5-c]-pyrimidine one and grafting a lipophilic hydrophobic group (=NH-8, –N=CHPh-9) or halogen (F > Cl > Br) on the 4-position of the phenyl group at the 7-position of the 7H-benzo[h]chromeno-[2,3-d]pyrimidine moiety is more beneficial than a CH2CN group at the 2-position of a 14H-benzo[h]chromeno[3,2-e][1,2,4]-triazolo[1,5-c]pyrimidine. Compounds 7a,i,h,g,m,b,n,d,o, 8a, 7c,e,f,j, 8b, 7k,l, 8c were found to be the most potent against S. epidermidis, with inhibitory effects ranging from 11.9 to 28.5 µg/mL compared to standard antibiotics ampicillin and streptomycin (28.6 ± 0.5 and 29.1 ± 0.3 µg/mL), suggesting that the 14H-benzo[h]chromeno[3,2-e][1,2,4]triazolo[1,5-c]pyrimidine nucleus was more effective than the 7H-benzo[h]chromeno[2,3-d]pyrimidine nucleus. Moreover, the non-substituted triazolo ring at the 2-postion is better than a hydrophobic moiety like a CO2Et, Ph, Me and CH2CN group. In addition, compounds 7k,j,l and 8a–c) showed good activity against B. subtilis with inhibitory effects ranging 12.2–25.1 µg/mL as compared to the standard antibiotics ampicillin and streptomycin (29.4 ± 0.7 and 30.1 ± 0.9 µg/mL), while compounds 7k,j,l and 8c,b,a with inhibitory effects ranging 12.2–24.0 µg/mL, exhibited good activity against B. pumilus as compared to the standard antibiotics ampicillin and streptomycin (29.9 ± 0.3 and 30.8 ± 0.4 µg/mL). These results implied that 14H-benzo[h]chromeno[3,2-e][1,2,4]triazolo[1,5-c]pyrimidine nucleus with hydrophobic group CH2CN group at 2-position was more beneficial than 7H-benzo[h]chromeno[2,3-d]pyrimidine nucleus with a lipophilic hydrophobic groups (=NH-8, –N=CHPh-9). Furthermore, compounds 8a–c exhibited good activities against P. aeruginosa, with inhibitory effects ranging from 19.3–23.3 µg/mL, as compared to the standard antibiotics ampicillin and streptomycin (26.3 ± 0.3 and 24.3 ± 0.8 µg/mL), while compounds 8c,b,a and 7k,j,l showed high activities against E. coli, with inhibitory effects ranging from 20.7–27.8 µg/mL as compared to the standard antibiotics ampicillin and streptomycin (28.5 ± 0.1 and 25.6 ± 0.4 µg/mL), suggesting that a 7H-benzo[h]chromeno[2,3-d]pyrimidine nucleus with a lipophilic hydrophobic group (=NH-8, –N=CHPh-9) was more effective than a 14H-benzo[h]chromeno[3,2-e][1,2,4]-triazolo[1,5-c]pyrimidine nucleus with a hydrophobic CH2CN group at the 2-position. Finally, compounds 7l,k,j, 7j–l and 8c,b,a)with inhibitory effects ranging from 12.1–12.3, 19.3–21.2, 22.1–23.8 and 21.7–22.9 µg/mL, respectively, showed high activity against A. fumigatus and C. albicans as compared to the standard antibiotics mycostatine (29.5 ± 0.1 and 27.1 ± 0.1 µg/mL) and clotrimazole (26.1 ± 0.1 and 23.1 ± 0.3 µg/mL), respectively, implying that the 14H-benzo[h]chromeno[3,2-e][1,2,4]-triazolo[1,5-c]pyrimidine nucleus with a hydrophobic CH2CN group at the 2-position was more active than a 7H-benzo[h]chromeno[2,3-d]pyrimidine nucleus with a lipophilic hydrophobic group (=NH-8, –N=CHPh-9), while compounds 8a–c and 7l,k,j with inhibitory effects between 16.8–19.5 and 18.2–23.3 µg/mL, respectively, are more effective against S. cerevisiae as compared to the standard antibiotic mycostatine (24.1 ± 0.3 µg/mL) and compounds 8a,b and 7l (16.8–18.2 µg/mL) are more active than the standard antibiotic clotrimazole (18.3 ± 0.1 µg/mL). The rest of the compounds showed almost equipotent and moderate activities or were inactive.
3. Experimental Section
3.1. General Information
Commercial-grade solvents and reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA) and used without further purification. Melting points were measured with a Stuart Scientific (Stone, Staffordshire, UK) apparatus and are uncorrected. IR spectra were determined as KBr pellets on a Jasco FT/IR 460 plus spectrophotometer (Jasco, Tokoyo, Japan). 1H-NMR (500 MHz) and 13C-NMR (125 MHz) spectra were recorded using a Bruker AV 500 MHz spectrometer (Bruker, Billerica, MA, USA). Chemical shifts (δ) are expressed in parts per million (ppm). The 1H-NMR and 13C-NMR spectra of the compounds are provided in the Supplementary Material. The MS were measured using a Shimadzu GC/MS-QP5050A spectrometer (Shimadzu, Tokoyo, Japan). Elemental analyses were carried out at the Regional Centre for Mycology & Biotechnology (RCMP, Al-Azhar University, Cairo, Egypt) and the results were within ±0.3% of calculated values. Analytical thin layer chromatography (TLC) on silica gel precoated F254 plates (Merck, Billerica, MA, USA) was used to check the purity of the final compounds and intermediates.
3.2. Synthesis
3.2.1. Starting Materials
2-Amino-4-(4-fluoro/chloro/bromophenyl)-6-methoxy-4H-benzo[h]chromene-3-carbonitriles 4a–c, 2-ethoxymethyleneamino-4-(4-fluoro/chloro/bromophenyl)-6-methoxy-4H-benzo[h]chromene-3-carbonitriles 5a–c and 9-amino-7-(4-fluoro/chloro/bromophenyl)-5-methoxy-8-imino-7H-benzo[h]-chromeno[2,3-d]pyrimidines 6a–c were prepared by reported methods ([34,35,36,37], [29,30,31] and [29,30,31], respectively).
