Abstract
(±)cis-2-(4-Methoxyphenyl)-3-hydroxy/methoxy-2,3-dihydro-1,5-benzothiazepin-4[5H/5-chloroacetyl/5-(4'-methylpiperazino-1')acetyl]-ones have been synthesized by the condensation of 2-aminobenzene thiols with methyl(±)trans-3-(4-methoxyphenyl)glycidate in xylene. The synthesized compounds have been characterized by elemental analyses and spectral data and screened for their antimicrobial activity.
Introduction
The importance of the 1,5-benzothiazepine nucleus has been well proved as illustrated by a large number of patents, as chemotherapeutic agents, available on it. A number of biological activities have been associated with it, such as antihypertensive [1,2], antiasthemic [2,3], analgesic [4], cardiovascular [5], platelate aggregation inhibitor and Ca antagonist [2]. Recently Ahmed et al [6,7] patented 1,5-benzothiazepine derivatives as potential anticancer drugs.
With this in mind, some new 1,5-benzothiazepine derivatives have been synthesized in search of better therapeutic agents, by a convenient single pot method.
Results and Discussion
Synthesis
(±)cis-2-(4-Methoxyphenyl)-3-hydroxy-2,3-dihydro-1,5-benzothiazepin-4(5H)-ones (3) have been synthesized by the condensation of 2-aminobenzenethiols (1) with methyl (±)trans-3-(4-methoxyphenyl)glycidate (2) in xylene at 160°C for 16-20 hrs under a nitrogen atmosphere. Compounds 3 on methylation with dimethyl sulphate and alkali yielded (±)cis-2-(4-methoxyphenyl)-3-methoxy-2,3-dihydro-1,5-benzothiazepin-4(5H)-ones (4). Compounds 4 on treatment with chloroacetylchloride gave (±)cis-2-(4-methoxyphenyl)-3-methoxy-2,3-dihydro-1,5-benzothiazepin-4-(5-chloroacetyl)-ones (5) which in turn afforded (±)-cis-2,3-dihydro-1,5-benzothiazepin-4-[5-(4'-methylpiperazino-1')acetyl]-ones (6), on treatment with N-methylpiperazine. (Scheme 1).
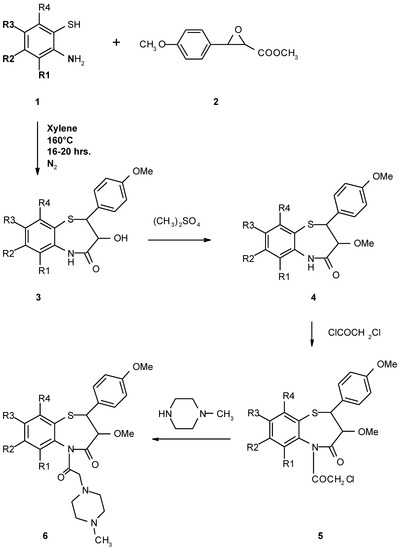
Scheme 1.
The physical data of compounds 1 to 6 is given in Table-1.

Table 1.
Characterization data of 1,5-benzothiazepine derivatives.
In the IR spectra of compounds 3, a broad band in the range 3600-3110 cm-1 was obtained due to the merged N-H and O-H stretching vibrations. Compounds 4 showed a less broad band, in the region 3180-3140 cm-1 due to the presence of only the N-H group. This band was absent in compounds 5 and 6.
The N-H bending vibrations were observed as a sharp medium to strong band at 1540-1500 cm-1 in compounds 3 and 4. The C-S-C linkage of the seven membered ring caused a weak and sharp absorption band at 800-760 cm-1 in all the compounds. The C=O group was observed as a strong and sharp band at 1660-1600 cm-1 in these compounds. The C-O-C linkage showed two sharp and strong bands at 1360-1340 cm-1 and 1070-1020 cm-1 due to asymmetric and symmetric vibrations respectively. The C-H (aliphatic and aromatic), C=C, C-Cl, stretching vibrations were observed at their usual positions. (Table 2).

Table 2.
The IR spectral data of 1,5-benzothiazepine derivatives.
In the 1H NMR spectra of compounds 3 the -OH proton was found to be at δ 8.17-10.00 ppm. The proton of the - NH group was observed at δ 8.10-8.25 ppm, as a broad singlet. Two characteristic doublets at δ 2.35-2.92 ppm (J=8 Hz) and δ 3.05-3.44 ppm (J=8 Hz) were assigned to cis-protons at C-2 and C-3. All the compounds showed a complex multiplet of aromatic protons in the range δ 6.00-7.90 ppm. Protons of the methoxy group were observed at δ 3.32-3.54 ppm. The piperazine protons were observed at d 4.10-4.60 ppm in compounds 6. The methyl protons, whenever present, were observed at their usual position (δ 1.65-2.30 ppm) as a singlet (Table-3).

Table 3.
The 1H NMR spectral data of 1,5-benzothiazepine derivatives in δ ppm.
Antimicrobial activity
All the synthesized compounds were screened for their in vivo antimicrobial activity against the bacteria S. aureus, E. coli and fungi Aspergillus niger, Aspergillus flavus, Curvularia lunata and Fusarium moniliformae at a concentration of 100 μg/disc in agar media. A single disc method of Bauer et al [8] was employed using Streptomycin and Mycostatin as the reference compounds in antibacterial and antifungal activity respectively. The data are given in Table-4.

