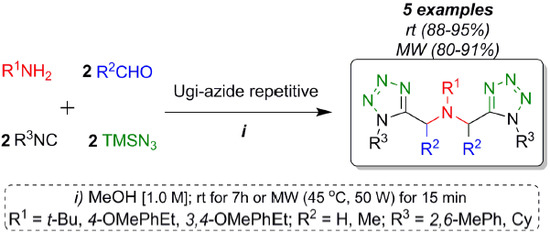
Synthesis of Novel bis-1,5-Disubstituted-1H-Tetrazoles by an Efficient Catalyst-Free Ugi-Azide Repetitive Process
Abstract
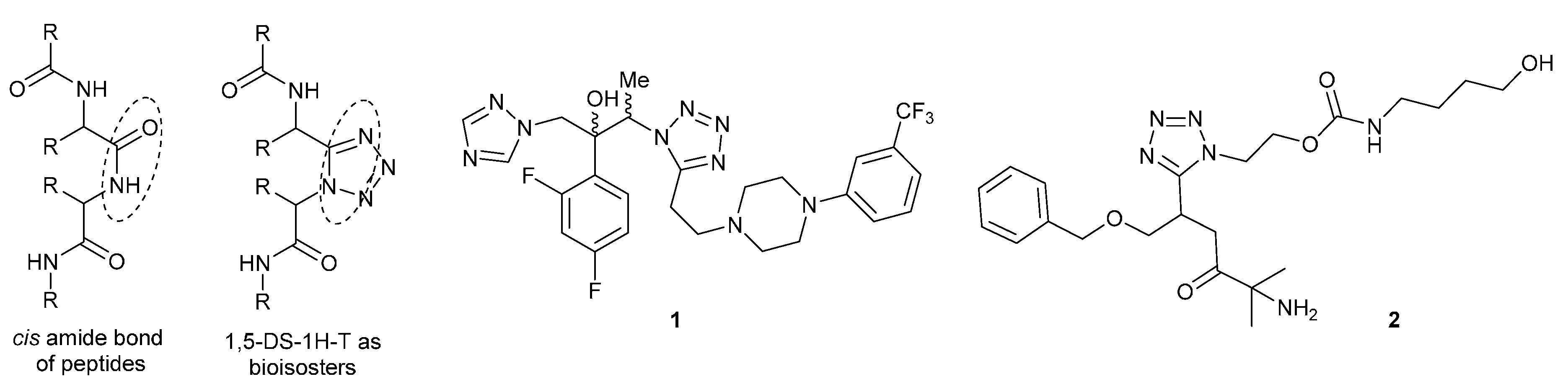
:1. Introduction
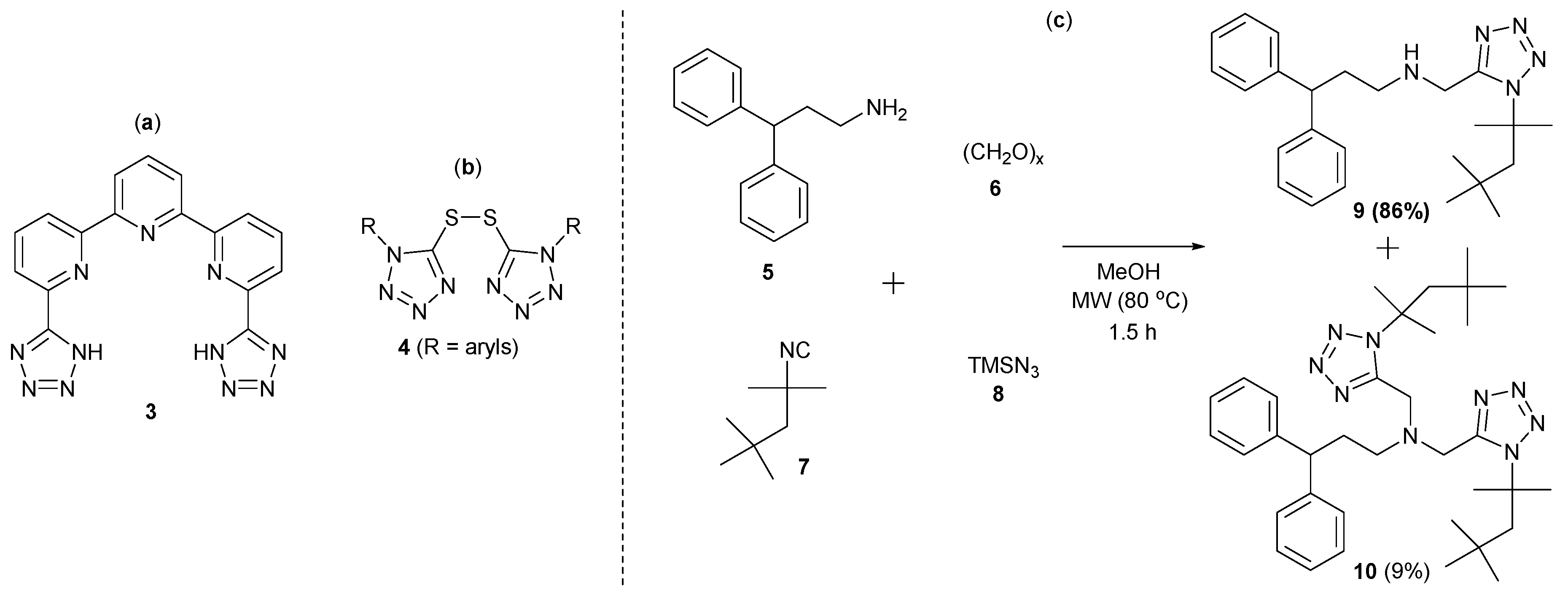
2. Results and Discussion
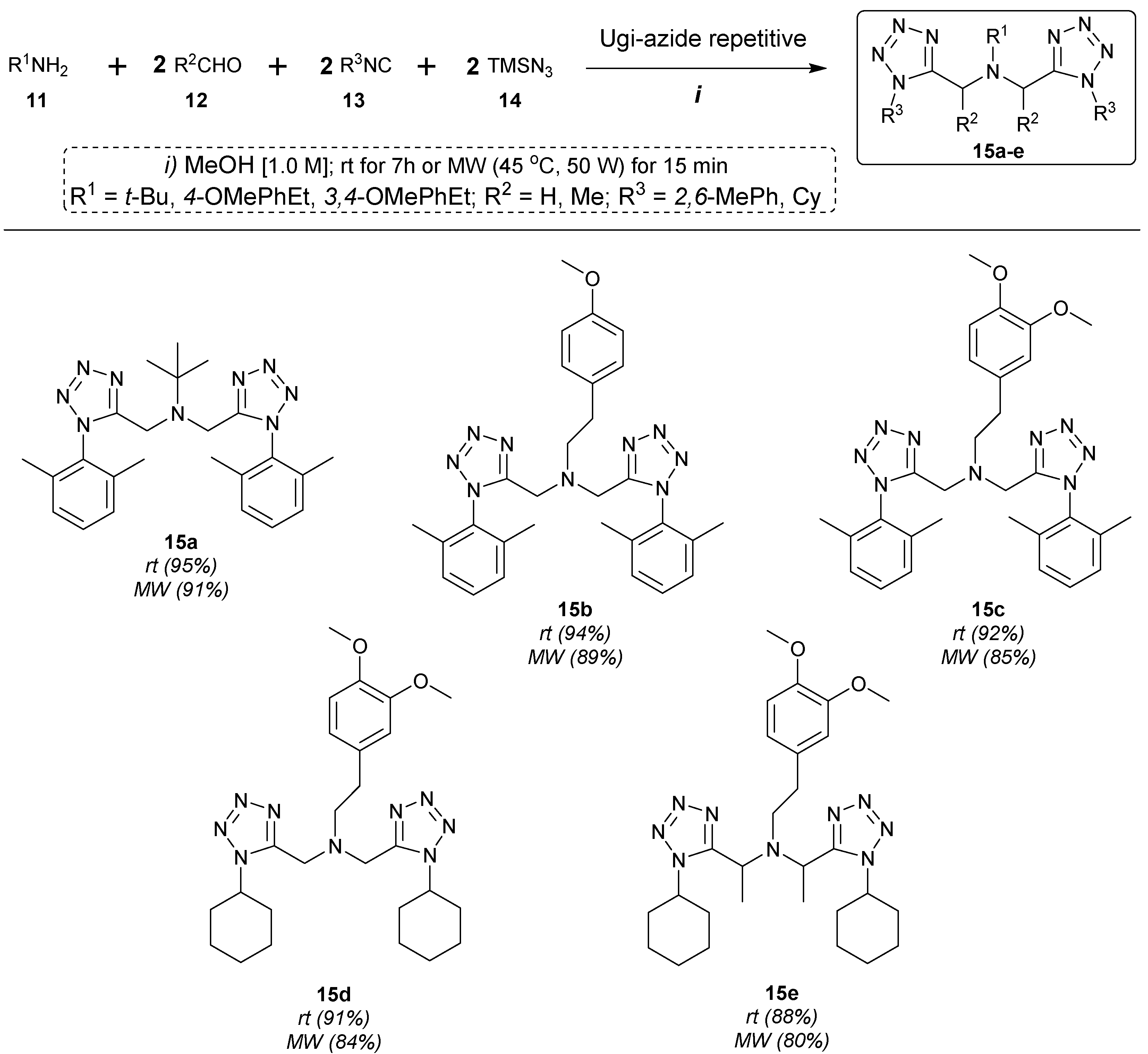
3. Experimental Section
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zabrocki, J.; Smith, G.D.; Dunbar, J.B., Jr.; Iijima, H.; Marshall, G.R. Conformational mimicry. 1. 1,5-Disubstituted tetrazole ring as surrogate for the cis amide bond. J. Am. Chem. Soc. 1988, 110, 5875–5880. [Google Scholar]
- Upadhayaya, R.S.; Jain, S.; Sinha, N.; Kishore, N.; Chandra, R.; Arora, S.K. Synthesis of novel substituted tetrazoles having antifungal activity. Eur. J. Med. Chem. 2004, 39, 579–592. [Google Scholar]
- Davulcu, A.H.; McLeod, D.D.; Li, J.; Katipally, K.; Littke, A.; Doubleday, W.; Xu, Z.; McConlogue, C.W.; Lai, C.J.; Gleeson, M.; et al. Process research and development for a tetrazole-based growth hormone secretagogue (GHS) pharmaceutical development candidate. J. Org. Chem. 2009, 74, 4068–4079. [Google Scholar]
