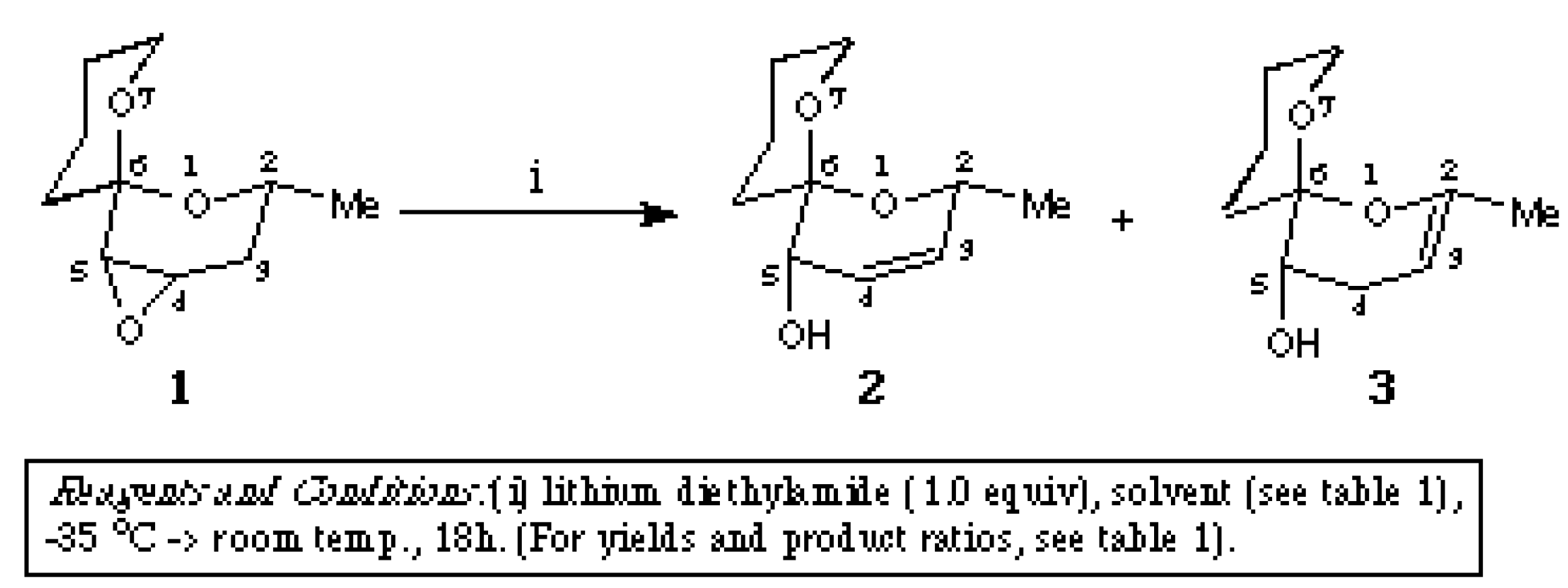
The reaction and the product ratio shown in the scheme was reported [1]. The two products (2 [1] and 3) were separated by flash chromatography using hexane-ethyl acetate (6:4) as eluent to afford the title compound [2R*,5S*,6S*]-2-Methyl-1,7-dioxaspiro[5.5]undec-2-en-5-ol (3 in the scheme) as a colourless oil (20 mg, 16%).
High Res. MS calc. for C10H16O3 M+ 184.10994, found: M+ 184.10981.
IR (film) cm-1 3680-3100 (br, s, OH), 2942, 2850 (s, C-H), 1689 (m, C=C), 1091 (s, C-O).
1H-NMR (400 MHz, CDCl3) 1.48 (1H, ddd, J11ax,11eq 13.4, J11ax,10ax 13.4 and J11ax,10eq4.5 Hz, 11ax-H), 1.57-1.70 (4H, m, 9-CH2 and 10-CH2), 1.80 (3H, s, Me), 1.85-1.94 (1H, m, 11eq-H), 2.05-2.09 (1H, m, 4eq-H), 2.39-2.45 (1H, m, 4ax-H), 3.57 (1H, t, J5,4 3.5 Hz, 5-H), 3.69 (1H, dt, J8eq,8ax 11.4 and J8eq,9 2.1 Hz, 8eq-H), 3.83 (1H, ddd, J8ax,8eq 11.4 , J8ax,9ax 11.4 and J8ax,9eq 3.1 Hz, 8ax-H), 4.47-4.49 (1H, m, 3-H).
13C-NMR (100 MHz, CDCl3) 18.0, 25.1, 26.4, 29.6 (CH2, C-4, C-9, C-10 and C-11), 19.6 (CH3, Me), 61.8 (CH2, C-8), 68.2 (CH, C-5), 93.3 (CH, C-3), 97.1 (quat, C-6), 147.1 (quat, C-2).
EI-MS 184 (M+, 5%), 167 (M+ -OH, 100), 125 (35), 111 (30), 101 (15), 98 (20), 55 ( 25), 43 (70).
Supplementary materials
Supplementary File 1Supplementary File 2Acknowledgment
The authors gratefully acknowledge financial support from the Australian Research Council and The University of Sydney.
References
- Brimble, M. A.; Johnston, A. D. Molecules 1997, 2, M15.
- Sample Availability: No sample available.
© 1997 MDPI. All rights reserved