Abstract
This study addressed the design and syntheses of diverse ligands, which were then successfully treated with Ni (II) ion to afford a series of nickel complexes. α-Chloroformylarylhydrazine hydrochlorides 6 contain two different functional groups. One is a strong nucleophile, and the other is a good electrophile. Therefore, it can be designed to react with several reagents to obtain diverse derivatives which can be used as ligands for metal complexes. Furthermore, benzimidazole and salicylaldehyde can provide electron donor sites, N and O electron donors, separately. Hence, the starting materials α-chloroformylarylhydrazine hydrochlorides 6 were first treated with 2-(aminomethyl)-benzimidazole (7) to give the corresponding semicarbazides 8. Then, the semicarbazides 8 reacted with various substituted salicylaldehydes 9–11 to afford the desired substituted-salicylaldehyde 2-aryl-4-substituted semicarbazones 12–14, which could coordinate with nickel (II) ion to give the corresponding nickel complexes 15–17.
1. Introduction
The design and synthesis of metal-organic frameworks have attracted much attention from chemists. Thiosemicarbazones, semicarbazones and their metal complexes have been extensively studied in recent years, mainly because of their potential biological properties [1,2,3]. However, less attention has been devoted to the synthesis of the structurally analogous semicarbazones and their metal complexes. Semicarbazones are readily available and can coordinate to the metal ion either as neutral or deprotonated ligands through two or three donor atoms. In order to obtain novel ligands containing semicarbazone moieties, adequate precursors should first be designed and investigated. 3-Arylsydnones 1 could be cleaved and hydrolyzed to α-formylarylhydrazine hydrochloride intermediates by hydrochloric acid, and then the intermediates were sequentially converted to 3-arylhydrazine hydrochlorides 2 [4]. However, 3-phenyl-4-methylsydnone (3) was only hydrolyzed to α-acetylphenylhydrazine (4) by hydrochloric acid [5]. According to the above result, we considered treating 3-arylsydnones 1 with N-chlorosuccinimde (NCS) to obtain 3-aryl-4-chlorosydnones 5 which could further react with hydrochloric acid to afford α-chloroformylarylhydrazine hydrochlorides 6, as shown in Scheme 1. The precursors 6 would be expected to react with appropriate amines and aldehydes to afford various Schiff-bases which contained the desired semicarbazone moieties.
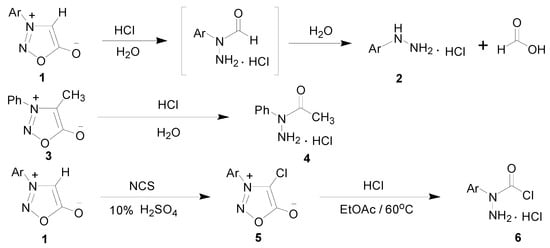
Scheme 1.
The preparation of α-chloroformylarylhydrazine hydrochlorides 6 from 3-aryl-4-chlorosydnones 5.
α-Chloroformylarylhydrazine hydrochlorides 6 contained two different functional groups. One is a very strong nucleophile, and the other is a good electrophile [6]. Precursors 6 could be treated with several reagents to give diverse derivatives [7]. We have already done abundant research on numerous aspects of 3-arylsydnone derivatives [8,9,10,11,12,13,14,15], and this is the first work conducted to utilize the starting materials 6, which are derived from the decomposition of sydnone compounds, to synthesize diverse ligands and transition metal complexes.
2. Results and Discussion
2.1. Synthetic Chemistry
Starting materials 6, since they contain acyl chloride groups, are very active and moisture-sensitive. We thus first dealt with the acyl chloride group of precursors 6 in the novel ligands design, and then dealt with the amino group to obtain the desired ligands. The benzimidazole scaffold is a useful structural motif for imparting chemical functionality to biologically active molecules [16,17,18,19,20], and many metal complexes with benzimidazole moieties display a wide range of special activities [21,22,23]. 2-(Aminomethyl)benzimidazole (7) can provide N,N two electron donor atoms, therefore, the starting materials 6a–c were first treated with benzimidazole 7 at 0 °C in the presence of triethylamine to give the corresponding 2-aryl-4-[(1H-benzo[d]imidazol-2-yl)methyl]semicarbazides 8a–c, as shown in Scheme 2. Because the starting materials 6 have a tendency to dimerize, the desired reactions must be carried out at 0 °C, otherwise, the efforts would fail. All the semicarbazides were synthesized in good yields and analytically pure. Among them, single crystals of 8a and 8b were suitable for X-ray structural analyses. Figure 1 and Figure 2 display the ORTEP drawings of semicarbazides 8a and 8b. Based on the X-ray data, semicarbazide 8b was crystallized with a water molecule, and formed hydrogen bonds between the N4 atom and H2O because the N4 and O2 distance was 2.724 Å.
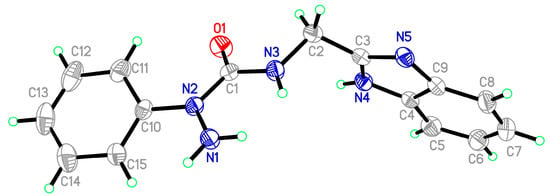
Figure 1.
ORTEP drawing of 2-phenyl-4-[(1H-benzo[d]imidazol-2-yl)methyl]semicarbazide (8a).
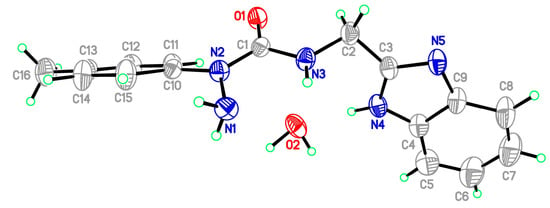
Figure 2.
ORTEP drawing of 2-(4-methylphenyl)-4-[(1H-benzo[d]imidazol-2-yl)methyl]-semicarbazide (8b).
It was considered that the novel semicarbazides could be reacted with various salicylaldehydes to form the well-known hydrazone Schiff bases. Salicylaldehyde hydrazone Schiff base have long received considerable attention for their fascinating chemical behavior and biological activity. Of interest to chemists is the coordination ability of salicylaldehyde hydrazone ligand through the imine nitrogen and phenoxy oxygen electron-donating atoms that allow it to serve as a multidentate ligand in structural assemblies [24,25,26,27,28,29]. In this study, the synthesized semicarbazides 8a–c were treated with various substituted salicylaldehydes, such as salicylaldehyde (9), 5-chlorosalicylaldehyde (10) and 4-methoxysalicylaldehyde (11) to afford a series of corresponding substituted-salicylaldehyde 2-aryl-4-[(1H-benzo[d]imidazol-2-yl)methyl]semicarbazones 12a–14c. The semicarbazones 12a–14c with salicylaldehyde-acylhydrazone moieties could coordinate with nickel (II) metal ion to give novel transition nickel (II) complexes 15a–17c as shown in Scheme 2. The novel synthesized ligands 12–14 and metal complexes 15–17 were identified by IR, NMR, ESIMS, EA and X-ray crystallography.
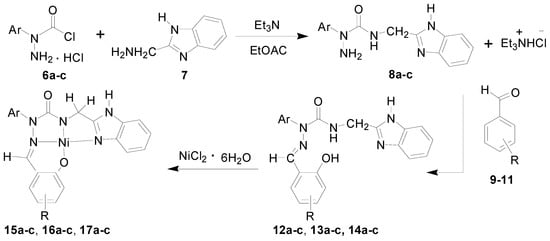
Scheme 2.
Novel Nickel (II) complexes 15–17 derived from diverse semicarbazone ligands 12–14. a: Ar = C6H5; b: Ar = p-CH3C6H4; c: Ar = p-CH3OC6H4; 9, 12, 15: R = H; 10, 13, 16: R = 5-Cl; 11, 14, 17: R = 4-OCH.
2.2. Spectroscopy Studies of Ligands and Metal Complexes
2.2.1. IR and NMR Studies
Based on the IR studies, the O-H and N-H stretching frequencies observed at around 3400 and 3200 cm−1 in free semicarbazones 12–14 are found to be absent in the complexes 15–17. The result confirms the deprotonation of these ligands upon metal complexation. Similarly, in the NMR studies, the signals at approximately 8.4 ppm (triplet, J = 5.6 Hz, 1H, NH) and about 10.0 ppm (singlet, 1H, OH) were originally assigned to the NH and OH protons of the semicarbazone. However, the signals are not found in the spectra of the nickel complexes 15–17. In addition, the NMR signal of CH2 in the semicarbazones is at about 4.6 ppm (doublet, J = 5.6 Hz, 2H), and is split into a doublet by coupling with the neighboring NH proton. However, the NMR signal of CH2 is a singlet without any neighboring NH proton coupling in the metal complexes. Figure 3 and Figure 4 show the NMR spectrum of ligand 13a and the corresponding complex 16a, respectively. All the above results indicated that semicarbazone ligands 12–14 served as deprotoned ligands after losing two protons from the NH and OH groups upon metal complexation, and 2-(aminomethyl)benzimidazole (7) and substituted-salicylaldehydes 9–11 might provide electron donor sites, N, N and O electron donor atoms.