- Sarvary, A.; Maleki, A. A review of synthesis of 1,5-disubstituted tetrazole derivatives. Mol. Divers. 2014. [Google Scholar] [CrossRef]
- Bräse, S.; Gil, C.; Knepper, K.; Zimmermann, V. Organic azides: An exploiting diversity of a unique class of compounds. Angew. Chem. Int. Ed. 2005, 44, 5188–5240. [Google Scholar]
- Himo, F.; Demko, Z.P.; Noodleman, L.; Sharpless, K.B. Mechanism of tetrazole formation by addition of azide to nitriles. J. Am. Chem. Soc. 2002, 124, 12210–12216. [Google Scholar]
- Scriven, E.F.V.; Turnbull, K. Azides: Their preparation and synthetic uses. Chem. Rev. 1988, 88, 297–368. [Google Scholar]
- El Kaim, L.; Grimaud, L. Beyond the Ugi reaction: Less conventional interactions between isocyanides and iminium species. Tetrahedron 2009, 65, 2153–2171. [Google Scholar]
- Giraud, M.; Andreiadis, E.S.; Fisyuk, A.S.; Demadrille, R.; Pécaut, J.; Imbert, D.; Mazzanti, M. Efficient sensitization of lanthanide luminescence by tetrazole-based polydentate ligands. Inorg. Chem. 2008, 47, 3952–3954. [Google Scholar]
- Waisser, K.; Adamec, J.; Kunes, J.; Kaustova, J. Antimycobacterial 1-aryl-5-benzylsulfanyl tetrazoles. Chem. Pap. 2004, 58, 214–219. [Google Scholar]