3.2.2. General Procedure for the Synthesis of Compounds 7a–c
A mixture of aminoimino compound 6a–c (3.88, 4.04, or 4.49 g, respectively, 0.01 mol) and formic acid or methyl formate (0.46 g or 0.6 g, 0.01 mol) in dry benzene (30 mL) was refluxed for 5 h. The solvent was extracted and the resulting products were recrystallized from ethanol/1,4-dioxane to give 7a–c. The physical data of the compounds 7a–c are as follows:
14-(4-Fluorophenyl)-12-methoxy-14H-benzo[h]chromeno[3,2-e][1,2,4]triazolo[1,5-c]pyrimidine (7a). Pale yellow crystals. Yield: 79%; m.p. 262–263 °C; IR (KBr, cm−1): ν 3053, 3010 (CH-arom.), 2959, 2848 (CH-aliph.), 1652 (C=N); 1H-NMR (DMSO-d6) δ 3.87 (s, 3H, OCH3), 5.34 (s, 1H, H-14), 6.68–8.22 (m, 11H, Ar-H, H-2, H-5); 13C-NMR (DMSO-d6) δ 39.02 (C-14), 55.75 (CH3), 98.75 (C-14a), 103.59 (C-13), 115.22 (Ar-C), 120.66 (C-13a), 121.65 (C-8), 124.02 (C-11), 124.39 (C-10), 126.18 (C-11a), 127.37 (C-9), 129.71 (C-8a), 129.77 (Ar-C), 136.67 (C-5), 140.25 (Ar-C), 141.36, (C-8b), 146.79 (C-14b), 151.42 (C-12), 155.91 (C-2), 160.01 (Ar-C), 161.94 (C-6a); MS (m/z), 398 (M+, 11.46) with a base peak at 278 (100); C23H15FN4O2 (398.39): calcd; % C: 69.34, % H: 3.80, % N: 14.06; found; % C: 69.40, % H: 3.84, % N: 14.10.
14-(4-Chlorophenyl)-12-methoxy-14H-benzo[h]chromeno[3,2-e][1,2,4]triazolo[1,5-c]pyrimidine (7b). Pale yellow crystals. Yield: 92%; m.p. 242–243 °C; IR (KBr, cm−1): ν 3071, 3011 (CH-arom.), 2953, 2912, 2862, 2828 (CH-aliph.), 1651 (C=N); 1H-NMR (DMSO-d6) δ 3.86 (s, 3H, OCH3), 5.34 (s, 1H, H-14), 6.68–8.22 (m, 10H, Ar-H, H-5), 8.34 (s, 1H, H-2); 13C-NMR (DMSO-d6) δ 39.91 (C-14), 55.73 (CH3), 98.73 (C-14a), 103.51 (C-13), 120.66 (C-13a), 121.64 (C-8), 124.00 (C-11), 124.41 (C-10), 126.20 (C-11a), 127.37 (C-9), 128.28 (C-8a), 128.38 (Ar-C), 129.77 (Ar-C), 133.20 (Ar-C), 137.37 (C-5), 141.64 (Ar-C), 143.37 (C-8b), 148.77 (C-14b), 151.42 (C-12), 155.92 (C-2), 166.92 (C-6a); MS (m/z), 416 (M+ + 2, 19.25), 414 (M+, 4.67) with a base peak at 62 (100); C23H15ClN4O2 (414.84): calcd; % C: 66.59, % H: 3.64, % N: 13.51; found; % C: 66.64, % H: 3.70, % N: 13.56.
14-(4-Bromophenyl)-12-methoxy-14H-benzo[h]chromeno[3,2-e][1,2,4]triazolo[1,5-c]pyrimidine (7c). Pale yellow crystals. Yield: 83%; m.p. 232–233 °C; IR (KBr, cm−1): ν 3071, 3007 (CH-arom.), 2962, 2915, 2895 (CH-aliph.), 1652 (C=N); 1H-NMR (DMSO-d6) δ 3.87 (s, 3H, OCH3), 5.32 (s, 1H, H-14), 6.67–8.22 (m, 10H, Ar-H, H-5), 8.95 (s, 1H, H-2); 13C-NMR (DMSO-d6) δ 40.03 (C-14), 55.75 (CH3), 96.96 (C-14a), 103.63 (C-13), 120.03 (Ar-C), 120.67 (C-13a), 121.66 (C-8), 124.01 (C-11), 124.44 (C-10), 126.21 (C-11a), 127.38 (C-9), 130.16 (C-8a), 131.39 (Ar-C), 133.53 (Ar-C), 137.56 (C-5), 141.70, (C-8b), 143.24 (Ar-C), 151.44 (C-14b), 152.44 (C-12), 155.94 (C-2), 161.44 (C-6a), 163.94 (C-6a); MS (m/z), 460 (M+ + 2, 6.03), 458 (M+, 7.68) with a base peak at 279 (100); C23H15BrN4O2 (459.29): calcd; % C: 60.15, % H: 3.29, % N: 12.20; found; % C: 60.20, % H: 3.34, % N: 12.27.
3.2.3. General Procedure for the Preparation of Compounds 7d–f
A mixture of aminoimino compounds 6a–c (3.88, 4.04, or 4.49 g, respectively, 0.01 mol) and acetyl chloride or acetic anhydride (30 mL) was refluxed for 2 h. The solvent was extracted and the resulting product was recrystallized from 1,4-dioxan to give 7d–f. The physical data of the compounds 7d–f are as follows:
14-(4-Fluorophenyl)-2-methyl-12-methoxy-14H-benzo[h]chromeno[3,2-e][1,2,4]triazolo[1,5-c]pyrimidine (7d). Colourless crystals. Yield: 81%; m.p. 252–253 °C; IR (KBr, cm−1): ν 3068, 3035, 3006 (CH-arom.), 2967, 2938, 2848 (CH-aliph.), 1623 (C=N); MS (m/z), 412 (M+, 77.98) with a base peak at 318 (100); C24H17FN4O2 (412.42): calcd; % C: 69.89, % H: 4.15, % N: 13.59; found; % C: 69.94, % H: 4.17, % N: 13.61.
14-(4-Chlorophenyl)-2-methyl-12-methoxy-14H-benzo[h]chromeno[3,2-e][1,2,4]triazolo[1,5-c]pyrimidine (7e). Pale yellow crystals. Yield: 89%; m.p. 238–239 °C; IR (KBr, cm−1): ν 3071, 3025 (CH-arom.), 2970, 2937, 2845 (CH-aliph.), 1632 (C=N); 1H-NMR (DMSO-d6) δ 2.46 (s, 3H, CH3), 3.91 (s, 3H, OCH3), 5.71 (s, 1H, H-14), 6.68–8.35 (m, 9H, Ar-H), 9.59 (s, 1H, H-5); 13C-NMR (DMSO-d6) δ 14.28 (CH3), 40.05 (C-14), 55.88 (CH3), 103.76 (C-13), 117.75 (C-13a), 119.77 (C-14a), 121.75 (C-8), 124.04 (C-11), 124.59 (C-10), 126.38 (C-11a), 127.57 (C-9), 128.28 (C-8a), 129.51 (Ar-C), 130.64 (Ar-C), 135.92 (Ar-C), 136.52 (C-5), 139.47 (C-8b), 146.01 (Ar-C), 148.54 (C-14b), 152.76 (C-12), 153.91 (C-2), 165.18 (C-6a), 151.44 (C-14b), 152.38 (C-12), 155.94 (C-2), 161.94 (C-6a), 163.90 (C-6a); MS (m/z), 430 (M+ + 2, 19.45), 428 (M+, 57.92) with a base peak at 51 (100); C24H17ClN4O2 (428.87): calcd; % C: 67.21, % H: 4.00, % N: 13.06; found; % C: 67.30, % H: 4.09, % N: 13.10.