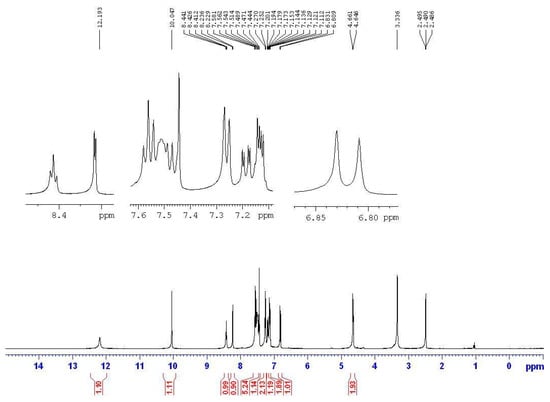
Figure 3.
The NMR spectrum of semicarbazone 13a.
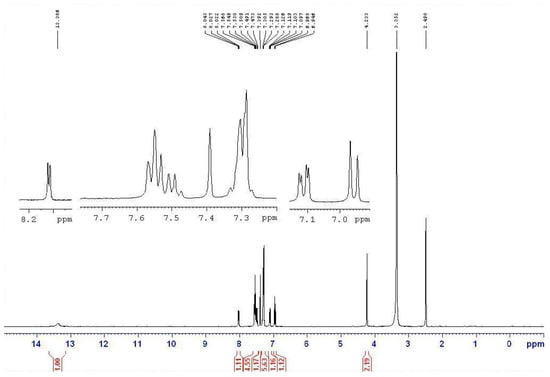
Figure 4.
The NMR spectrum of nickel complex 16a derived from semicarbazone 13a.
2.2.2. MS Study
To further confirm the molecular formula of nickel complexes 15–17, Fourier-Transfer Mass Analyzer was used to get the low resolution and high resolution ESI mass spectral data. The obtained analysis results definitely confirmed the molecular formulas of the synthesized complexes 15–17. The low-resolution ESIMS spectra of all nickel complexes 15a–c and 17a–c show distinct m/z [M+H]+ and [M+2+H]+ peak patterns because the relative isotope abundances of elemental nickel are 58Ni (68.0769%), 60Ni (26.2231%), 61Ni (1.1399%), 62Ni (3.6435%) and 64Ni (0.9256%). Besides, the relative isotope abundances of the element chlorine are 35Cl (75.76%) and 37Cl (24.24%), nickel complexes 16a–c containing one chlorine element would show more complicated MS peak patterns.
2.2.3. X-ray Study of Ligands and Complexes
All the new semicarbazones and complexes were synthesized in good yields and analytically pure. Among them, the crystals of 12a and 16a were suitable for X-ray structure analyses. Figure 5 and Figure 6 show the ORTEP drawings of semicarbazone 12a, and Ni complex 16a, respectively. Based on the ORTEP drawing of semicarbazone 12a (Figure 5), we confirm that the electron donor sites N(1), N(3), N(5) of free semicarbazone ligands 12–14 show a Z, Z configuration about N(2)-C(1), C(2)-C(3), and the hydroxyl oxygen is anti to the imine nitrogen N(1). However, the nickel complex 16a was crystallized with the salicylaldehyde hydroxyl oxygen syn to the imine nitrogen, as shown in Figure 6. According to the X-ray diffraction analyses, during metal complexation, semicarbazones 12–14 behave as tetradentate and deprotonated ligands after losing two protons from the OH and NH groups (Scheme 2), and form one six- and two five-membered chelate rings around the central metal through a set of donor atoms that consists of the salicylaldehyde hydroxyl oxygen, imine nitrogen, and two nitrogens of 2-(aminomethyl)benzimidazole (Figure 6).
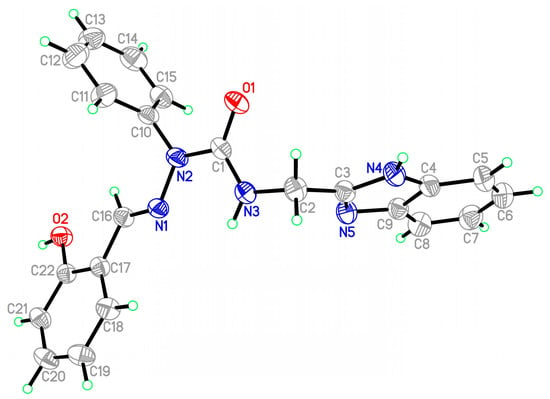
Figure 5.
ORTEP drawing of salicylaldehyde 2-phenyl-4-[(1H-benzo[d]imidazol-2-yl)methyl]semicarbazone (12a).
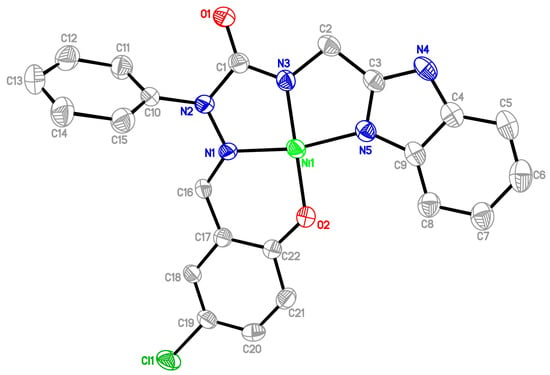
Figure 6.
ORTEP drawing of Ni complex of 5-chlorosalicylaldehyde 2-phenyl-4-[(1H-benzo[d]imidazol-2-yl)methyl]semicarbazone (16a).
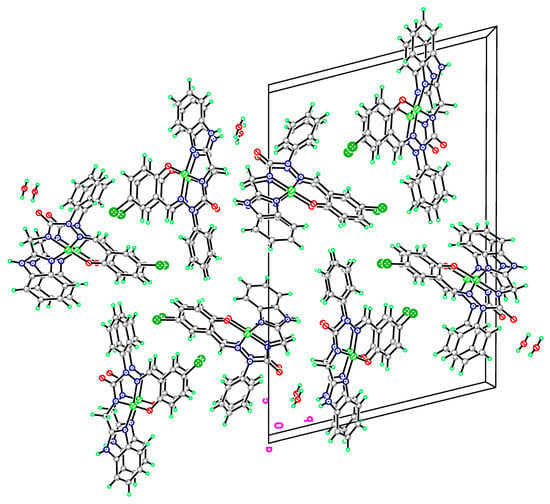
Figure 7.
The packing diagram of complex 16a crystallized with water molecule.
From the X-ray analysis of nickel complex 16a, the bond lengths (Å) Ni-N(1), Ni-N(5), Ni-N(3), Ni-O(2) were 1.835(5), 1.885(5), 1.819(5), 1.834(4), and the bond angles (°) involving nickel N(3)-Ni-N(1), N(1)-Ni-O(2), N(3)-Ni-O(2), N(3)-Ni-N(5), N(5)-Ni-O(2), N(1)-Ni-N(5) were 83.5(2), 96.4(2), 178.8(2), 84.5(2), 95.6(2), 167.9(2). The result indicates that the tricyclic N,N,N,O ring system forms a nearly square planar structure around the nickel atom, and contributes to the stability of the complex 16a. The ORTEP drawings of the metal complexes and diffraction data showed that the synthesized semicarbazones were tetradentate and dideprotoned ligands upon metal complexation. The nickel complex 16a was crystallized with a water molecule, but there is no hydrogen bond between the N4 atom and H2O. Figure 7 displays the packing diagram of complex 16a. The crystallographic data of semicarbazides 8a and 8b are summarized in Table 1. Table 2 lists the crystallographic data of semicarbazone 12a and complex 16a.

Table 1.
