Relative Stability of cis- and trans-Hydrindanones
Abstract
:1. Introduction
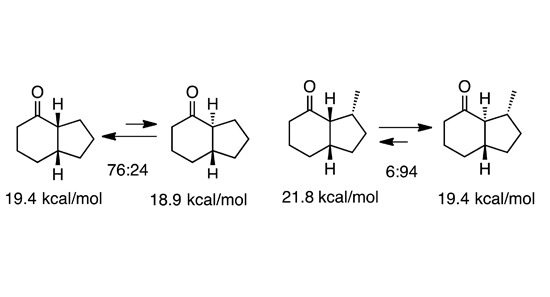
2. Results and Discussion
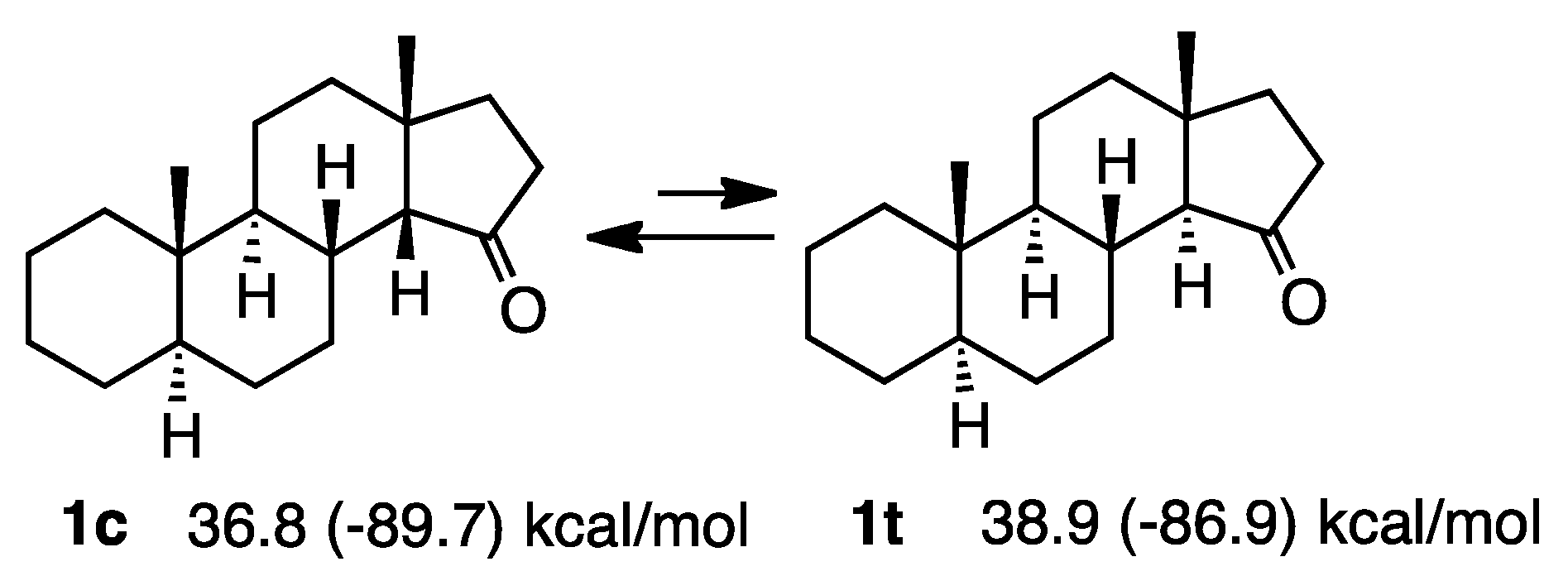
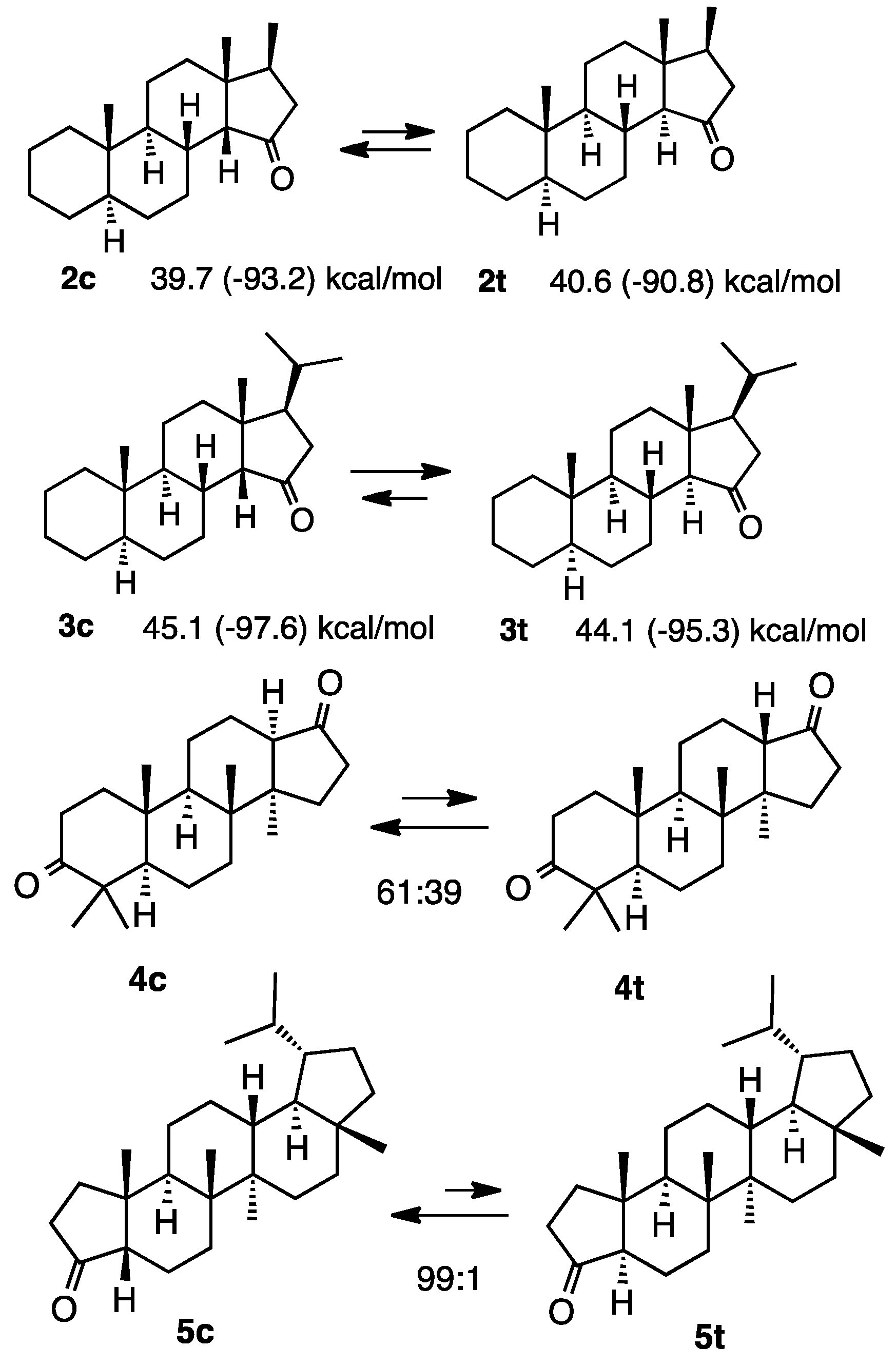
2.1. Steroid Ketones
2.2. Simple 4-Hydrindanones
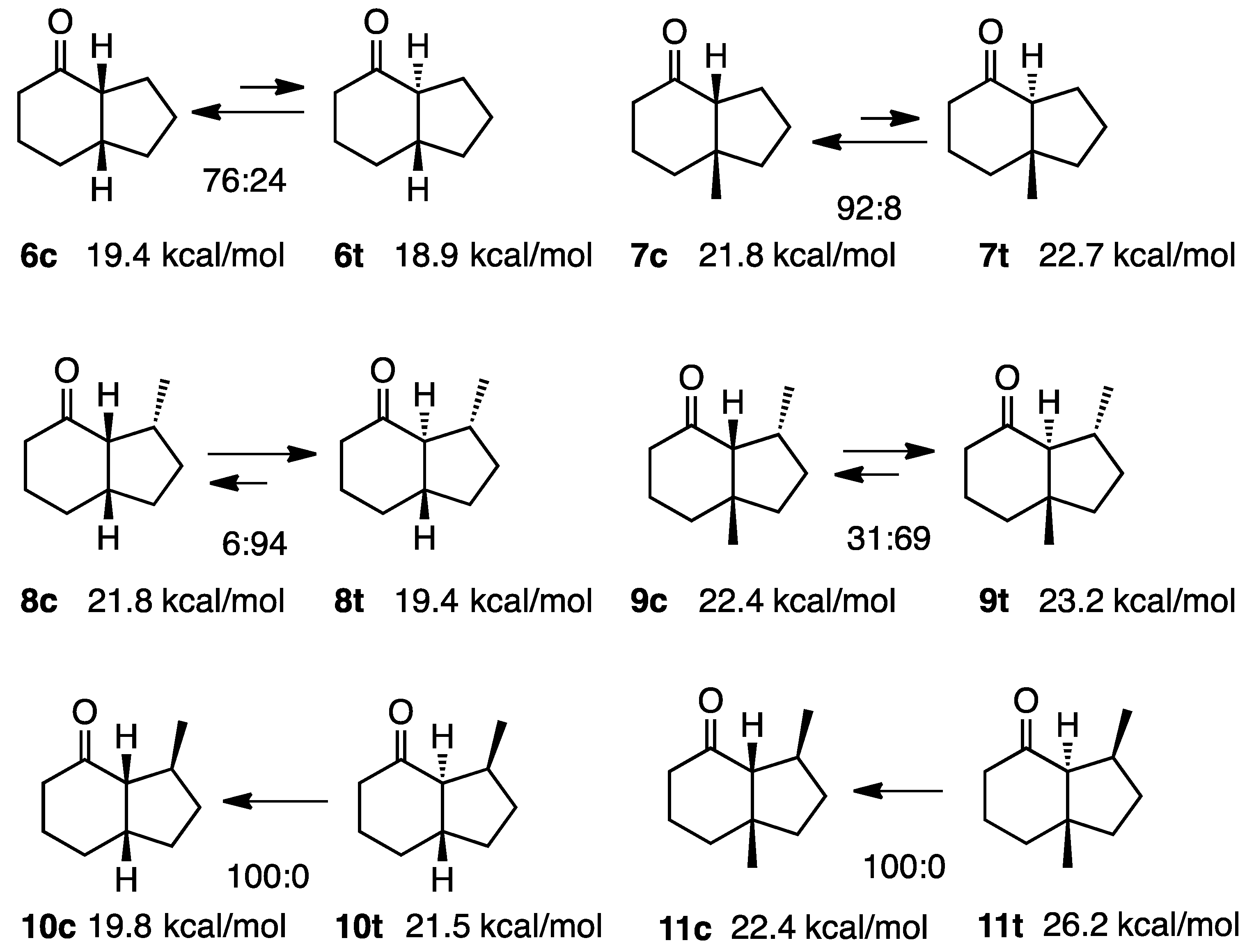
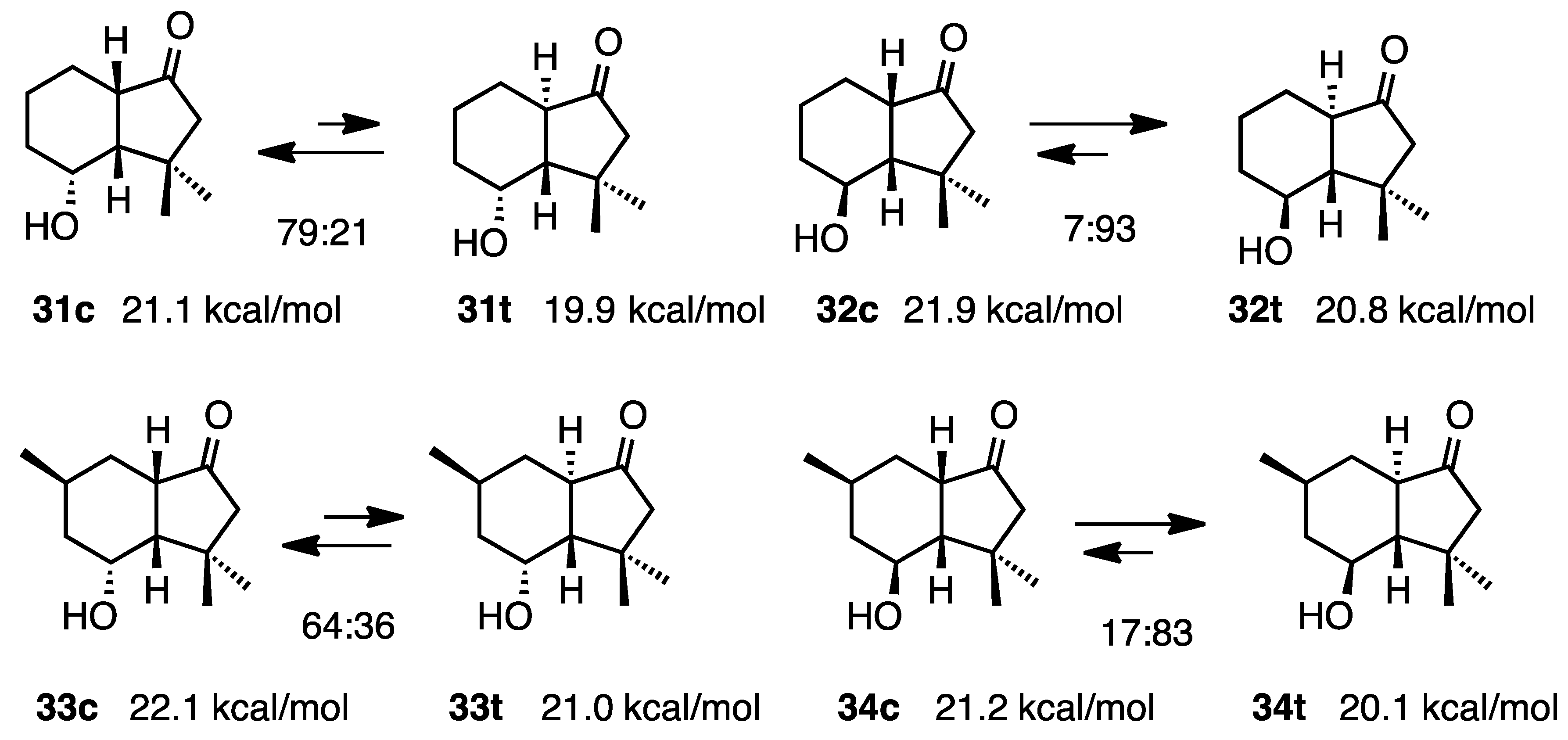
2.3. Dimethyhydrindanones
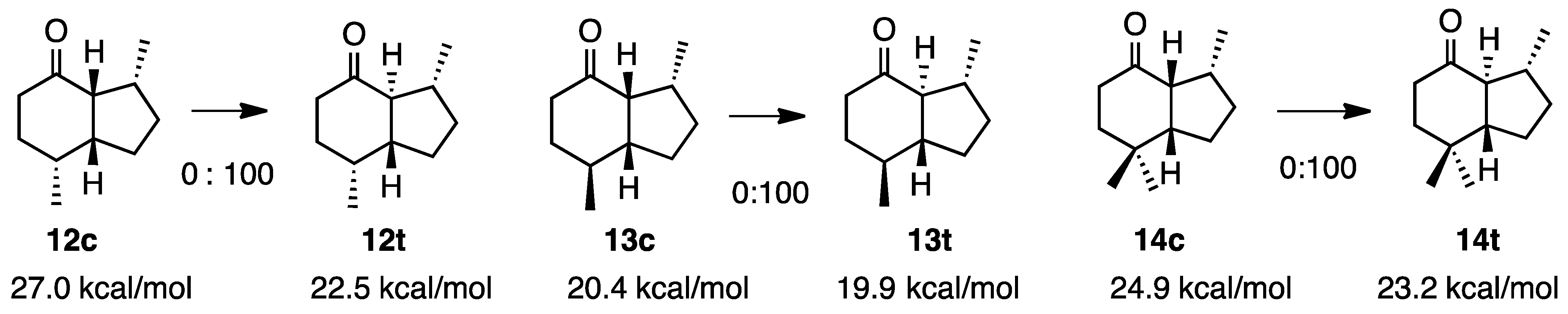
2.4. Isopropylketones
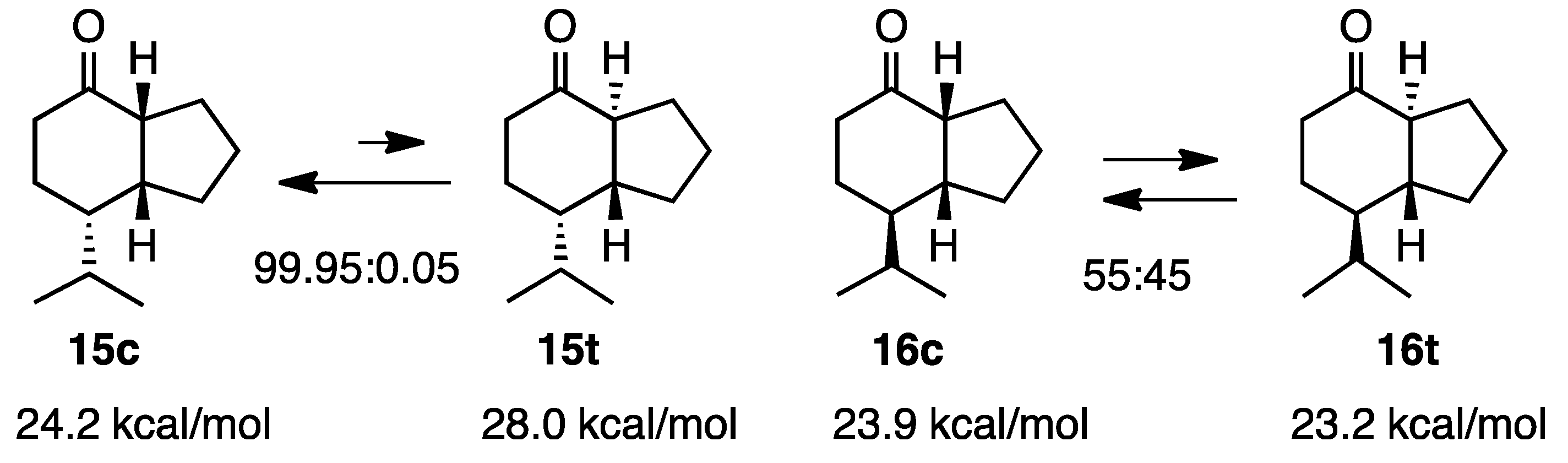
2.5. Simple 1-Hydrindanones

2.6. 4-Hydroxy-1-hydrindanones
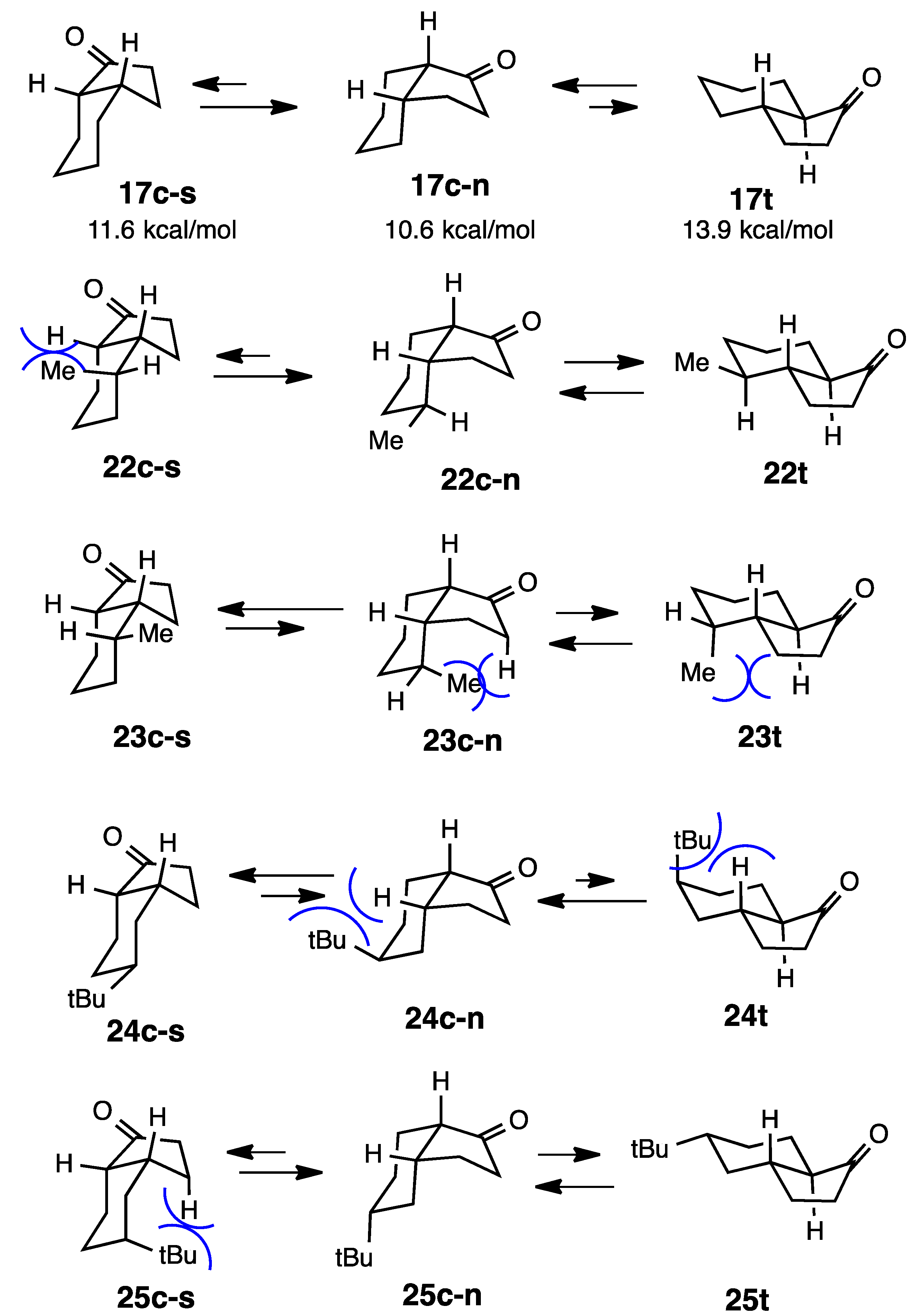
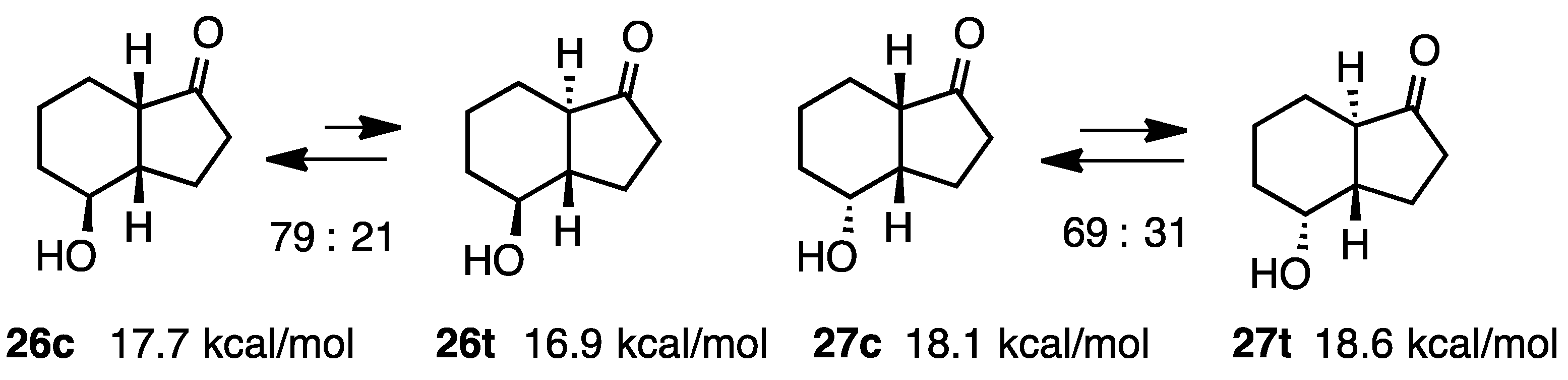