14-(4-Bromophenyl)-2-methyl-12-methoxy-14H-benzo[h]chromeno[3,2-e][1,2,4]triazolo[1,5-c]pyrimidine (7f). Colourless crystals. Yield: 84%; m.p. 243–244 °C; IR (KBr, cm−1): ν 3060, 3021, 3006 (CH-arom.), 2958, 2932, 2848 (CH-aliph.), 1637 (C=N); 1H-NMR (DMSO-d6) δ 2.41 (s, 3H, CH3), 3.97 (s, 3H, OCH3), 5.33 (s, 1H, H-14), 6.17–8.45 (m, 9H, aromatic), 9.76 (s, 1H, H-5); 13C-NMR (DMSO-d6) δ 14.53 (CH3), 38.58 (C-14), 55.99 (CH3), 96.80 (C-14a), 103.39 (C-13), 120.08 (Ar-C), 120.57 (C-13a), 121.73 (C-8), 124.05 (C-11), 124.56 (C-10), 128.91 (C-11a), 129.62 (C-9), 130.24 (C-8a), 131.14 (Ar-C), 134.25 (Ar-C), 137.62 (C-5), 143.90 (C-8b), 144.55 (Ar-C), 151.70 (C-14b), 156.57 (C-12), 158.48 (C-2), 163.90 (C-6a); MS (m/z), 474 (M+ + 2, 12.19), 472 (M+, 13.44) with a base peak at 75 (100); C24H17BrN4O2 (473.32): calcd; % C: 60.90, % H: 3.62, % N: 11.84; found; % C: 60.96, % H: 3.66, % N: 11.88.
3.2.4. General Procedure for the Preparation of Compounds 7g–i
A mixture of aminoimino compounds 6a–c (3.88, 4.04, or 4.49 g, respectively, 0.01 mol) and diethyl oxalate (1.46 g, 0.01 mol) in ethanol (30 mL) was refluxed for 2 h. The solvent was extracted and the resulting product was recrystallized from ethanol/1,4-dioxane to give 7g–i. The physical data of the compounds 7g–i are as follows:
2-Ethoxycarbonyl-14-(4-fluorophenyl)-12-methoxy-14H-benzo[h]chromeno[3,2-e][1,2,4]-triazolo[1,5-c]-pyrimidine (7g). Yellow crystals. Yield: 75%; m.p. 299–300 °C; IR (KBr, cm−1): ν 3067 (CH-arom.), 2995, 2987, 2939 (CH-aliph.), 1639 (C=N), 1743 (CO); 1H-NMR (DMSO-d6) δ 1.36 (t, 3H, CH3, J = 7.2 Hz), 3.93 (s, 3H, OCH3), 4.42 (q, 2H, CH2, J = 7.2 Hz), 5.97 (s, 1H, H-14), 6.63–8.64 (m, 9H, Ar-H), 9.82 (s, 1H, H-5); 13C-NMR (DMSO-d6) δ 14.00 (CH3), 37.95 (C-14), 55.98 (CH3), 61.92 (CH2), 102.68 (C-13), 103.55 (C-13a), 115.73 (Ar-C), 117.90 (C-14a), 121.79 (C-8), 123.89 (C-11), 124.62 (C-10), 126.68 (C-11a), 127.95 (C-9), 128.30 (C-8a), 129.95 (Ar-C), 138.62 (C-5), 139.95 (Ar-C), 141.32 (C-8b), 153.06 (C-14b), 154.57 (C-12), 157.43 (C-2), 159.46 (C-6a), 160.53 (Ar-C), 164.55 (CO); MS (m/z), 470 (M+, 17.39) with a base peak at 260 (100); C26H19FN4O4 (470.45): calcd; % C: 66.38, % H: 4.07, % N: 11.91; found; % C: 66.42, % H: 4.11, % N: 12.00.
2-Ethoxycarbonyl-14-(4-chlorophenyl)-12-methoxy-14H-benzo[h]chromeno[3,2-e][1,2,4]-triazolo[1,5-c]-pyrimidine (7h). Colourless powder. Yield: 87%; m.p. 248–249 °C; IR (KBr, cm−1): ν 3071 (CH-arom.), 2979, 2945, 2920 (CH-aliph.), 1625 (C=N), 1747 (CO); MS (m/z), 488 (M+ + 2, 1.15), 486 (M+, 3.52) with a base peak at 111 (100); C26H19ClN4O4 (486.91): calcd; % C: 64.14, % H: 3.93, % N: 11.51; found; % C: 64.09, % H: 3.89, % N: 11.47.
2-Ethoxycarbonyl-14-(4-bromophenyl)-12-methoxy-14H-benzo[h]chromeno[3,2-e][1,2,4]-triazolo[1,5-c]pyri-midine (7i). Colourless needles. Yield: 80%; m.p. 288–289 °C; IR (KBr, cm−1): ν 3067, 3025, 3001 (CH-arom.), 2948, 2845 (CH-aliph.), 1626 (C=N), 1747 (CO); 1H-NMR (DMSO-d6) δ 1.52 (t, 3H, CH3, J = 7.2 Hz), 3.95 (s, 3H, OCH3), 4.59 (q, 2H, CH2, J = 7.2 Hz), 5.93 (s, 1H, H-14), 6.43–8.51 (m, 9H, aromatic), 9.24 (s, 1H, H-5); 13C-NMR (DMSO-d6) δ 14.46 (CH3), 40.84 (C-14), 56.05 (CH3), 63.02 (CH2), 102.70 (C-13), 103.49 (C-13a), 116.04 (C-14a), 121.80 (C-8), 121.47 (Ar-C), 122.47 (C-11), 125.00 (C-10), 125.99 (C-11a), 126.86 (C-9), 127.90 (C-8a), 130.50 (Ar-C), 132.12 (Ar-C), 139.20 (C-5), 139.43 (C-8b), 142.16 (Ar-C), 153.54 (C-14b), 155.06 (C-2), 153.81 (C-12), 158.78 (C-6a), 159.93 (CO); MS (m/z), 532 (M+ + 2, 1.19), 530 (M+, 1.24) with a base peak at 76 (100); C26H19BrN4O4 (531.36): calcd; % C: 58.77, % H: 3.60, % N: 10.54; found; % C: 58.81, % H: 3.66, % N: 10.60.