Crystal data of semicarbazides 8a and 8b.
| Compounds | 8a | 8b |
|---|---|---|
| Diffractometer | Nonius Kappa CCD | Nonius Kappa CCD |
| Formula | C15H15N5O | C16H17N5O·H2O |
| Formula weight | 281.32 | 313.36 |
| Crystal system | Monoclinic | Monoclinic |
| Space group | P2(1)/c | P2(1)/c |
| a/Å | 14.0940(3) | 15.3794(7) |
| b/Å | 10.0209(2) | 8.2222(5) |
| c/Å | 10.1067(2) | 13.8159(7) |
| α/° | 90.00 | 90 |
| β/° | 104.088(2) | 104.331(5) |
| γ/° | 90.00 | 90 |
| V/Å3 | 1384.48(5) | 1692.69(15) |
| Z | 4 | 4 |
| Dcalc (g·cm−3) | 1.350 | 1.230 |
| F000 | 592 | 664 |
| Absorption coefficient (mm−1) | 0.090 | 0.085 |
| Crystal size/mm | 0.25 × 0.20 × 0.15 | 0.25 × 0.25 × 0.15 |
| Temperature (K) | 295(2) | 295(2) |
| θrange, deg | 1.49–27.49 | 2.83–27.50 |
| Reflections collected | 18599 | 19743 |
| Independent reflections | 3161[R(int) = 0.0522] | 3879 [R(int) = 0.0412] |
| Refinement method | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 |
| Final R indices [I > 2.00σ(I)] | R1 = 0.0595, wR2 = 0.1625 | R1 = 0.0828, wR2 = 0.2736 |
| R indices (all data) | R1 = 0.0947, wR2 = 0.1888 | R1 = 0.1071, wR2 = 0.2861 |
| GoF | 1.263 | 1.051 |
3. Experimental Section
3.1. General Information
All melting points were determined on an England Electrothermal Digital Melting Point apparatus and were uncorrected. IR spectra were recorded on a Mattson/Satellite 5000 FT-IR spectrophotometer. Mass spectra were measured on a high-resolution JEOL JMS-700 mass spectrometer and a Bruker APEX II FT-MS. 1H-NMR spectra were run on a Bruker AV 400 NMR spectrometer, using TMS as an internal standard. 13C-NMR spectra were recorded out with complete 1H decoupling and assignments were made through additional DEPT experiments. Elemental analyses were taken with an Elementar Vario EL-III Analyzer. X-ray crystallography was performed on a Nonius CAD4 Kappa Axis XRD instrument. α-Chloroformylarylhydrazine hydrochlorides 6a–c were prepared from the corresponding 3-aryl-4-chlorosydnones 5a–c according to the literature [6].

Table 2.
Crystal data of semicarbazone 12a and complex 16a.
| Compounds | 12a | 16a |
|---|---|---|
| Diffractometer | Nonius Kappa CCD | Nonius Kappa CCD |
| Formula | C22H19N5O2·C2H5OH | [Ni2(C22H16N5O2Cl)2]·H2O |
| Formula weight | 431.49 | 971.13 |
| Crystal system | Triclinic | Triclinic |
| Space group | P-1 | P-1 |
| a/Å | 10.3571(7) | 5.2360(4) |
| b/Å | 10.3772(8) | 16.6535(9) |
| c/Å | 10.7518(7) | 25.6203(16) |
| α/° | 100.618(6) | 75.822(5) |
| β/° | 96.497(5) | 88.784(6) |
| γ/° | 90.357(6) | 87.121(5) |
| V/Å3 | 1128.06(14) | 2163.2(2) |
| Z | 2 | 2 |
| Dcalc (g·cm−3) | 1.270 | 1.491 |
| F000 | 456 | 996 |
| Absorption coefficient (mm−1) | 0.700 | 1.052 |
| Crystal size/mm | 0.25 × 0.15 × 0.10 | 0.2 × 0.1 × 0.02 |
| Temperature (K) | 295(2) | 295(2) |
| θrange, deg | 4.30–67.93 | 3.03–27.50 |
| Reflections collected | 10692 | 17218 |
| Independent reflections | 4069 [R(int) = 0.0287] | 9910 [R(int) = 0.0584] |
| Refinement method | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 |
| Final R indices [I > 2.00σ(I)] | R1 = 0.0626, wR2 = 0.1871 | R1 = 0.0898, wR2 = 0.2252 |
| R indices (all data) | R1 = 0.0778, wR2 = 0.2127 | R1 = 0.1419, wR2 = 0.2457 |
| GoF | 1.013 | 1.306 |
3.2. Syntheses of 2-aryl-4-[(1H-benzo[d]imidazol-2-yl)methyl]semicarbazides 8a–c
To an ice-cooled solution of α-chloroformyl phenylhydrazine hydrochloride (6a, 207.1 mg, 1.0 mmol) in ethyl acetate (2 mL), an ice-cooled solution of 2-(aminomethyl)benzimidazole dihydrochloride hydrate (7, 242.2 mg, 1.1 mmol) in ethyl acetate (2 mL) was slowly added. Then, triethylamine (454.5 mg, 4.5 mmol) was added dropwise to the above solution. The mixed solution was stirred at 0 °C for about 6–7 h until the reaction was complete. The precipitating solid was first collected by filtration, and the organic filtrate was kept for the next step. First, the filtered solid was added to cold water (5 mL) with stirring and filtered to remove the dissolved triethylamine hydrochloride salt, then 261.5 mg of white crude product was obtained. Next, the organic filtrate was evaporated to near dryness and cold 2-propanol (1 mL) was added to precipitate a solid after stirring and filtration, and 21.2 mg of white solid were thus obtained. All the solid products were combined and recrystallized from dichloromethane/2-propanol to afford 221.9 mg (0.79 mmol, yield 79%) of 8a as white crystals. The chemical and physical spectral characteristics of these products 8a–c are given below.
2-Phenyl-4-[(1H-benzo[d]imidazol-2-yl)methyl]semicarbazide (8a): White crystals from CH2Cl2/(CH3)2CHOH; yield 79%; mp 172–174 °C; IR (KBr): 3343, 3327, 3283, 3198, 1649, 1597, 1519, 1417, 1291, 1178, 1011, 984, 837, 747 cm−1; 1H-NMR (DMSO-d6): δ 4.53 (d, J = 5.6 Hz, 2H, CH2), 5.28 (s, 2H, NH2), 7.02 (t, J = 8.0 Hz, 1H, ArH), 7.10–7.16 (m, 2H, imidazole-H), 7.28 (t, J = 8.0 Hz, 2H, ArH), 7.45–7.55 (m, 2H, imidazole-H), 7.62 (d, J = 8.0 Hz, 2H, ArH), 7.95 (t, J = 5.6 Hz, 1H, NH), 12.17 (s, 1H, NH); FABMS: m/z (%) = 282 ([M+H]+, 100), 281 (M+, 10), 174 (90), 131 (37). Anal. Calcd for C15H15N5O: C, 64.04; H, 5.37; N, 24.89. Found: C, 64.00; H, 5.35; N, 24.90. X-ray analytical data is listed in Table 1. Further details have been deposited at the Cambridge Crystallographic Data Center and allocated the deposition number CCDC 959866.
2-(4-Methylphenyl)-4-[(1H-benzo[d]imidazol-2-yl)methyl]semicarbazide (8b): White crystals from CH2Cl2/(CH3)2CHOH; yield 73%; mp 162–164 °C; IR (KBr): 3421, 3382, 3332, 3192, 1646, 1510, 1438, 1274, 1168, 1015, 996, 812, 742 cm−1; 1H-NMR (DMSO-d6): δ 2.25 (s, 3H, CH3), 4.52 (d, J = 5.6 Hz, 2H, CH2), 5.22 (s, 2H, NH2), 7.08 (d, J = 8.4 Hz, 2H, ArH), 7.12–7.14 (m, 2H, imidazole-H), 7.48 (d, J = 8.4 Hz, 2H, ArH) 7.49–7.56 (m, 2H, imidazole-H), 7.88 (t, J = 5.6 Hz, 1H, NH), 12.16 (s, 1H, NH); FABMS: m/z (%) = 296 ([M+H]+, 100), 295 (M+, 5), 174 (84), 131 (45). Anal. Calcd for C16H17N5O·H2O: C, 61.33; H, 6.11; N, 22.35. Found: C, 61.28; H, 6.09; N, 22.40. X-ray analytical data is listed in Table 1. Further details have been deposited at the Cambridge Crystallographic Data Center and allocated the deposition number CCDC 959867.
