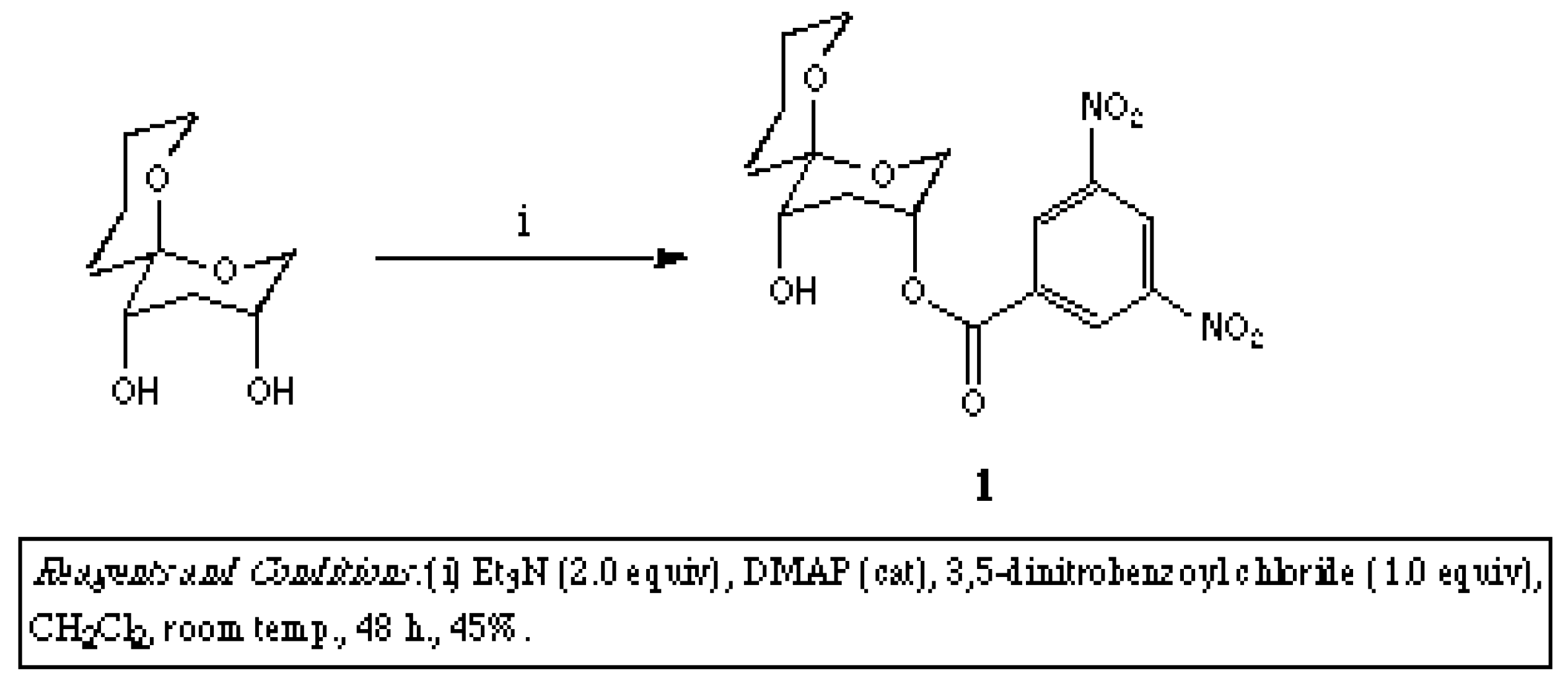
To a solution of [3R*,5S8,6S*]-1,7-dioxaspiro[5.5]undecane-3,5-diol (50 mg, 0.27 mmol) in dichloromethane (5.0 ml), was added triethylamine (56 mg, 0.55 mmol), 4-dimethylaminopyridine (~1 mg), and 3,5-dinitrobenzoyl chloride (64 mg, 0.28 mmol) and the resultant solution allowed to stand at room temperature for 48 h. Removal of the solvent at reduced pressure gave a pale yellow oil, that was purified by flash chromatography using hexane-ethyl acetate (6:4) as eluent to afford the title compound (1) (46 mg, 45%) as pale yellow plates.
M.p. 54-56 deg.C.
IR (Nujol) cm-1 3680-3100 (br, s, OH), 1723 (s, C=O), 1110, 1083 (s, C-O).
High Res. MS calc. for C16H18N2O9 M+ 382.1012, found: M+, 382.0988.
1H-NMR (400 MHz, CDCl3) 1.35 (1H, ddd, J11ax,11eq 13.4, J11ax,10ax 13.4 and J11ax,10eq 4.8 Hz, 11ax-H), 1.41-1.72 (4H, m, 9-CH2 and 10-CH2), 2.01 (1H, dt, J11eq,11ax 13.4 and J11eq,10 2.8 Hz, 11eq-H), 2.09 (1H, dddd, J4eq,4ax 15.3, J4eq,5 2.5, J4eq,3 2.5 and J4eq,2eq 2.5 Hz, 4eq-H), 2.12 (1H, m, OH), 2.35 (1H, ddd, J4ax,4eq 15.3, J4ax,5 3.6 and J4ax,3 3.6 Hz, 4ax-H), 3.44 (1H, m, 5-H), 3.56-3.67 (2H, m, 8-CH2), 3.85 (1H, ddd, J2ax,2eq 13.3, J2ax,3 2.5 and J2ax,4ax 2.5 Hz, 2eq-H), 3.95 (1H, dd, J2eq,2ax 13.3 and J2eq,3 1.9 Hz, 2eq-H), 5.14 (1H, m, 3-H), 9.07-9.14 (3H, m, Ar-H).
13C-NMR (100 MHz, CDCl3) 18.0, 24.8, 29.5, 30.8 (CH2, C-4, C-9, C-10 and C-11), 61.4, 61.5 (CH2, C-2 and C-8), 68.9 (CH, C-3), 70.6 (CH, C-5), 96.5 (quat, C-6), 122.4 (CH, C-4'), 129.4 (CH, C-2' and C-6'), 133.9 (quat , C-1'), 148.6 148.7 (quat, C-3' and C-5'), 162.0 (quat, C=O).
CI-MS 382 (M+, 2%), 295 (40), 213 (30), 195 (80), 179 (20), 149 (60), 114 (38), 101 (100), 83 (33), 75 (65), 69 (90), 59 (20), 55 (80), 41 (59).
Supplementary materials
Supplementary File 1Supplementary File 2Acknowledgment
The authors gratefully acknowledge financial support from the Australian Research Council and The University of Sydney.
- Sample Availability: No sample available.
© 1997 MDPI. All rights reserved.