3.2.5. General Procedure for the Preparation of Compounds 7j–l
A mixture of aminoimino compounds 6a–c (3.88, 4.04, or 4.49 g, respectively, 0.01 mol) and ethyl cyanoacetate (1.13 g, 0.01 mol) in ethanol (30 mL) was refluxed for 2 h. (TLC monitoring). The solvent was extracted and the resulting product was recrystallized from ethanol/1,4-dioxane to give 7j–l. The physical data of the compounds 7j–l are as follows:
2-Cyanomethyl-14-(4-fluorophenyl)-12-methoxy-14H-benzo[h]chromeno[3,2-e][1,2,4]-triazolo[1,5-c]-pyrimidine (7j). Pale yellow crystals. Yield: 90%; m.p. >300 °C; IR (KBr, cm−1): ν 3065, 3026 (CH-arom.), 2983, 2940 (CH-aliph.), 2260 (CN), 1631 (C=N); 1H-NMR (DMSO-d6) δ 3.45 (s, 2H, CH2), 3.93 (s, 3H, OCH3), 5.62 (s, 1H, H-14), 6.63–8.32 (m, 9H, aromatic), 8.55 (s, 1H, H-5); 13C-NMR (DMSO-d6) δ 25.07 (CH2), 38.06 (C-14), 55.97 (CH3), 97.29 (C-13), 102.71 (C-13a), 116.50 (Ar-C), 117.41 (CN), 117.84 (C-14a), 120.64 (C-8), 121.79 (C-11), 123.90 (C-10), 124.63 (C-11a), 126.67 (C-9), 127.92 (C-8a), 130.00 (Ar-C), 138.65 (C-5), 140.36 (Ar-C), 151.34 (C-8b), 152.32 (C-14b), 153.37 (C-2), 159.14 (C-12), 160.80 (Ar-C), 167.38 (C-6a); MS (m/z), 437 (M+, 54.05) with a base peak at 252 (100); C25H16FN5O2 (437.43): calcd; % C: 68.64, % H: 3.69, % N: 16.01; found; % C: 68.70, % H: 3.74, % N: 16.08.
2-Cyanomethyl-14-(4-chlorophenyl)-12-methoxy-14H-benzo[h]chromeno[3,2-e][1,2,4]-triazolo[1,5-c]-pyrimidine (7k). Pale yellow powder. Yield: 83%; m.p. 240–241 °C; IR (KBr, cm−1): ν 3062, 3012 (CH-arom.), 2962, 2905, 2890 (CH-aliph.), 2258 (CN), 1624 (C=N); MS (m/z), 455 (M+ + 2, 8.15), 453 (M+, 25.25) with a base peak at 343 (100); C25H16ClN5O2 (453.88): calcd; % C: 66.16, % H: 3.55, % N: 15.43; found; % C: 66.55, % H: 3.49, % N: 15.38.
2-Cyanomethyl-14-(4-bromophenyl)-12-methoxy-14H-benzo[h]chromeno[3,2-e][1,2,4]-triazolo[1,5-c]-pyrimidine (7l). Colourless needles. Yield: 75%; m.p. 232–233 °C; IR (KBr, cm−1): ν 3077 (CH-arom.), 2959, 2925 (CH-aliph.), 2255 (CN), 1626 (C=N); 1H-NMR (DMSO-d6) δ 3.45 (s, 2H, CH2), 3.93 (s, 3H, OCH3), 5.61 (s, 1H, H-14), 6.63–8.26 (m, 9H, aromatic), 8.56 (s, 1H, H-5); 13C-NMR (DMSO-d6) δ 25.06 (CH2), 40.04 (C-14), 55.98 (CH3), 96.93 (C-13), 102.64 (C-13a), 117.45 (CN), 117.83 (C-14a), 120.53 (Ar-C), 120.79 (C-8), 121.79 (C-11), 123.88 (C-10), 124.62 (C-11a), 126.72 (C-9), 127.95 (C-8a), 129.88 (Ar-C), 131.76 (Ar-C), 136.96 (C-5), 141.71 (Ar-C), 151.41 (C-8b), 152.35 (C-14b), 153.38 (C-2), 159.18 (C-12), 167.38 (C-6a); MS (m/z), 499 (M+ + 2, 32.02), 497 (M+, 33.62) with a base peak at 342; C25H16BrN5O2 (498.33): calcd; % C: 60.25, % H: 3.24, % N: 14.05; found; % C: 60.31, % H: 3.37, % N: 14.10.
3.2.6. General Procedure for the Preparation of Compounds 7m–o
Method A: A mixture of aminoimino compound 6a–c (3.88, 4.04, or 4.49 g, respectively, 0.01 mol) and benzoyl chloride (0.01 mol) in dry benzene (30 mL) was refluxed for 5 h. The solvent was removed under reduced pressure and the resulting solids were recrystallized from ethanol/1,4-dioxane to give 7m–o.
Method B: A mixture of compounds 8a–c (4.76, 4.92 or 5.37 g, respectively, 0.01 mol), dioxane (20 mL) and piperidine (0.5 mL) was refluxed for 2 h. The solvent was extracted and the resulting product was recrystallized from ethanol/1,4-dioxane to give 7m–o in 60% yield (as verified by m.p., mixed m.p., identical IR and MS spectrum).
The physical data of the compounds 7m–o are as follows:
14-(4-Fluorophenyl)-2-phenyl-12-methoxy-14H-benzo[h]chromeno[3,2-e][1,2,4]triazolo[1,5-c]pyrimidine (7m). Colourless needles. Yield: 67%; m.p. 248–249 °C; IR (KBr, cm−1): ν 3058, 3001 (CH-arom.), 2967, 2941 (CH-aliph.), 1625 (C=N); 1H-NMR (DMSO-d6) δ 3.90 (s, 3H, OCH3), 5.96 (s, 1H, H-14), 6.85–8.35 (m, 14H, aromatic), 9.69 (s, 1H, H-5); 13C-NMR (DMSO-d6) δ 40.03 (C-14), 55.88 (CH3), 101.24 (C-13), 103.72 (C-13a), 115.22 (Ar-C), 117.30 (C-14a), 120.66 (C-8), 121.75 (C-11), 124.07 (C-10), 124.69 (C-11a), 126.50 (C-9), 127.64 (C-8a), 128.28 (Ar-C), 129.03 (Ar-C), 129.65 (Ar-C), 130.09 (Ar-C), 130.92 (Ar-C), 138.15 (C-5), 140.24 (Ar-C), 151.89 (C-8b), 153.13 (C-14b), 154.00 (C-12), 160.09 (C-2), 162.03 (Ar-C), 165.50 (C-6a); MS (m/z), 474 (M+, 54.05) with a base peak at 279 (100); C29H19FN4O2 (474.49): calcd; % C: 73.41, % H: 4.04, % N: 11.81; found; % C: 73.48, % H: 4.11, % N: 11.98.
14-(4-Chlorophenyl)-2-phenyl-12-methoxy-14H-benzo[h]chromeno[3,2-e][1,2,4]triazolo-[1,5-c]pyrimidine (7n). Colourless needles. Yield: 66%; m.p. 295–296 °C; IR (KBr, cm−1): ν 3062, 3021 (CH-arom.), 2954, 2895 (CH-aliph.), 1621 (C=N); MS (m/z), 492 (M+ + 2, 3.13), 490 (M+, 9.42) with a base peak at 104 (100); C29H19ClN4O2 (490.94): calcd; % C: 70.95, % H: 3.90, % N: 11.41; found; % C: 71.00, % H: 3.94, % N: 11.45.
14-(4-Bromophenyl)-2-phenyl-12-methoxy-14H-benzo[h]chromeno[3,2-e][1,2,4]triazolo-[1,5-c]pyrimidine (7o). Colourless needles. Yield: 67%; m.p. 298–299 °C; IR (KBr, cm−1): ν 3046 (CH-arom.), 2979, 2945 (CH-aliph.), 1621 (C=N); MS (m/z), 536 (M+ + 2, 6.23), 534 (M+, 5.15) with a base peak at 433 (100); C29H19BrN4O2 (535.39): calcd; % C: 65.06, % H: 3.58, % N: 10.46; found; % C: 65.13, % H: 3.62, % N: 10.57.