2-(4-Methoxyphenyl)-4-[(1H-benzo[d]imidazol-2-yl)methyl]semicarbazide (8c): White crystals from CH2Cl2/(CH3)2CHOH; yield 74%; mp 168–169 °C; IR (KBr): 3369, 3292, 3252, 3192, 1642, 1454, 1245, 1274, 1170, 1033, 977, 833, 743 cm−1; 1H-NMR (DMSO-d6): δ 3.72 (s, 3H, CH3O), 4.51 (d, J = 5.6 Hz, 2H, CH2), 5.20 (s, 2H, NH2), 6.86 (d, J = 8.8 Hz, 2H, ArH), 7.11–7.18 (m, 2H, imidazole-H), 7.46 (d, J = 8.8 Hz, 2H, ArH), 7.49–7.54 (m, 2H, imidazole-H), 7.80 (t, J = 5.6 Hz, 1H, NH), 12.15 (s, 1H, NH); FABMS: m/z (%) = 312 ([M+H]+, 100), 311 (M+, 4), 174 (92), 131 (100); HRMS (FAB): m/z [M+H]+ calcd for C16H18N5O2: 312.1460; found: 312.1462. Anal. Calcd for C16H17N5O2: C, 61.72; H, 5.50; N, 22.49. Found: C, 61.61; H, 5.51; N, 22.35.
3.3. Syntheses of Substituted-Salicylaldehyde 2-Aryl-4-[(1H-benzo[d]imidazol-2-yl)methyl] semicarbazones 12a–14c
To a solution of 2-phenyl-4-(1H-benzimidazole-2-ylmethyl)semicarbazide (8a, 562.6 mg, 2.0 mmol) in absolute ethanol (6 mL), salicylaldehyde (9, 268.7 mg, 2.2 mmol) was added. The mixed solution was stirred at room temperature for about 8 h until the reaction was complete. The precipitated white powder (679.9 mg) was collected by filtration and washed with cold hexane/ethyl acetate (3:1). The collected solid was recrystallized from dichloromethane/ethanol to afford 602.5 mg (1.56 mmol, yield 78%) of 12a as white powder. The chemical and physical spectral characteristics of these products 12a–c, 13a–c, 14a–c are given below.
Salicylaldehyde 2-phenyl-4-[(1H-benzo[d]imidazol-2-yl)methyl]semicarbazone (12a): White crystals from CH2Cl2/EtOH; yield 78%; mp 243–244 °C; IR (KBr): 3415, 3230, 3213, 3060, 1668, 1605, 1517, 1450, 1272, 748 cm−1; 1H-NMR (DMSO-d6): δ 4.65 (d, J = 5.6 Hz, 2H, CH2), 6.80–6.85 (m, 2H, salicyl-H), 7.09–7.21 (m, 3H, imidazole-H, salicyl-H), 7.26 (d, J = 7.6 Hz, 2H, ArH), 7.42–7.61 (m, 6H, imidazole-H, =C-H, 3ArH), 8.12 (d, J = 7.6 Hz, 1H, salicyl-H), 8.24 (t, J = 5.6 Hz, 1H, NH), 9.75 (s, 1H, OH), 12.19 (s, 1H, NH); FABMS: m/z (%) = 386 ([M+H]+, 100), 385 ((M+, 10), 212 (30), 174 (51); HRMS (FAB): m/z [M+H]+ calcd for C22H20N5O2: 386.1617; found: 386.1715. Anal. Calcd for C22H19N5O2: C, 68.56; H, 4.97; N, 18.17. Found: C, 68.46; H, 4.96; N, 18.13. X-ray analytical data is listed in Table 2. Further details have been deposited at the Cambridge Crystallographic Data Center and allocated the deposition number CCDC 959868.
Salicylaldehyde 2-(4-methylphenyl)-4-[(1H-benzo[d]imidazol-2-yl)methyl]semicarbazone (12b): White powder from CH2Cl2/EtOH; yield: 81%; mp 226–227 °C; IR (KBr): 3419, 3375, 3180, 3057, 2927, 1665, 1605, 1525, 1456, 1265, 746 cm−1; 1H-NMR (DMSO-d6): δ 2.38 (s, 3H,CH3), 4.63 (d, J = 6.0 Hz, 2H, CH2), 6.78–6.86 (m, 2H, salicy-H), 7.08–7.20 (m, 5H, ArH, imidazole-H, salicyl-H), 7.35 (d, J = 8.0 Hz, 2H, ArH), 7.44–7.55 (m, 3H, imidazole-H, =C-H), 8.11 (d, J = 8.0 Hz, 1H, salicyl-H), 8.21 (t, J = 6.0 Hz, 1H, NH), 9.77 (s, 1H, OH), 12.18 (s, 1H, NH); 13C-NMR (DMSO-d6): δ = 21.04, 57.99, 111.46, 116.12, 118.50, 119.40, 120.76, 121.23, 121.87, 126.61, 129.86, 130.62, 130.85, 133.88, 134.51, 135.17, 138.47, 143.34, 153.55, 155.56, 156.11; FABMS: m/z (%) = 400 ([M+H]+, 100), 399 (M+, 8), 226 (58), 174 (83); HRMS (FAB): m/z [M+H]+ calcd for C23H22N5O2: 400.1774; found: 400.1772. Anal. Calcd for C23H21N5O2: C, 69.16; H, 5.30; N, 17.53. Found: C, 69.06; H, 5.31; N, 17.56.
Salicylaldehyde 2-(4-methoxyphenyl)-4-[(1H-benzo[d]imidazol-2-yl)methyl]semicarbazone (12c): White powder from CH2Cl2/EtOH; yield: 80%; mp 202–203 °C; IR (KBr): 3418, 3357, 3183, 3061, 2935, 1664, 1606, 1524, 1447, 1253, 744 cm−1; 1H-NMR (DMSO-d6): δ 3.82 (s, 3H, CH3O), 4.63 (d, J = 6.0 Hz, 2H, CH2), 6.78–6.87 (m, 2H, salicyl-H), 7.09 (d, J = 8.4 Hz, 2H, ArH), 7.11–7.15 (m, 3H, imidazole-H, salicyl-H), 7.18 (d, J = 8.4 Hz, 2H, ArH), 7.43–7.55 (m, 3H, =C-H, imidazole-H,), 8.10 (d, J = 7.6 Hz, 1H, salicyl-H), 8.19 (t, J = 6.0 Hz, 1H, NH), 9.80 (s, 1H, OH), 12.18 (s, 1H, NH); FABMS: m/z (%) = 416 ([M+H]+, 100), 410 (M+, 4); HRMS (FAB): m/z [M+H]+ calcd for for C23H22N5O3: 416.1723; found: 416.1723. Anal. Calcd for C23H21N5O3: C, 66.49; H, 5.09; N, 16.86. Found: C, 66.40; H, 5.07; N, 16.81.
5-Chlorosalicylaldehyde 2-phenyl-4-[(1H-benzo[d]imidazol-2-yl)methyl]semicarbazone (13a): White powder from CH2Cl2/EtOH; yield 75%; mp 231–232 °C; IR (KBr): 3419, 3210, 3065, 2937, 1675, 1624, 1518, 1423, 1284, 1275, 1196, 1108, 1032, 814, 743, 696 cm−1; 1H-NMR (DMSO-d6): δ 4.65 (d, J = 5.6 Hz, 2H, CH2), 6.81 (d, J = 8.8 Hz, 1H, salicyl-H), 7.11–7.15 (m, 2H, imidazole-H), 7.18 (dd, J = 8.8, 2.8 Hz, 1H, salicyl-H), 7.26 (d, J = 7.6 Hz, 2H, ArH), 7.44–7.58 (m, 6H, 3ArH, imidazole-H, =C-H), 8.23 (d, J = 2.8 Hz, 1H, salicyl-H), 8.42 (t, J = 5.6 Hz, 1H, NH), 10.03 (s, 1H, OH), 12.18 (s, 1H, NH); MS (EI, 30ev): m/z (%) = 421 ([M+2]+, 2), 419 (M+, 6), 248 ([M+2-C7N2H5CH2NCO]+, 15), 246 (M+-C7N2H5CH2NCO, 46), 174 (C7N2H5CH2NHCO, 100) 173 (C7N2H5CH2NCO, 41); HRMS (EI): m/z [M]+ calcd for C22H18N5O235Cl: 419.1149; found: 419.1152. Anal. Calcd for C22H18N5O2Cl: C, 62.93; H, 4.32; N, 16.68. Found: C, 62.82; H, 4.31; N, 16.72.