Table 4.
Antimicrobial activity of 1,5-benzothiazepine derivatives: Zone of inhibition (mm) (activity index)*.
Experimental
General
All the melting points were determined in open capillary tubes and are uncorrected. The IR spectra were recorded on a Perkin Elmer-833 grating infrared spectrophotometer in KBr pallets.
The 1H NMR spectra were scanned on a FX 90Q Jeol spectrometer (90 MHz) in CDCl3/DMSO-d6 using TMS as the internal standard. The puritiy of the compounds synthesized was checked by TLC using silica gel-G as adsorbent.
(±)-cis-2-(4-Methoxyphenyl)-3-hydroxy-8-phenoxy/-7-chloro-8-methyl/-9-chloro-6-methoxy-2,3-dihydro-1,5-benzothiazepin-4(5H)-one (3)
Methyl (±)-trans-3-(4-methoxyphenyl)glycidate (2) (0.01 mol) was added slowly to a stirred mixture of substituted-2-aminobenzenethiol (1) (0.01 mol) in xylene at 160°C under a nitrogen atmosphere. The stirring was continued for a further 16-20 hrs, maintaining the temperature and the nitrogen atmosphere. The reaction mixture was cooled to room temperature and ethanol was added. The product, thus separated from the mother liquor was collected by filtration, washed with ethanol and recrystallized from DMF.
(±)-cis-2-(4-Methoxyphenyl)-3-methoxy-8-phenoxy/-7-chloro-8-methyl/-9-chloro-6-methoxy-2,3-dihydro-1,5-benzothiazepin-4(5H)-one (4)
(±)-cis-2-(4-Methoxyphenyl)-3-hydroxy-8-phenoxy/-7-chloro-8-methyl/-9-chloro-6-methoxy-2,3-dihydro-1,5-benzothiazepin-4(5H)-one (3) (0.005 mol), sodium dithionite (0.25 g) and 10% ethanolic potassium hydroxide solution (55 ml) were mixed with dimethyl sulphate (0.014 mol). The reaction mixture was refluxed for 5 hrs and filtered. The filtrate was poured into ice cold water. The precipitate obtained was filtered, dried and recrystallized from benzene.
(±)-cis-2-(4-Methoxyphenyl)-3-methoxy-8-phenoxy/-7-chloro-8-methyl/-9-chloro-6-methoxy-2,3-dihydro-1,5-benzothiazepin-4(5-chloroacetyl)-one (5)
A solution of (±)-cis-2-(4-methoxyphenyl)-3-methoxy-8-phenoxy/-7-chloro-8-methyl/-9-chloro-6-methoxy-2,3-dihydro-1,5-benzothiazepin-4(5H)-one (4) (0.005 mol) in dry benzene (100 ml) was added to a solution of chloroacetyl chloride (0.008 mol) in dry benzene (2.5 ml) with stirring. The reaction mixture was refluxed on a water bath for 4 hrs, cooled. Benzene was chilled and then triturated with petroleum ether (60-80°). The product thus obtained was recrystallized from ethanol.
(±)-cis-2-(4-Methoxyphenyl)-3-methoxy-8-phenoxy/-7-chloro-8-methyl/-9-chloro-6-methoxy-2,3-dihydro-1,5-benzothiazepin-4[5-(4'-methylpiperazino-1')acetyl]-one (6)
A solution of 5 (0.005 mol) in dry benzene (7.5 ml) was treated with N-methylpiperazine (0.013 mol). The resulting solution was subsequently refluxed on a water bath for 5 hrs and cooled. The hydrochloride salt of the unreacted amine was removed by filtration. The benzene layer was washed well with water to remove traces of the unreacted amine. It was then dried over anhydrous sodium sulphate and filtered. Benzene was removed from the filtrate under reduced pressure and the residual solid was recrystallize from ethanol.
Acknowledgmentt
Authors wish to thank Head, Department of Chemistry for the facilities.
References
- Salim, H. A.; Abdul, R.; Mohamed, S.; Dahab, G. M. J. Appl. Toxicol. 1993, 13(2), 85, Chem. abstr. 1993, 118, 204993.
- Lochead, A.; Muller, J. C.; Hoornaert, C.; Denys, C. Eur. Pat. Appl. EP320,362; (ClA61 K31/55), 14 Jun 1989. FR Appl 87/17, 044, 08 Dec 1987. Chem Abstr. 1990, 112, 172330.
- Ben, H.; Ruben, S.; Melissa, S.; Kenneth, M. Life Sci. 1992, 51(26), 2049, Chem. Abstr. 1993, 118, 52185.
- Miranda, H. F.; Pelissier, T.; Sierralta, F. Gen. Pharmacol. 1993, 24(1), 201, Chem. Abstr. 1993, 118, 22558. [PubMed]
- Hiroki, Y.; Koichi, F.; Yasuo, S.; Takuro, K.; Hiroyuki, K.; Hiroshi, N. Eur. Pat. Appl. EP 353, 032; (ClCO7D281/10), 31 Jan 1990. JP Appl. 88/185, 097, 25 Jul 1988. Chem. Abstr. 1991, 114, 122434.
- Ahmed, N. K. Can. Pat. Appl. CA 2,030, 159; (Cl.A61k31/55), 23 May 1991. US Appl. 441, 083, 22 Nov. 1989.
- Ahmed, N. K. Eur. Pat. Appl. EP430, 036; (ClA61k31/55), 05 Jun 1991. US Appl. 440, 121, 22 Nov 1989. Chem. Abstr. 1992, 116, 717.
- Bauer, A. W.; Kibby, W. M. M.; Sherris, J. C.; Turk, M. Am. J. Clin. Path. 1966, 45(4), 493. [PubMed]
- Samples Availability: Available from the authors.
© 1997 MDPI. All rights reserved