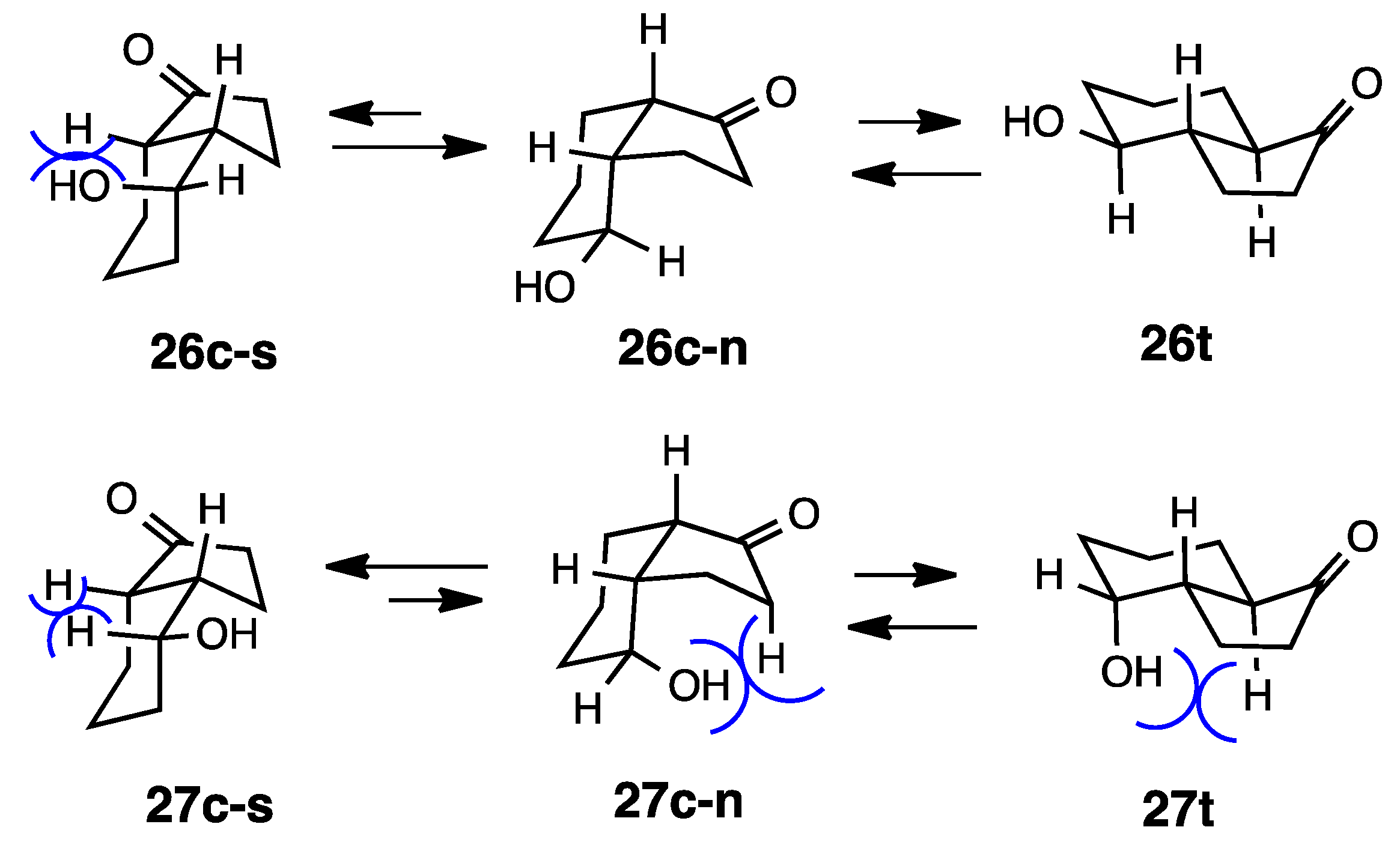
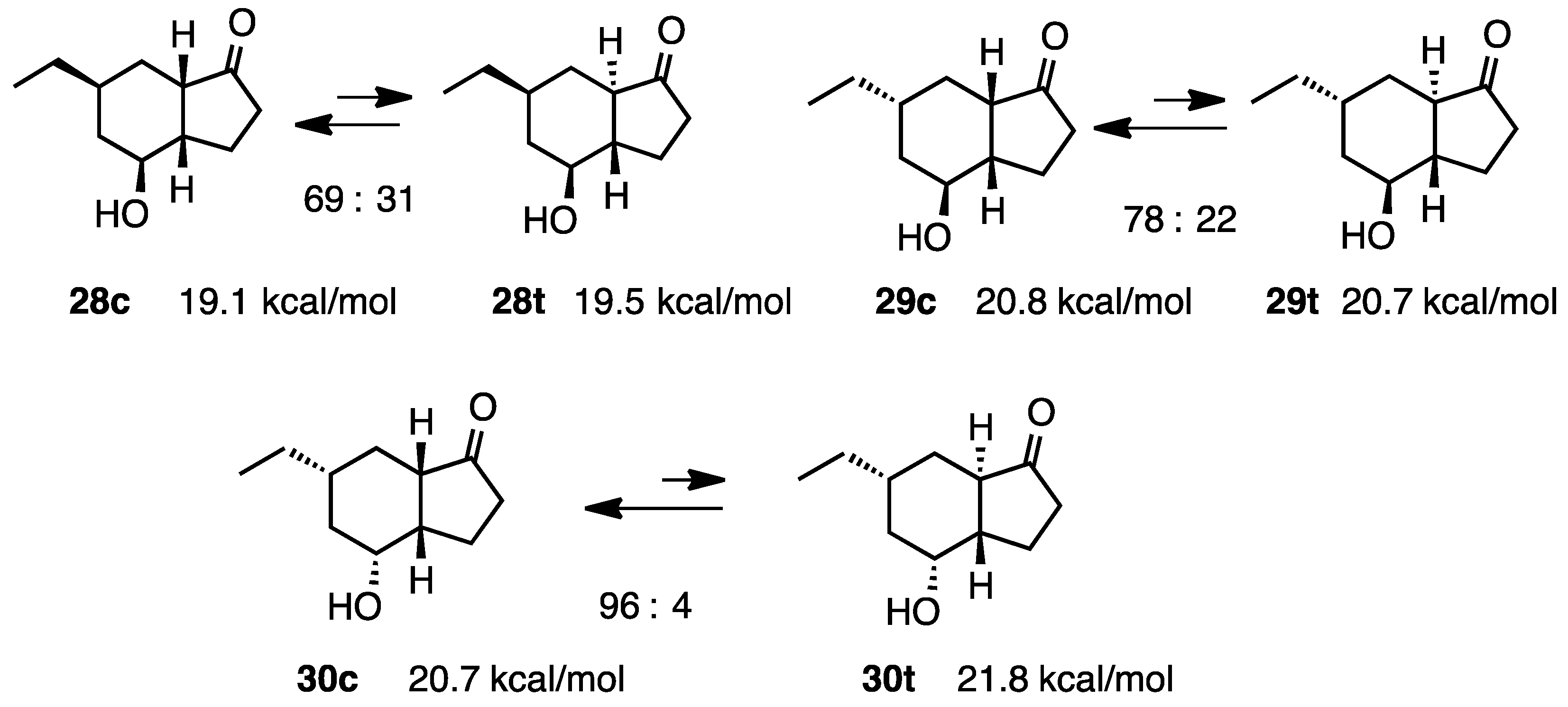
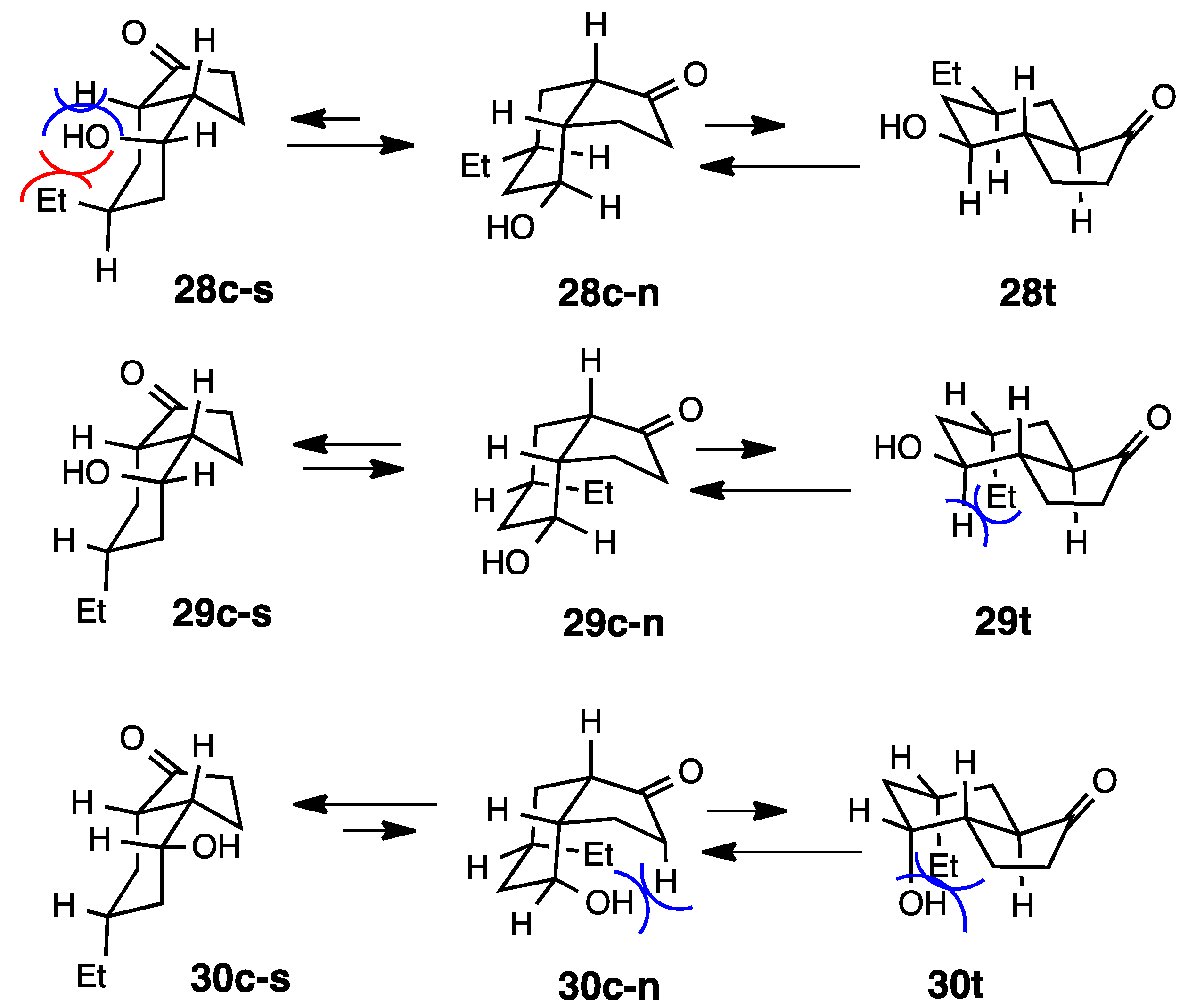
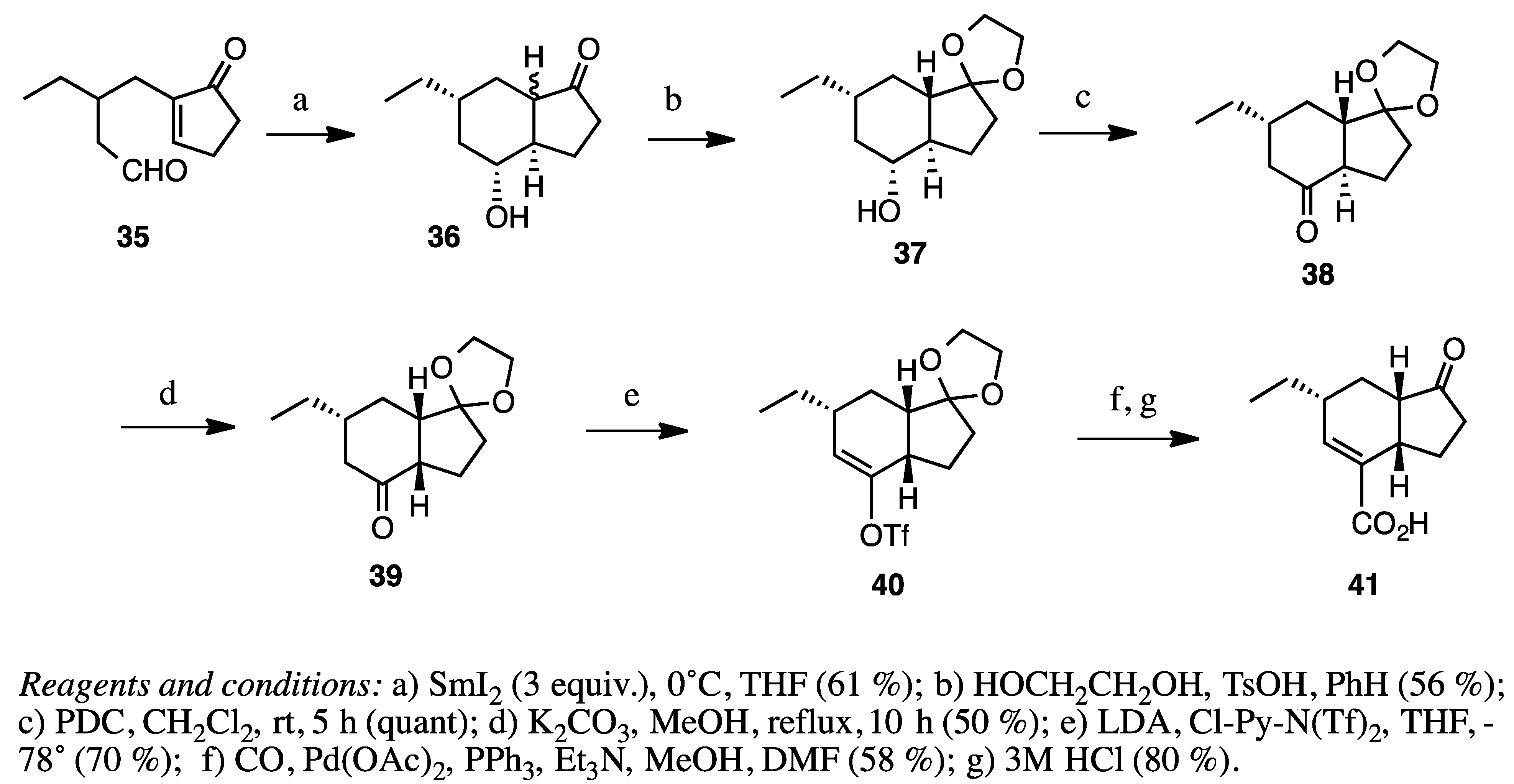
2.7. Fused with a Cyclobutane Ring
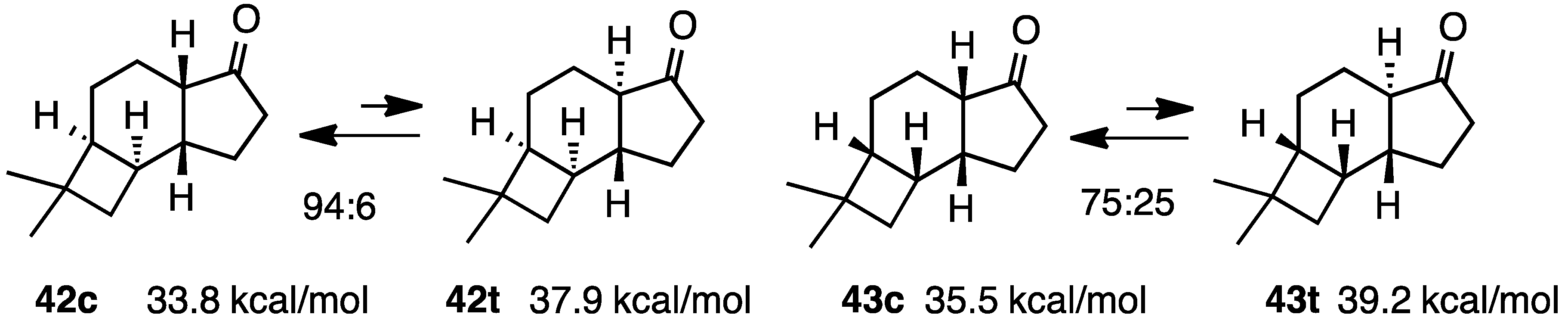
3. Conclusions
Acknowledgments
Conflicts of Interest
References and Notes
- Defaut, B.; Parsons, T.B.; Spencer, N.; Male, L.; Kariuki, B.M.; Grainger, R.S. Synthesis of the trans-hydrindane core of dictyoxetane. Org. Biomol. Chem. 2012, 10, 4926–4932. [Google Scholar] [CrossRef] [PubMed]
- Yue, G.; Huang, X.; Liu, B. Progress in the total syntheses of trans-hydrindane-containing terpenoids. Chin. J. Org. Chem. 2013, 33, 1167–1185. [Google Scholar] [CrossRef]