5-Chlorosalicylaldehyde 2-(4-methylphenyl)-4-[(1H-benzo[d]imidazol-2-yl)methyl]semicarbazone (13b): White powder from CH2Cl2/EtOH; yield 76%; mp 229–230 °C; IR (KBr): 3405, 3196, 3063, 2924, 1666, 1606, 1510, 1425, 1273, 1206, 1107, 1029, 820, 743, 655 cm−1; 1H-NMR (DMSO-d6): δ 2.38 (s, 3H, CH3), 4.64 (d, J = 5.6 Hz, 2H, CH2), 6.82 (d, J = 8.8 Hz, 1H, salicyl-H), 7.09–7.15 (m, 4H, ArH, imidazole-H), 7.18 (dd, J = 8.8, 2.4 Hz, 1H, salicyl-H), 7.35 (d, J = 8.0 Hz, 2H, ArH), 7.45–7.55 (m, 3H, imidazole-H, =C-H), 8.21 (d, J = 2.4 Hz, 1H, salicyl-H), 8.38 (t, J = 5.6 Hz, 1H, NH), 10.04 (s, 1H, OH), 12.17 (s, 1H, NH); MS (EI, 30ev): m/z (%) = 435 ([M+2]+, 2), 433 (M+, 5), 262 ([M+2-C7N2H5CH2NCO]+, 36), 260 (M+-C7N2H5CH2NCO, 100), 174 (C7N2H5CH2NHCO, 79) 173 (C7N2H5CH2NCO, 42); HRMS (EI): m/z [M]+ calcd for C23H20N5O235Cl: 433.1306; found: 433.1308. Anal. Calcd for C23H20N5O2Cl: C, 63.67; H, 4.65; N, 16.14. Found: C, 63.65; H, 4.63; N, 16.10.
5-Chlorosalicylaldehyde 2-(4-methoxyphenyl)-4-[(1H-benzo[d]imidazol-2-yl)methyl]semicarbazone (13c): White powder from CH2Cl2/EtOH; yield 82%; mp 245–246 °C; IR (KBr): 3403, 3206, 3072, 2935, 1662, 1606, 1508, 1422, 1283, 1249, 1182, 1029, 821, 751, 653 cm−1; 1H-NMR (DMSO-d6): δ 3.81 (s, 3H, CH3O), 4.63 (d, J = 6.0 Hz, 2H, CH2), 6.82 (d, J = 8.8 Hz, 1H, salicyl-H), 7.05–7.20 (m, 7H, 4ArH, imidazole-H, salicyl-H), 7.42–7.58 (m, 3H, imidazole-H, =C-H), 8.21 (d, J = 2.4 Hz, 1H, salicyl-H), 8.36 (t, J = 6.0 Hz, 1H, NH), 10.04 (s, 1H, OH), 12.16 (s, 1H, NH); MS (EI, 30ev): m/z (%) = 451 ([M+2]+, 2), 449 (M+, 6), 278 ([M+2-C7N2H5CH2NCO]+, 39), 276 (M+-C7N2H5CH2NCO, 100), 174 (C7N2H5CH2NHCO, 71) 173 (C7N2H5CH2NCO, 38); HRMS (EI): m/z [M]+ calcd for C23H20N5O335Cl: 449.1255; found: 449.1258. Anal. Calcd for C23H20N5O3Cl: C, 61.40; H, 4.48; N, 15.57. Found: C, 61.49; H, 4.46; N, 15.60.
4-Methoxysalicylaldehyde 2-phenyl-4-[(1H-benzo[d]imidazol-2-yl)methyl]semicarbazone (14a): White powder from CH2Cl2/EtOH; yield 80%; mp 218–219 °C; IR (KBr): 3417, 3231, 3019, 2942, 2870, 1666, 1614, 1523, 1436, 1292, 1202, 1031, 745 cm−1; 1H-NMR (DMSO-d6): δ 3.70 (s, 3H, OCH3), 4.62 (d, J = 6.0 Hz, 2H, CH2), 6.35 (d, J = 2.4 Hz, 1H, salicyl-H), 6.43 (dd, J = 8.8, 2.4 Hz, 1H, salicyl-H), 7.11–7.24 (m, 2H, imidazole-H), 7.25 (d, J = 7.2 Hz, 2H, ArH), 7.44–7.63 (m, 6H, 3ArH, imidazole-H, =C-H), 8.02 (d, J = 8.8 Hz, 1H, salicyl-H), 8.17 (t, J = 6.0 Hz, 1H, NH), 9.84 (s, 1H, OH), 12.17 (s, 1H, NH); MS (EI, 30ev): m/z (%) = 415 (M+, 2), 242 (M+-C7N2H5CH2NCO, 100), 173 (C7N2H5CH2NCO, 25); HRMS (EI): m/z [M]+ calcd for C23H21N5O3: 415.1644; found: 415.1642. Anal. Calcd for C23H21N5O3: C, 66.49; H, 5.09; N, 16.86. Found: C, 66.52; H, 5.08; N, 16.79.
4-Methoxysalicylaldehyde 2-(4-methylphenyl)-4-[(1H-benzo[d]imidazol-2-yl)methyl]semicarbazone (14b): White powder from CH2Cl2/EtOH; yield 81%; mp 198–199 °C; IR (KBr): 3418, 3174, 3143, 3059, 2963, 1643, 1609, 1511, 1438, 1291, 1237, 1208, 1031, 740 cm−1; 1H-NMR (DMSO-d6): δ 2.37 (s, 3H, CH3), 3.71 (s, 3H, OCH3), 4.63 (d, J = 5.6 Hz, 2H, CH2), 6.36 (d, J = 2.4 Hz, 1H, salicyl-H), 6.43 (dd, J = 8.8, 2.4 Hz, 1H, salicyl-H), 7.10–7.14 (m, 4H, ArH, imidazole-H), 7.34 (d, J = 8.0 Hz, 2H, ArH), 7.45–7.60 (m, 3H, =C-H, imidazole-H), 8.00 (d, J = 8.8 Hz, 1H, salicyl-H), 8.12 (t, J = 5.6 Hz, 1H, NH), 9.88 (s, 1H, OH), 12.17 (s, 1H, NH); MS (EI, 30ev): m/z (%) = 429 (M+, 1), 256 (M+-C7N2H5CH2NCO, 100), 173 (C7N2H5CH2NCO, 37); HRMS (EI): m/z [M]+ calcd for C24H23N5O3: 429.1801; found: 429.1802. Anal. Calcd for C24H23N5O3: C, 67.12; H, 5.40; N, 16.31. Found: C, 67.01; H, 5.38; N, 16.34.
4-Methoxysalicylaldehyde 2-(4-methoxyphenyl)-4-[(1H-benzo[d]imidazol-2-yl)methyl]semicarbazone (14c): White powder from CH2Cl2/EtOH; yield 84%; mp 170–171 °C; IR (KBr): 3404, 3190, 3059, 2837, 1657, 1609, 1510, 1441, 1295, 1250, 1205, 1031, 743 cm−1; 1H-NMR (DMSO-d6): δ 3.70 (s, 3H, OCH3), 3.81 (s, 3H, CH3O), 4.61 (d, J = 5.6 Hz, 2H, CH2), 6.36 (d, J = 2.4 Hz, 1H, salicyl-H), 6.42 (dd, J = 8.8, 2.4 Hz, 1H, salicy-H), 7.05–7.20 (m, 6H, ArH, imidazole-H), 7.40–7.55 (m, 3H, imidazole-H, =C-H), 7.98 (d, J = 8.8 Hz, 1H, salicyl-H ), 8.09 (t, J = 5.6 Hz, 1H, NH), 9.92 (s, 1H, OH), 12.16 (s, 1H, NH); MS (EI, 30ev): m/z (%) = 445 (M+, 1), 272 (M+-C7N2H5CH2NCO, 100), 173 (C7N2H5CH2NCO, 23); HRMS (EI): m/z [M]+ calcd for C24H23N5O4: 445.1750; found: 445.1751. Anal. Calcd for C24H23N5O4: C, 64.71; H, 5.20; N, 15.72. Found: C, 64.66; H, 5.18; N, 15.79.