3.2.7. General Procedure for the Preparation of Compounds 8a–c
A mixture of aminoimino compounds 6a–c (3.88, 4.04, or 4.49 g, respectively, 0.01 mol) and benzaldehyde (1.06 g, 0.01mol) in ethanol (30 mL) and piperidine (0.5 mL) was refluxed for 2 h. The solvent was extracted and the resulting product was recrystallized from 1,4-dioxane to give the open chain products 8a–c. The physical data of the compounds 8a–c are as follows:
9-Benzylideneamino-7-(4-fluorophenyl)-5-methoxy-8-imino-7H-benzo[h]chromeno[2,3-d]pyrimidine (8a). Pale yellow crystals. Yield: 66%; m.p. 257–258 °C; IR (KBr, cm−1): ν 3204 (NH), 3055, 3000 (CH-arom.), 2951, 2850 (CH-aliph.), 1631 (C=N); 1H-NMR (DMSO-d6) δ 3.98 (s, 3H, OCH3), 6.13 (s, 1H, H-7), 6.80–8.26 (m, 14H, aromatic), 8.21 (s, 1H, H-10), 8.41 (N=CH), 11.03 (bs, 1H, NH); 13C-NMR (DMSO-d6) δ 38.52 (C-7), 56.19 (CH3), 96.78 (C-7a), 103.38 (C-6), 116.57 (Ar-C), 118.82 (C-6a), 121.74 (C-1), 124.01 (C-4), 124.54 (C-3), 126.33 (C-4a), 126.74 (C-2), 127.54 (C-1a), 128.91 (Ar-C), 129.62 (Ar-C), 130.23 (Ar-C), 132.64 (Ar-C), 134.64 (Ar-C), 137.55 (C-1b), 144.51 (C-5), 144.88 (Ar-C), 151.65 (C-11a), 156.54 (N=CH), 158.42 (C-10), 160.98 (Ar-C), 163.83 (C-8); MS (m/z), 476 (M+, 99.45) with a base peak at 380 (100); C29H21FN4O2 (476.50): calcd; % C: 73.10, % H: 4.44, % N: 11.76; found; % C: 73.14, % H: 4.47, % N: 11.81.
9-Benzylideneamino-7-(4-chlorophenyl)-5-methoxy-8-imino-7H-benzo[h]chromeno[2,3-d]pyrimidine (8b). Colourless powder. Yield: 86%; m.p. 260–261 °C; IR (KBr, cm−1): ν 3214 (NH), 3065, 3010 (CH-arom.), 2971, 2860 (CH-aliph.), 1629 (C=N); 1H-NMR (DMSO-d6) δ 3.97 (s, 3H, OCH3), 6.18 (s, 1H, H-7), 6.82–8.29 (m, 14H, aromatic), 8.21 (s, 1H, H-10), 8.42 (N=CH), 11.04 (bs, 1H, NH); 13C-NMR (DMSO-d6) δ 38.54 (C-7), 55.99 (CH3), 96.79 (C-7a), 103.36 (C-6), 118.80 (C-6a), 121.76 (C-1), 124.02 (C-4), 124.52 (C-3), 126.36 (C-4a), 126.50 (Ar-C), 126.76 (C-2), 127.56 (C-1a), 128.91 (Ar-C), 129.44 (Ar-C), 129.62 (Ar-C), 131.64 (Ar-C), 131.98 (Ar-C), 132.23 (Ar-C), 137.60 (C-1b), 144.53 (C-5), 146.88 (Ar-C),151.66 (C-11a), 156.58 (N=CH), 158.44 (C-10), 163.88 (C-8); MS (m/z), 494 (M+ + 2, 2.07), 492 (M+, 5.29) with a base peak at 385 (100); C29H21ClN4O2 (492.96): calcd; % C: 70.66, % H: 4.29, % N: 11.37; found; % C: 70.70, % H: 4.33, % N: 11.42.
9-Benzylideneamino-7-(4-bromophenyl)-5-methoxy-8-imino-7H-benzo[h]chromeno[2,3-d]pyrimidine (8c). Pale yellow crystals. Yield: 89%; m.p. 274–275 °C; IR (KBr, cm−1): ν 3216 (NH), 3040 (CH-arom.), 2937, 2894 (CH-aliph.), 1650 (C=N); 1H-NMR (DMSO-d6) δ 3.97 (s, 3H, OCH3), 6.17 (s, 1H, H-7), 6.17–8.27 (m, 14H, aromatic), 8.28 (s, 1H, H-10), 8.44 (N=CH), 11.06 (bs, 1H, NH); 13C-NMR (DMSO-d6) δ 38.54 (C-7), 55.99 (CH3), 96.77 (C-7a), 103.37 (C-6), 118.80 (C-6a), 120.57(Ar-C), 121.73 (C-1), 124.03 (C-4), 124.53 (C-3), 126.35 (C-4a), 126.77 (C-2), 127.55(C-1a), 128.50 (Ar-C), 128.91 (Ar-C), 129.44 (Ar-C), 129.62 (Ar-C), 131.64 (Ar-C), 134.23 (Ar-C), 137.59 (C-1b), 143.88 (Ar-C), 144.54 (C-5), 151.68 (C-11a), 156.57 (N=CH), 158.45 (C-10), 163.87 (C-8); MS (m/z), 538 (M+ + 2, 4.61), 536 (M+, 4.70) with a base peak at 433 (100); C29H21BrN4O2 (537.41): calcd; % C: 64.81, % H: 3.94, % N: 10.43; found; % C: 64.77, % H: 3.99, % N: 10.48.
3.2.8. Synthesis of Compounds 12a–c
A mixture of aminoimino compounds 6a–c (3.88, 4.04, or 4.49 g, respectively, 0.01 mol) and ethyl chloroformate (1.08 g, 0.01 mol) in dry benzene (30 mL) was refluxed for 2 h. The solid product was collected by filtration and recrystallized from ethanol/1,4-dioxane to give 12a–c. The physical data of the compounds 12a–c are as follows:
3-Ethoxycarbonyl-14-(4-fluorophenyl)-12-methoxy-14H-benzo[h]chromeno[3,2-e][1,2,4]triazolo[1,5-c]-pyrimidine-2-one (12a). Yellow crystals. Yield: 45%; m.p. 241–242 °C; IR (KBr, cm−1): ν 3051 (CH-arom.), 2983, 2950, 2880 (CH-aliph.), 1645 (C=N), 1721 (CO), 1770 (CO ester); 1H-NMR (DMSO-d6) δ 1.35 (t, 3H, CH3, J = 7.2 Hz), 3.89 (s, 3H, OCH3), 4.44 (q, 2H, CH2, J = 7.2 Hz), 5.59 (s, 1H, H-14), 6.82–8.29 (m, 9H, aromatic), 9.35 (s, 1H, H-5); 13C-NMR (DMSO-d6) δ 13.96 (CH3), 40.03 (C-14), 55.92 (CH3), 64.47 (CH2), 98.45 (C-14a), 103.55 (C-13), 115.38 (Ar-C), 117.65 (C-13a), 120.51 (C-8), 121.79 (C-11), 123.88 (C-10), 124.63 (C-11a), 127.84 (C-9), 130.14 (C-8a), 130.19 (Ar-C), 137.46 (C-8b), 139.59 (Ar-C), 140.51 (C-5), 148.77 (C-12), 152.21 (C-6a), 155.11 (Ar-C), 155.21 (C-14b), 157.39 (CO), 160.32 (CO); MS (m/z), 486 (M+, 2.44) with a base peak at 320 (100); C26H19FN4O5 (486.45): calcd; % C: 64.20, % H: 3.94, % N: 11.52; found; % C: 64.24, % H: 3.99, % N: 11.56.