- Allinger, N.L.; Tribble, M.T. Conformational analysis-LXXX. The hydrindanone ring system. Tetrahedron 1972, 28, 1191–1202. [Google Scholar] [CrossRef]
- Goto, H.; Osawa, E. Corner flapping: A simple and fast algorithm for exhaustive generation of ring conformations. J. Am. Chem. Soc. 1989, 111, 8950–8951. [Google Scholar] [CrossRef]
- Goto, H.; Osawa, E. An efficient algorithm for searching low-energy conformers of cyclic and acyclic molecules. J. Chem. Soc. Perkin Trans. 2 1993, 187–198, MMFF94S and BARISTA Ver. 1.3.2 interface software were used in these experiments. [Google Scholar]
- Peterson, P.E.; Leffew, R.L.B.; Jensen, B.L. Studies of the ketone obtained from the ozonolysis of vitamin D. Molecular mechanics calculations for it and related hydrindanones. J. Org. Chem. 1986, 51, 1948–1954. [Google Scholar] [CrossRef]
- Cicero, B.L.; Weisbuch, F.; Dana, G. Conformational analysis and stability of substituted 4-hydrindanones. A thermodynamic and magnetic resonance (1H and 13C) study. J. Org. Chem. 1981, 46, 914–919. [Google Scholar] [CrossRef]
- Kobayashi, M.; Yasuzawa, T.; Kyogoku, Y.; Kido, M.; Kitagawa, I. Three new ent-valerenane sesquiterpenes from an Okinawan soft coral. Chem. Pharm. Bull. 1982, 30, 3431–3434. [Google Scholar] [CrossRef]