- Schaffert, E.S.; Höfner, G.; Wanner, K.T. Aminomethyltetrazoles as potential inhibitors of the γ-aminobutyric acid transporters mGAT1–mGAT4: Synthesis and biological evaluation. Bioorg. Med. Chem. 2011, 19, 6492–6504. [Google Scholar]
- Cano, P.A.; Islas-Jácome, A.; González-Marrero, J.; Yépez-Mulia, L.; Calzada, F.; Gámez-Montaño, R. Synthesis of 3-tetrazolylmethyl-4H-chromen-4-ones via Ugi-azide and biological evaluation against Entamoeba histolytica, Giardia lamblia and Trichomonas vaginalis. Bioorg. Med. Chem. 2014, 22, 1370–1376. [Google Scholar]
- Cárdenas-Galindo, L.E.; Islas-Jácome, A.; Alvarez-Rodríguez, N.V.; El Kaim, L.; Gámez-Montaño, R. Synthesis of 2-tetrazolylmethyl-2,3,4,9-tetrahydro-1H-β-carbolines by a one pot Ugi-azide/Pictet-spengler process. Synthesis 2014, 46, 49–56. [Google Scholar]
- Gordillo-Cruz, R.E.; Rentería-Gómez, A.; Islas-Jácome, A.; Cortes-García, C.J.; Díaz-Cervantes, E.; Robles, J.; Gámez-Montaño, R. Synthesis of 3-tetrazolylmethyl-azepino[4,5-b]indol-4-ones in two reaction steps: (Ugi-azide/N-acylation/SN2)/free radical cyclization and docking studies to a 5-Ht6 model. Org. Biomol. Chem. 2013, 11, 6470–6476. [Google Scholar]