3-Ethoxycarbonyl-14-(4-chlorophenyl)-12-methoxy-14H-benzo[h]chromeno[3,2-e][1,2,4]triazolo[1,5-c]-pyrimidine-2-one (12b). Colourless needles. Yield: 43%; m.p. 200–201 °C; IR (KBr, cm−1): ν 3020 (CH-arom.), 2992, 2970, 2860 (CH-aliph.), 1641 (C=N), 1740 (CO), 1759 (CO ester); 1H-NMR (DMSO-d6) δ 1.35 (t, 3H, CH3, J = 7.2 Hz), 3.89 (s, 3H, OCH3), 4.44 (q, 2H, CH2, J = 7.2 Hz), 5.59 (s, 1H, H-14), 6.82–8.29 (m, 9H, aromatic), 9.35 (s, 1H, H-5); 13C-NMR (DMSO-d6) δ 13.98 (CH3), 40.04 (C-14), 55.94 (CH3), 64.50 (CH2), 98.38 (C-14a), 103.51 (C-13), 117.33 (C-13a), 120.53 (C-8), 121.81 (C-11), 123.89 (C-10), 124.69 (C-11a), 126.70 (Ar-C), 127.88 (C-9), 128.55 (C-8a), 130.15 (Ar-C), 131.78 (Ar-C), 137.49 (C-8b), 140.59 (C-5), 142.29 (Ar-C), 148.80 (C-12), 152.25 (C-6a), 155.12 (C-14b), 155.23 (CO), 157.40 (CO); MS (m/z), 504 (M+ + 2, 5.24), 502 (M+, 16.36) with a base peak at 320 (100); C26H19ClN4O5 (502.91): calcd; % C: 62.09, % H: 3.81, % N: 11.14; found; % C: 62.03, % H: 3.77, % N: 11.17.
3-Ethoxycarbonyl-14-(4-bromophenyl)-12-methoxy-14H-benzo[h]chromeno[3,2-e][1,2,4]-triazolo[1,5-c]pyrimidine-2-one (12c). Colourless powder. Yield: 49%; m.p. 200–201 °C; IR (KBr, cm−1): ν 3068 (CH-arom.), 2975, 2937, 2885 (CH-aliph.), 1646 (C=N), 1736 (CO), 1773 (CO ester); 1H-NMR (DMSO-d6) δ 1.34 (t, 3H, CH3, J = 7.2 Hz), 3.89 (s, 3H, OCH3), 4.44 (q, 2H, CH2, J = 7.2 Hz), 5.57 (s, 1H, H-14), 6.81–8.28 (m,9H, aromatic), 9.35 (s, 1H, H-5); 13C-NMR (DMSO-d6) δ 13.98 (CH3), 40.05 (C-14), 55.93 (CH3), 64.50 (CH2), 98.31 (C-14a), 103.49 (C-13), 117.23 (C-13a), 120.53 (Ar-C), 121.81 (C-8), 123.87 (C-11), 124.68 (C-10), 126.69 (C-11a), 127.78 (C-9), 130.32 (C-8a), 130.52 (Ar-C), 131.46 (Ar-C), 137.48 (C-8b), 140.58 (C-5), 142.70 (Ar-C), 148.79 (C-12), 152.25 (C-6a), 155.11 (C-14b), 155.21 (CO), 157.37 (CO); MS (m/z), 548 (M+ + 2, 10.14), 546 (M+, 10.90) with a base peak at 320 (100); C26H19BrN4O5 (547.36): calcd; % C: 57.05, % H: 3.50, % N: 10.24; found; % C: 57.11, % H: 3.55, % N: 10.27.
3.3. Antimicrobial Assay
All the newly synthesized compounds 7a–o, 8a–c and 12a–c were screened for their in vitro antimicrobial activity at 25 µg/mL to determine the zone of inhibition against four Gram-positive bacteria: Staphylococcus aureus (RCMB 000106), Staphylococcus epidermidis (RCMB 000107), Bacillis subtilis (RCMB 000108), Bacillus pumilus (RCMB 000109), and two Gram-negative pathogenic bacteria: Pseudomonas aeruginosa (RCMB 000102), Escherichia coli (RCMB 000103) using two standard antibiotics (ampicillin, streptomycin) as reference drugs, and three fungi: Aspergillus fumigatus (RCMB 002003), Candida albicans (RCMB 005002) and Saccharomyces cerevisiae (RCMB 006002) using two standard antibiotics (mycostatine, clotrimazole) as reference drugs. The activities of these compounds were tested by agar diffusion method using Mueller-Hinton agar medium for bacteria and Sabouraud’s agar medium for fungi [31,32]. The tested compounds were dissolved in N,N-dimethylformamide (DMF) to give a solution of 1 mg·mL−1. The inhibition zones (diameter of the hole) were measured in millimeters (6 mm) at the end of an incubation period of 48 h at 28 °C; N,N-dimethylformamide showed no inhibition zone.
4. Conclusions
In conclusion, several 14-(4-halophenyl)-12-methoxy-14H-benzo[h]chromeno[3,2-e][1,2,4]-triazolo[1,5-c]pyrimidine and 7H-benzo[h]chromeno[2,3-d]pyrimidine derivatives were synthesized in good yields, starting from 4H-benzo[h]chromene derivatives. The structures of compounds 7, 8 and 12 were established on the basis of IR, 1H-NMR, 13C-NMR and MS data. A pharmacological study has been performed in order to evaluate the effects of substituents on the antibacterial and antifungal activities. Compounds 12a–c did not show any antimicrobial activity against any of the tested bacteria and fungi, while compounds 7a,i,h,g,m,b,n,d,o,c,e,f showed high to good activities only against Staphylococcus epidermidis as compared to ampicillin and streptomycin. In contrast, compounds 7j–l and 8a–b showed high activity against all tested microorganisms. The rest of compounds showed almost equipotent and moderate activities or were inactive. The structure-activity relationship (SAR) study revealed that the antimicrobial activity of 14H-benzo[h]chromeno[3,2-e][1,2,4]triazolo[1,5-c]-pyrimidine nucleus was more affected by the lipophilicity of the non-substituent or 2-substituents than by lipophilic hydrophobic groups (=NH-8, –N=CHPh-9) on the 7H-benzo[h]-chromeno[2,3-d]pyrimidine nucleus and the 14H-benzo[h]chromeno[3,2-e][1,2,4]triazolo[1,5-c]-pyrimidine nucleus was more beneficial than the 7H-benzo[h]chromeno[2,3-d]pyrimidine nucleus for the antimicrobial activity.