3.4. Syntheses of Ni Complexes of Substituted-salicylaldehyde 2-Aryl-4-[(1H-benzo[d]imidazol-2-yl)methyl] Semicarbazones 15a–17c
To a hot solution of salicylaldehyde 2-phenyl-4-[(1H-benzo[d]imidazol-2-yl)methyl] semicarbazone (12a, 385.4 mg, 1.0 mmol) in 95% ethanol (8 mL, 60 °C), a green hot solution of nickel(II) chloride hexahydrate (356.6 mg, 1.5 mmol) in distilled water (4 mL, 60 °C) was added dropwise. The orange mixture appeared cloudy and some solid precipitated out. The mixture was still heated at 60 °C and stirred for about 4–5 days until the reaction was complete. The precipitated orange powder (298.5 mg) was collected by filtration and washed with cold water and cold ethanol. The collected powder was recrystallized from dichloromethane/ethanol to afford 264.5 mg (0.60 mmol, yield 60%) of 15a as golden-yellow crystals. The chemical and physical spectral characteristics of the Ni complexes 15a–c, 16a–c, 17a–c are given below.
Ni complex of salicylaldehyde 2-phenyl-4-[(1H-benzo[d]imidazol-2-yl)methyl]semicarbazone (15a): Golden-yellow crystals from CH2Cl2/EtOH; yield 60%; mp 221–223 (dec) °C; IR (KBr): 3183, 3056, 2917, 1658, 1603, 1455, 1365, 1317, 1205, 1150, 1108, 839, 738, 593 cm−1; 1H-NMR (DMSO-d6): δ 4.24 (s, 2H, CH2), 6.48 (t, J = 8.0 Hz, 1H, salicyl-H), 6.95 (d, J = 8.0 Hz, 1H, salicyl-H), 7.08–7.16 (m, 2H, imidazole-H), 7.26–7.40 (m, 5H, 2ArH, =C-H, salicyl-2H), 7.45–7.60 (m, 4H, 3ArH, imidazole-H), 8.06–8.12 (m, 1H, imidazole-H), 13.37 (s, 1H, NH); MS (ESI): m/z (%) = 442 ([M+H]+, 100), 444 ([M+2+H]+, 28); HRMS (ESI): m/z [M+H]+ calcd for 58NiC22H18N5O2: 442.0814; found: 442.0816. Anal. Calcd for C22H17N5O2Ni: C, 59.77; H, 3.88; N, 15.84. Found: C, 59.68; H, 3.89; N, 15.79.
Ni complex of salicylaldehyde 2-(4-methylphenyl)-4-[(1H-benzo[d]imidazol-2-yl)methyl]semicarbazone (15b): Golden-yellow powder from CH2Cl2/EtOH; yield 66%; mp 242–244 (dec) °C; IR (KBr): 3167, 3024, 2910, 2839, 1649, 1604, 1451, 1363, 1317, 1203, 1148, 1098, 813, 735, 589 cm−1; 1H-NMR (DMSO-d6): δ 2.38 (s, 3H, CH3), 4.23 (s, 2H, CH2), 6.47 (t, J = 8.0 Hz, 1H, salicy-H), 6.95 (d, J = 8.0 Hz, 1H, salicyl-H), 7.05–7.20 (m, 4H, 2ArH, imidazole-H), 7.29–7.36 (m, 5H, 2ArH, =C-H, salicyl-H), 7.56 (d, J = 5.6 Hz, 1H, imidazole-H), 8.09 (d, J = 5.6 Hz, 1H, imidazole-H), 13.36 (s, 1H, NH); MS (ESI): m/z (%) = 456 ([M+H]+, 100), 458 ([M+2+H]+, 32); HRMS (ESI): m/z [M+H]+ calcd for 58NiC23H20N5O2: 456.0970; found: 456.0973. Anal. Calcd for C23H19N5O2Ni: C, 60.57; H, 4.20; N, 15.35. Found: C, 60.48; H, 4.19; N, 15.36.
Ni complex of salicylaldehyde 2-(4-methoxyphenyl)-4-[(1H-benzo[d]imidazol-2-yl)methyl]semicarbazone (15c): Orange-red crystals from CH2Cl2/EtOH; yield 62%; mp 219–221 (dec) °C; IR (KBr): 3113, 3034, 2963, 2907, 2837, 1650, 1605, 1448, 1364, 1315, 1248, 1204, 1148, 1092, 821, 735, 589 cm−1; 1H-NMR (DMSO-d6): δ 3.82 (s, 3H, CH3O), 4.23 (s, 2H, CH2), 6.47 (t, J = 8.0 Hz, 1H, salicyl-H), 6.95 (d, J = 8.0 Hz, 1H, salicyl-H), 7.05–7.16 (m, 4H, 2ArH, imidazole-H), 7.23 (d, J = 8.8 Hz, 2H, ArH), 7.29–7.36 (m, 3H, salicyl-H, =C-H), 7.56 (dd, J = 6.4, 2.4 Hz, 1H, imidazole-H), 8.09 (dd, J = 6.4, 2.4 Hz, 1H, imidazole-H), 13.37 (s, 1H); MS (ESI): m/z (%) = 472 ([M+H]+, 100), 474 ([M+2+H]+, 35); HRMS (ESI): m/z [M+H]+ for 58NiC23H20N5O3: 472.0920; found: 472.0923. Anal. Calcd for C23H19N5O3Ni: C, 58.51; H, 4.06; N, 14.83. Found: C, 58.46; H, 4.05; N, 14.79.
Ni complex of 5-chlorosalicylaldehyde 2-phenyl-4-[(1H-benzo[d]imidazol-2-yl)methyl]semicarbazone (16a): Orange-red needles from CH2Cl2/EtOH; yield 80%; mp 341–343 (dec) °C; IR (KBr): 3117, 3059, 2894, 1663, 1593, 1536, 1465, 1433, 1369, 1312, 1292, 1184, 1047, 813, 756, 698, 601 cm−1; 1H-NMR (DMSO-d6): δ 4.24 (s, 2H, CH2), 6.96 (d, J = 8.8 Hz, 1H, salicyl-H), 7.10 (dd, J = 8.8, 2.8 Hz, 1H, salicyl-H), 7.28–7.34 (m, 5H, 2ArH, imidazole-2H, salicyl-H), 7.40 (s, 1H, =C-H), 7.47–7.57 (m, 4H, 3ArH, imidazole-H), 8.03–8.05 (m, 1H, imidazole-H), 13.38 (s, 1H, NH); MS (ESI): m/z (%) = 476 ([M+H]+, 100), 478 ([M+2+H]+, 55); HRMS (ESI): m/z [M+H]+ for 58NiC22H17N5O235Cl: 476.0424; found: 476.0425. Anal. Calcd for C22H16N5O2ClNi: C, 55.45; H, 3. 39; N, 14.70. Found: C, 55.48; H, 3.40; N, 14.74. X-ray analytical data is listed in Table 2. Further details have been deposited at the Cambridge Crystallographic Data Center and allocated the deposition number CCDC 959869.
Ni complex of 5-chlorosalicylaldehyde 2-(4-methylphenyl)-4-[(1H-benzo[d]imidazol-2-yl)methyl]semicarbazone (16b): Orange powder from CH2Cl2/EtOH; yield 62%; mp 362–364 (dec) °C; IR (KBr): 3107, 3050, 2916, 1661, 1592, 1537, 1461, 1434, 1367, 1293, 1183, 1045, 814, 754, 598 cm−1; 1H-NMR (DMSO-d6): δ 2.38 (s, 3H, CH3), 4.24 (s, 2H, CH2), 6.96 (d, J = 9.2 Hz, 1H, salicyl-H), 7.11 (dd, J = 9.2, 2.8 Hz, 1H, salicyl-H), 7.17 (d, J = 8.4 Hz, 2H, ArH), 7.29–7.36 (m, 5H, salicyl-H, imidazole-2H, 2ArH), 7.39 (s, 1H, =C-H), 7.54–7.59 (m, 1H, imidazole-H), 8.01–8.07 (m, 1H, imidazole-H), 13.37 (s, 1H, NH); MS (ESI): m/z (%) = 490 ([M+H]+, 100), 492 ([M+2+H]+, 65); HRMS (ESI): m/z [M+H]+ for 58NiC23H19N5O235Cl: 490.0581; found: 490.0582. Anal. Calcd for C23H18N5O2ClNi: C, 56.31; H, 3.70; N, 14.28. Found: C, 56.22; H, 3.71; N, 14.24.