- Tori, M.; Ikawa, M.; Sagawa, T.; Furuta, H.; Sono, M.; Asakawa, Y. Synthesis of isovalerenenol, a sesquiterpene alcohol isolated from a soft coral and their stability of related hydrindanone derivatives. Tetrahedron 1996, 30, 9999–10010. [Google Scholar] [CrossRef]
- Galemmo, R.A., Jr.; Paquette, L.A. Contrasting directed-aldol reactivity of a pair of epimeric trimethyl silyl enol ethers. J. Org. Chem. 1985, 50, 1768–1770. [Google Scholar] [CrossRef]
- Matlin, A.R.; Agosta, W.C. Four isomeric 7-isopropyloctahydro-4H-inden-4-ones. J. Chem. Soc. Perkin Trans. 1 1987, 365–368. [Google Scholar]
- House, H.O.; Rasmusson, G.H. Perhydroindanone derivatives. II. Stability relationships. J. Org. Chem. 1963, 28, 31–34. [Google Scholar] [CrossRef]
- Fringuelli, F.; Pizzo, F.; Taticchi, A.; Halls, T.D.J.; Wenkert, E. Diels-Alder reactions of cycloalkenones. 1. Preparation and structure of the adducts. J. Org. Chem. 1982, 47, 5056–5065. [Google Scholar] [CrossRef]
- Jones, T.K.; Denmark, S.E. Silicon-directed Nazarov reactions III. Stereochemical and mechanistic considerations. Helv. Chim. Acta 1983, 66, 2397–2411. [Google Scholar] [CrossRef]