Acknowledgments
The authors profoundly thank the Regional Center for Mycology & Biotechnology (RCMP), Al-Azhar University for carrying out the antitumor study and Elemental analyses.
Author Contributions
A.M.E., A.M.F. and R.M.O. conceived and designed the experiments; A.M.A. performed the experiments; R.M.O., F.F.A., T.H.A., A.M.F. and A.M.E. analyzed the data, wrote the paper and edited English language. All authors discussed the results and commented on the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Saini, M.S.; Kumar, A.; Dwivedi, J.; Singh, R. A Review: Biological Significances of Heterocyclic Compounds. Int. J. Pharm. Sci. Res. 2013, 4, 66–77. [Google Scholar]
- Li, Q.-Z.; Nie, X.-Y.; Liang, J. Novel Coumarin and 4H-Chromene Derivatives Containing 4,5-Dihydropyrazole Moiety: Synthesis and Antibacterial Activity. Lett. Drug Des. Discov. 2011, 8, 558–561. [Google Scholar]
- Liu, X.-H.; Liu, J.-X.; Bai, L.-S.; Lan, G.-L.; Pan, C.-X. Novel dihydropyrazole Derivatives Linked with 4H-Chromene: Microwave-Promoted Synthesis and Antibacterial Activity. Lett. Drug. Des. Discov. 2010, 7, 487–490. [Google Scholar] [CrossRef]
- Foroumadi, A.; Emami, S.; Sorkhi, M.; Nakhjiri, M.; Nazarian, Z.; Heydar, S.; Ardestani, S.; Poorrajab, F.; Shafiee, A. Chromene-Based Synthetic Chalcones as Potent Antileishmanial Agents: Synthesis and Biological Activity. Chem. Biol. Drug Des. 2010, 75, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Narender, T.; Shweta Gupta, S. A convenient and biogenetic type synthesis of few naturally occurring chromeno dihydrochalcones and their in vitro antileishmanial activity. Bioorg. Med. Chem. Lett. 2009, 14, 3913–3916. [Google Scholar] [CrossRef] [PubMed]
- Sabry, N.M.; Mohamed, H.M.; Khattab, E.S.A.E.H.; Motlaq, S.S.; El-Agrody, A.M. Synthesis of 4H chromene, coumarin, 12H-chromeno[2,3-d]pyrimidine derivatives and some of their antimicrobial and cytotoxicity activities. Eur. J. Med. Chem. 2011, 46, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Rampa, A.; Bisi, A.; Belluti, F.; Gobbi, S.; Piazzi, L.; Valenti, P.; Zampiron, A.; Caputo, A.; Varani, K.; Borea, P.A.; Carrara, M. Homopterocarpanes as bridged triarylethylene analogues:Synthesis and antagonistic effects in human MCF-7 breast cancer cells. IL Farmco 2005, 60, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Magedov, I.V.; Manpadi, M.; Evdokimov, N.M.; Elias, E.M.; Rozhkova, E.; Ogasawara, M.A.; Bettale, J.D.; Przheval’skii, N.M.; Rogelj, S; Kornienko, A. Antiproliferative and apoptosis inducing properties of pyrano[3,2-c]pyridones accessible by a onestep multicomponent synthesis. Bioorg. Med Chem. Lett. 2007, 17, 3872–3876. [Google Scholar] [CrossRef] [PubMed]
- Singh, O.M.; Devi, N.S.; Thokchom, D.S.; Sharma, G.J. Novel 3-alkanoyl/aroyl/heteroaroyl-2H-chromene-2-thiones: Synthesis and evaluation of their antioxidant activities. Eur. J. Med. Chem. 2010, 45, 2250–2257. [Google Scholar] [CrossRef] [PubMed]
- Vukovic, N.; Sukdolak, S.; Solujic, S.; Niciforovic, N. Substituted imino and amino derivatives of 4-hydroxycoumarins as novel antioxidant, antibacterial and antifungal agents: synthesis and in vitro assessments. Food Chem. 2010, 120, 1011–1018. [Google Scholar] [CrossRef]
- Tandon, V.K.; Vaish, M.; Jain, S.; Bhakuni, D.S.; Srimal, R.C. Synthesis, carbon-13 NMR and hypotensive action of 2,3-dihydro-2,2-dimethyl-4H-naphtho [1,2-b] pyran-4-one. Indian J. Pharm. Sci. 1991, 53, 22–23. [Google Scholar]
- Mahmoodi, M.; Aliabadi, A.; Emami, S.; Safavi, M.; Rajabalian, S.; Mohagheghi, M.A.; Khoshzaban, A.; Samzadeh-Kermani, A.; Lamei, N.; Shafiee, A. Synthesis and in vitro Cytotoxicity of Polyfunctionalized 4-(2-Arylthiazol-4-yl)-4H-Chromemes. Arch. Pharm. Chem. 2010, 343, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Endo, S.; Matsunaga, T.; Kuwata, K.; Zhao, H.-T.; El-Kabbani, O.; Kitade, Y.; Hara, A. Chromene-3-carboxamide derivatives discov ered from virtual screening as potent inhibitors of the tumor maker, AKR1B10. Bioorg. Med. Chem. 2010, 18, 2485–2490. [Google Scholar] [CrossRef] [PubMed]
- Tseng, T.-H.; Chuang, S.-K.; Hu, C.-C.; Chang, C.-F.; Huang, Y.-C.; Lin, C.-W.; Lee, Y.-J. The synthesis of morusin as a potent antitumor agent. Tetrahedron 2010, 66, 1335–1340. [Google Scholar] [CrossRef]
- El-Agrody, A.M.; Fouda, A.M.; Khattab, E.S.A.E.H. Synthesis, antitumor activity of 2-amino-4H-benzo[h]chromene derivatives and Structure-activity relationships of the 3- and 4-positions. Med. Chem. Res. 2013, 22, 6105–6120. [Google Scholar] [CrossRef]
- Bruhlmann, C.; Ooms, F.; Carrupt, P.; Testa, B.; Catto, M.; Leonetti, F.; Altomare, C.; Cartti, A. Coumarins derivatives as dual inhibitors of acetylcholinesterase and monoamine oxidase. J. Med. Chem. 2001, 44, 3195–3198. [Google Scholar] [CrossRef] [PubMed]