Ni complex of 5-chlorosalicylaldehyde 2-(4-methoxyphenyl)-4-[(1H-benzo[d]imidazol-2-yl)methyl]semicarbazone (16c): Orange feathers from CHCl3/EtOH; yield 60%; mp 383–385 (dec) °C; IR (KBr): 3103, 3048, 2989, 2888, 1659, 1593, 1512, 1462, 1435, 1366, 1308, 1255, 1182, 1046, 816, 753, 664, 599 cm−1; 1H-NMR (DMSO-d6): δ 3.84 (s, 3H, CH3O), 4.25 (s, 2H, CH3), 6.98 (d, J = 8.8 Hz, 1H, salicyl-H), 7.08–7.14 (m, 3H, 2ArH, salicyl-H), 7.23 (d, J = 8.8 Hz, 2H, ArH), 7.31–7.34 (m, 2H, imidazole-2H), 7.35 (d, J = 2.8 Hz, 1H, salicyl-H), 7.39 (s, 1H, =C-H), 7.55–7.61 (m, 1H, imidazole-H), 8.03–8.09 (m, 1H, imidazole-H), 13.40 (s, 1H, NH); MS (ESI): m/z (%) = 506 ([M+H]+, 100), 508 ([M+2+H]+, 60); HRMS (ESI): m/z [M+H]+ for 58NiC23H19N5O335Cl: 506.0530; found: 506.0533. Anal. Calcd for C23H18N5O3ClNi: C, 54.53; H, 3.58; N, 13.82. Found: C, 54.42; H,3.57; N, 13.79.
Ni complex of 4-methoxysalicylaldehyde 2-phenyl-4-[(1H-benzo[d]imidazol-2-yl)methyl]semicarbazone (17a): Orange-red needles from CHCl3/EtOH; yield 68%; mp 325–326 (dec) °C; IR (KBr): 3181, 3051, 2911, 2840, 1642, 1604, 1435, 1370, 1218, 1126, 1049, 826, 724, 539 cm−1; 1H-NMR (DMSO-d6): δ 3.74 (s, 3H, OCH3), 4.22 (s, 2H, CH2), 6.14 (dd, J = 8.8, 2.4 Hz, 1H, salicyl-H), 6.46 (d, J = 2.4 Hz, 1H, salicyl-H), 7.01 (d, J = 8.8 Hz, 1H, salicyl-H), 7.26 (s, 1H, =C-H), 7.28–7.34 (m, 4H, 2ArH, imidazole-2H), 7.44–7.58 (m, 4H, 3ArH, imidazole-H), 8.08 (dd, J = 6.0, 3.2 Hz, 1H, imidazole-H), 13.35 (s, 1H, NH); MS (ESI): m/z (%) = 472 ([M+H]+, 100), 474 ([M+2+H]+, 30); HRMS (ESI): m/z [M+H]+ for 58NiC23H20N5O3: 472.0920; found: 472.0917. Anal. Calcd for C23H19N5O3Ni: C, 58.51; H, 4.06; N, 14.83. Found: C, 58.46; H, 4.04; N, 14.76.
Ni complex of 4-methoxysalicylaldehyde 2-(4-methylphenyl)-4-[(1H-benzo[d]imidazol-2-yl)methyl]semicarbazone (17b): Orange-red needles from CHCl3/EtOH; yield 63%; mp 318–319 (dec) °C; IR (KBr): 3186, 3066, 2914, 2834, 1643, 1606, 1438, 1372, 1221, 1118, 1038, 827, 745, 537 cm−1; 1H-NMR (DMSO-d6): δ 2.37 (s, 3H, CH3), 3.74 (s, 3H, OCH3), 4.21 (s, 2H, CH2), 6.13 (d, J = 8.8 Hz, 1H, salicyl-H), 6.45 (s, 1H, salicyl-H), 7.01 (d, J = 8.8 Hz, 1H, salicyl-H), 7.16 (d, J = 7.6 Hz, 2H, ArH), 7.23 (s, 1H, =C-H), 7.28–7.40 (m, 4H, 2ArH, imidazole-H), 7.56 (brs, 1H, imidazole-H), 8.07 (brs, 1H, imidazole-H), 13.35 (s, 1H, NH); MS (ESI): m/z (%) = 486 ([M+H]+, 100), 488 ([M+2+H]+, 35); HRMS (ESI): m/z [M+H]+ for 58NiC24H22N5O3: 486.1076; found: 486.1078. Anal. Calcd for C24H21N5O3Ni: C, 59.30; H, 4.35; N, 14.41. Found: C, 59.18; H, 4.37; N, 14.35.
Ni complex of 4-methoxysalicylaldehyde 2-(4-methoxyphenyl)-4-[(1H-benzo[d]imidazol-2-yl)methyl]semicarbazone (17c): Yellow powder from CH2Cl2/EtOH; yield 64%; mp 335–336 (dec) °C; IR (KBr): 3118, 3051, 2905, 2836, 1654, 1606, 1440, 1372, 1219, 1105, 1043, 824, 744, 542 cm−1; 1H-NMR (DMSO-d6): δ 3.74 (s, 3H, OCH3), 3.81 (s, 3H, CH3O), 4.21 (s, 2H, CH2), 6.13 (dd, J = 8.8, 1.2 Hz, 1H, salicyl-H), 6.44 (d, J = 1.2 Hz, 1H, salicyl-H), 7.01 (d, J = 8.8 Hz, 1H, salicyl-H), 7.06 (d, J = 8.8 Hz, 2H, ArH), 7.14–7.21 (m, 3H, 2ArH, =C-H), 7.30–7.32 (m, 2H, imidazole-H), 7.56 (dd, J = 6.0, 2.8 Hz, 1H, imidazole-H), 8.07 (dd, J = 6.0, 2.8 Hz, 1H, imidazole-H), 13.34 (s, 1H, NH); MS (ESI): m/z (%) = 502 ([M+H]+, 100), 504 ([M+2+H]+, 30); HRMS (ESI): m/z [M+H]+ for 58NiC24H22N5O4: 502.1025; found: 502.1023. Anal. Calcd for C24H21N5O4Ni: C, 57.41; H, 4.22; N, 13.95. Found: C, 57.45; H, 4.23; N, 13.92.
4. Conclusions
In conclusion, this is the first work conducted to investigate the coordination chemistry of novel ligands containing the α-chloroformylarylhydrazine, benzimidazole and salicylaldehyde moieties. α-Chloroformylarylhydrazine hydrochlorides 6 were obtained through ring opening of 3-aryl-4-chlorosydnones with conc. hydrochloric acid. The starting materials 6 contained two special functional groups and had been proved to be good potential precursors of important ligands for metal complex formation in the study. The starting materials 6 were first treated with 2-(aminomethyl)benzimidazole (7) to give the corresponding semicarbazides 8. Then, the semicarbazides 8 reacted with various substituted salicylaldehydes 9–11 to afford the corresponding semicarbazones 12–14 which could coordinate with nickel (II) metal ions in a N,N,N,O-tetradentate coordination mode. Novel synthesized ligands and complexes were characterized by IR, NMR, ESIMS, EA and X-ray diffraction. Based on the analytical results, the most reasonable structure for the nickel complexes is nearly square planar. Semicarbazones 12–14 behave as tetradentate and deprotonated ligands after losing two protons from the OH and NH groups, and form one six- and two five-membered chelate rings around the central nickel metal through a set of donor atoms that consists of the salicylaldehyde hydroxyl oxygen, imine nitrogen, and two nitrogens of 2-(aminomethyl)benzimidazole. In summary, this is the first study to describe an efficient method of obtaining a series of novel tetradentate semicarbazone ligands and nickel complexes with benzimidazole and acylhydrazone moieties from the synthesized precursors, which were derived from acid hydrolysis of sydnone compounds.
Acknowledgments
Financial support of this work by the National Science Council of the Republic of China (NSC 99-2113-M-218-001) and Southern Taiwan University of Science and Technology is highly appreciated.