- Sono, M.; Nakashiba, Y.; Nakashima, K.; Tori, M. Cyclization into hydrindanones using samarium diiodide. J. Org. Chem. 2000, 65, 3099–3106. [Google Scholar] [CrossRef] [PubMed]
- Sono, M.; Hashimoto, A.; Nakashima, K.; Tori, M. Total synthesis of coronafacic acid through 6-endo-trig mode intramolecular cyclization of an enone-aldehyde to a hydrindanone using samarium(II) iodide. Tetrahedron Lett. 2000, 41, 5115–5118. [Google Scholar] [CrossRef]
- Denmark, S.E.; Habermas, K.L.; Hite, G.A. Silicon-directed Nazarov cyclizations. Part V. Substituent and heteroatom effects on the reaction. Helv. Chim. Acta 1988, 71, 168–194. [Google Scholar] [CrossRef]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tori, M. Relative Stability of cis- and trans-Hydrindanones. Molecules 2015, 20, 1509-1518. https://doi.org/10.3390/molecules20011509
Tori M. Relative Stability of cis- and trans-Hydrindanones. Molecules. 2015; 20(1):1509-1518. https://doi.org/10.3390/molecules20011509
Chicago/Turabian StyleTori, Motoo. 2015. "Relative Stability of cis- and trans-Hydrindanones" Molecules 20, no. 1: 1509-1518. https://doi.org/10.3390/molecules20011509
APA StyleTori, M. (2015). Relative Stability of cis- and trans-Hydrindanones. Molecules, 20(1), 1509-1518. https://doi.org/10.3390/molecules20011509