- Kesten, S.R.; Heffner, T.G.; Johnson, S.J.; Pugsley, T.A.; Wright, J.L.; Wise, D.L. Design, Synthesis, and Evaluation of Chromen-2- ones as Potent and Selective Human Dopamine D4 Antagonists. J. Med. Chem. 1999, 42, 3718–3725. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-S.; Khil, L.-Y.; Chae, S.-H.; Kim, D.; Lee, B.-H.; Hwang, G.-S.; Moon, C.-H.; Chang, T.-S.; Moon, C.-K. Effects of DK-002, a synthesized(6aS,cis)-9,10-Dimethoxy-7,11b-dihydro-indeno[2,1-c]chromene-3,6a-diol, on platelet activity. Life Sci. 2006, 78, 1091–1097. [Google Scholar] [CrossRef] [PubMed]
- Coudert, P.; Coyquelet, J.M.; Bastide, J.; Marion, Y.; Fialip, J. Synthesis and anti-allergic properties of N-arylnitrones with furo-pyran structure. Ann. Pharm. Fr. 1988, 46, 91–96. [Google Scholar] [PubMed]
- El-Sayed, A.T.; Ibrahim, M.A. Synthesis and Antimicrobial Activity of Chromone- linked-2-Pyridone Fused with 1,2,4-Triazoles, 1,2,4-Triazines and 1,2,4-Triazepines Ring Systems. J. Braz. Chem. 2010, 21, 1006–1007. [Google Scholar]
- Narasimhan, B.; Kumar, P.; Sharma, D. Biological activities of hydrazide derivatives in the new millennium. Acta Pharm. Sci. 2010, 52, 169–180. [Google Scholar]
- Keri, R.S.; Hosamani, K.M.; Shingalapur, R.V.; Hugar, M.H. Analgesic, anti-pyretic and DNA cleavage studies of novel pyrimidine derivatives of coumarin moiety. Eur. J. Med. Chem. 2010, 45, 2597–2605. [Google Scholar] [CrossRef] [PubMed]
- Sashidhara, K.V.; Kumar, M.; Modukuri, R.K.; Srivastava, A.; Puri, A. Discovery and synthesis of novel substituted benzocoumarins as orally active lipid modulating agents. Bioorg. Med Chem. Lett. 2011, 21, 6709–6713. [Google Scholar] [CrossRef] [PubMed]
- Hiramoto, K.; Nasuhara, A.; Michiloshi, K.; Kato, T.; Kikugawa, K. DNA strand-breaking activity and mutagenicity of 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one (DDMP), a Maillard reaction product of glucose and glycine. Mutat. Res. 1997, 395, 47–56. [Google Scholar] [CrossRef]
- El-Agrody, A.M.; El-Hakium, M.H.; Abd El-Latif, M.S.; Fekry, A.H.; Sayed, S.M.; El-Gareab, K.A. Synthesis of Pyrano[2,3-d]pyrimidine and Pyrano[3,2-e][1,2,4]triazolo[2,3-c]pyri- midine Derivatives with Promising Antimicrobial Activities. Acta Pharm. 2000, 50, 111–120. [Google Scholar]
- Bedair, A.H.; Emam, H.A.; El-Hady, N.A.; Ahmed, K.A.R.; El-Agrody, A.M. Synthesis and Antimicrobial Activities of Novel Naphtho[2,1-b]pyran, Pyrano[3,2-d]pyrimidine and Pyrano-[3,2-e][1,2,4]triazolo-[2,3-c]pyrimidine Derivatives. Il Farmaco 2001, 56, 965–973. [Google Scholar] [CrossRef]
- Khafagy, M.M.; Abd El-Wahab, A.H.F.; Eid, F.A.; El-Agrody, A.M. Synthesis of Halogen Derivatives of Benzo[h]cheromene and Benzo[a]anthracene with Promising Antimicrobial Activities. Il Farmaco 2002, 57, 715–722. [Google Scholar] [CrossRef]
- El-Agrody, A.M.; Sabry, N.M.; Motlaq, S.S. Synthesis of some new 2-substituted 12H-chromeno[3,2-e][1,2,4]triazolo[1,5-c]pyrimidine, 3-ethoxycarbonyl-12H-chromeno[3,2-e]-[3,2-e][1,2,4]triazolo[1,5-c]pyrimidine-2-one, ethyl 2-formylamino/acetylamino-4H-chromene-3-carboxylate and some of their ntimicrobial activities. J. Chem. Res. 2011, 35, 77–83. [Google Scholar]
- Halawa, A.H.; Fouda, A.M.; Al-Dies, A.M.; El-Agrody, A.M. Synthesis, Biological Evaluation and Molecular Docking Studies of 4Hbenzo[h]chromenes, 7H-benzo[h]chromeno-[2,3-d]pyrimidines as Antitumor Agents. Lett. Drug. Des. Discov. 2016, 13, 77–88. [Google Scholar] [CrossRef]
- El-Agrody, A.M.; Halawa, A.H.; Fouda, A.M.; Al-Dies, A.M. The anti-proliferative activity of novel 4H-benzo[h]chromenes, 7H-benzo[h]chromeno[2,3-d]pyrimidines and the structure-activity relationships of the 2-,3-positions and fused rings at the 2,3-positions. J. Saudi Chem. Soc. 2016. [Google Scholar] [CrossRef]
- El-Agrody, A.M.; Fouda, A.M.; Al-Dies, A.M. Studies on the synthesis, in vitro antitumor activity of 4H-benzochromene, 7H-benzo[h]chromeno[2,3-d]pyrimidine derivatives and Structure activity relationships of the 2-,3- and 2,3-positions. Med. Chem. Res. 2014, 23, 3187–3199. [Google Scholar] [CrossRef]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by agar dilution. Clin. Microbiol. Infect. 2000, 6, 509–515. [Google Scholar]
- National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 5th ed.; National Committee for Clinical Laboratory Standards (NCCLS): Wayne, PA, USA, 2000; Approved Standard M7-A5. [Google Scholar]
- Al-Dies, A.M.; Amr, A.-G.E.; El-Agrody, A.M.; Chia, T.S.; Fun, H.-K. 2-Amino-4-(4-fluorophenyl)-6-methoxy-4H-benzo[h]chromene-3-carbonitrile. Acta Cryst. 2012, E68, 1934–1935. [Google Scholar] [CrossRef] [PubMed]
- Al-Sehemi, A.G.; Irfan, A.; El-Agrody, A.M. Synthesis, characterization and DFT study of 4H-benzo[h]chromene derivatives. J. Mol. Struct. 2012, 1018, 171–175. [Google Scholar] [CrossRef]
- Al-Dies, A.M.; El-Agrody, A.M.; Al-Omar, M.A.; Amr, A.-G.E.; Ng, S.W.; Tiekink, E.R.T. 2-Amino-4-(4-bromophenyl)-6-methoxy-4H-benzo[h]chromene-3-carbonitrile. Acta Cryst. 2013, E69, 0480–0481. [Google Scholar] [CrossRef] [PubMed]
- El-Agrody, A.M.; Al-Dies, A.M.; Fouda, A.M. Microwave assisted synthesis of 2-amino-6-methoxy-4H-benzo[h]chromene derivatives. Eur. J. Chem. 2014, 5, 133–137. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds (7a–o), (8a–c) and (12a–c) are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).