Author Contributions
Mei-Hsiu Shih planned and supervised the project and wrote the manuscript. Yu-Yuan Xu carried out the syntheses of complexes and participated in the characterization of all synthesized compounds. Yu-Sheng Yang carried out the syntheses of ligands and performed their physical and chemical characterization. Tzu-Ting Lin performed the syntheses of starting materials and some ligands. All the authors have read and approved the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ho, J.; Lee, W.Y.; Koh, K.J.T.; Lee, P.P.F.; Yan, Y.K. Rhenium(I) tricarbonyl complexes of salicylaldehyde semicarbazones: Synthesis, crystal structures and cytotoxicity. J. Inorg. Biochem. 2013, 19, 10–20. [Google Scholar] [CrossRef]
- Garbelini, E.R.; Martin, M.G.M.B.; Back, D.F.; Evans, D.J.; Müller-Santos, M.; Ribeiro, R.R.; Lang, E.S.; Nunes, F.S. Synthesis, characterization and chemical properties of 1-((E)-2-pyridinylmethylidene) semicarbazone manganese(II) and iron(II) complexes. J. Mol. Struct. 2012, 1008, 35–41. [Google Scholar] [CrossRef]
- Shih, M.H.; Chen, J.C.; Ling, G.L.; Lin, T.T.; Sun, M.H. Novel synthesis of palladium (II) complexes derived from 3-arylsydnone-4-carbaldehyde N(4)-phenylthiosemicarbazones and biological activity. J. Pharm. Pharmacol. 2014, 66, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Aziz, S.; Cockerill, A.F.; Tillett, J.G. Nucleophilic catalysis. Part III. Acid-catalysed hydrolyses of 3-phenylsydnone and 3-m-nitrophenylsydnone. J. Chem. Soc. (B) 1970, 416–420. [Google Scholar]
- Kenner, J.; Mackay, K. α-Acyl hydrazines. Nature 1947, 160, 465–466. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.N.; Wu, M.H.; Chen, S.P.; Li, T.P.; Hung, C.Y.; Yeh, M.Y. Syntheses of α-haloformylaryl-hydrazines and their self-dimerizations. J. Chin. Chem. Soc. 1994, 41, 849–856. [Google Scholar]
- Kuo, W.F.; Lee, C.Y.; Yeh, M.Y. Syntheses of 4-(5-oxo-1,2,4-triazol-3-yl)-sydnones and 4-(4-arylamino-5-oxo-1,2,4-triazol-3-yl)-sydnones from sydnone derivatives and their fragments. J. Chin. Chem. Soc. 2000, 47, 227–240. [Google Scholar]
- Shih, M.H. Studies on the syntheses of heterocycles from 3-arylsydnone-4-carbohydroximic acid chlorides with N-arylmaleimides, [1,4]naphthoquinone and aromatic amines. Tetrahedron 2002, 58, 10437–10445. [Google Scholar] [CrossRef]
- Shih, M.H.; Yeh, M.Y. Access to the syntheses of sydnonyl-substituted α,β-unsaturated ketones and 1,3-dihydro-indol-2-ones by modified Knoevenagel reaction. Tetrahedron 2003, 59, 4103–4111. [Google Scholar] [CrossRef]
- Shih, M.H. A concise synthetic method for sydnonyl-substituted pyrazoline derivatives. Synthesis 2004, 1, 26–32. [Google Scholar] [CrossRef]
- Shih, M.H.; Ke, F.Y. Syntheses and evaluation of antioxidant activity of sydnonyl substituted thiazolidinone and thiazoline derivatives. Bioorg. Med. Chem. 2004, 12, 4633–4643. [Google Scholar] [CrossRef] [PubMed]
- Shih, M.H.; Yeh, M.Y.; Lee, M.J.; Su, Y.S. Efficient syntheses of 3-(3-arylsydnon-4-yl)triazole derivatives. Synthesis 2004, 17, 2877–2885. [Google Scholar] [CrossRef]
- Shih, M.H.; Wu, C.L. Efficient syntheses of thiadiazoline and thiadiazole derivatives by the cyclization of 3-aryl-4-formylsydnone thiosemicarbazones with acetic anhydride and ferric chloride. Tetrahedron 2005, 61, 10917–10925. [Google Scholar] [CrossRef]
- Shih, M.H.; Tsai, C.H.; Wang, Y.C.; Shieh, M.Y.; Lin, G.L.; Wei, C.Y. Microwave-assisted synthesis of sydnonyl-substituted imidazoles. Tetrahedron 2007, 63, 2990–2999. [Google Scholar] [CrossRef]
- Shih, M.H.; Su, Y.S.; Wu, C.L. Syntheses of aromatic substituted hydrazino-thiazole derivatives to clarify structural characterization and antioxidant activity between 3-arylsydnonyl and aryl substituted hydrazino-thiazoles. Chem. Pharm. Bull. 2007, 58, 1126–1135. [Google Scholar] [CrossRef]
- Tonelli, M.; Simone, M.; Tasso, B.; Novelli, F.; Boido, V.; Sparatore, F.; Paglietti, G.; Pricl, S.; Giliberti, G.; Blois, S.; et al. Antiviral activity of benzimidazole derivatives. II. Antiviral activity of 2-phenylbenzimidazole derivatives. Bioorg. Med. Chem. 2010, 18, 2937–2953. [Google Scholar] [CrossRef] [PubMed]
- Dettmann, S.; Szymanowitz, K.; Wellner, A.; Schiedel, A.; Müller, C.E.; Gust, R. 2-Phenyl-1-[4-(2-piperidine-1-yl-ethoxy)benzyl]-1H-benzimidazoles as ligands for the estrogen receptor: Synthesis and pharmacological evaluation. Bioorg. Med. Chem. 2010, 18, 4905–4916. [Google Scholar] [CrossRef] [PubMed]
- Refaat, H.M. Synthesis and anticancer activity of some novel 2-substituted benzimidazole derivatives. Eur. J. Med. Chem. 2010, 45, 2949–2956. [Google Scholar] [CrossRef] [PubMed]
- Shaharyar, M.; Abdullah, M.M.; Bakht, M.A.; Majeed, J. Pyrazoline bearing benzimidazoles: Search for anticancer agent. Eur. J. Med. Chem. 2010, 45, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Hranjec, M.; Starčević, K.; Pavelić, S.K.; Lučin, P.; Pavelić, K.; Zamola, G.K. Synthesis, spectroscopic characterization and antiproliferative evaluation in vitro of novel Schiff bases related to benzimidazoles. Eur. J. Med. Chem. 2011, 46, 2274–2279. [Google Scholar] [CrossRef] [PubMed]
- Małecki, J.G.; Kruszynski, R.; Mazurak, Z. Synthesis, spectroscopic and structural characterizations of two new complexes of ruthenium with 2-(hydroxymethyl)benzimidazole and 1,10-phenanthroline ligands. Polyhedron 2009, 28, 3891–3898. [Google Scholar] [CrossRef]
- Lumb, I.; Hundal, M.S; Mathur, P.; Corbella, M.; Aliaga-Alcalde, N.; Hundal, G. First report on a dinuclear Cu(II) complex based on pyridine dicarboxamido ligand having benzimidazole moieties in the amide side arms: Synthesis, structure and magnetic properties of [Cu(GBPA)]2·4H2O, GBPA=N,N'-bis (2-methylbenzimidazolyl)-pyridine-1,3-dicarboxamide. Polyhedron 2012, 36, 85–91. [Google Scholar] [CrossRef]
- Abdel Ghani, N.T.; Mansour, A.M. Novel palladium(II) and platinum(II) complexes with 1H-benzimidazol-2-ylmethyl-N-(4-bromo-phenyl)-amine: Structural studies and anticancer activity. Eur. J. Med. Chem. 2012, 47, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.; Kar, P.; Ianelli, S.; Ghosh, A. Isolation of a novel intermediate during unsymmetrical to symmetrical rearrangement of a tetradentate Schiff base ligand in a manganese(III) complex: Catalytic activity of the rearranged product towards alkene epoxidation. Inorg. Chim. Acta 2011, 365, 318–324. [Google Scholar] [CrossRef]
- Shit, S.; Rosair, G.; Mitra, S. A new tetranuclear copper(II) Schiff base complex containing Cu4O4 cubane core: Structural and spectral characterizations. J. Mol. Struct. 2011, 991, 79–83. [Google Scholar] [CrossRef]
- Hong, M.; Yin, H.D.; Chen, S.W.; Wang, D.Q. Synthesis and structural characterization of organotin(IV) compounds derived from the self-assembly of hydrazone Schiff base series and various alkyltin salts. J. Organomet. Chem. 2010, 695, 653–662. [Google Scholar] [CrossRef]
- Mathew, N.; Kurup, M.R.P. Synthesis and characterization of Mo(VI) complexes derived from ONO donor acylhydrazones. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2011, 78, 1424–1428. [Google Scholar] [CrossRef]
- Ahmadi, M.; Mague, J.T.; Akbari, A.; Takjoo, R. Dianion N1,N4-bis(salicylidene)-S-allyl-thiosemicarbazide complexes: Synthesis, structure, spectroscopy and thermal behavior. Polyhedron 2012, 42, 128–134. [Google Scholar] [CrossRef]
- Ahmad, J.U.; Räisänen, M.T.; Nieger, M.; Leskelä, M.; Repo, T. A facile synthesis of mixed ligand Cu(II) complexes with salicylaldehyde and salicylaldimine ligands and their X-ray structural characterization. Inorg. Chim. Acta 2012, 384, 275–280. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 13a–b, 15a–c, 16b, 17a, 17b are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).