- Sample Availability: Samples of the compounds 15a–e are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cárdenas-Galindo, L.E.; Islas-Jácome, A.; Colmenero-Martínez, K.M.; Martínez-Richa, A.; Gámez-Montaño, R. Synthesis of Novel bis-1,5-Disubstituted-1H-Tetrazoles by an Efficient Catalyst-Free Ugi-Azide Repetitive Process. Molecules 2015, 20, 1519-1526. https://doi.org/10.3390/molecules20011519
Cárdenas-Galindo LE, Islas-Jácome A, Colmenero-Martínez KM, Martínez-Richa A, Gámez-Montaño R. Synthesis of Novel bis-1,5-Disubstituted-1H-Tetrazoles by an Efficient Catalyst-Free Ugi-Azide Repetitive Process. Molecules. 2015; 20(1):1519-1526. https://doi.org/10.3390/molecules20011519
Chicago/Turabian StyleCárdenas-Galindo, Luís E., Alejandro Islas-Jácome, Karla M. Colmenero-Martínez, Antonio Martínez-Richa, and Rocío Gámez-Montaño. 2015. "Synthesis of Novel bis-1,5-Disubstituted-1H-Tetrazoles by an Efficient Catalyst-Free Ugi-Azide Repetitive Process" Molecules 20, no. 1: 1519-1526. https://doi.org/10.3390/molecules20011519
APA StyleCárdenas-Galindo, L. E., Islas-Jácome, A., Colmenero-Martínez, K. M., Martínez-Richa, A., & Gámez-Montaño, R. (2015). Synthesis of Novel bis-1,5-Disubstituted-1H-Tetrazoles by an Efficient Catalyst-Free Ugi-Azide Repetitive Process. Molecules, 20(1), 1519-1526. https://doi.org/10.3390/molecules20011519